Introduction
Breast masses are one of the most common diseases in
women. According to the 2020 GLOBOCAN cancer estimates, female
breast cancer is the fifth leading cause of cancer mortality
worldwide with 685,000 deaths; it also ranks first for incidence
among women in the vast majority of countries (1). Therefore, an increasing number of
surgical procedures are performed on patients with breast masses
that may be breast cancer. With the continuous development of
minimally invasive breast technology and focus on the aesthetic
needs of patients, minimally invasive resection using the
ultrasound (US)-guided Mammotome system has been widely used for
the accurate biopsy of suspicious lesions or removal of benign
breast masses (2,3), which is particularly efficient for
multifocal, tiny and impalpable breast lesions. It has the
advantages of being minimally invasive, resulting in tiny scars,
having a high accuracy and good performance, and being safe while
providing real-time visualization (4,5).
Despite these advantages, it is nonetheless an invasive procedure
and varying degrees of breast tissue injury can occur. Therefore,
the precise preoperative assessment of breast masses is imperative
when performing Mammotome minimally invasive resection, as it helps
to avoid unnecessary biopsy or surgery.
The Breast Imaging Reporting and Data System
(BI-RADS) (6) is a tool developed
by the American College of Radiology (ACR). It has unified and
standardized the risk stratification of breast masses in terms of
breast lesion characteristics observed in different types of
imaging, including mammography, US and magnetic resonance imaging.
This standardized system is used for the identification of
pathology based on various medical imaging methods, which
facilitates reproducibility and consistency and decreases the
subjectivity of breast mass diagnosis. It provides a means of
communication between radiologists and surgeons via a lexicon of
feature descriptors, structured reports based on assessment
categories and management recommendations, and a framework for
collecting and auditing data (7).
Although the US BI-RADS classification unifies the
descriptive terms and risk stratification of the lesions, the
terminology used to describe breast US findings is evolving, and
the diversity of this terminology may cause confusion. Therefore,
its practical application efficacy varies and an abundance of
large-sample data is required to verify and improve US BI-RADS,
adapt to changes in the practice of breast imaging and improve its
practical use for interpreting physicians. Although the US BI-RADS
classification has been used for the diagnosis of breast masses for
several decades, few studies have combined minimally invasive
breast surgery with US BI-RADS classification. In the present
study, ultrasonography was used to guide the Mammotome minimally
invasive system and to assess and classify the breast lesions.
Minimally invasive breast surgery performed using the US-guided
Mammotome system was combined with US BI-RADS in a large sample of
patients with breast masses. The preoperative US BI-RADS
classification and postoperative pathology results were
retrospectively analyzed to explore the effectiveness of applying
this classification in the preoperative evaluation of breast
masses.
Materials and methods
Patients and breast masses
The study was approved by the Institutional Review
Committee of Tongji Hospital of Huazhong University of Science and
Technology (Wuhan, China). All patients gave their consent for the
presentation of their data in this publication. They had
preoperative conversations with the surgeons and provided written
informed consent to participate in the study. A total of 1,028
patients with 1,341 breast masses who underwent minimally invasive
resection with a US-guided Mammotome device from September 2018 to
February 2019 were selected. All patients were female, ranging from
16 to 75 years in age, with a median age of 40 years. The maximum
diameter of the breast masses ranged from 0.3 to 3.2 cm, with a
median of 1.5 cm. The exclusion criteria were as follows: i) The
mass was close to the nipple, and breast-feeding or avoidance of
breast duct damage was necessary; ii) the mass was close to the
marginal area of the breast, with minimal normal breast tissue
around the mass; iii) the mass was too close to the skin and the
risk of cutting off the skin was too high; iv) patients with
bleeding tendency, blood coagulation disorder and associated
disorders; v) patients with serious systemic diseases who were
unable to tolerate surgery; and vi) masses with previous
intervention affecting the judgment of US images.
Instruments and methods. Breast
ultrasonography and body marking
All breast ultrasonography was performed with a
Mindray DC-8 color Doppler US diagnostic system (Mindray Medical
International Ltd.), equipped with L12-3E linear-array transducers.
Generally, the patients assumed a supine position and completely
exposed the mass-containing breast. However, if necessary, the
patients were placed in a semi-lateral position that was suitable
for minimally invasive resection using the Mammotome device.
Conventional US images of the breast lesions were acquired and
saved, including B-mode and color Doppler flow mode images. The
lesion position and B-mode US characteristics, including size,
shape, echo pattern, margin, growth orientation, posterior
features, calcifications, presence of architectural distortion and
duct changes were recorded. Vascularity was classified into three
patterns, namely absent, internal vascularity and vessels in the
rim, by color Doppler flow according to US BI-RADS. At the end of
the examination, the location of the breast lesion was marked on
the skin.
Minimally invasive resection using a
US-guided Mammotome
All minimally invasive surgical procedures were
conducted using a vacuum-assisted Mammotome biopsy system (Devicor
Medical Products, Inc.) with the following components: 8G Mammotome
rotary cutter, control handle, vacuum suction pump and associated
software (Mammotome EX SCMSW5). While undergoing routine
sterilization, the patient was placed in a supine or semi-lateral
position with their ipsilateral arm lifted up and then draped with
a surgical towel. A moderate anesthetic (local anesthesia, 1%
lidocaine ≤200 mg.) was administered subcutaneously and underneath
the posterior breast space in the surgical area. A ~3-mm incision
was made in the predetermined location, which allowed for the
proper insertion of the 8G Mammotome needle. The needle was placed
underneath the deep surface of the breast mass by US guidance at an
appropriate angle so that the breast mass was just inside the
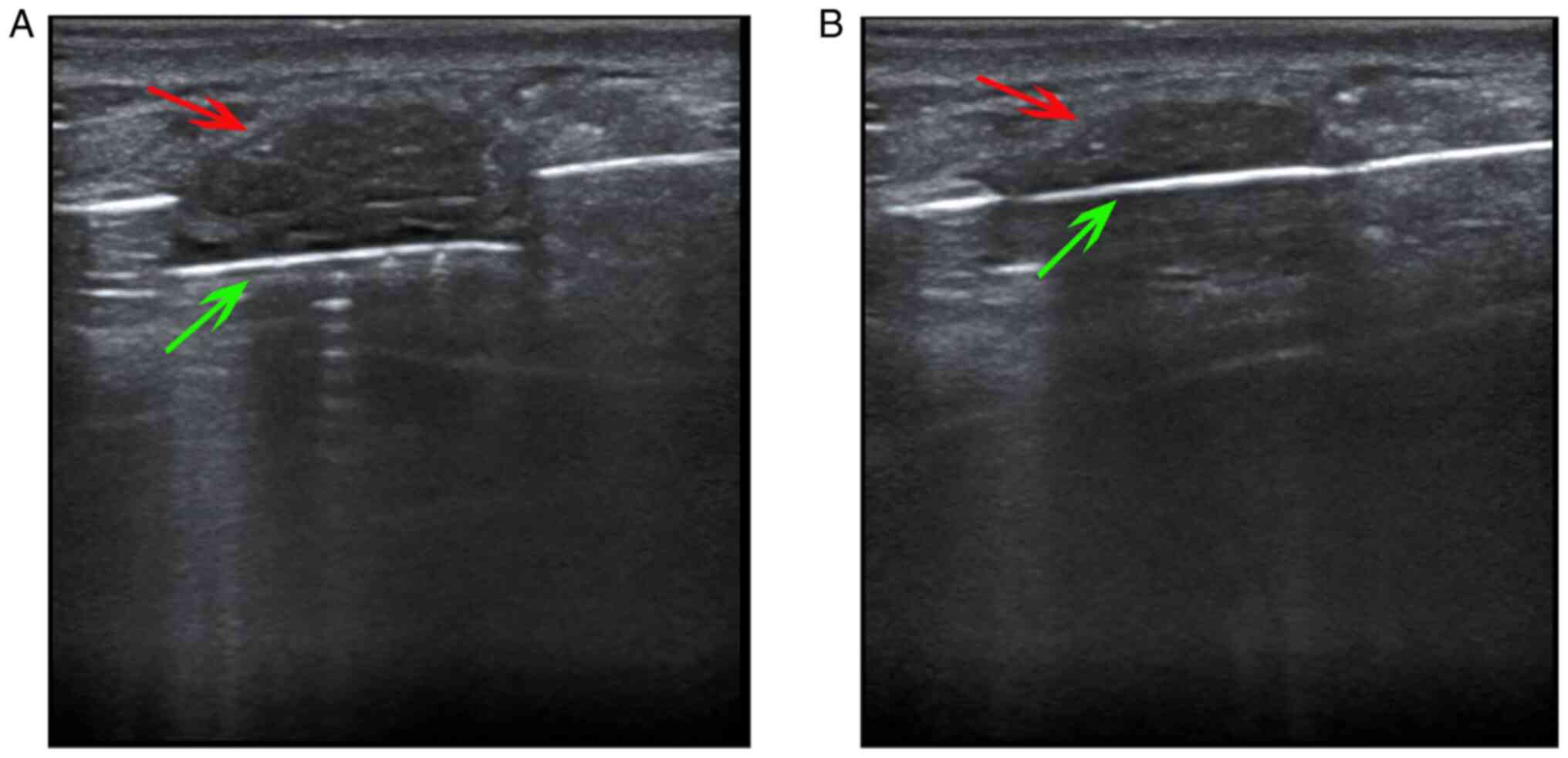
groove of the needle (Fig. 1).
Repeated rotary cutting was performed to remove the aspirated
lesion tissue until no residual lesions were detected in the US
images. After completion of the resection, hemostasis was performed
in the surgical area to stop bleeding. Compression bandages were
applied to all patients for 72 h following the procedure.
US BI-RADS classification. Breast mass
classification was based on the latest edition of the US BI-RADS
recommendations of the ACR (8).
Two physicians with >10 years of breast US experience determined
the US BI-RADS classification. If the analysis results were
inconsistent, the two physicians discussed the results together
until a consensus was reached. According to the US BI-RADS
management recommendations, category 3 lesions should have a short
(6-month) follow-up interval or continued surveillance, while
category 4 lesions require biopsy for tissue diagnosis. As there is
a marked difference in the treatment of category 3 and 4 lesions by
clinicians, category 3 lesions were defined as benign and lesions
of category 4 and above were defined as malignant in the present
study.
Statistical analysis
SPSS19.0 (IBM Corp.) statistical analysis software
was used to analyze the diagnostic efficacy of US BI-RADS
classification in breast masses that underwent minimally invasive
resection using the Mammotome system. To detect statistical
differences in lesion characteristics, χ2-test was used
for shape while Fisher's exact test was used for other
characteristics. The specificity, sensitivity, accuracy, positive
predictive value and negative predictive value were calculated by
comparison with pathology results. The receiver operating
characteristic (ROC) curve for the US BI-RADS classification in the
diagnosis of breast masses subjected to minimally invasive surgery
was constructed, and the area under curve (AUC) was calculated.
Results
Lesion position and US
characteristics
The position and main ultrasonographic features of
benign and malignant lesions were analyzed, and the results are
shown in Table I. Significant
statistical differences in shape, orientation, margin, posterior
features, calcifications and architectural distortion were detected
between benign and malignant lesions (P<0.001). However, no
significant difference in position, echo pattern, duct changes and
vascularity was found.
 | Table IPosition and ultrasound
characteristics of benign and malignant breast masses removed by
ultrasound-guided Mammotome-assisted minimally invasive
resection. |
Table I
Position and ultrasound
characteristics of benign and malignant breast masses removed by
ultrasound-guided Mammotome-assisted minimally invasive
resection.
| Lesion
characteristics | Benign, n | Malignant, n | P-value |
|---|
| Position | | | 0.182 |
|
Upper outer
quadrant | 667 | 12 | |
|
Upper inner
quadrant | 307 | 9 | |
|
Lower outer
quadrant | 175 | 8 | |
|
Lower inner
quadrant | 158 | 5 | |
| Shape | | | <0.001 |
|
Oval | 987 | 10 | |
|
Round | 124 | 9 | |
|
Irregular | 196 | 15 | |
| Orientation | | | <0.001 |
|
Parallel | 1,197 | 20 | |
|
Not
parallel | 110 | 14 | |
| Margin | | | <0.001 |
|
Circumscribed | 1,201 | 11 | |
|
Not
circumscribed | 106 | 23 | |
| Echo pattern | | | 1.000 |
|
Hypoechoic | 1,286 | 34 | |
|
Isoechoic | 9 | 0 | |
|
Complex
cystic and solid | 12 | 0 | |
| Posterior
features | | | <0.001 |
|
No posterior
features | 901 | 19 | |
|
Enhancement | 369 | 0 | |
|
Shadowing | 29 | 15 | |
|
Combined
pattern | 8 | 0 | |
| Calcifications | | | <0.001 |
|
Microcalcifications | 0 | 13 | |
|
No
microcalcifications | 1,307 | 21 | |
| Architectural
distortion | | | <0.001 |
|
Yes | 0 | 9 | |
|
No | 1,307 | 25 | |
| Duct changes | | | 0.207 |
|
Yes | 8 | 1 | |
|
No | 1,299 | 33 | |
| Vascularity | | | 0.162 |
|
Absent | 536 | 11 | |
|
Internal
vascularity | 277 | 12 | |
|
Vessels in
the rim | 494 | 11 | |
Pathology results
Among the 1,028 patients with 1,341 breast masses
who underwent minimally invasive resection, there were 1,307 benign
lesions, including adenosis, fibroadenosis, sclerosing adenosis,
fibrocystic breast disease, adenosis with fibroadenomatous nodules,
fibroadenoma, intraductal papilloma, benign phyllodes tumor,
inflammatory lesions and other benign lesions. There were 34
malignant lesions, including ductal carcinoma in situ and
invasive carcinoma. The detailed pathology results of this study
are summarized in Table II and
typical pathological images are displayed in Fig. 2.
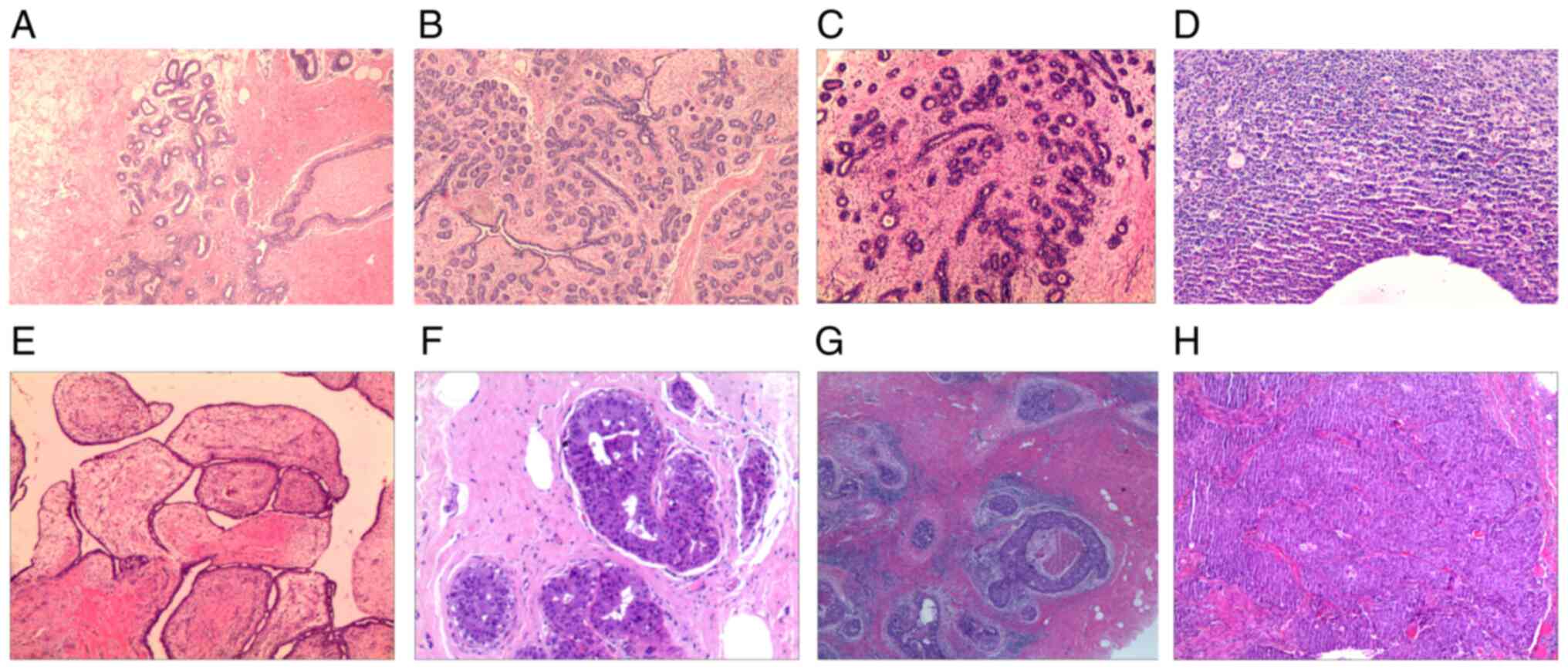 | Figure 2Representative pathology images. (A)
Breast adenosis (magnification, x40), (B) breast adenosis with
fibroadenomatous nodules (magnification, x40), (C) breast
fibroadenoma (magnification, x40), (D) breast inflammatory lesion
(magnification, x100), (E) breast benign phyllodes tumor
(magnification, x40), (F) intraductal papilloma (magnification,
x100), (G) ductal carcinoma in situ (magnification, x40) and
(H) invasive carcinoma (magnification, x40). Hematoxylin and eosin
staining. |
 | Table IIPathological results of breast masses
removed by ultrasound-guided Mammotome-assisted minimally invasive
resection. |
Table II
Pathological results of breast masses
removed by ultrasound-guided Mammotome-assisted minimally invasive
resection.
| Pathology | n (%) |
|---|
| Adenosis | 343 (25.58) |
| Fibroadenosis | 57 (4.25) |
| Sclerosing
adenosis | 6 (0.45) |
| Fibrocystic breast
disease | 5 (0.37) |
| Adenosis with
fibroadenomatous nodules | 709 (52.87) |
| Fibroadenoma | 138 (10.29) |
| Intraductal
papilloma | 29 (2.16) |
| Benign phyllodes
tumor | 5 (0.37) |
| Inflammatory
lesion | 3 (0.22) |
| Other benign
lesions | 12 (0.89) |
| Invasive
carcinoma | 23 (1.72) |
| Ductal carcinoma
in situ | 11 (0.82) |
Comparative analysis of US BI-RADS
classification and pathology results
Taking pathology results as the gold standard,
according to the US BI-RADS classification, there were 1,091
category 3 masses, of which 1091 (100.00%) were benign and 0
(0.00%) were malignant. There were also 188 category 4a masses with
181 benign (96.28%) and 7 malignant (3.72%), 50 category 4b masses
with 35 benign (70.00%) and 15 malignant (30.00%), 10 category 4c
masses with 0 benign (0.00%) and 10 malignant (100.00%), and 2
category 5 masses with 0 benign (0.00%) and 2 malignant (100.00%)
(Table III).
 | Table IIIComparative analysis of US BI-RADS
classification and pathological results of breast masses removed by
ultrasound-guided Mammotome minimally invasive resection |
Table III
Comparative analysis of US BI-RADS
classification and pathological results of breast masses removed by
ultrasound-guided Mammotome minimally invasive resection
| | US BI-RADS
classification, n (%) | |
|---|
| Pathology
results | Category 3 | Category 4a | Category 4b | Category 4c | Category 5 | Total, n (%) |
|---|
| Benign | 1,091 (100.00) | 181 (96.28) | 35 (70.00) | 0 (0.00) | 0 (0.00) | 1,307 (97.46) |
| Malignant | 0 (0.00) | 7 (3.72) | 15 (30.00) | 10 (100.00) | 2 (100.00) | 34 (2.54) |
| Total | 1,091 (81.36) | 188 (14.02) | 50 (3.73) | 10 (0.75) | 2 (0.15) | 1,341 |
Diagnostic efficacy of US BI-RADS
classification in patients with breast masses undergoing
Mammotome-assisted resection
The specificity, sensitivity, accuracy, positive
predictive value and negative predictive value of the US BI-RADS
classification in the diagnosis of the patients were 83.47, 100.00,
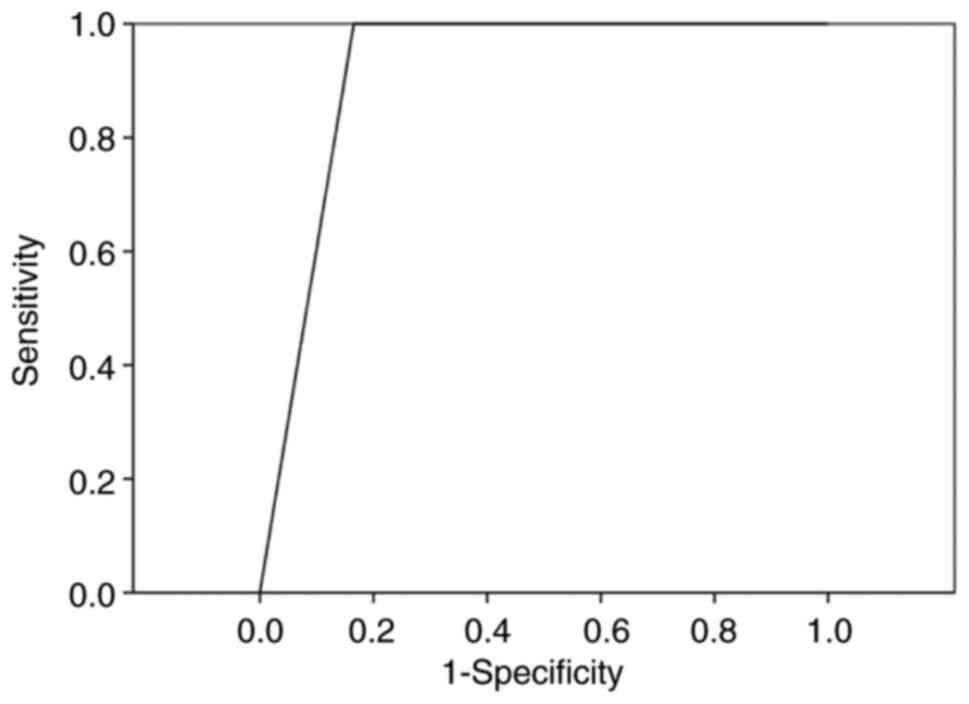
83.89, 13.60 and 100.00%, respectively (Table IV). The AUC calculated from the
corresponding ROC curve was 0.917 (Fig. 3).
 | Table IVDiagnostic efficacy of the ultrasound
Breast Imaging Reporting and Data System in breast masses
undergoing Mammotome-assisted minimally invasive resection. |
Table IV
Diagnostic efficacy of the ultrasound
Breast Imaging Reporting and Data System in breast masses
undergoing Mammotome-assisted minimally invasive resection.
| Statistic | Result, % |
|---|
| Specificity | 83.47 |
| Sensitivity | 100.00 |
| Accuracy | 83.89 |
| PPV | 13.60 |
| NPV | 100.00 |
Discussion
With the increasing prevalence of breast disease
screening and health consciousness of the population, the early
detection rate of breast masses has increased significantly. The
risk of breast cancer has also increased markedly for several
reasons, including lifestyle and diet changes, as well as genetic,
environmental and drug-associated factors (9). Subsequently, greater numbers of
patients experience psychological anxiety, such as carcinophobia,
due to the presence of breast masses, and choose to find out the
pathological nature of their lesions and undergo surgical
resection. Thus, it is very important for the surgeon to provide a
reasonable therapy plan. A meta-analysis (10) comparing Mammotome vacuum-assisted
biopsy with open excision for benign breast lesions indicated that
the procedure using a hand-held Mammotome device was advantageous
with respect to the size of the skin incision, intraoperative blood
loss, surgery and healing times, scar size, wound infection and
cosmetic breast deformity, with additional advantages including
real-time and dynamic observation, high accuracy, minimal pain
during the procedure, high patient satisfaction, a smaller incision
and improved aesthetic appearance when compared with that achieved
using conventional open surgery (11,12).
Among the 1,341 breast masses in the present study, 1,328 lesions
were completely removed under US guidance via the Mammotome
minimally invasive system; there were only 13 lesions that could
not be completely removed due to hard calcifications in the lesions
that abraded the Mammotome needle, causing the surgeon to switch to
a conventional open surgical approach.
The BI-RADS has been designed by the ACR to
standardize the reporting of breast imaging findings, reduce
uncertainty in the interpretation of images and the recommendations
based on them, and facilitate outcome monitoring. The US BI-RADS
classification provides consistent and standardized terminology for
the assessment of breast masses using feature descriptors including
mass, shape, orientation, margin, internal echo pattern, posterior
echo features, calcification, associated features and special cases
(8). Several feature descriptors
were analyzed in the present study, and some US features were found
to overlap between benign and malignant lesions. There were also
statistically significant differences in shape, orientation,
margin, posterior features, microcalcifications and architectural
distortion; however, the position, echo pattern, duct changes and
vascularity did not significantly differ. These findings indicate
that the accurate assessment of breast masses requires the analysis
of multiple feature descriptors rather than any single
characteristic. In the present study, there were 1,091 category 3
masses, all of which were benign, and the negative predictive value
was 100.00%. This is consistent with the 0-2% likelihood of cancer
for US BI-RADS category 3 lesions, indicating that the US BI-RADS
system had high diagnostic accuracy for category 3 lesions in the
present study. Furthermore, the sensitivity was 100.00% and all the
pathological malignant lesions were classified as category 4 or
above, indicating that no pathological malignant tumors were
misdiagnosed using US BI-RADS and the diagnostic efficacy of US
BI-RADS in the diagnosis of true positive lesions was good. The
specificity and accuracy were 83.47 and 83.89%, respectively, and
the AUC was 0.917, which further validated the good preoperative
diagnostic performance of US BI-RADS. However, the positive
predictive value was only 13.60% due to false positives in the
category 4a and 4b lesions in the present study. This upgraded
classification may be due to lesion features such as irregular
shape, different sized calcifications and posterior shadowing. The
low positive predictive value and the presence of false positive
cases can be compensated for by combining US BI-RADS with
elastography or contrast-enhanced US (13-17).
Furthermore, given its high accuracy, cost effectiveness, low
complication rate and convenience, core needle biopsy is the most
common method used for impalpable and palpable lesions (18), and most suspicious lesions undergo
an initial core needle biopsy first. Therefore, in the present
study category 4c and 5 lesions were rare and the majority of
lesions that underwent minimally invasive resection using a
Mammotome device were category 3. The diagnosis of typical benign
category 3 lesions using the US BI-RADS classification was found to
be relatively easy and accurate, and provides satisfactory
diagnostic efficiency according to the results of the present
study.
In summary, the present study demonstrates that the
US BI-RADS classification has good preoperative diagnostic
effectiveness for breast masses undergoing US-guided
Mammotome-assisted minimally invasive resection, particularly for
category 3 lesions and true positive lesions. Therefore, it can
effectively help surgeons to make reasonable decisions for
subsequent therapy and is recommended for further clinical use.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Hubei Province
Health and Family Planning Scientific Research Project (grant no.
WJ2017M081).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YP and HW organized and designed the study. HW
performed US guidance work during the study and wrote the
manuscript. YZ recorded and collected the raw data. QW and HW
confirm the authenticity of all the raw data and performed the data
analysis. YP and HW performed the statistical analysis and reviewed
the study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Committee of Tongji Hospital of Huazhong University of Science and
Technology (approval no. TJ-IRB2021916). All patients provided
written informed consent to participate.
Patient consent for publication
All patients gave their consent for the presentation
of their data and materials in this publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fang M, Liu G, Luo G and Wu T: Feasibility
and safety of image-guided vacuum-assisted breast biopsy: A
PRISMA-compliant systematic review and meta-analysis of 20 000
population from 36 longitudinal studies. Int Wound J. 16:1506–1512.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li SJ, Hao XP, Hua B, Wang JD and Fan ZM:
Chinese Society of Breast Surgery. Clinical practice guidelines for
ultrasound-guided vacuum-assisted breast biopsy: Chinese Society of
Breast Surgery (CSBrS) practice guidelines 2021. Chin Med J (Engl).
134:1390–1392. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li X, Gao H, Xu M, Wu Y and Gao D: Breast
papillary lesions diagnosed and treated using ultrasound-guided
vacuum-assisted excision. BMC Surg. 20(204)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pistolese CA, Castrignano A, Ricci F,
Meucci R, Croce G, Mondillo M, Collura A, Perretta T and Floris R:
Ultrasound-Guided vacuum-assisted biopsy in small breast: A
cost-saving solution. Clin Breast Cancer. 19:e352–e357.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
D'Orsi CJ, Mendelson EB and Morris EA: ACR
BI-RADS® Atlas, Breast Imaging Reporting and Data
System. American College of Radiology, Reston, VA, 2013.
|
|
7
|
Spak DA, Plaxco JS, Santiago L, Dryden MJ
and Dogan BE: BI-RADS(R) fifth edition: A summary of
changes. Diagn Interv Imaging. 98:179–190. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mendelson EB and Berg WA: ACR
BI-RADS® Ultrasound. In: ACR BI-RADS® Atlas,
Breast Imaging Reporting and Data System. American College of
Radiology, Reston, VA, 2013.
|
|
9
|
Lukasiewicz S, Czeczelewski M, Forma A,
Baj J, Sitarz R and Stanislawek A: Breast cancer-epidemiology, risk
factors, classification, prognostic markers, and current treatment
strategies-an updated review. Cancers (Basel).
13(4287)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ding B, Chen D, Li X, Zhang H and Zhao Y:
Meta analysis of efficacy and safety between mammotome minimally
invasive operation and open excision for benign breast tumor. Zhong
Nan Da Xue Xue Bao Yi Xue Ban. 38:291–300. 2013.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
11
|
Papathemelis T, Heim S, Lux MP, Erhardt I,
Scharl A and Scharl S: Minimally invasive breast fibroadenoma
excision using an ultrasound-guided vacuum-assisted biopsy device.
Geburtshilfe Frauenheilkd. 77:176–181. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jiang Y, Lan H, Ye Q, Jin K, Zhu M, Hu X,
Teng L, Cao F and Lin X: Mammotome(R) biopsy system for
the resection of breast lesions: Clinical experience in two
high-volume teaching hospitals. Exp Ther Med. 6:759–764.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cantisani V, David E, Barr RG, Radzina M,
de Soccio V, Elia D, De Felice C, Pediconi F, Gigli S, Occhiato R,
et al: US-Elastography for breast lesion characterization:
Prospective comparison of US BIRADS, strain elastography and shear
wave elastography. Ultraschall Med. 42:533–540. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zheng X, Li F, Xuan ZD, Wang Y and Zhang
L: Combination of shear wave elastography and BI-RADS in
identification of solid breast masses. BMC Med Imaging.
21(183)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ding Z, Liu W, He N, Ma X, Fu L and Ye L:
Value of ultrasound elastography combined with contrast-enhanced
ultrasound and micro-flow imaging in differential diagnosis of
benign and malignant breast lesions. Am J Transl Res.
13:13941–13949. 2021.PubMed/NCBI
|
|
16
|
Cheng M, Tong W, Luo J, Li M, Liang J, Pan
F, Pan J, Zheng Y and Xie X: Value of contrast-enhanced ultrasound
in the diagnosis of breast US-BI-RADS 3 and 4 lesions with
calcifications. Clin Radiol. 75:934–941. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Janu E, Krikavova L, Little J, Dvorak K,
Brancikova D, Jandakova E, Pavlik T, Kovalcikova P, Kazda T and
Valek V: Prospective evaluation of contrast-enhanced ultrasound of
breast BI-RADS 3-5 lesions. BMC Med Imaging. 20(66)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Park HL, Kim KY, Park JS, Shin JE, Kim HR,
Yang B, Kim JY, Shim JY, Shin EA and Noh SM: Clinicopathological
analysis of ultrasound-guided vacuum-assisted breast biopsy for the
diagnosis and treatment of breast disease. Anticancer Res.
38:2455–2462. 2018.PubMed/NCBI View Article : Google Scholar
|