Introduction
Chronic high-salt (HS) intake is closely associated
with elevated blood pressure (BP) level and target organ injuries
(1,2). In addition, ample evidence has
demonstrated that excessive salt intake could also adversely affect
cardiac functional and structural remodeling via BP-independent
mechanisms (3,4). Previous studies showed that
uncontrolled oxidative stress is a key pathophysiological process
during the development of heart failure (5), presumably via reactive oxygen species
(ROS)-mediated apoptosis, leading to cardiomyocyte loss, and
ultimately, left ventricular (LV) dysfunction in murine models
(6,7). In response to chronic HS challenge,
the heart initially manifests robust hypertrophic growth and
interstitial fibrosis, followed by LV maladaptive remodeling, if
stress persists. However, the exact mechanism governing the
transition from compensated LV hypertrophy to a decompensated state
during chronic HS challenge is not fully understood.
Autophagy is an essential, life-sustaining renewal
process that involves the degradation of cell constituents, such as
long-lived proteins and organelles, when subjected to cellular
stress or during certain stages of development. Basal constitutive
autophagy is indispensable for maintaining cellular homeostasis,
whereas this process is upregulated in response to stressors, such
as nutrient or growth factor deprivation, hypoxia and ischemia. In
other settings, however, dysfunction in the autophagy pathway has
been implicated in a range of pathological situations, such as
neurodegenerative disorders, skeletal myopathy, cancer and
infectious diseases (8).
Previously, alterations in autophagy activity have
been implicated as a key pathological process in hypertensive LV
remodeling and pressure overload (transverse aortic constriction;
TAC) induced heart failure models (9-11).
To the best of our knowledge, the autophagic change and its
association with high-salt (HS) intake-accelerated progression of
LV dysfunction has not been investigated. Therefore, the present
study was designed to address this issue, as well as the potential
molecular basis underlying this functional transition in
HS-diet-fed spontaneously hypertensive rats (SHR). The present
study would provide a novel mechanism to explain HS intake-induced
cardiac remodeling.
Materials and methods
Animals
A total of seven-week-old male SHR and Wistar Kyoto
rats (WKY) weighing 200-250 g were obtained from Shanghai
Laboratory Animal Center of the Chinese Academy of Science
(Shanghai, China), and received humane care in compliance with the
Guide for the Care and Use of Laboratory Animals (NIH Pub. no.
85-23, revised 1996), which was approved by the Institutional
Ethics Review Board of the Characteristic Medical Center of the
Chinese People's Armed Police Forces (Tianjin, China; approval
number: 2012-0005). Rats were maintained in a 12/12-h dark/light
cycle in air-conditioned rooms (23±1˚C, 55±5% humidity) and were
acclimated to the local conditions for 1 week before the study. At
8 weeks of age, rats were divided randomly (n=12 for each group),
and then given either a low-salt (LS; 0.5% NaCl) or HS diet (8%
NaCl) for 8, 12 or 16 weeks. All rats were permitted free access to
chow and tap water.
Invasive hemodynamic measurements
A total of 8, 12 and 16 weeks after dietary
intervention, the rats were anesthetized (sodium pentobarbital, 60
mg/kg, intraperitoneally) for invasive hemodynamics using a 2F high
fidelity micro-tip pressure catheter (SPR-320; Millar Instruments
Ltd.) to measure systolic and diastolic BP (SBP and DBP), LV
end-diastolic pressure (LVEDP), maximal slope of systolic pressure
increment (+dP/dtmax), and diastolic pressure
decrement (-dP/dtmin) as previously described by
the authors (12).
Cardiac pathological evaluation
Animals were euthanized with an overdose of sodium
pentobarbital (100 mg/kg, intraperitoneally) after in vivo
hemodynamic measurement, and then were rapidly perfused via
inferior vena cava with precooled physiological saline for 5 min.
The hearts were quickly removed, sectioned, and prepared for
paraffin embedding and cryo-section. Routine staining techniques in
paraffin embedded tissue (7-µm) included hematoxylin and eosin
(H&E) staining (25˚C, 15 min) and Masson's trichrome staining
for evaluating interstitial collagen deposition. Tetraethyl
rhodamine isothiocyanate-conjugated wheat germ agglutinin
(Invitrogen; Thermo Fisher Scientific, Inc.) plus
4,6-diamidino-2-phenylindole (DAPI; 5 mg/ml; Vector Laboratories,
Inc.) staining was used to measure cardiomyocyte cross-sectional
area, which was examined with a fluorescent microscope (80i; Nikon
Corporation) as previously described by the authors (13). All image analysis was performed in
a blinded manner by Image Pro Plus (version 4.5; Media Cybernetics,
Inc.).
For detecting lipid droplet, Oil Red O staining was
used in cryo-sectioned heart tissue (10 µm), and the sections were
fixed with 10% buffered formalin (25˚C, 24 h) and stained with Oil
Red O working solution (25˚C, 30 min). Hematoxylin was used for
counterstaining. The perirenal adipose tissue was removed and
smeared over a slide serving as the positive controls. For
detecting sulfated proteoglycans, paraffin-embedded sections were
stained with toluidine blue. Alcian blue/periodic acid-Schiff
(AB-PAS) was used in paraffin-embedded sections to detect acidic
sulfated mucins (AB positive), O-glycosides (PAS positive) and
sialic acid (PAS positive) as previously described (14).
Transmission electron microscopy was performed for
evaluating myocardial ultrastructure. Briefly, the samples from LV
were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH
7.2; 4˚C, 24 h), post-fixed in 1.0% OsO4, dehydrated in alcohol and
acetone solution, embedded in Epon812, sectioned with LKB
ultramicrotome, and stained with uranyl acetate followed by lead
citrate, then observed with a transmission electron microscope.
Cardiac tissue ashing procedure
A total of 8 weeks after dietary intervention, when
LV hemodynamic measurement was finished, rat hearts were harvested.
The blood was collected from superior vena cava for measurement of
plasma [Na+], [K+] and [Cl-]. The
right ventricle was dissected away, and the left ventricle was
weighted to determine the wet weight (WW). Then the hearts were
desiccated at 90˚C for 72 h and their dry weights (DW) were
determined. Because the weight was unchanged with further drying,
the difference between WW and DW was considered as tissue water
content. The tissues were then ashed at 200˚C, 400˚C for 24 h at
each temperature level and 600˚C for a further 48 h and then were
dissolved in 20 ml 10% HNO3. [Na+] and
[K+] were measured by inductively-coupled plasma
emission spectrometer (ICP, IRIS Intrepid II XSP, Thermo Electron
Corporation). [Cl-] was measured by titration with 0.1 N
silver nitrate (15).
Cell culture and treatments
H9c2, a rat ventricular myoblast cell line (Shanghai
Cell Bank, Chinese Academy of Science) was maintained at 37˚C and
5% CO2 in Dulbecco's modified Eagle's medium (HyClone;
Cytiva) supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 1% glutamine. H9c2 cells were
seeded onto six-well plates and incubated with culture media
containing NaCl (50 mM) or mannitol (100 mM), which yielded
hypertonic state, equaling 400 mOsm/l, and treated with or without
ROS scavenger N-acetylcysteine (NAC; 0.5 and 2 mM). Normal culture
medium (isotonic, 300 mOsm/l) served as the control for the
experiment. After exposure for desired time periods, the monolayer
cultures were washed with D-Hanks and the cells were removed by
0.25% trypsin.
Measurement of apoptosis,
intracellular free [Na+] and ROS in H9c2 cells
Intracellular free Na+ concentration
([Na+]) was detected using the dye CoroNa™ Green (cat.
no. C36676; Invitrogen; excitation 488 nm, emission at 516 nm) as
previously described (16).
Briefly, the H9c2 cells were cultured in six-well plates and
treated with a NaCl concentration gradient (final concentrations
were 25, 50, 100 and 150 mmol/l) for 24 h; after adding CoroNa
Green (5 µmol/l), the fluorescence signal was detected by flow
cytometry (Cytomic FC500; Beckman-Coulter, Inc.) and analyzed by
FlowJo software (version 7.6.1; TreeStar Inc.).
ROS production was detected using the dye
2,7-dichlorofluorescein diacetate (DCFH-DA; MilliporeSigma). The
H9c2 cells were pretreated with or without ROS scavenger NAC (0.5,
2 mM) for 1 h followed by incubation with NaCl (50 mM), mannitol
(100 mM) or control medium for 90 min. Cells were removed by 0.25%
trypsin with culture medium without FBS. DCFH-DA (10 µmol/l) was
added to the H9c2 cells and incubated at 37˚C for 30 min in dark.
Analysis of apoptosis was performed by an Annexin V-fluorescein
isothiocyanate (FITC)/Propidium Iodide (PI) staining kit
(BioLegend, Inc.). Briefly, H9c2 cells were seeded onto six-well
plates and incubated with increased medium mannitol (100 mM), NaCl
(50 mM) and NaCl (50 mM) supplemented with ROS scavenger NAC (0.5,
2 mM) for 24 h. Thereafter, the cells were harvested and washed
twice with D-Hanks, centrifuged (4˚C, 350 x g, 5 min) and added 1X
binding buffer (500 µl), Annexin V-FITC (2.5 µl) and PI (5 µl),
incubated at room temperature in dark for 15 min then filtrated and
kept on ice for an immediate detection by a FC500 flow
cytometer.
Detection of autophagosomes in H9c2
cells
H9c2 cells were washed and fixed with 4%
paraformaldehyde at 20˚C for 15 min. Then the cells were blocked in
fetal bovine serum for 20 min and stained with primary antibodies
against LC3 (1:500; cat. no. NB100-2220; Novus Biologicals, LLC)
for 2 h at 37˚C. After washing four times with PBS for 5 min, cells
were incubated with rhodamine red-conjugated secondary antibodies
(1:100; cat. no. CW0161; Beijing Kangwei Century Biotechnology Co.,
Ltd.) for 1 h at 37˚C and the nuclei were counterstained with DAPI
(1:100, Vector Laboratories, Inc.) for 20 min. Then images were
examined with a fluorescent microscope (80i; Nikon Corporation).
The median fluorescence intensity of LC3 positive area was
determined by Image Pro Plus software (version 4.5; Media
Cybernetics, Inc.).
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. Briefly, the H9c2 cells were washed twice
with D-Hanks and lysed with 1 ml of TRIzol®.
Subsequently, total RNA was extracted by adding chloroform and
subsequent centrifugation (4˚C, 12,000 x g, 5 min) and
precipitation with isopropyl alcohol. The RNA concentration and
purity were measured by NanoDrop 2000C (Thermo Fisher Scientific,
Inc.) and the ration of optical density value (260/280 nm) was at
1.8 to 2.0. Total RNA (2 µg) was reverse-transcribed into cDNA
using a reverse transcription kit (Promega Corporation) in 25-µl
reaction volumes according to the manufacturer's instructions.
Transcript expression levels were quantified by SYBR Green RT-qPCR
using an ABI PRISM 7300 sequence detector system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Primers used for PCR
analysis are shown in Table I. The
conditions for amplification were as follows: 95˚C for 10 min,
followed by 40 cycles at 95˚C for 15 sec and 60˚C for 60 sec. The
relative expression levels were calculated using the
2-ΔΔCq method (17).
 | Table IPrimers used for reverse
transcription-quantitative PCR analysis. |
Table I
Primers used for reverse
transcription-quantitative PCR analysis.
| Primer name | Sequence
(5'-3') | Product length
(bp) | Tm (˚C) |
|---|
| β-actin | F:
TCTGTGTGGATTGGTGGCTCT | 115 | 60 |
| | R:
AGAAGCATTTGCGGTGCAC | | |
| Beclin1 | F:
CTCCTGTGGAATGGAATGA | 168 | 49 |
| | R:
ACAACGGCAACTCCTTAG | | |
| LC3b | F:
AAGGCTGAAGTCCAAGTG | 192 | 50 |
| | R:
GAAGTGGCTGTATGTCTGT | | |
| ATG9A | F:
GGCAGAAGAGATGGCAGGACAG | 170 | 57 |
| | R:
TGGGAGGATGGGCAGAAGATGG | | |
| ATG16L1 | F:
TGGACAGTAGGAACAGACA | 86 | 50 |
| | R:
CAGCGAATGACAGGTGAG | | |
Protein extraction and western blot
analysis
For in vivo experiments, the heart tissue was
homogenized in the RIPA lysis buffer containing 50 mM Tris (pH
7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1%
SDS, with PMSF (100:1), incubated on ice for 30 min to obtain the
whole-cell protein extraction, followed by centrifugation at 4˚C
and 12,000 x g for 10 min. For in vitro examinations, the
H9c2 cells were washed and centrifuged then lysed in RIPA buffer
for 30 min on ice to obtain the protein extraction. The protein
concentration was determined with BCA protein assay kit (Thermo
Fisher Scientific, Inc.), following the manufacturer's instructions
with bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.)
as the standard. For western blotting, equal amounts of protein
samples (50 µg) were added denaturing buffer and subjected to a 10
min boil at 99.5˚C. Following SDS-PAGE on 10 or 15% polyacrylamide
gels, proteins were transferred onto PVDF membranes using the
Trans-Blot Turbo blotting system (Bio-Rad Laboratories, Inc.) and
incubated in 5% skim milk in PBST (PBS containing 0.1% v/v Tween
20) for 2 h at 37˚C, then respectively probed with primary
antibodies against lysosome-associated membrane protein 1 (LAMP1;
1:1,000; cat. no. sc-17768; Santa Cruz Biotechnology, Inc.), light
chain 3LC3; 1:1,000; cat. no. NB100-2220), autophagy-related gene
(ATG) 9 (1:1,000; cat. no. NB110-56893), ATG5 (1:500; cat. no.
NB110-53818), Beclin1 (1:8,000; cat. no. NB110-87318), ATG16L1
(1:1,000; cat. no. NB110-60928; all from Novus Biologicals, LLC),
GAPDH (1:2,000; cat. no. ab181602; Abcam) at 4˚C overnight. After
washing four times with PBST for 15 min, membranes were incubated
with horseradish peroxidase-conjugated secondary antibodies (goat
anti-rabbit IgG, cat. no. 6721, 1:2,000; goat anti-mouse IgG, cat.
no. 6789, 1:2,000; Abcam) for 40 min at 37˚C. After washing fourth
with PBST for 15 min, protein signals were detected by enhanced
chemiluminescence method. The protein signal was amplified with
Western Chemiluminescent Horseradish Peroxidase Substrate
(MilliporeSigma) and detected using a Chemi DOC XRS+ Imaging System
(Bio-Rad Laboratories, Inc.). Densitometric analyses were performed
with Image J software (version 1.41o; National Institutes of
Health).
Statistical analysis
All data are presented as the mean ± SEM.
Statistical analysis was determined by independent sample unpaired
t-test or one-way analysis of variance (ANOVA) followed by
Newman-Keuls post hoc test using the GraphPad Prism v5 (GraphPad
Prism Software Inc.). Two-tailed P<0.05 was considered to
indicate a statistically significant difference.
Results
Transition from compensated LV
hypertrophy to decompensation during HS intake in SHR
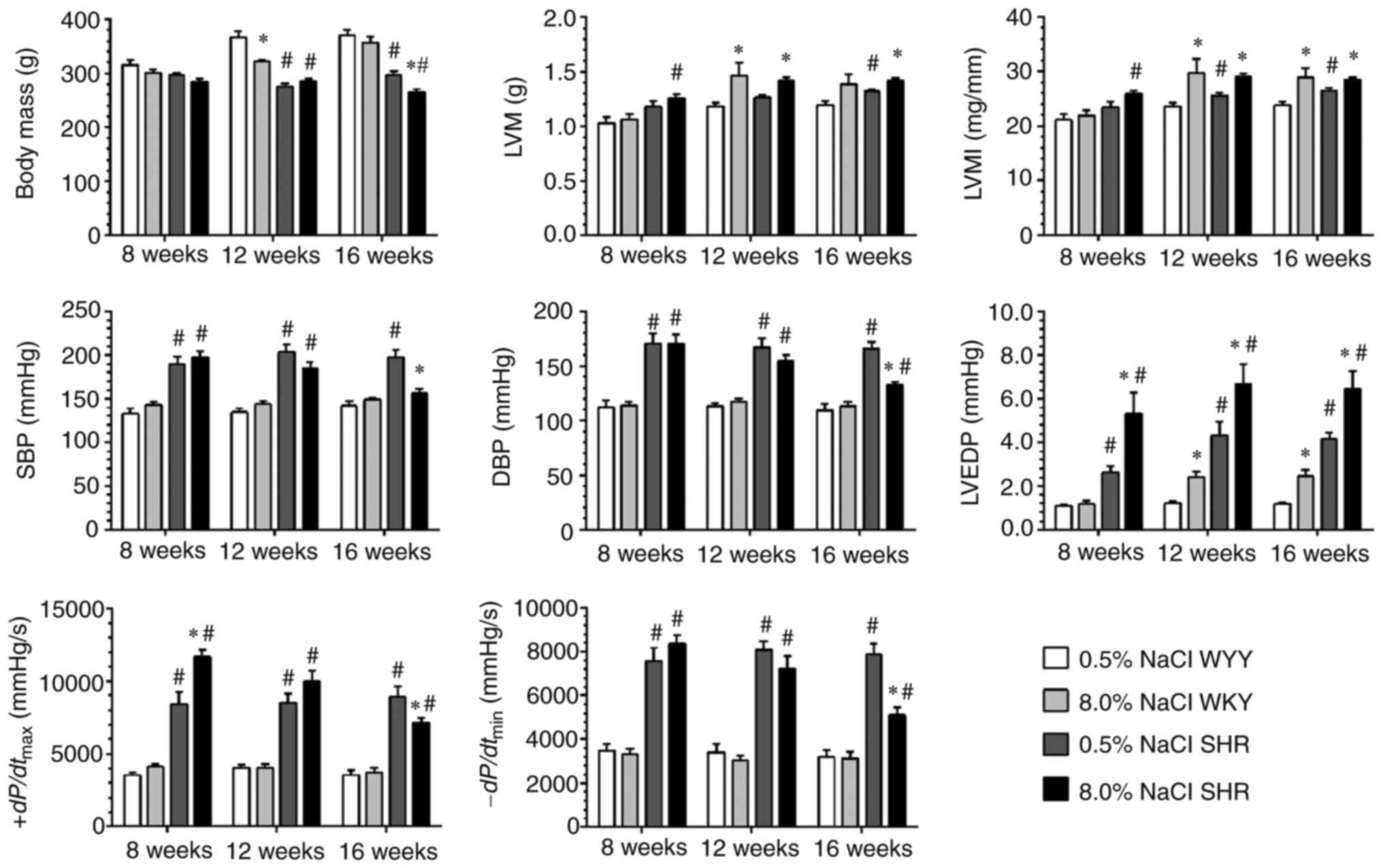
Body mass was comparable among all of the groups
after 8 weeks of dietary intervention (Fig. 1). Throughout the 16 weeks of
dietary intervention, there was a significant decrease in body
weight in the SHR fed with the HS diet, which indicates a
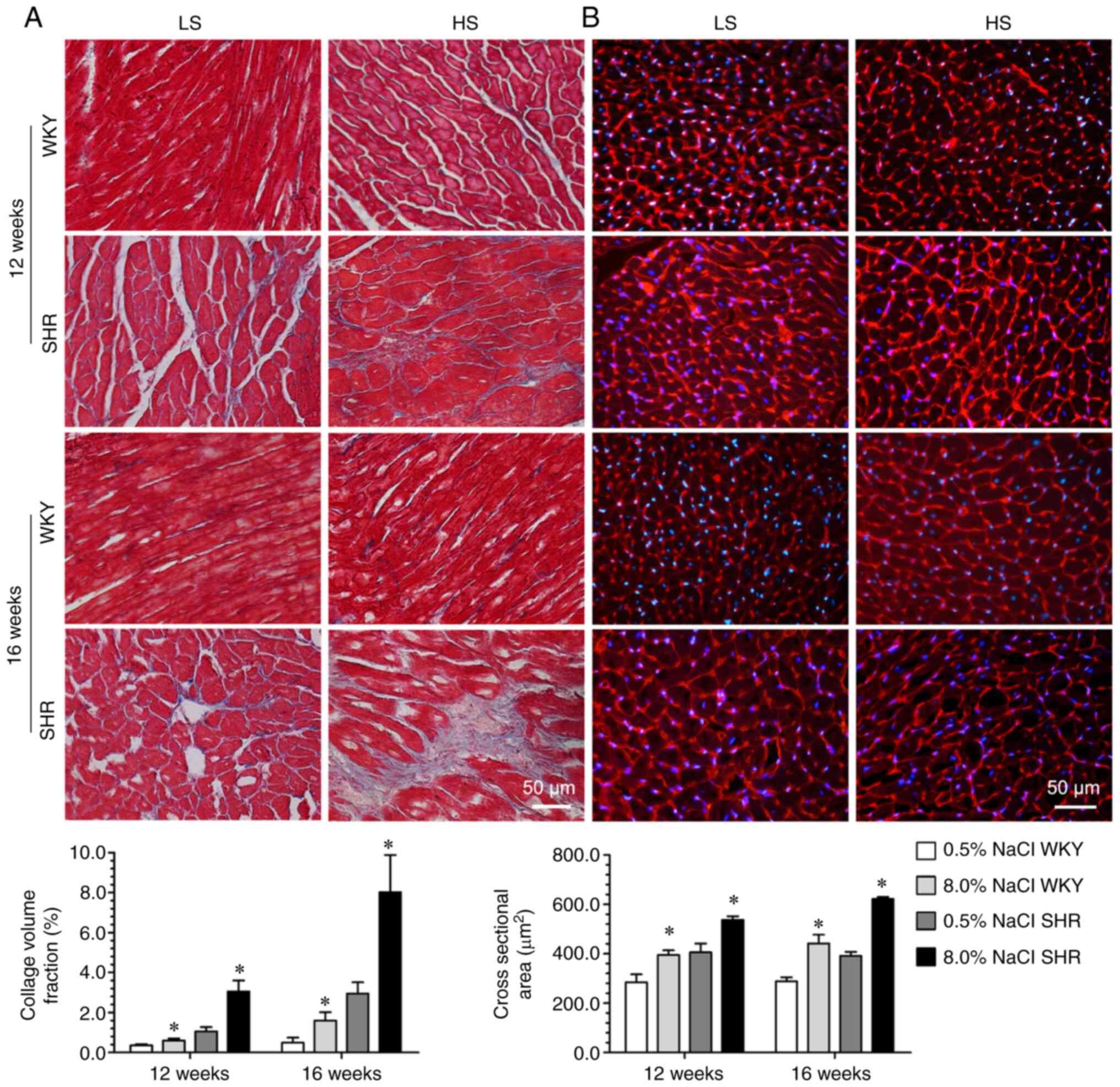
deteriorating health status. Moreover, the HS diet also led to an
increase in LV mass, cardiomyocyte hypertrophy and collagen
deposition in SHR and WKY compared with their LS-diet-fed
counterparts (Figs. 1 and 2). Invasive LV hemodynamic analysis
revealed that the HS diet progressively impaired the LV systolic
(SBP and +dp/dtmax) and diastolic (LVEDP and
-dp/dtmin) functions of the SHR in a
time-dependent manner (Fig. 1).
These results demonstrated that a transition from compensated LV
hypertrophy to decompensation occurred when the HS diet
intervention persisted for 12 weeks in the SHR. A time-dependent
increase in collagen deposition and LVEDP was also noted in the
LS-fed SHR and HS-fed WKY rats, but an obvious deterioration of the
LV systolic and diastolic functions was not observed during this
period in these two groups (Figs.
1 and 2).
Chronic HS Intake induces myocardial
autophagic vacuolization in SHR
After 12 weeks of dietary intervention, massive
vacuole-like structures were observed in LV tissue from HS-diet-fed
SHR, compared with LS-diet-fed SHR (Fig. 3A and B). To examine the potential reasons
accounting for these structures, various staining methods and TEM
were performed for pathological examinations (Fig. 3). The results showed that these
cardiac vacuoles observed in the HS-fed SHR under light microscopy
are due to autophagic vacuolization (double-membrane vesicles
containing cytoplasmic organelles). Depending on the engulfed
structures (from protein aggregates, intracellular organelles to
pathogens), the size of the autophagosomes can range from 0.5 to 10
µm in diameter (18). As revealed
in Fig. 3, the size of these
vacuole-like structures, observed under light microscopy, could be
even larger than 20 µm in diameter; this finding has previously
been reported in humans (19), and
may be due to the fusion of the autophagosomes.
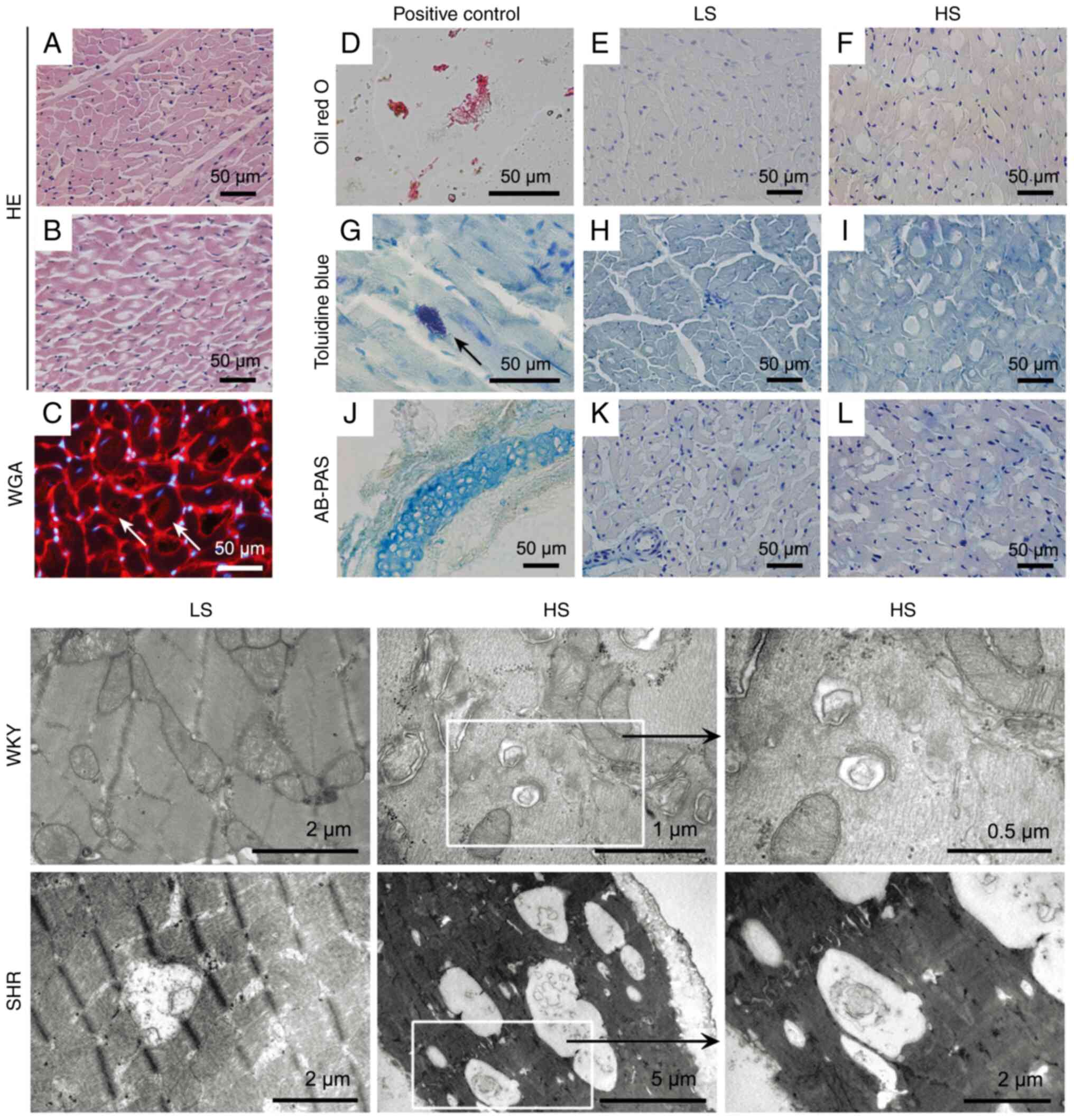 | Figure 3Pathological evaluation of cardiac
vacuoles induced by HS intake using different staining methods
(upper panel) and transmission electron microscopy (lower panel).
(A and B) H&E staining of representative heart sections from
LS- (A, 0.5% NaCl) and HS- (B, 8.0% NaCl) fed SHR for 12 weeks,
respectively. (C) A heart section from HS- (B, 8.0% NaCl) fed SHR
using wheat germ agglutinin conjugated by tetraethyl rhodamine
isothiocyanate (red) to reveal the cell membrane (the nuclei were
counterstained by 4'-6-diamidino-2-phenylindole, blue). The arrows
indicate that these vacuole-like structures are inside the
cardiomyocytes. (D-F) Oil red O staining revealed that these
cardiac vacuoles are not fatty droplets (D: rat abdominal adipose
tissue smearing was used as the positive controls.) (G-I) Toluidine
blue staining revealed that the vacuoles are also negative for
glycosaminoglycan. (G) shows a typical interstitial mast cell
(arrow) positive for toluidine blue. (J-L) AB-PAS staining, which
is used to detect acidic sulfated mucins (AB), O-glycosides (PAS)
and sialic acid (PAS), was also negative for cardiac vacuoles. (J)
The rat tracheal cartilage (AB-PAS positive, blue) was used as the
positive control. Scale bars in the upper panel indicate 50 µm. The
lower panel shows representative transmission electron microscopic
images from LS- and HS-fed rats after 12 weeks of dietary
intervention. There are numerous double-membrane vesicles
containing cytoplasmic organelles characteristic of autophagy,
particularly in the HS-fed SHR. HS, high-salt; LS, low-salt; WKY,
Wistar Kyoto rats; SHR, spontaneously hypertensive rats. Alcian
blue/periodic acid-Schiff. |
A time-dependent of cardiac autophagic
change accompanies LV functional deterioration in SHR during HS
Intake
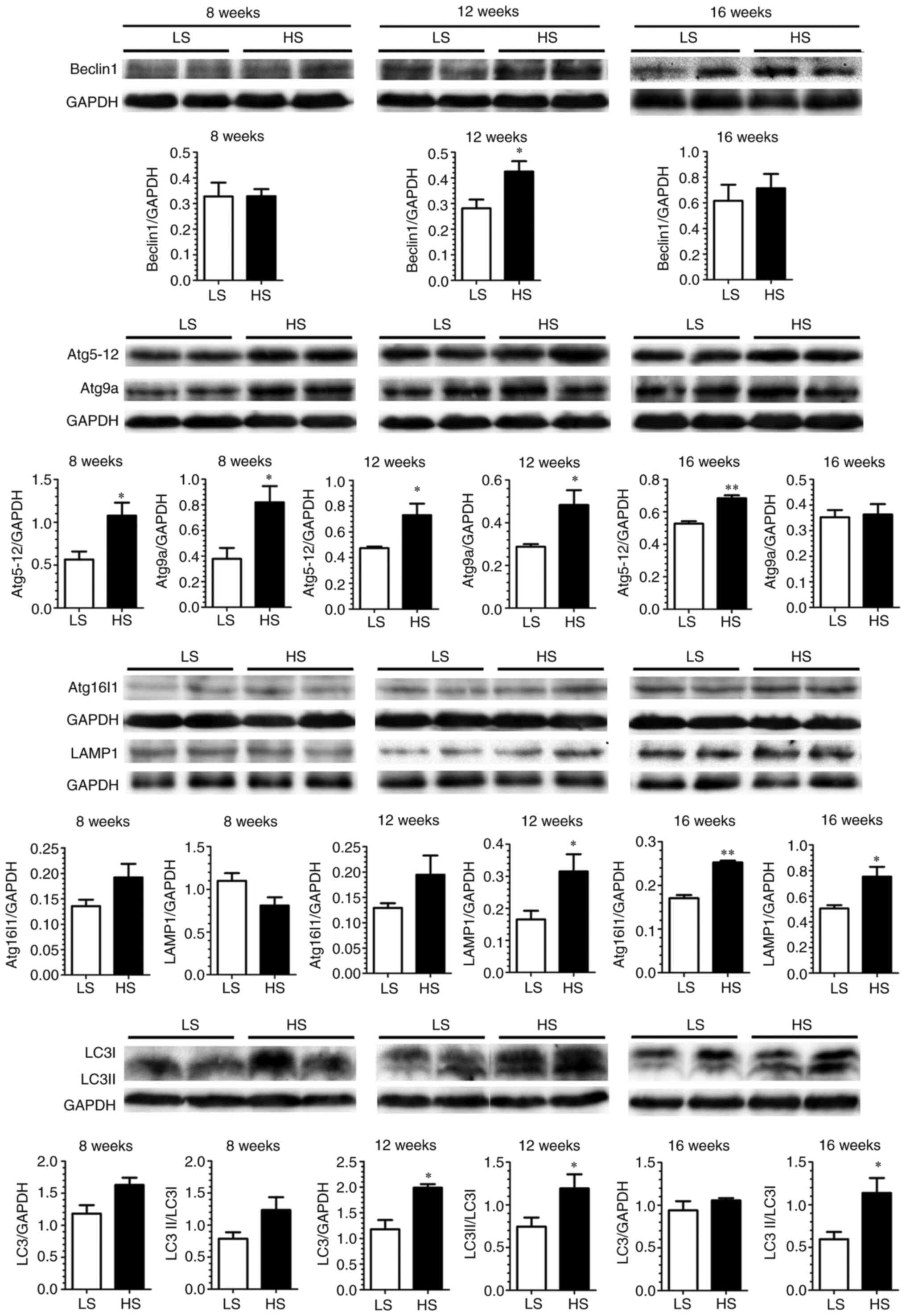
Next, autophagy-associated key proteins in LV tissue
from SHR sacrificed at different time points were examined by
western blotting (Fig. 4). The
results demonstrated a global change of the autophagy since SHR was
fed by HS diet for 12 weeks. This change persisted to 16 weeks in
SHR on the HS diet, as shown by upregulated Beclin 1/ATG6 (required
for autophagy induction and nucleation), ATG16-ATG5-ATG12 and
LC3-II (two complexes required for phagophore formation and
autophagosome expansion), LAMP1 (maker for lysosome fusion with the
autophagosome to form an autolysosome) and ATG9 (necessary for most
ATG protein recycle). These results clearly demonstrated a temporal
and spatial association between autophagic change and LV function
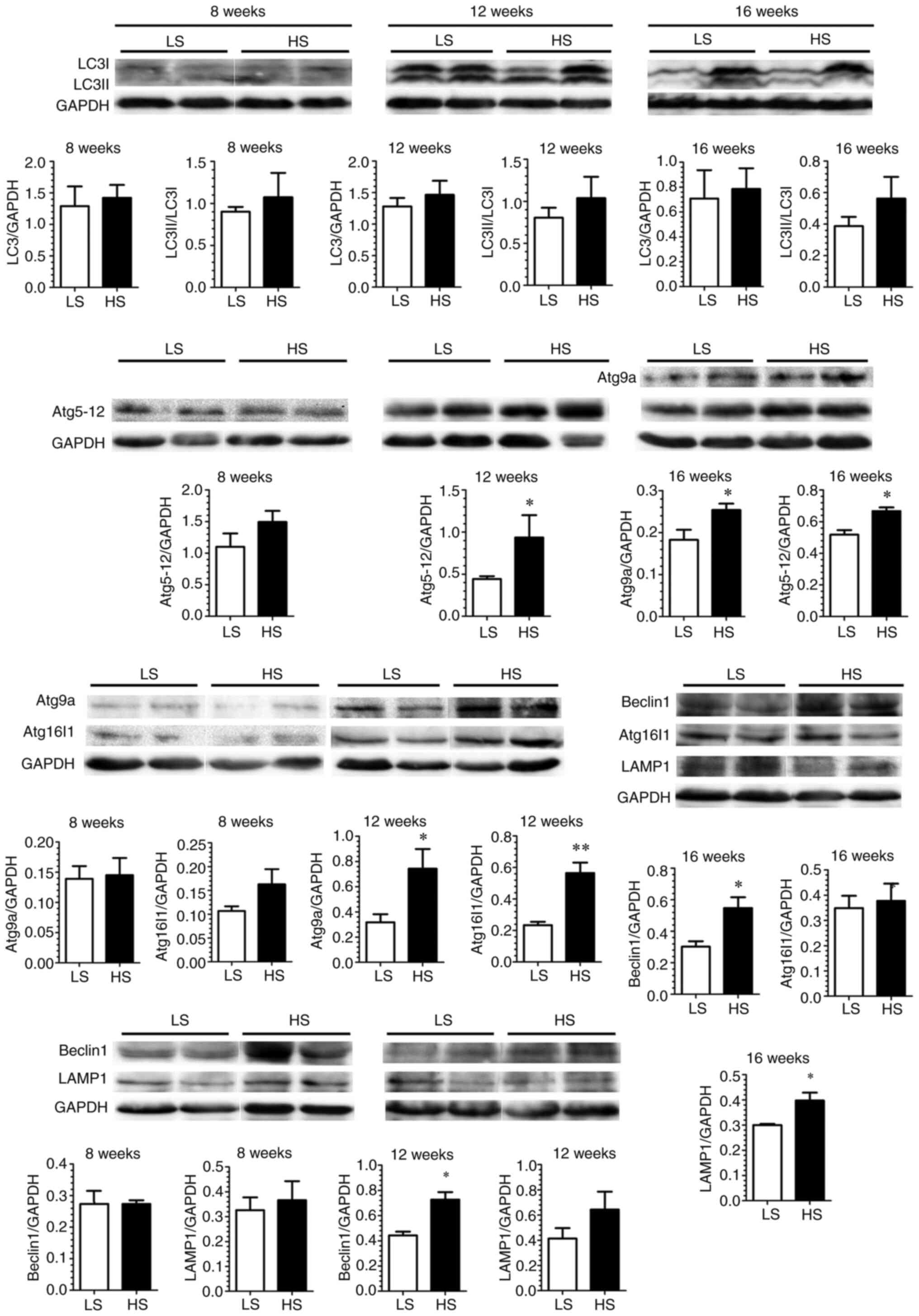
deterioration in the HS-fed SHR. Similarly, but to a lesser extent
(not global), autophagic change was observed in the HS-fed WKY
(Fig. 5).
Na+ leak-induced cytosolic
[Na+] elevation contributes to ROS-dependent autophagic
change in H9c2 Cardiomyocytes
Increasing evidence shows that chronic HS intake
could induce Na+ accumulation in the interstitial space
without a commensurate retention of water, which yields increased
tonicity (18,20,21).
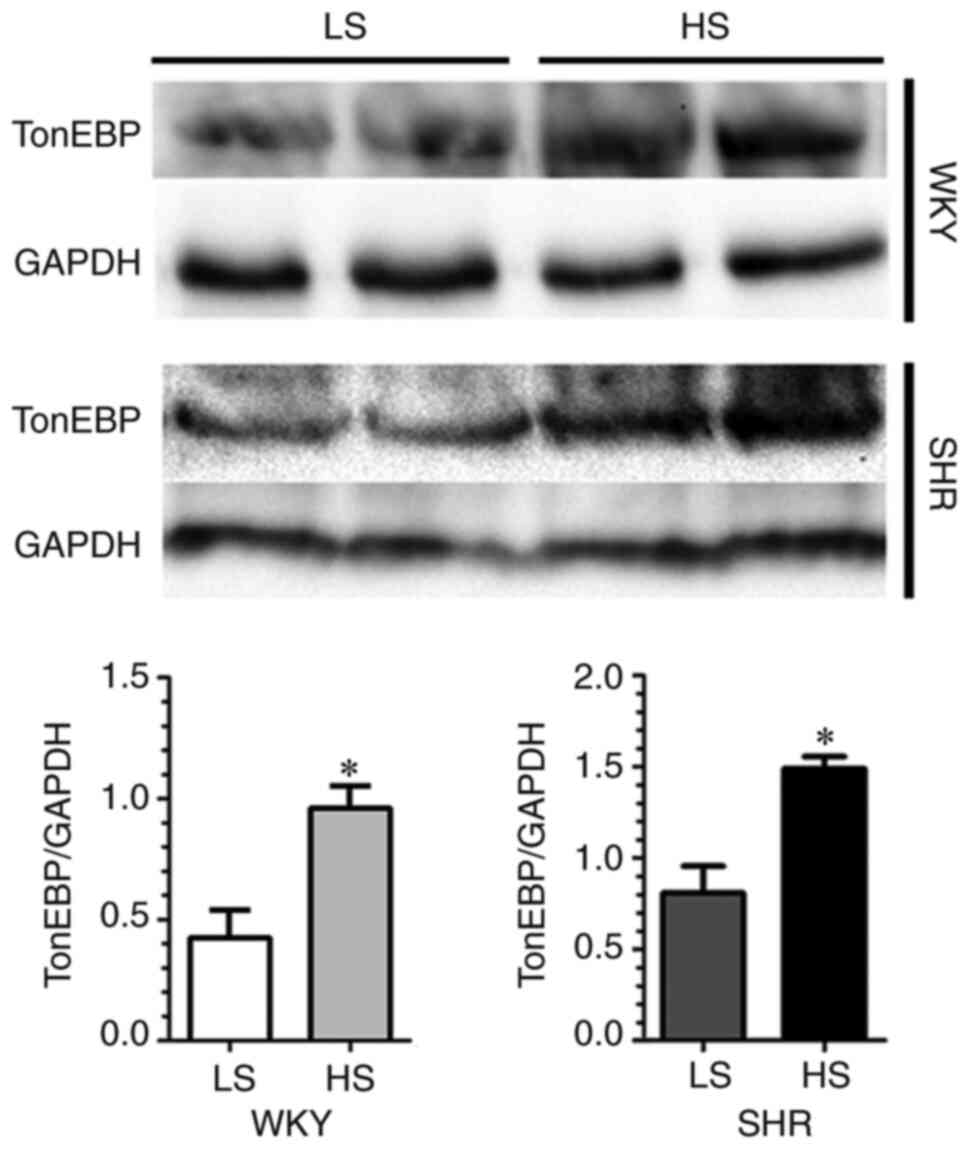
As shown in Fig. 6, upregulation
of myocardial TonEBP was also demonstrated in the HS-fed WKY and
SHR. In addition, using LV ashing samples, LV tissue composition
analysis of Na+, K+ and water was performed
in SHR after dietary intervention for 8 weeks. The results revealed
that cardiac extracellular [Na+] was markedly higher
than plasma level (215.0±2.0 mmol/l vs. 139.1±1.84 mmol/l) in
LS-diet-fed SHR, and this difference was further increased in
HS-diet-fed SHR (237.0±6.0 mmol/l, Table II). To examine the impact of
increased extracellular [Na+] on the autophagic response
in rat H9c2 cardiomyocytes, an additional NaCl concentration
gradient was added in the culture medium. It was identified that
there was a proportional increase of cytosolic [Na+] as
extracellular [Na+] increased (Fig. 7A), indicating a Na+ leak
into the cytosol when the cells were exposed to high
[Na+]. A previous study showed that HS loading with a
diet containing 8.0% NaCl in Sprague-Dawley rats for 2 weeks led to
an increase of [Na+] ~40 mM in the skin compared with
serum [Na+] (15).
Based on the relationship between extracellular and intracellular
[Na+] in H9c2 cells and cardiac extracellular
[Na+] (Fig. 7A and
Table II), an additional 50 mM
NaCl was thus used in the culture medium, which equals 400 mOsm/l
(serum osmolality is ~300 mOsm/l), to simulate the extracellular
hyperosmolarity induced by chronic HS intake in the subsequent
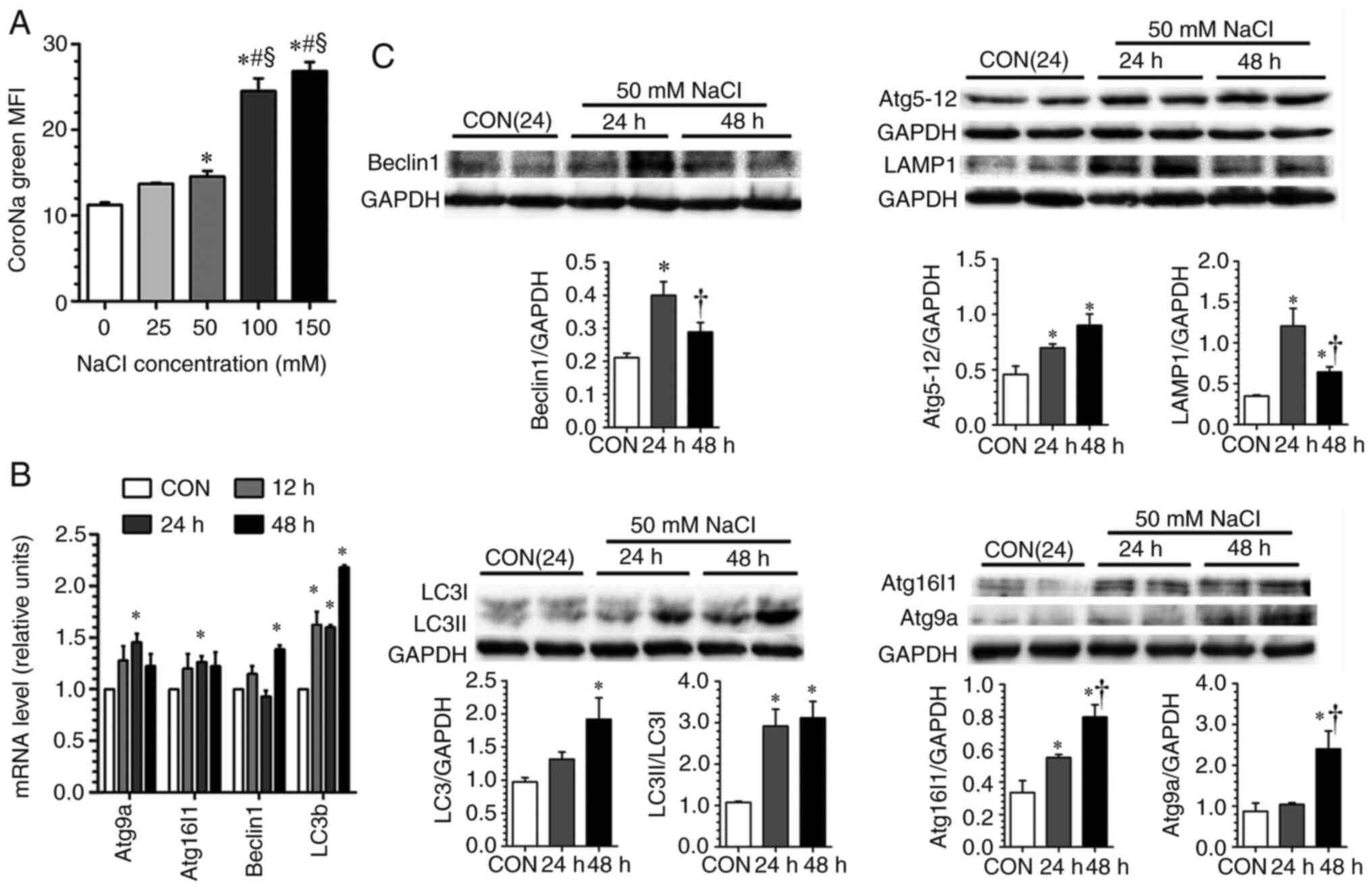
in vitro studies. It was found that incubation with an
additional 50 mM NaCl induced an upregulation of key components of
autophagy at both the mRNA and protein levels (Fig. 7B and C).
 | Table IIElectrolytes and water distribution
in plasma and heart tissue of SHR. |
Table II
Electrolytes and water distribution
in plasma and heart tissue of SHR.
| Electrolytes and
water distribution indexes | Low-salt diet | High-salt diet |
|---|
| Plasma
[Na+] (mmol/l) | 139.1±1.84 | 141.1±0.92 |
| Plasma
[K+] (mmol/l) | 4.17±0.21 | 3.94±0.18 |
| Cardiac muscle
water (ml/g DW) | 3.526±0.020 |
3.700±0.057a |
| Cardiac muscle
K+/cardiac muscle water (mmol/ml) | 0.075±0.002 |
0.063±0.005a |
| Cardiac muscle
Na+/cardiac muscle water (mmol/ml) | 0.102±0.002 |
0.135±0.007a |
| Cardiac muscle
K+ (mmol/g DW) | 0.265±0.008 | 0.231±0.017 |
| Cardiac muscle
Na+ (mmol/g DW) | 0.359±0.006 |
0.499±0.034a |
| Cardiac muscle
extracellular fluid (ml/g WW) | 0.370±0.011 |
0.448±0.027a |
| Cardiac muscle
intracellular fluid (ml/g WW) | 0.409±0.011 |
0.339±0.026a |
| Cardiac muscle
Na+/cardiac muscle extracellular fluid (mmol/ml) | 0.215±0.002 |
0.237±0.006a |
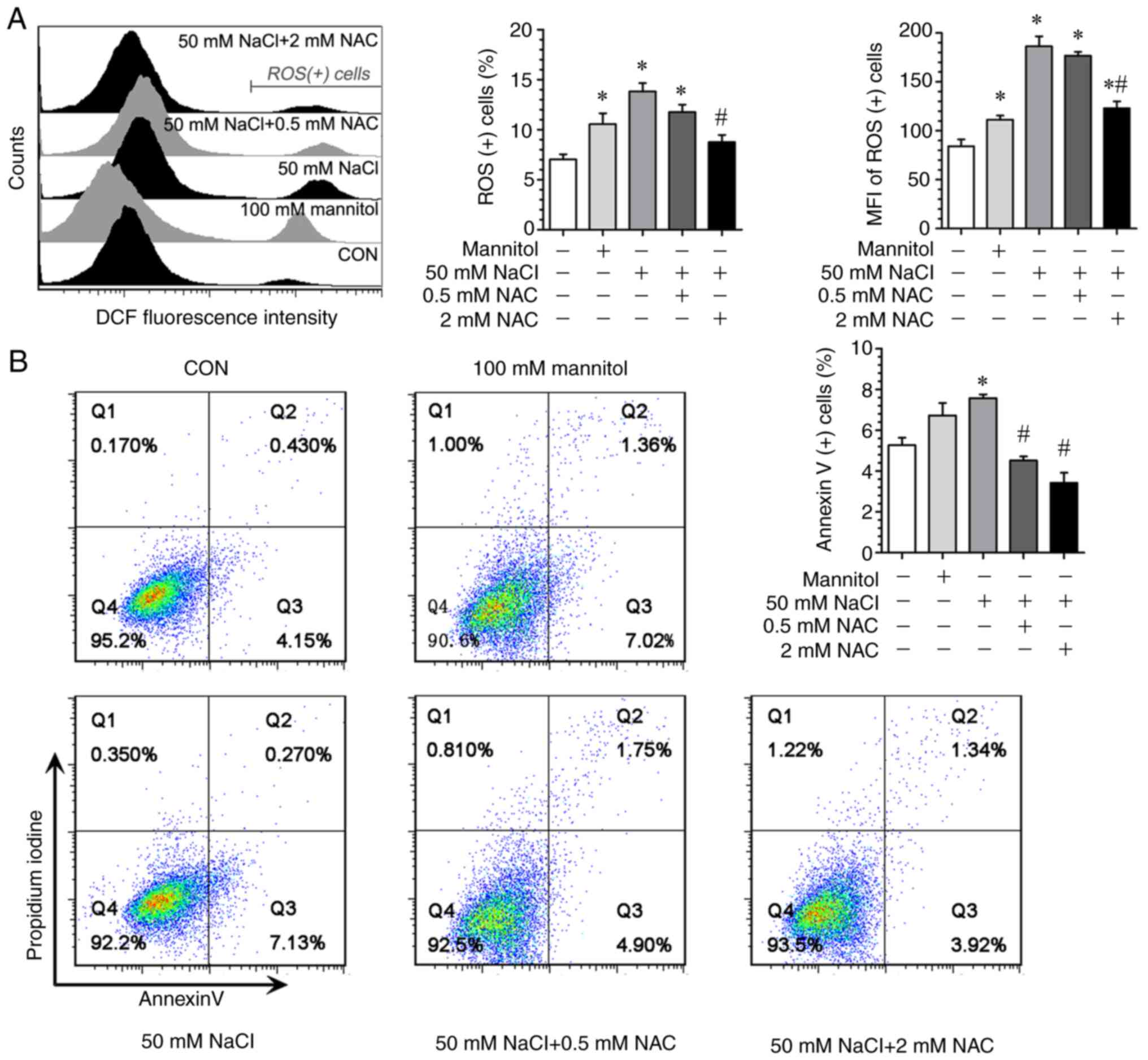
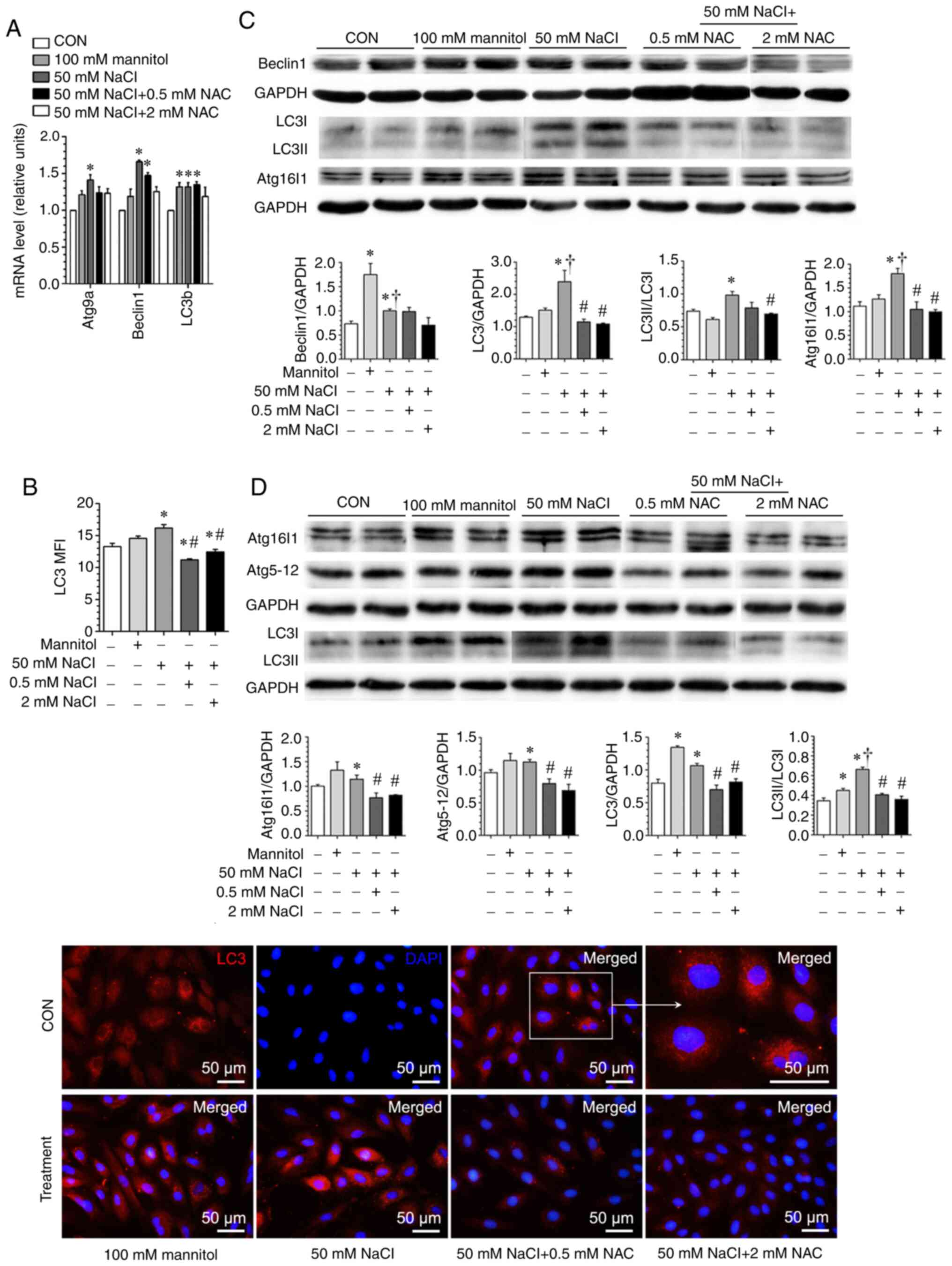
In addition, it was identified that the exposure of
H9c2 cells to an additional 50 mM NaCl in the culture medium led to
an enhanced intracellular ROS production (Fig. 8A), as well as increased
pro-apoptotic response (Fig. 8B).
When NAC was used as a ROS scavenger, the high NaCl-induced
upregulation of pro-apoptotic response was significantly attenuated
(Fig. 8B). Similarly, when
exposure to the high extracellular NaCl occurred, there was an
upregulation of key autophagy components both at mRNA and protein
levels, as well as increased autophagosome formation (Fig. 9), which could be suppressed by NAC,
implying that high [Na+]-induced autophagic change is
ROS-dependent. Notably, when 100 mM mannitol (400 mOsm/l) was used
as a positive control at the same extracellular hyperosmolality
with 50 mM NaCl, the magnitude of ROS generation (Fig. 8) and the pattern of key autophagic
component upregulation were different from those achieved by 50 mM
NaCl, providing evidence to indicate that high
[Na+]-induced changes in H9c2 cells are not entirely
dependent on extracellular osmolality (Fig. 9A-D).
Discussion
In the present study, for the first time, it was
demonstrated that chronic dietary HS intake could induce LV
autophagic change and myocardial autophagic vacuolization in SHR.
More importantly, there is a temporal coincidence between the
transition from compensated to decompensated LV hypertrophy and
cardiomyocyte autophagic change, which indicates an important role
of the myocardial autophagy during the development of hypertensive
heart remodeling. In addition, it was showed that chronic HS
intake-induced myocardial interstitial hypertonicity, the majority
of which is harbored by Na+, via a Na+
leak-induced cytosolic [Na+] elevation, contributes to
autophagic change in cardiomyocytes in a ROS-dependent manner.
Cardiomyocyte vacuolization is a common pathological
finding observed in a variety of pathological conditions, including
hypertrophic cardiomyopathy (22),
idiopathic dilated cardiomyopathy (19), arrhythmogenic right ventricular
cardiomyopathy/dysplasia (23),
and chronic and acute coronary ischemia (24). The content inside these
intracellular vacuole-like structures is quite different depending
on the underlying pathological mechanisms. Nonetheless, the
existence of myocardial vacuolization is a sign of worsening LV
function, and is associated with poor prognosis (25). In the present study, it was found
that there are more vacuoles in HS-loaded SHR compared with their
LS-diet-fed counterparts or WKY rats, indicating a HS
intake-related phenomenon. After careful investigations by
different staining methods and TEM examination, these cardiac
vacuoles observed in the HS-fed SHR under light microscopy were
proven to be autophagic vacuolization.
Previously, autophagy has been implicated as a key
process during hypertension-related heart disease, whose impact on
LV remodeling is determined by three factors, i.e., basal autophagy
level, stress severity and duration (26). The importance of basal autophagic
activity has been well defined by Atg-modified mice. Zhu
et al (27) demonstrated
that the partial suppression of autophagy using Beclin
1+/- mice blunted pressure overload TAC-induced LV
adverse remodeling (27).
Conversely, Beclin 1 overexpression accentuated load-induced
LV pathological remodeling (27).
Another study using an Atg5 loss-of-function mice, in which
constitutive autophagy is near-completely inactivated, demonstrated
a deterioration of LV function in a TAC model (28). These lines of evidence support the
notion that, in the basal state, constitutive levels of autophagy
are required for cell survival, while the uncontrolled activation
of autophagy during sustained stress eliminates essential cellular
elements and provokes cell death (26).
In terms of the roles of stress severity and
duration in initiating myocardial autophagy, the present model
could provide novel insights to elucidate their interactions. The
first description of hypertension-related cardiac autophagy dates
to a 1984 study, in which obvious autophagic vacuoles with
lysosomal activity were observed in SHR over 20 months of age
(29). The effectiveness of
dietary intervention with different NaCl contents (0.5, 4.0 and 8%)
in inducing LV dysfunction in SHR was previously compared
systematically (12), since it is
time consuming to induce hypertensive LV dysfunction in SHR [an
overt cardiac dysfunction occurs at age of 52 to 90 weeks (30)]. It was revealed that 8 to 12 weeks
after 8% salt-loading is the key time window in which a transition
from compensated to decompensated LV hypertrophy occurs (12). Based on these findings, the dietary
intervention was extended to 16 weeks and a global change of
myocardial autophagy was demonstrated at 12 weeks after HS
challenge, which persisted to 16 weeks. Notably, a temporal and
spatial coincidence of progressive deterioration of LV systolic and
diastolic dysfunction was also observed during this period.
Therefore, the present study demonstrated the kinetics and
association between autophagic change and LV remodeling in
HS-diet-fed SHR. Notably, an overt LV dysfunction and the global
activation of ATG components were not observed in the HS-fed WKY
rats, indicating the inadequacy of stress severity (HS intake
per se, without hemodynamic overload) and/or stress
duration.
To explore the potential mechanism underlying the
excessive change of myocardial autophagy, the hypothesis that
chronic HS intake may lead to myocardial interstitial
hyperosmolality was examined. The plasma [Na+] is
normally maintained within a narrow range by a rigorous control
system. However, the results from previous studies (15,31,32),
have expanded this concept by showing that chronic dietary high
salt intake leads to Na+ storage in the extracellular
space without a commensurate retention of water, which could yield
a hypertonicity state in the interstitium. Additionally, in
cartilage, the negative charge density of glycosaminoglycans
contributes to the local interstitial [Na+] at 450 mM,
which far exceeds the serum [Na+] (32). In the present study, using similar
procedure, it was identified that myocardial interstitial
[Na+] is ~200 mM, and will be increased after chronic HS
intake. Moreover, a proportional increase of cardiomyocyte TonEBP
expression in response to hyperosmolality has been demonstrated
previously (33). In the present
study, using heart tissue ashing procedure, it was found that LV
interstitial [Na+] is over 200 mM, and will be increased
after chronic HS intake. Moreover, TonEBP (or the nuclear factor of
activated T cells 5, a well-characterized transcription factor
induced by osmotic stress) expression was increased in HS-diet-fed
rats, thus providing evidence supportive of myocardial interstitial
hyperosmolality.
Next, it was examined whether increased
extracellular [Na+] may have a direct impact on
myocardial autophagic change. Because the role of oxidative stress
in regulating LV autophagic change is well characterized (34,35),
the hypothesis that ROS generation may be the bridge linking
extracellular hypertonicity and autophagic change was examined. A
dose-dependent cytosolic [Na+] elevation was revealed as
extracellular [Na+] increased, which induced the
upregulation of key autophagy components at both the mRNA and
protein levels in a ROS-dependent manner. A previous study revealed
that in the failing heart, elevated cytosolic [Na+]
promotes ROS formation by reducing mitochondrial Ca2+
uptake via the Na+/Ca2+ exchanger (36). Considering the close relationship
between HS intake and myocardial oxidative stress (37), it is conceivable that this
cytosolic [Na+] elevation-triggered ROS production would
work in tandem with hemodynamic stress in a vicious cycle to
promote LV dysfunction. Because a variety of channels and/or
exchangers, such as voltage-gated sodium channels and transient
receptor potential channels (non-selective cation channels), are
responsible for the transmembrane Na+ influx, the
precise mechanism for this ‘Na+ leak’, when exposed to
extracellular [Na+] that is significantly elevated above
the plasma level, remains to be elucidated in future studies.
Although autophagy is implicated as a key
pathological event during hypertensive ventricular remodeling, to
the best of our knowledge, our current understanding of myocardial
autophagy in this setting is mainly derived from the LV pressure
overloaded model (TAC in mice) (26). The reason may be ascribed to the
time-consuming nature of bone fide hypertensive models, such
as SHR. Though the LV autophagic change and its association with
the hypertensive LV remodeling induced by chronic HS intake were
observed in SHR, whether autophagy is upregulated in this
pathophysiological process needs further studies to be
confirmed.
In conclusion, the present study demonstrated that
the myocardial autophagic change may participate in the maladaptive
LV remodeling induced by chronic HS intake in SHR, and revealed a
novel mechanism by which interstitial hypertonicity-induced
cytosolic [Na+] elevation triggers ROS-dependent
autophagic change. The present results strengthen the notion that
autophagy regulation may have therapeutic potential in
cardiovascular disease, and provides a novel mechanism for HS
intake-induced LV remodeling.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81600328), the National Key
R&D Program of China (grant nos. 2017YFC1307600 and
2017YFC1307602) and the Tianjin Municipal Science and Technology
Committee (grant nos. 16JCQNJC11800, 15ZXJZSY00010 and
16ZXMJSY00130).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YB performed the experiments and wrote the
manuscript. GHY conceptualized the study design and wrote the
manuscript. SBC and WC analyzed the data and revised the
manuscript. YB and ZZG performed the experiments and provided
material support. XZ and YML conceptualized the study and
interpreted the data, and confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All of the studies were performed in agreement with
the national and international laws and policies and were approved
by the Institutional Animal Care and Ethics Committee of
Characteristic Medical Center of the Chinese People's Armed Police
Forces (Tianjin, China; approval no. 2012-0005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee SS, McGrattan A, Soh YC, Alawad M, Su
TT, Palanisamy UD, Hussin AM, Kassim ZB, Ghazali ANB, Stephan BC,
et al: Feasibility and acceptability of a dietary intervention to
reduce salt intake and increase high-nitrate vegetable consumption
in malaysian middle-aged and older adults with elevated blood
pressure: Findings from the DePEC-nutrition trial. Nutrients.
14(430)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pires NM, Igreja B and Soares-da-Silva P:
Antagonistic modulation of SIK1 and SIK2 isoforms in high blood
pressure and cardiac hypertrophy triggered by high-salt intake.
Clin Exp Hypertens. 43:428–435. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hao J, Chang L, Wang D, Ji C, Zhang S, Hou
Y and Wu Y: Periplocin alleviates cardiac remodeling in
DOCA-salt-induced heart failure rats. J Cardiovasc Transl Res 26:
10.1007/s12265-022-10277-2, 2022.
|
|
4
|
Hsu A, Duan Q, McMahon S, Huang Y, Wood
SA, Gray NS, Wang B, Bruneau BG and Haldar SM: Salt-inducible
kinase 1 maintains HDAC7 stability to promote pathologic cardiac
remodeling. J Clin Invest. 130:2966–2977. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang X, Zhang G, Dasgupta S, Niewold EL,
Li C, Li Q, Luo X, Tan L, Ferdous A, Lorenzi PL, et al: ATF4
protects the heart from failure by antagonizing oxidative stress.
Circ Res. 131:91–105. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang Y and Epelman S: Cardiac macrophages,
reactive oxygen species, and development of left ventricular
dysfunction. JACC Basic Transl Sci. 2:699–701. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wen JJ, Porter C and Garg NJ: Inhibition
of NFE2L2-antioxidant response element pathway by mitochondrial
reactive oxygen species contributes to development of
cardiomyopathy and left ventricular dysfunction in chagas disease.
Antioxid Redox Signal. 27:550–566. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Klionsky DJ, Petroni G, Amaravadi RK,
Baehrecke EH, Ballabio A, Boya P, Pedro JM, Cadwell K, Cecconi F,
Choi AMK, et al: Autophagy in major human diseases. EMBO J.
40(e108863)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nishida K and Otsu K: Autophagy during
cardiac remodeling. J Mol Cell Cardiol. 95:11–18. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang L, Gao JY, Ma J, Xu X, Wang Q, Xiong
L, Yang J and Ren J: Cardiac-specific overexpression of
metallothionein attenuates myocardial remodeling and contractile
dysfunction in l-NAME-induced experimental hypertension: Role of
autophagy regulation. Toxicol Lett. 237:121–132. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li MH, Zhang YJ, Yu YH, Yang SH, Iqbal J,
Mi QY, Li B, Wang ZM, Mao WX, Xie HG and Chen SL: Berberine
improves pressure overload-induced cardiac hypertrophy and
dysfunction through enhanced autophagy. Eur J Pharmacol. 728:67–76.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang GH, Zhou X, Ji WJ, Zeng S, Dong Y,
Tian L, Bi Y, Guo ZZ, Gao F, Chen H, et al: Overexpression of
VEGF-C attenuates chronic high salt intake-induced left ventricular
maladaptive remodeling in spontaneously hypertensive rats. Am J
Physiol Heart Circ Physiol. 306:H598–H609. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang GH, Zhou X, Ji WJ, Liu JX, Sun J,
Dong Y, Jiang TM and Li YM: VEGF-C-mediated cardiac
lymphangiogenesis in high salt intake accelerated progression of
left ventricular remodeling in spontaneously hypertensive rats.
Clin Exp Hypertens. 39:740–747. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ma Y, Shi L, Zhao K and Zheng C: lncRNA
FR215775 regulates Th2 differentiation in murine allergic rhinitis.
J Immunol Res. 2022(7783481)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Machnik A, Neuhofer W, Jantsch J, Dahlmann
A, Tammela T, Machura K, Park JK, Beck FX, Müller DN, Derer W, et
al: Macrophages regulate salt-dependent volume and blood pressure
by a vascular endothelial growth factor-C-dependent buffering
mechanism. Nat Med. 15:545–552. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Bortner CD, Sifre MI and Cidlowski JA:
Cationic gradient reversal and cytoskeleton-independent volume
regulatory pathways define an early stage of apoptosis. J Biol
Chem. 283:7219–7229. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kumar S, Javed R, Mudd M, Pallikkuth S,
Lidke KA, Jain A, Tangavelou K, Gudmundsson SR, Ye C, Rusten TE, et
al: Mammalian hybrid pre-autophagosomal structure HyPAS generates
autophagosomes. Cell. 184:5950–5969 e5922. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vigliano CA, Meckert PM, Diez M, Favaloro
LE, Cortes C, Fazzi L, Favaloro RR and Laguens RP: Cardiomyocyte
hypertrophy, oncosis, and autophagic vacuolization predict
mortality in idiopathic dilated cardiomyopathy with advanced heart
failure. J Am Coll Cardiol. 57:1523–1531. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim EJ, Choi MJ, Lee JH, Oh JE, Seo JW,
Lee YK, Yoon JW, Kim HJ, Noh JW and Koo JR: Extracellular
fluid/intracellular fluid volume ratio as a novel risk indicator
for all-cause mortality and cardiovascular disease in hemodialysis
patients. PLoS One. 12(e0170272)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wiig H, Luft FC and Titze JM: The
interstitium conducts extrarenal storage of sodium and represents a
third compartment essential for extracellular volume and blood
pressure homeostasis. Acta Physiol (Oxf): Nov 28, 2018 (Epub ahead
of print).
|
|
22
|
Lopes LR, Garcia-Hernandez S, Lorenzini M,
Futema M, Chumakova O, Zateyshchikov D, Isidoro-Garcia M,
Villacorta E, Escobar-Lopez L, Garcia-Pavia P, et al: Alpha-protein
kinase 3 (ALPK3) truncating variants are a cause of autosomal
dominant hypertrophic cardiomyopathy. Eur Heart J. 42:3063–3073.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pitsch M, Kant S, Mytzka C, Leube RE and
Krusche CA: Autophagy and endoplasmic reticulum stress during onset
and progression of arrhythmogenic cardiomyopathy. Cells.
11(96)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sun W, Lu H, Dong S, Li R, Chu Y, Wang N,
Zhao Y, Zhang Y, Wang L, Sun L and Lu D: Beclin1 controls caspase-4
inflammsome activation and pyroptosis in mouse myocardial
reperfusion-induced microvascular injury. Cell Commun Signal.
19(107)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Frustaci A, Letizia C, Chimenti C, Verardo
R, Alfarano M, Scialla R, Bagnato G, Miraldi F, Sansone L and Russo
MA: Myocardial aldosterone receptor and aquaporin 1 up-regulation
is associated with cardiomyocyte remodeling in human heart failure.
J Clin Med. 10(4854)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang ZV, Rothermel BA and Hill JA:
Autophagy in hypertensive heart disease. J Biol Chem.
285:8509–8514. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu H, Tannous P, Johnstone JL, Kong Y,
Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA and Hill
JA: Cardiac autophagy is a maladaptive response to hemodynamic
stress. J Clin Invest. 117:1782–1793. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nakai A, Yamaguchi O, Takeda T, Higuchi Y,
Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et
al: The role of autophagy in cardiomyocytes in the basal state and
in response to hemodynamic stress. Nat Med. 13:619–624.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Tomanek RJ, Trout JJ and Lauva IK:
Cytochemistry of myocardial structures related to degenerative
processes in spontaneously hypertensive and normotensive rats. J
Mol Cell Cardiol. 16:227–237. 1984.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pfeffer JM, Pfeffer MA, Fishbein MC and
Frohlich ED: Cardiac function and morphology with aging in the
spontaneously hypertensive rat. Am J Physiol. 237:H461–H468.
1979.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schafflhuber M, Volpi N, Dahlmann A,
Hilgers KF, Maccari F, Dietsch P, Wagner H, Luft FC, Eckardt KU and
Titze J: Mobilization of osmotically inactive Na+ by growth and by
dietary salt restriction in rats. Am J Physiol Renal Physiol.
292:F1490–F1500. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Titze J and Machnik A: Sodium sensing in
the interstitium and relationship to hypertension. Curr Opin
Nephrol Hypertens. 19:385–392. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Navarro P, Chiong M, Volkwein K, Moraga F,
Ocaranza MP, Jalil JE, Lim SW, Kim JA, Kwon HM and Lavandero S:
Osmotically-induced genes are controlled by the transcription
factor TonEBP in cultured cardiomyocytes. Biochem Biophys Res
Commun. 372:326–330. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ren J, Wu NN, Wang S, Sowers JR and Zhang
Y: Obesity cardiomyopathy: Evidence, mechanisms, and therapeutic
implications. Physiol Rev. 101:1745–1807. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rabinovich-Nikitin I, Rasouli M, Reitz CJ,
Posen I, Margulets V, Dhingra R, Khatua TN, Thliveris JA, Martino
TA and Kirshenbaum LA: Mitochondrial autophagy and cell survival is
regulated by the circadian clock gene in cardiac myocytes during
ischemic stress. Autophagy. 17:3794–3812. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kohlhaas M, Liu T, Knopp A, Zeller T, Ong
MF, Bohm M, O'Rourke B and Maack C: Elevated cytosolic Na+
increases mitochondrial formation of reactive oxygen species in
failing cardiac myocytes. Circulation. 121:1606–1613.
2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Huang P, Shen Z, Yu W, Huang Y, Tang C, Du
J and Jin H: Hydrogen sulfide inhibits high-salt diet-induced
myocardial oxidative stress and myocardial hypertrophy in dahl
rats. Front Pharmacol. 8(128)2017.PubMed/NCBI View Article : Google Scholar
|