Introduction
Owing to the high similarity in clinical
manifestations and genetics between polypoid choroidal vasculopathy
(PCV) and age-related macular degeneration (AMD), PCV was
hypothesized to be an AMD subtype (1). However, unlike AMD, PCV is more
common in people of colour and lesions occur outside the vascular
arch or the nasal side of the optic disc, in addition to in the
macula. AMD, on the other hand, is common in Caucasians, and the
lesions are concentrated in the macula (2). The primary difference between the two
diseases is response to intravitreal anti-vascular endothelial
growth factor (anti-VEGF) drugs in patients with PCV patients is
not as good as that in AMD patients (2-4).
These findings suggest that the pathological mechanisms of PCV and
AMD differ.
To improve the treatment efficacy for PCV and AMD,
differences between the two diseases have been explored (2). The pathogenesis of both PCV and AMD
is associated with choroidal vessels (2). A typical PCV subtype with choroidal
thickness ≥257 µm has a significantly greater choroidal vascular
area in the macular and foveal regions compared with that in
typical AMD (5). The vortex
vessels, located in the middle layer of the eyeball wall, drain the
choroid. They are difficult to detect by direct observation but can
be observed more clearly with indocyanine green angiography (ICGA)
than with other non-invasive techniques (6). However, research on vortex veins
mostly transforms the ICGA image into binary image, and then
manually segment the quadrants based on the position of the vortex
vein to measure the brightness of each quadrant for research
(7). The present study aimed to
develop a method that is simpler and more suitable for clinical use
and easier for evaluating vortex vein filling.
Moreover, the present study aimed to describe the
differences in vortex vein engorgement and appearance between PCV,
AMD and healthy people of the same age using ICGA to reveal
differences in vortex vein anatomy between PCV and AMD.
Patients and methods
Patients
Patients orally agreed to the use of their data in
the present study. Ethical approval for this retrospective study
was obtained from the Institutional Review Board of the Zhongshan
Ophthalmic Centre (approval no. 2022KYPJ173). In total, 180
participants(mean age, 64.12±8.87 years; range, 52-75 years) were
recruited in this study, including 109 males and 71 females. All
participants underwent ICGA (SPECTRALIS Diagnostic Imaging
Platform; Heidelberg Engineering, Inc.) and optical coherence
tomography (OCT) (SPECTRALIS® OCT; Heidelberg
Engineering Inc.) between January 2018 and January 2022 at the
Zhongshan Ophthalmic Centre, Guangzhou, China.
The present study included 63 patients with PCV, 50
with AMD and 67 healthy control group. Based on the results of
fundus examination, OCT, fundus fluorescein angiography (FFA) and
ICGA, age- and sex-matched patients were grouped based on diagnosis
into PCV, AMD and healthy control group. Only one eye was included
for patients diagnosed with bilateral PCV or AMD. In healthy
participants, only the eye with the best-corrected visual acuity
(>20/16) was included.
The following exclusion criteria were adopted:
History of prior ocular surgery or trauma(excluded 15 PCV
patients); severe vitreous haemorrhage that may affect imaging
examination (excluded two PCV patients); any systemic disease that
may affect blood flow, such as diabetes mellitus or hypertension
(excluded one PCV patients and three AMD patients); central serous
chorioretinopathy (CSC); primary glaucoma; optic neuritis; retinal
vein occlusion; choroidal melanoma; retinal vasculitis; uveitis; an
epiretinal membrane that may affect ocular circulation (excluded
one PCV patients and five AMD patients) or moderate to high myopia
(defined as a spherical equivalent refractive error in phakic eyes
<-3.00 D) (excluded nine healthy participants).
We conducted another screening to exclude the cases
who only received monocular ICGA and OCT examination and included
44 cases of unilateral PCV and 18 cases of unilateral AMD. The
diseased eye was included in the PCV/AMD group, and the healthy
fellow eye was included in the PCV/AMD fellow eye group.
Following intravenous injection of 5 ml 25 mg ICG
(Dandong Yichuang Pharmaceutical Co., Ltd), ICGA images were
recorded. Early-stage images (5 min after dye injection) were
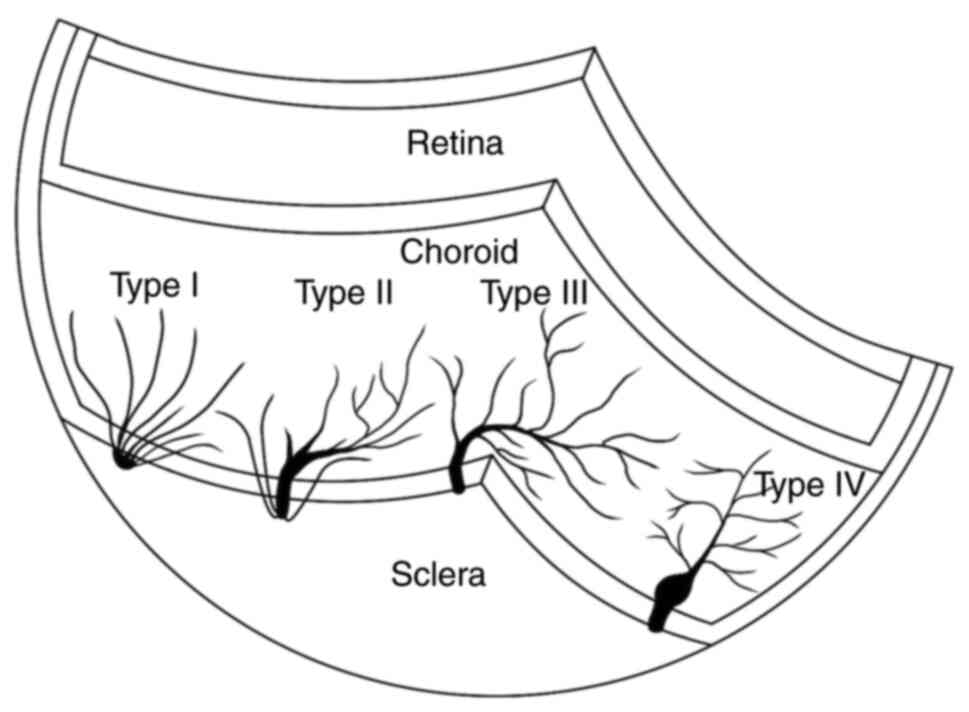
selected for analysis. The vortex veins were separated into four
categories according to a previous method (8). The branches of type I vortex veins do
not converge and pass directly through the sclera, whereas all
branches of type IV (complete with ampulla) converge to form the
ampulla, which is a complete vortex system. Type IV systems have a
larger root area due to the dilated ampulla (8). The fundus was divided into four
quadrants: Superior and inferior temporal and superior and inferior
nasal. Patient characteristics, such as sex, age, number, location
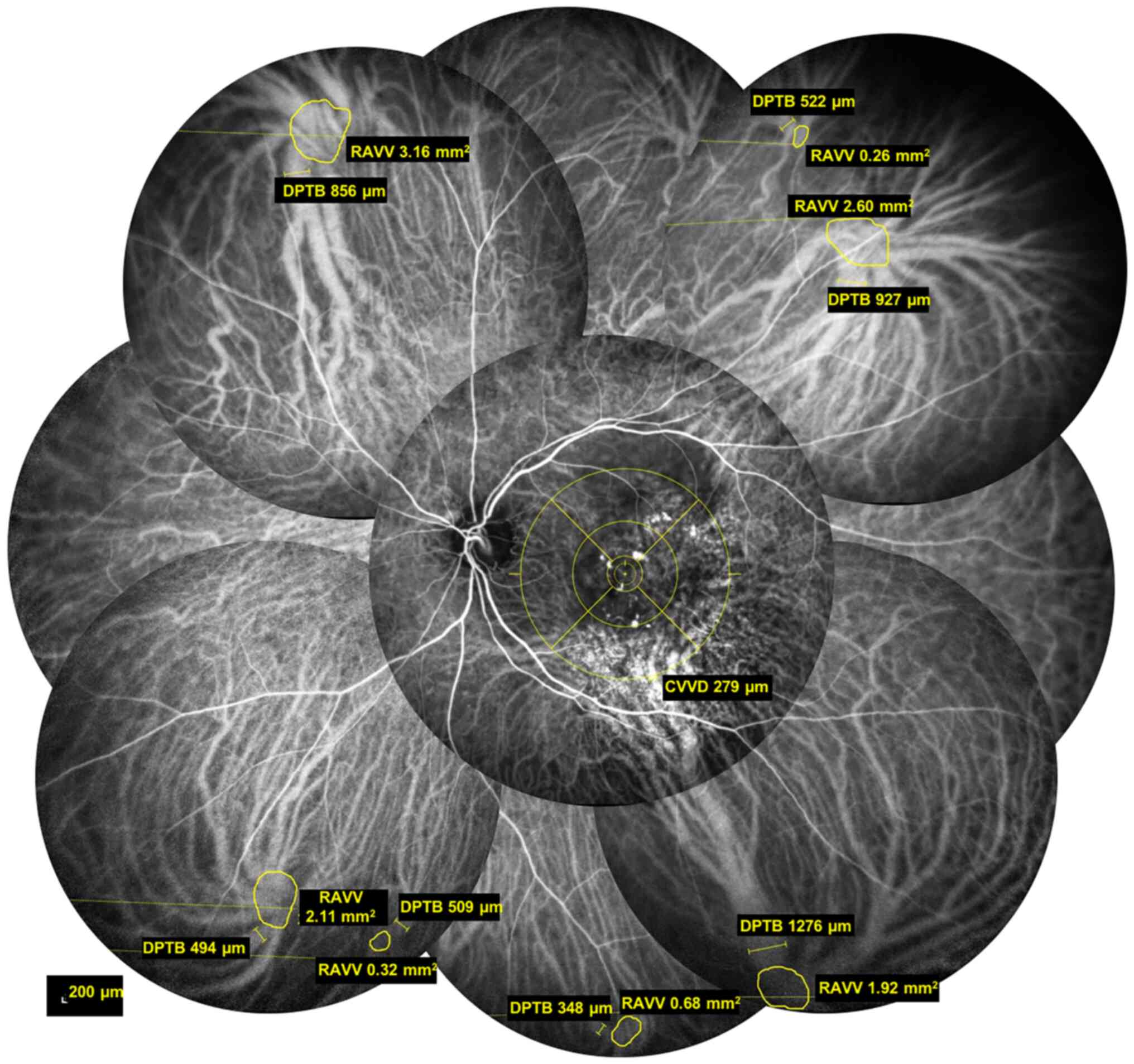
and type of vortex veins were recorded. The sketching tool of the
retinal device was used to mark the root area and diameter of the
thickest branch of each vortex vein (Fig. 1). The centre of a concentric circle
was placed on the macula, the thickest vortex vein branch
intersecting with the outermost circle was selected and its
diameter was measured and stored as the central vortex vein
diameter (CVVD). The ends of each vortex vein branch were connected
with a smooth curve and the area enclosed by the curve was defined
as the root area of the vortex vein (RAVV). The width of the
thickest first-order branch of the vortex vein was defined as the
diameter of the peripheral thickest branch (DPTB). The mean RAVV
(MRAVV) and MDPTB were calculated. Vortex vein anastomosis was
observed when vortex vein branches connected the two vortex vein
systems on IGCA. The percentage of eyes with vortex vein
anastomosis in each group was calculated and recorded as the
percentage of vortex vein anastomosis (PVVA). Subfoveal choroidal
thickness (SFCT) was measured using SPECTRALIS® OCT
device. All labelling was performed separately by two experienced
ophthalmologists (CXC and XMX) and the mean of the two measurements
was used as the final data.
Statistical analysis
One-way ANOVA was used to compare differences in
age, sex, number of vortex veins (NVV), CVVD, MRAVV, MDPTB, and
SFCT between PCV, AMD and healthy controls, as well as to compare
differences in the NVV between the four quadrants. When one-way
ANOVA indicated a significant difference between PCV, AMD and
healthy groups, the least-significant-difference test was used to
perform pairwise comparisons. One-way ANOVA was used to compare PCV
group differences in NVV, MRAVV, and MDPTB within each quadrant,
followed by post hoc Tukey's test was used to perform pairwise
comparisons. χ2 test was used to compare the differences
in the proportions of subretinal haemorrhages and four types of
vortex veins in each quadrant of the PCV group and differences in
the proportions of the four types of vortex veins and PVVA among
the PCV, AMD and healthy controls. Paired t test was used to
compare affected and healthy eyes in patients with PCV/AMD.
Receiver operating characteristic (ROC) curve of the CVVD, MRAVV
and MDPTB were drawn between polypoid choroidal vasculopathy and
age-related macular degeneration group. Area under the curve (AUC)
for CVVD distinguished between PCV and AMD. Data are presented as
the mean ± standard deviation. We repeated the independent
experiment twice. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using SPSS for Windows (version 17.0; SPSS, Inc.).
Results
The present study included 180 participants,
including 63 patients with PCV, 50 with AMD and 67 healthy
age-matched controls. There were no significant differences in sex
or age between the three groups (P>0.05; data not shown). A
total of 44 contralateral healthy eyes of patients with PCV were
included in the PCV fellow eye group. A total of 18 contralateral
healthy eyes of patients with AMD were included in the AMD fellow
eye group.
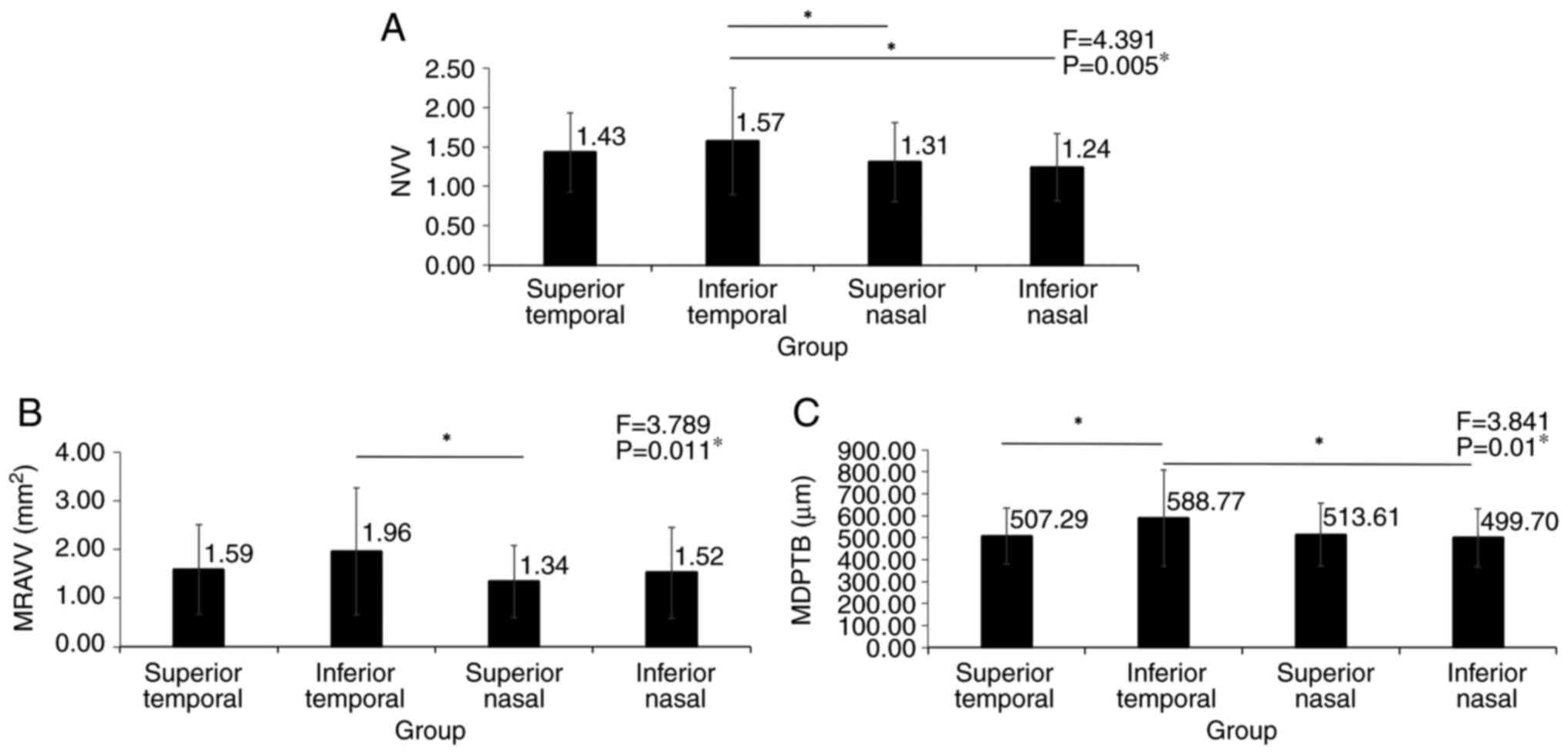
Differences in NVV, CVVD, MRAVV, MDPTB, SFCT and
PVVA were compared (Fig. 2). There
were no significant differences in NVV and SFCT between the three
groups (P>0.05; Fig. 2A and
E). CVVD in the PCV group was
significantly increased by 1.24-fold compared with that in the
healthy control group and by 1.14-fold compared with that in the
AMD group (P<0.05; Fig. 2B).
MRAVV in the PCV group was significantly decreased compared with
that in the healthy controls (P=0.004; Fig. 2C). MDPTB in the PCV group was
significantly wider compared with that in the AMD group (P=0.013;
Fig. 2D). PVVA in the PCV group
was significantly increased compared with that in healthy controls
(P=0.006; Fig. 2F).
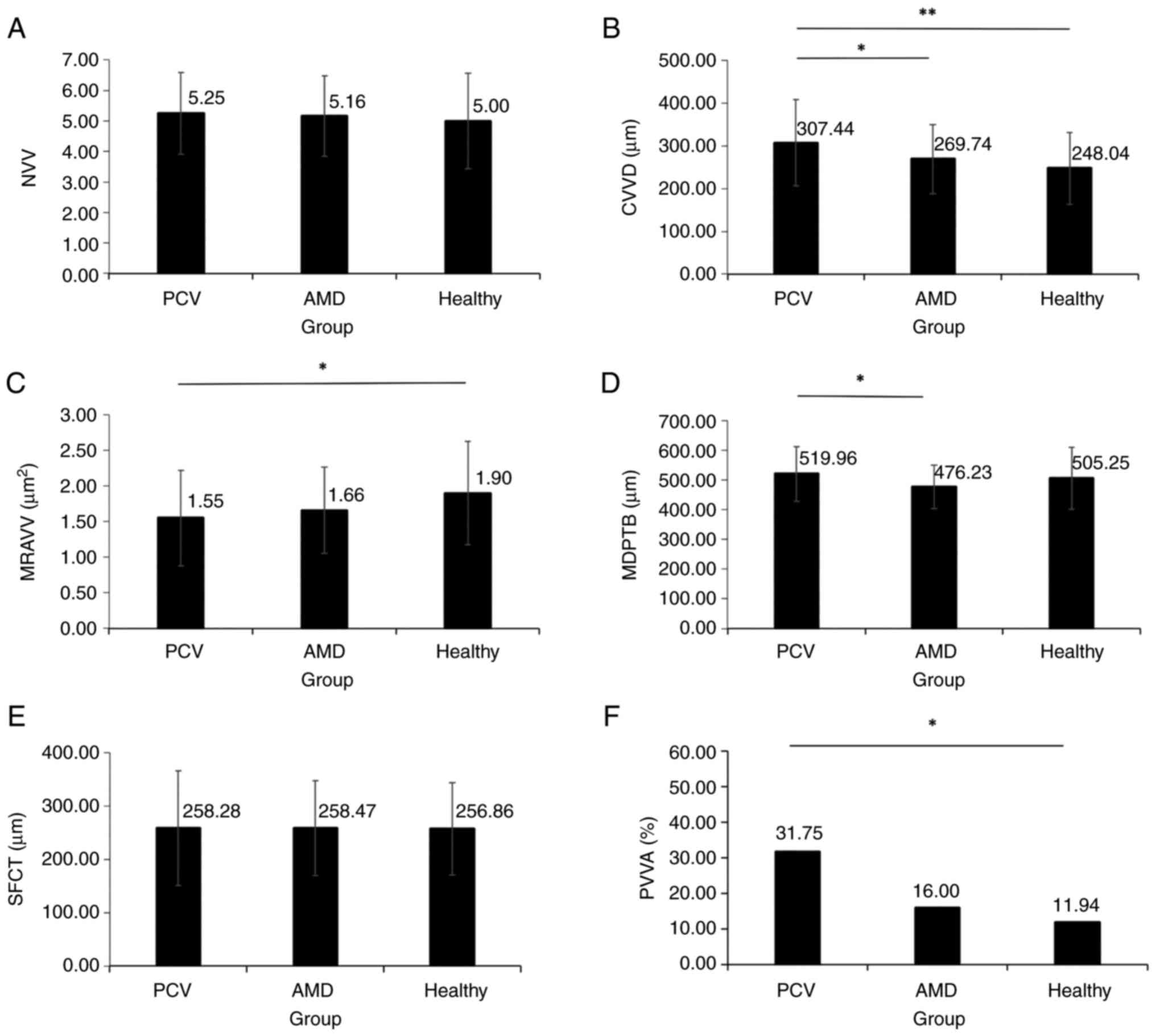 | Figure 2NVV, CVVD, MRAVV, MDPTB, SFCT and PVVA
among PCV, AMD and healthy groups. (A) There were no significant
differences in NVV between three groups. (B) CVVD of the PCV group
is significantly wider than that of the AMD group and the healthy
group. (C) MRAVV of the PCV group is significantly larger than that
of the healthy group. (D) MDPTB of the PCV group is significantly
wider than that of the AMD group. (E) There were no significant
differences in SFCT between groups. (F) PVVA in the PCV group is
significantly higher than that of the healthy group. NVV, number of
vortex veins; CVVD, central vortex vein diameter; PCV, polypoid
choroidal vasculopathy; AMD, age-related macular degeneration;
MRAVV, mean root area of the vortex vein; MDPTB, mean diameter of
the peripheral thickest branch; SFCT, subfoveal choroidal
thickness; PVVA, percentage of the vortex vein anastomosis.
(*P<0.05, **P<0.001). |
PCV group showed the lowest proportion of type IV
vortex veins (complete with ampulla), while the proportion of type
I (absent) vortex veins was highest; this was significantly
different between the PCV, AMD and healthy controls (P<0.001;
Table I). NVV in the inferior
temporal quadrant of the PCV group was increased compared with that
in the AMD group (P=0.034) and NVV in the superior temporal and
nasal and inferior nasal quadrants showed no significant
differences between groups (P>0.05; Table II).
 | Table IDistribution of vortex vein types in
each group. |
Table I
Distribution of vortex vein types in
each group.
| | Group, n (%) | |
|---|
| Characteristic | PCV, 63 (35.00) | AMD, 50 (27.78) | Healthy controls, 67
(37.22) | χ2 | P-value |
|---|
| Type I (vortex vein
absent) | 108 (25.96) | 82 (15.05) | 102 (14.43) | 27.520 | <0.001 |
| Type II
(incomplete) | 115 (27.64) | 177 (32.48) | 194 (27.44) | 4.380 | 0.112 |
| Type III
(complete) | 110 (26.44) | 165 (30.28) | 201 (28.43) | 1.706 | 0.426 |
| Type IV (complete
with ampulla) | 83 (19.95) | 121 (22.20) | 210 (29.70) | 16.319 | <0.001 |
| Total | 416 (100.00) | 545 (100.00) | 707 (100.00) | - | - |
 | Table IINumber of vortex veins in each
quadrant. |
Table II
Number of vortex veins in each
quadrant.
| | Group, n (%) | |
|---|
| Quadrant | PCV, 63.00
(35.00) | AMD, 50.00
(27.78) | Healthy controls,
67.00 (37.22) | F-value | P-value |
|---|
| Superior
temporal | 1.43±0.50 | 1.29±0.54 | 1.37±0.67 | 0.841 | 0.433 |
| Inferior
temporal | 1.57±0.68 | 1.28±0.45 | 1.39±0.58 | 3.448 | 0.034 |
| Superior nasal | 1.31±0.50 | 1.36±0.63 | 1.21±0.54 | 1.062 | 0.348 |
| Inferior nasal | 1.24±0.43 | 1.28±0.50 | 1.09±0.56 | 2.261 | 0.107 |
NVV, MRAVV and MDPTB of the PCV group were the
largest in the inferior temporal region, with significant
differences between quadrants (P<0.05; Fig. 3). The mean number of polyps in the
PCV group was 3.37±3.47 (range, 0-15). In 15 eyes, polypoid lesions
could not be observed due to severe subretinal haemorrhage on ICGA.
Subretinal haemorrhage was absent in 24 and present in 39
participants in the PCV group, all of which involved the posterior
pole macular area. Subretinal haemorrhages involved both superior
and inferior temporal regions in seven eyes, and the posterior pole
and peripheral parts in two eyes. The proportion of type I vortex
veins in the PCV group was significantly different between
quadrants (P<0.001; Table
III). Among the PCV participants, subretinal haemorrhage was
observed in 47.62% of the inferior temporal and 23.81% in the
superior temporal quadrants, with significant differences between
the quadrants (P<0.001; Table
III).
 | Table IIIProportion of type of vortex veins
(complete with ampulla) and subretinal haemorrhage in participants
with polypoid choroidal vasculopathy in each quadrant. |
Table III
Proportion of type of vortex veins
(complete with ampulla) and subretinal haemorrhage in participants
with polypoid choroidal vasculopathy in each quadrant.
| | Quadrant, n
(%) | |
|---|
| Characteristic | Superior
temporal | Inferior
temporal | Superior nasal | Inferior nasal | Total | χ2 | P-value |
|---|
| Type I (vortex vein
absent) | 42 (36.21) | 33 (30.56) | 14 (15.05) | 19 (19.19) | 116 (100.00) | 23.847 | <0.001 |
| Type II
(incomplete) | 26 (24.07) | 29 (26.85) | 29 (26.85) | 31 (28.70) | 108 (100.00) | 0.604 | 0.895 |
| Type III
(complete) | 26 (27.96) | 27 (29.03) | 28 (30.11) | 29 (31.18) | 93 (100.00) | 0.258 | 0.968 |
| Type IV (complete
with ampulla) | 22 (18.97) | 19 (17.59) | 22 (23.66) | 20 (20.20) | 99 (100.00) | 0.412 | 0.938 |
| Subretinal
haemorrhage | 15 (23.81) | 30 (47.62) | 2 (3.17) | 3 (4.76) | 63 (100.00) | 51.198 | <0.001 |
Considering that the area under the curve of CVVD
was largest, with its' highest availability and clinical
practicability, CVVD was selected as the indicator to reveal the
difference between PCV and AMD. Cut-off value of CVVD is 252.5 µm
and sensitivity, specificity and AUC of 88.3%, 54.0% and 0.70
respectively (Fig. S1). CVVD in
the AMD group was significantly increased compared with that in the
AMD fellow eye group (P=0.024; Table
IV). Among the 18 participants with unilateral AMD, there were
no significant differences in NVV, MRAVV, MDPTB, SFCT and PVVA
between the affected and fellow eyes (P>0.05; Table IV). There were no significant
differences in NVV, CVVD, MRAVV, MDPTB, SFCT and PVVA between the
affected and fellow eyes in the PCV group (P>0.05; Table V).
 | Table IVDifferences in vortex veins between
affected and fellow eye in patients with age-related macular
degeneration. |
Table IV
Differences in vortex veins between
affected and fellow eye in patients with age-related macular
degeneration.
| | Groups, n (%) | |
|---|
| Parameter | Affected eye, 18
(10.00) | Fellow eye, 18
(10.00) | T-value | P-value |
|---|
| Number of vortex
veins | 5.16±1.39 | 5.11±1.52 | 0.136 | 0.893 |
| Central vortex vein
diameter, µm | 305.53±109.37 | 249.79±82.35 | 2.464 | 0.024a |
| Mean root area of
the vortex vein, mm2 | 1.64±0.60 | 1.53±0.45 | 0.703 | 0.491 |
| Mean diameter of
the peripheral thickest branch, µm | 475.22±63.00 | 479.36±69.57 | -0.214 | 0.833 |
| Subfoveal choroidal
thickness, µm | 256.00±107.74 | 268.36±127.26 | -0.543 | 0.599 |
| Vortex vein
anastomosis, % | 5.56 | 11.11 | -0.566 | 0.579 |
 | Table VDifferences in vortex veins between
affected and fellow eye in patients with polypoid choroidal
vasculopathy. |
Table V
Differences in vortex veins between
affected and fellow eye in patients with polypoid choroidal
vasculopathy.
| | Group, n (%) | |
|---|
| Parameter | Affected eye, 44
(24.44) | Fellow eye, 44
(24.44) | T-value | P-value |
|---|
| Number of vortex
veins | 5.38±1.38 | 5.21±1.66 | 0.692 | 0.493 |
| Central vortex vein
diameter, µm | 296.71±83.12 | 313.02±66.02 | -1.217 | 0.230 |
| Mean root area of
the vortex vein, mm2 | 1.47±0.62 | 1.57±0.72 | -0.878 | 0.385 |
| Mean diameter of
the peripheral thickest branch, µm | 506.30±88.95 | 518.93±102.88 | -0.693 | 0.492 |
| Subfoveal choroidal
thickness, µm | 257.46±109.22 | 252.68±77.83 | 0.319 | 0.752 |
| Vortex vein
anastomosis, % | 27.27 | 22.73 | 0.628 | 0.533 |
Discussion
The present study aimed to identify differences in
venous system drainage patterns between PCV, AMD and healthy eyes.
The branches of the vortex veins in the PCV group were
significantly expanded in the posterior pole and periphery compared
with those in the AMD group because the CVVD and MDPTB were
significantly increased in the PCV group. If the CVVD was >252.5
µm on ICGA, the diagnosis was more likely to be PCV than AMD,
indicating that CVVD may serve as a point of differentiation
between PCV and AMD. The dilation of the vortex vein was asymmetric
in patients with PCV and the inferior temporal vortex vein was more
engorged. The appearance of the venous drainage system in patients
with PCV was different from that in the healthy control group. Type
IV vortex veins were least common, while type I veins were most
common in PCV eyes; these may point to the anatomical basis for the
pathogenesis of PCV.
Although typical PCV and AMD are not difficult to
differentiate using colour fundus photography, OCT, FFA and ICGA
(9), there are clinical cases that
are difficult to distinguish, requiring new points of
differentiation. The present results indicated that CVVD can be
used to distinguish between PCV and AMD, with a cut-off value of
252.5 µm. Gupta et al (5)
reported no significant differences in choroidal thickness and
vascular area between patients with PCV and those with typical AMD
after adjusting for age and hypertension. Luo et al
(10) found that the
choriocapillaris flow density of neovascular AMD fellow eyes is
significantly decreased compared with that in PCV and control eyes.
Takahashi et al (11) found
no significant difference in SFCT between neovascular AMD and PCV
but demonstrated that the ratio of the large choroidal vessel layer
thickness to SFCT is significantly increased in eyes with PCV
compared with that in eyes with typical neovascular AMD.
The present study indicated that PCV vortex vein
branches were significantly dilated and compensatory vortex vein
anastomosis was formed. The CVVD and PVVA of the PCV group were
significantly increased compared with that in healthy controls. PCV
vortex vein branches were dilated at the posterior pole and
peripheral choroid. PVVA in the PCV group was significantly
increased compared with that in the healthy controls. Chung et
al (12) found that PCV eyes
are more prone to vortex venous congestion than normal eyes using
ICGA, suggesting that vortex venous congestion may be associated
with PCV pathogenesis. Choroidal hypertension causes secondary
dilation of the vortex veins, which are compensatory anastomoses
that decrease intraluminal pressure (13). If the disease progresses, the
terminal blood vessels bulge locally and manifest as polypoid
lesions (12,14). Qiu et al (15) found statistically significant
differences in the proportion of diffuse collateral circulation
between PCV, neovascular AMD, central serous chorioretinopathy
(CSC) and healthy controls. During the progression of pachychoroid
spectrum disease, hyperaemia of the vortex veins may gradually
improve with the development of anastomosis between the superior
and inferior vortex veins (16).
Matsumoto et al (17) found
that neovascular AMD patients with pachychoroid are significantly
younger and include a higher proportion of males and increased SFCT
and frequency of macular vortex vein anastomoses compared with
patients with neovascular AMD without pachychoroid. Relative
choroidal thickening resulting from enlargement of deep choroidal
veins might be related to the mechanism of internal choroidal and
retinal pigment epithelium damage (18,19).
Intravitreal injection of anti-VEGF may also improve vision by
decreasing the focus and macular edema. Ryu et al (20) found that choroidal vascular density
(CVD) in PCV is correlated positively with choroidal thickness and
choroidal hyperpermeability and that a thicker choroid and
increased CVD are also associated with poor treatment response to
anti-VEGF injections. Subfoveal choroidal watershed zones are
associated with foveal PCV growth, suggesting that PCV arises
because of aberrant choroidal circulation (2,21).
Combined photodynamic therapy and intravitreal bevacizumab
significantly thin the total choroid in patients with PCV after 3
months of treatment and an increase in pachy vessel diameter and
choroidal vascularity has been observed in 31.33% of eyes with
recurrence of PCV (22).
Here, patients with PCV not only had extended
engorged vortex veins but also different appearances of venous
drainage systems compared with those in healthy controls. The four
types of vortex veins are illustrated in Figs. 4 and 5. Although vortex vein branches in the
PCV group showed dilation in both the posterior pole and periphery,
the MRAVV was significantly decreased compared with that in the
healthy controls. The vortex root area depended not only on the
degree of vortex dilatation but also on the appearance of the vein.
In the present study, the proportion of type IV was the lowest
while the proportion of type I drainage systems was the highest in
the PCV group, which was significantly different between the three
groups. This finding explains why the PCV group had the lowest
MRAVV. The vortex ampulla and intrascleral portion of the vortex
veins regulate the choroidal outflow site by flow resistance and
may be involved in choroidal venous congestion (23,24).
The non-expandable intrascleral route of vortex veins limits the
upregulation of choroidal venous outflow (25). Although vortex veins converge, the
effects on intraluminal pressure are different from pressure that
arises when the veins first penetrate the sclera and after
penetrating the sclera. The ampulla region of the vortex vein
resembles a cistern, which relieves luminal pressure within the
vortex vein. The vortex vein drains from the choroid to the outside
of the eye through the sclera. The vortex vein segment passing
through the sclera has poor diastolic ability, resulting in high
luminal pressure of the vortex vein in the intrachoroidal segment.
The ampullary region relieves high intraluminal pressure (13,26).
The total number of vortex veins in patients with PCV was not
significantly different compared with that in healthy controls, but
the low proportion of type IV vortex veins and a high proportion of
type I vortex veins, which would lead to increased intraluminal
pressure in the vortex veins, may be related to the pathogenesis of
PCV.
The present study found that choroidal vessels are
asymmetric in each ocular quadrant and extended engorged vortex
veins are more likely to occur in the inferior-temporal quadrant in
PCV, which may be associated with the pathogenic mechanism
underlying PCV. The inferior temporal NVV, MRAVV and MDPTB of the
PCV group were significantly increased compared with those of the
other groups and vortex veins were unevenly distributed among the
four quadrants in the PCV group. Subretinal haemorrhage appeared in
the inferior and the superior temporal quadrant in 47.62 and 23.81%
of the patients in the PCV group, respectively, with significant
differences between the quadrants in this group. Similar to the
present study, Chung et al (12) found that vortex venous congestion
in PCV eyes is most frequently distributed in the inferior,
followed by the superior, temporal quadrant. This suggests that PCV
lesions tend to occur in the inferior temporal region, which is
closely related to the distribution of the vortex veins. In the PCV
group, the proportion of type I vortex veins on the temporal side
was significantly increased compared with that on the nasal side,
with the highest proportion occurring in the superior temporal and
the second highest in the inferior temporal region, which might be
associated with the predominance of PCV bleeding in the inferior
temporal region. Due to gravity, the inferior temporal vortex vein
fills with more blood compared with the superior temporal vein.
Therefore, submacular haemorrhage is more likely to occur in the
inferior than in the superior temporal quadrant. Bacci et al
(27) reported asymmetric
choroidal drainage in the macula of 59% of pachychoroid eyes, with
choroidal vascular hyperpermeability (CVH) and vortex venous
anastomosis more prominent in areas with the greatest choroidal
thickness. By comparing the brightness of each quadrant on
ultra-widefield ICGA, Jung et al (7) found that the inferior temporal
quadrant was significantly brighter than the superior nasal
quadrant and eyes with CSC and thick choroidal pigment
epitheliopathy showed asymmetric choroidal venous outflow.
Imbalanced choroidal venous drainage and congestion of specific
vortex vein systems may lead to choroidal venous insufficiency,
characterized by regional choroidal thickening, CVH and remodelling
of venous drainage pathways (27).
The unbalanced expansion and congestion of vortex veins facilitate
their remodelling to form anastomotic branches, thereby decreasing
the local intraluminal pressure (13).
The present study compared vortex vein parameters in
the affected and fellow eyes of patients with unilateral PCV and
AMD. There were no significant differences in NVV, SFCT, MRAVV,
MDPTB or PVVA between the affected and healthy fellow eyes. This
suggested that in patients with PCV and AMD with unilateral onset,
similar anatomical changes are present in the vortex veins of the
affected and fellow eye; therefore, the healthy fellow eye may
develop into an affected eye. Wu et al (28) found that 70% of fellow eyes of
patients with unilateral PCV show CVH on ICGA. Yanagi et al
(29) found that ~1/5 fellow eyes
with unilateral neovascular AMD and PCV exhibit non-exudative
neovascularization. CNV occurs in 9% of fellow eyes in individuals
with unilateral PCV or aneurysmal type 1 neovascularization,
usually retinal pigment epithelium and outer retinal abnormalities
with pachy vessels (30). A study
that followed patients with unilateral PCV and CNV for up to 5
years found that 17% of fellow eyes developed PCV or CNV (31).
In conclusion, the present study describes various
parameters, such as the number, branch diameter, root area,
location, type and anastomosis of vortex veins, in PCV, AMD and
healthy control eyes, and proposed that CVVD, MRAVV and MDPTB are
parameters that quantitatively evaluate these vessels. Moreover, it
sheds light on the differences in the morphology and types of
vortex veins in PCV, AMD, and healthy individuals. The branches of
the vortex veins of the PCV were significantly dilated in the
posterior pole and periphery, while lower proportion of type IV
vortex veins may be pathognomonic for this condition. Based on the
present findings, CVVD may be a key distinguishing point between
AMD and PCV using ICGA. Patients with unilateral onset of PCV and
AMD showed similar vortex vein anatomy in affected and healthy
eyes, indicating the potential for the development of disease in
the fellow eye. The present study provides a basis for further
exploration of PCV pathogenesis based on vortex vein
dilatation.
Supplementary Material
ROC curve of the CVVD, MRAVV and MDPTB
between polypoid choroidal vasculopathy and age-related macular
degeneration group. CVVD, central vortex vein diameter; MDPTB, mean
diameter of the peripheral thickest branch; MRAVV, mean root area
of the vortex vein; ROC, receiver operating characteristic.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Bethune·Lumitin
Research Funding for the Young and Middle-aged Ophthalmologists
(grant no. BJ-LM2021014J).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CXC, XMX, TL, BQL, XHH, SSY, ZQL, QW, JLC, LL and YL
designed the study. CXC and XMX collected the patient data. CXC
analysed data and wrote the manuscript. All authors revised and
edited the manuscript. All authors have read and approved the final
manuscript. CXC, LL and YL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Patients orally agreed to the use of their data in
the present study. The study was approved by the Ethics Committee
of the Zhongshan Ophthalmic Centre (approval no. 2022KYPJ173).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen LJ: Genetic association of
age-related macular degeneration and polypoidal choroidal
vasculopathy. Asia Pac J Ophthalmol (Phila). 9:104–109.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wong CW, Yanagi Y, Lee WK, Ogura Y, Yeo I,
Wong TY and Cheung CMG: Age-related macular degeneration and
polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res.
53:107–139. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cheung CMG, Lai TYY, Ruamviboonsuk P, Chen
SJ, Chen Y, Freund KB, Gomi F, Koh AH, Lee WK and Wong TY:
Polypoidal choroidal vasculopathy: Definition, pathogenesis,
diagnosis, and management. Ophthalmology. 125:708–724.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Díaz-Villamarín X, Blánquez-Martínez D,
Pozo-Agundo A, Pérez-Gutiérrez AM, Muñoz-Ávila JI,
Antúnez-Rodríguez A, Fernández-Gómez AE, García-Navas P,
Martínez-González LJ and Dávila-Fajardo CL: Genetic variants
affecting anti-VEGF drug response in polypoidal choroidal
vasculopathy patients: A systematic review and meta-analysis. Genes
(Basel). 11(1335)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gupta P, Ting DSW, Thakku SG, Wong TY,
Cheng CY, Wong E, Mathur R, Wong D, Yeo I and Cheung CMG: Detailed
characterization of choroidal morphologic and vascular features in
age-related macular degeneration and polypoidal choroidal
vasculopathy. Retina. 37:2269–2280. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Verma A, Bacci T, Sarraf D, Freund KB and
Sadda SR: Vortex vein imaging: What can it tell us? Clin
Ophthalmol. 15:3321–3331. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jung JJ, Yu DJG, Ito K, Rofagha S, Lee SS
and Hoang QV: Quantitative assessment of asymmetric choroidal
outflow in pachychoroid eyes on ultra-widefield indocyanine green
angiography. Invest Ophthalmol Vis Sci. 61(50)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rutnin U: Fundus appearance in normal
eyes. I. The choroid. Am J Ophthalmol. 64:821–839. 1967.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Laude A, Cackett PD, Vithana EN, Yeo IY,
Wong D, Koh AH, Wong TY and Aung T: Polypoidal choroidal
vasculopathy and neovascular age-related macular degeneration: Same
or different disease? Prog Retin Eye Res. 29:19–29. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Luo M, Zhao X, Zhao N, Yuan M, Yang J, Dai
R and Chen Y: Comparison of choriocapillary flow density between
fellow eyes of polypoidal choroidal vasculopathy and neovascular
age-related macular degeneration. BMC Ophthalmol.
20(162)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Takahashi Y, Koizumi H, Hasegawa T, Izumi
T, Maruko I, Sonoda S, Sakamoto T and Iida T: Comparison of
subfoveal choroidal structures in typical neovascular age-related
macular degeneration and polypoidal choroidal vasculopathy. Jpn J
Ophthalmol. 62:576–583. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chung SE, Kang SW, Kim JH, Kim YT and Park
DY: Engorgement of vortex vein and polypoidal choroidal
vasculopathy. Retina. 33:834–840. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kishi S and Matsumoto H: A new insight
into pachychoroid diseases: Remodeling of choroidal vasculature.
Graefes Arch Clin Exp Ophthalmol. 260:3405–3417. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jeong A, Lim J and Sagong M: Choroidal
vascular abnormalities by ultra-widefield indocyanine green
angiography in polypoidal choroidal vasculopathy. Invest Ophthalmol
Vis Sci. 62(29)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qiu B, Zhang X, Li Z, Chhablani J, Fan H,
Wang Y and Xie R: Characterization of choroidal morphology and
vasculature in the phenotype of pachychoroid diseases by
swept-source OCT and OCTA. J Clin Med. 11(3243)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Matsumoto H, Hoshino J, Arai Y, Mukai R,
Nakamura K, Kikuchi Y, Kishi S and Akiyama H: Quantitative measures
of vortex veins in the posterior pole in eyes with pachychoroid
spectrum diseases. Sci Rep. 10(19505)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Matsumoto H, Hoshino J, Mukai R, Nakamura
K, Kishi S and Akiyama H: Clinical characteristics and pachychoroid
incidence in Japanese patients with neovascular age-related macular
degeneration. Sci Rep. 12(4492)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sakurada Y, Fragiotta S, Leong BCS, Parikh
R, Hussnain SA and Freund KB: Relationship between choroidal
vascular hyperpermeability, choriocapillaris flow density, and
choroidal thickness in eyes with pachychoroid pigment
epitheliopathy. Retina. 40:657–662. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gal-Or O, Dansingani KK, Sebrow D,
Dolz-Marco R and Freund KB: Inner choroidal flow signal attenuation
in pachychoroid disease: Optical coherence tomography angiography.
Retina. 38:1984–1992. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ryu G, Moon C, van Hemert J and Sagong M:
Quantitative analysis of choroidal vasculature in polypoidal
choroidal vasculopathy using ultra-widefield indocyanine green
angiography. Sci Rep. 10(18272)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shin MK, Lee JE, Byon IS and Park SW:
Choroidal watershed zone and growth of polypoidal choroidal
vasculopathy. Curr Eye Res. 42:252–259. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Baek J, Lee JH, Jeon S and Lee WK:
Choroidal morphology and short-term outcomes of combination
photodynamic therapy in polypoidal choroidal vasculopathy. Eye
(Lond). 33:419–427. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Spaide RF: CHOROIDAL BLOOD FLOW: Review
and potential explanation for the choroidal venous anatomy
including the vortex vein system. Retina. 40:1851–1864.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Spaide RF, Cheung CM, Matsumoto H, Kishi
S, Boon CJF, van Dijk EHS, Mauget-Faysse M, Behar-Cohen F, Hartnett
ME, Sivaprasad S, et al: Venous overload choroidopathy: A
hypothetical framework for central serous chorioretinopathy and
allied disorders. Prog Retin Eye Res. 86(100973)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Takahashi K and Kishi S: Remodeling of
choroidal venous drainage after vortex vein occlusion following
scleral buckling for retinal detachment. Am J Ophthalmol.
129:191–198. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yu PK, Tan PE, Cringle SJ, McAllister IL
and Yu DY: Phenotypic heterogeneity in the endothelium of the human
vortex vein system. Exp Eye Res. 115:144–152. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bacci T, Oh DJ, Singer M, Sadda S and
Freund KB: Ultra-widefield indocyanine green angiography reveals
patterns of choroidal venous insufficiency influencing pachychoroid
disease. Invest Ophthalmol Vis Sci. 63(17)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu H, Sugano Y, Itagaki K, Kasai A,
Shintake H and Sekiryu T: The characteristics of choriocapillaris
flow void in the unilateral polypoidal choroidal vasculopathy
fellow eyes. Sci Rep. 11(23059)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yanagi Y, Mohla A, Lee WK, Lee SY, Mathur
R, Chan CM, Yeo I, Wong TY and Cheung CMG: Prevalence and risk
factors for nonexudative neovascularization in fellow eyes of
patients with unilateral age-related macular degeneration and
polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci.
58:3488–3495. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Baek J, Cheung CMG, Jeon S, Lee JH and Lee
WK: Polypoidal choroidal vasculopathy: Outer retinal and choroidal
changes and neovascularization development in the fellow eye.
Invest Ophthalmol Vis Sci. 60:590–598. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kim K, Kim JM, Kim DG, Yu SY and Kim ES:
Five-year follow-up of unaffected fellow eyes in patients with
polypoidal choroidal vasculopathy. Ophthalmologica. 243:172–177.
2020.PubMed/NCBI View Article : Google Scholar
|