Cancer is one of the most serious fatal diseases,
with limited treatment response and unfavourable prognosis
(1). Currently, surgery,
radiotherapy, chemotherapy and immunotherapy are the primary
methods used in clinical practice for management of cancer and,
although they have been regarded as the four pillars of cancer
therapy (2), these therapeutic
modalities have shortcomings. For example, for surgical resection,
the incidence of surgical trauma and complications is high and
complete removal of all tumour tissue is not guaranteed (3). Tumour radiation resistance and
collateral radiation-induced damage to surrounding healthy tissue
limit the clinical application of radiotherapy. For chemotherapy
and immunotherapy, although new treatment targets and novel drugs
are increasingly studied, challenges, such as the low targeting
efficacy and intrinsic toxicity of these treatments, remain to be
overcome (4). In addition to these
four pillars of cancer therapy, more recent studies have focused on
developing and improving non-invasive and more patient-friendly
modalities with improved treatment efficacy and a lower incidence
of side effects: Among these, high-intensity focused ultrasound
(HIFU) is a promising approach (5-7).
The identification of the potential of HIFU for
clinical therapy dates to the 1950s when it was demonstrated to be
an alternative therapeutic procedure for central nervous system
disorder (8,9). When HIFU is absorbed by target tissue
such as tumour masses, the temperature of the tissue increases to
>55˚C, inducing cell death via local coagulative necrosis
(10-12)
to thermally ablate the tumour mass. HIFU can also induce the
generation of small gas bubbles inside the target tissue; sudden
collapse of these bubbles results in an increase in the local
pressure up to 2-3 kPa, thus causing severe damage to the
surrounding tissues (13,14). It has also been shown that HIFU
temporarily disrupts the blood-brain barrier (BBB), which aids in
delivery of therapeutics into the central nervous system (15). Currently, HIFU has been proven
successful in the treatment of numerous diseases such as
Parkinson's disease (16,17), essential tremor (18,19),
adenomyosis (20) and solid tumour
masses (21,22). However, due to the absorption
features of HIFU, the penetration of HIFU to deep tumour tissue is
severely limited and not sufficient for tumour ablation (23). While increasing HIFU irradiation
dosage is a potential strategy to increase efficacy, the collateral
damage caused to the surrounding normal tissue would also increase
(24). Furthermore, although HIFU
primarily results in a focused ablative effect in the targeted
tumour mass, off-target collateral damage occurs, resulting in
undesired tissue injury and burns, vasospasm and haemorrhaging,
impotence, incontinence, formation of atrial-oesophageal fistula
and off-site rib necrosis (25,26).
Nanoparticles (NPs) are now being adopted to overcome these
challenges to improve the clinical value of HIFU (27).
NPs are 10-500 nm in size and have previously been
reported to increase therapeutic efficacy whilst decreasing the
incidence of side effects (28).
These nanomedicines selectively accumulate in tumour tissues to
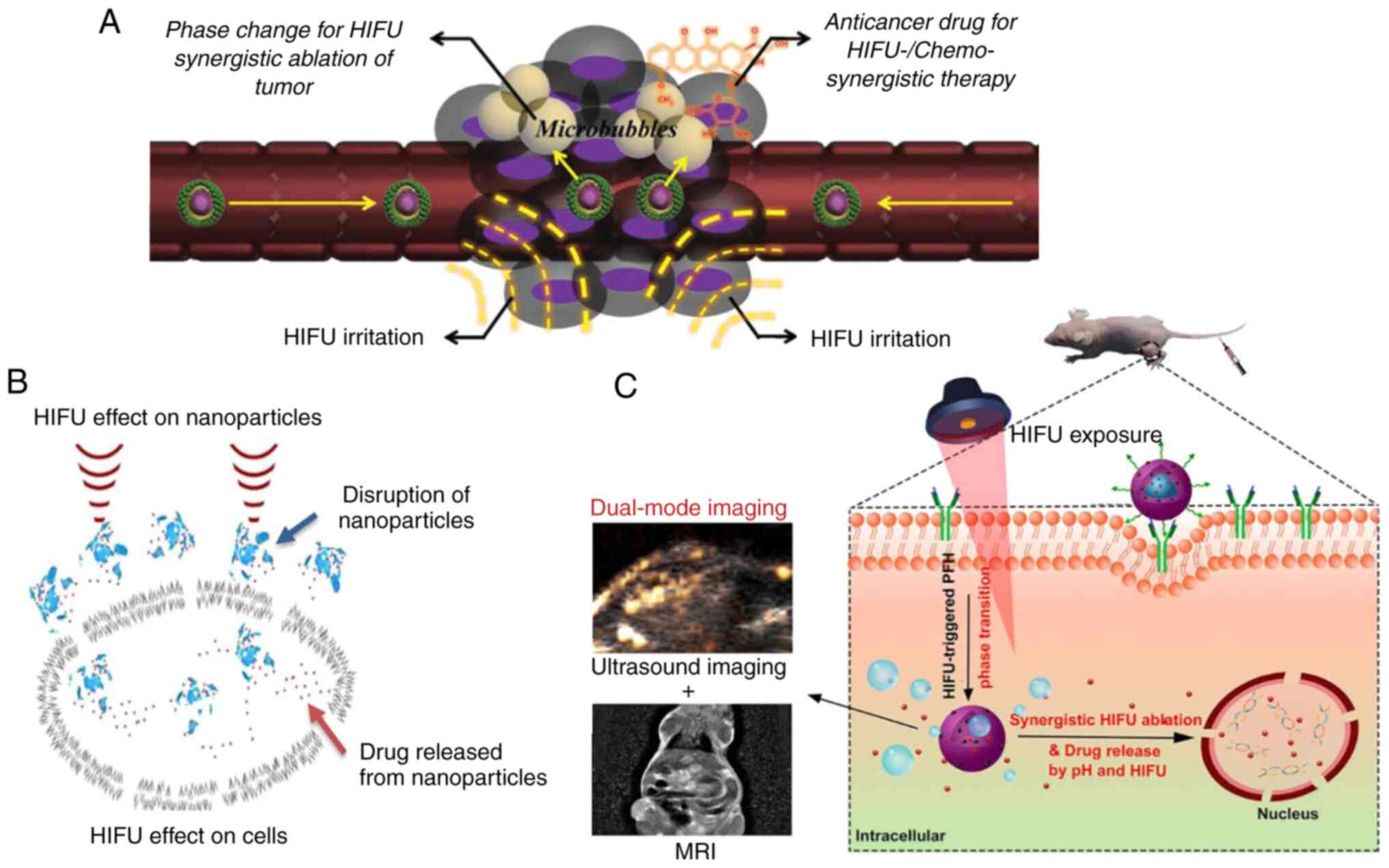
realize a selective and efficient therapeutic effect (Fig. 1A) (29). Furthermore, it has been indicated
that the adoption of NPs can effectively change the acoustic
environment (tissue structure, density, blood supply and functional
state on ultrasonic transmission and energy deposition during HIFU
treatment) of tumour tissues (30), making them more sensitive to HIFU,
resulting in greater ablative efficacy with the same or lower HIFU
irradiation doses. Additionally, since the first report on the
combination of HIFU with nanotechnology in 2000(31), it has been recognized that HIFU
induces target drug release from platforms such as NPs and
liposomes to enhance the ablative efficacy of HIFU and improve
safety (32); since then, studies
have attempted to design nanomedicines to improve the efficacy of
HIFU (33-36)
however, progress has not seen clinical translation. Additionally,
the promotion of theranostics also highlights novel opportunities
in this field. The present review aimed to provide an overview of
NPs in combination with HIFU for cancer treatment, including the
use of nanomedicines to increase the ablative efficacy of HIFU,
achieving greater synergic therapeutic efficacy and theranostics by
combining imaging probes and HIFU.
The combination of NPs and HIFU benefits cancer
treatment in multiple ways. By enhancing the permeability and
retention (EPR) effect, NPs selectively penetrate tumour tissues
and change the acoustic environment. NPs can enhance energy
deposition and magnify the thermal, mechanical and cavitation
effect via formation of microbubbles through a phase transition
(37), resulting in improved HIFU
ablation efficacy. Additionally, HIFU can alter vascular
permeability and disrupt blockade of overexpressed extracellular
matrix, thus enhancing the selective accumulation of NPs into
tumour tissue (38,39). In addition, HIFU physically induces
formation of cell membrane pores via sonoporation, enabling more
effective cellular internalization and accumulation of NPs
(Fig. 1B) (40). Furthermore, HIFU disrupts NPs to
trigger localized drug release at the target site (41), effectively decreasing the
off-target damage to normal tissue.
Lipid-based NPs such as liposomes and solid lipid
NPs, are phospholipid bilayer membranes that carry lipid-soluble
drugs with an inner core in which hydrophilic drugs can be loaded
(42). When constructed to be
thermosensitive, these lipid-based NPs respond to thermal changes
caused by HIFU, resulting in release of the loaded therapeutics at
the selected lesion site (43).
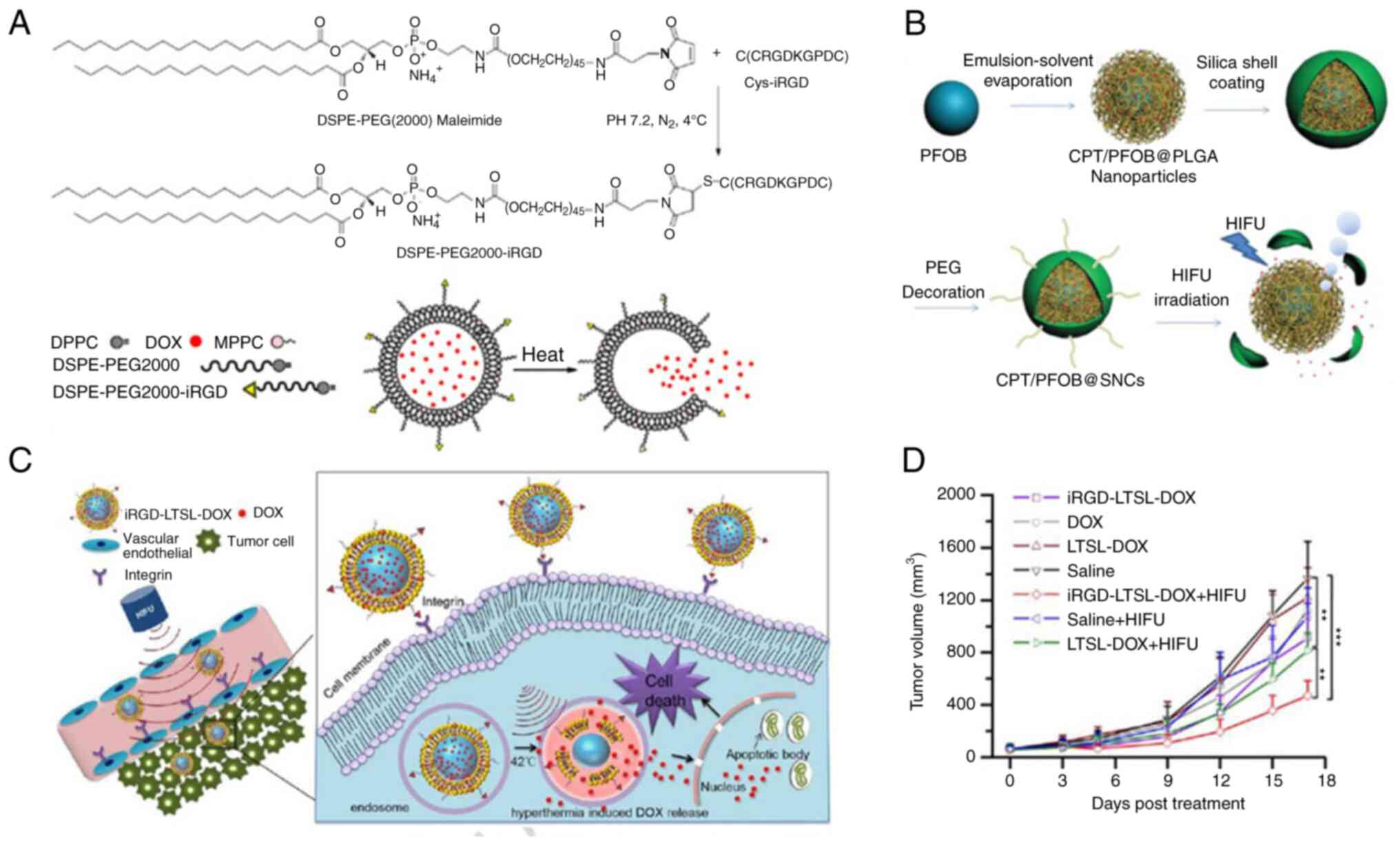
For example, Cha et al (44) and Deng et al (45) constructed liposomes sensitive to
low temperatures that contained the chemotherapeutic doxorubicin
(DOX). Following induction of hyperthermia caused by HIFU
irradiation, these liposomes selectively release encapsulated DOX
at the tumour tissue to increase their effective concentration in
the tumour cell nuclei, whilst keeping the concentration in the
general circulation low, thus effectively decreasing off-target
damage to normal tissues. These low temperature-sensitive liposomes
can be modified by internalised arginine-glycine-aspartic acid
(iRGD) to enhance the targeted delivery of iRGD to cancer and
tumour vascular cells (Fig. 2A and
C-D) (45). iRGD-modified liposomes allow longer
opportunities for HIFU irradiation and shorter HIFU exposure times,
effectively decreasing incidence of collateral damage such as skin
burns caused by long HIFU exposure.
In addition to use of HIFU as a tool to aid drug
delivery, another strategy for applying lipid-based nanomaterials
is to form nanobubbles to achieve enhanced tumour ablation
efficacy. Microbubbles have long been considered synergistic agents
for enhancing HIFU therapeutic efficacy (49-51).
However, traditional microbubbles are usually too large for tumour
tissue penetration and have short circulation times, limiting their
use in cancer treatment (49,52).
Thus, forming nano-size bubbles with improved tumour penetration
ability and increased stability during circulation is key for
improving the efficacy of these therapies. Hamano et al
(53) and VanOsdol et al
(54) formed nanobubble-based
liposomes. These echo-contrast gas or perfluoropentane-containing
liposomes were reported to achieve up to 4-5-fold greater drug
accumulation and release in tumour tissues compared with
nanomedicines or HIFU. Furthermore, these nanobubble-based
liposomes not only effectively increased HIFU ablation efficacy,
thus reducing irradiation time, but also encapsulated antitumour
genes, short interfering RNAs and chemotherapeutics to stimulate a
synergetic effect, further increasing their antitumour
efficacy.
Perfluorocarbon-containing nanomaterials are a
potential therapy that may solve the size and circulation problems
of microbubbles (55). By
incorporating liquid fluorocarbons into lipids or polymers, these
perfluorocarbon-containing nanomaterials shift from a liquid state
at room temperature to a gaseous state when temperature rises or
following irradiation of HIFU37). The gas released in tumour tissue
further triggers the formation of microbubbles, enhancing the
cavitation effect of HIFU ablation (56-58).
Since fluorocarbons have already penetrated the deep tumour tissue
via the EPR effect when it is in a liquid state with a nano size,
the microbubbles created following phase shift no longer exhibit
problems of short circulation times and low tumour tissue
penetrating rates, effectively increasing the therapeutic efficacy
of HIFU ablation. Studies have been designed based on
perfluorocarbon-containing nanomaterials applied for HIFU ablation
(Fig. 2B). Ashida et al
(59) prepared a phase-changing
nanodroplet from perfluoro-n-pentane (PFP),
perfluoro-n-hexane (PFH), dipalmitoyl-phosphatidylcholine,
dipalmitoyl-phosphatidic acid and pegylated
dipalmitoyl-phosphatidylethanol amine; use of these novel
nanomaterials together with HIFU irradiation resulted in moderate
tissue damage compared with histotripsy. This moderate damage is
sufficient to suppress tumour growth notably compared with HIFU
irradiation alone. In addition, compared with histotripsy, the
effect of combination therapy effectively decreases incidence of
collateral damage to surrounding normal tissues, reducing the
severity of side effects. Furthermore, addition of the
chemotherapeutic agent adriamycin further enhanced the
tumour-suppressing effects of this combination therapy: Tumour
regrowth rate was slowed by 1 week when adriamycin was used during
the 30-day observation time. However, the effect of repetitive
therapy management with longer observation periods should be
assessed to confirm the therapeutic effects of phase-changing
perfluorocarbon-containing nanodroplets. The choice of
perfluorocarbon is key when constructing HIFU-appliable
nanomaterials. Currently, the most commonly used perfluorocarbons
are PFP and PFH (60). The boiling
temperatures of other perfluorocarbons are usually either too low
or high to be applicable for clinical use. As boiling temperatures
also affect the phase-shifting temperature of constructed
nanomaterials (61,62), there remain challenges before these
can be used clinically. As the phase shifting temperature of PFP is
lower than that of PFH (>40 vs. >60˚C), PFP may be a better
choice for nanomaterial construction, as lower HIFU irradiation
doses can be used (63). Zhang
et al (64) constructed a
poly(lactide-co-glycolic acid) (PLGA) NP that incorporated PFP and
hematoporphyrin monomethyl ether (HMME) as synergistic agents (HMME
+ PFP/PLGA) for HIFU ablation. These agents were further modified
by streptavidin as a pre-targeting agent via a two-step
biotin-avidin technique. In addition to a lower HIFU irradiation
dosage required, the cavitation effect of HIFU, the sonodynamic
effect and vascular endothelial growth factor receptor-2 antibody
worked together to induce secondary necrosis surrounding the
initial HIFU ablation area, resulting in a greater synergetic
effect with less collateral damage to the normal tissue. This
method highlights the application of perfluorocarbon-containing
nanomaterials as HIFU synergetic agents for deep tumour ablation
and ablation of tumours with barriers along the HIFU beam path;
however, additional studies are needed before this method can be
used in clinical practice.
Magnetic nanomaterials, with their unique features
such as ease of manipulation using magnets and thermal
responsiveness to ultrasound and magnets, have potential as
effective sonosensitizers for HIFU cancer therapy (64). Sun et al (65,66),
You et al (67), Ho et
al (68) and Dibaji et
al (69) confirmed that
magnetic nanomaterials enhance the HIFU cavitation effect and thus
effectively increase tumour tissue destruction efficacy with a
lower HIFU exposure dose. According to Devarakonda et al
(70), the adoption of magnetic
nanomaterials (superparamagnetic iron oxide NPs; 0.047% w/v) halves
the HIFU irradiation dose required to obtain 13 mm3
tumour destruction volume, significantly reducing the side effects
caused by high HIFU doses (70).
They also discovered that the thermal enhancing efficacy of
magnetic nanomaterials was higher than that of gold NPs, which are
another HIFU hyperthermal candidate, making magnetic NPs clinically
preferable (71). However, the
mechanism by which magnetic NPs enhance HIFU cancer therapy remains
unclear. It has been suggested that magnetic NPs increase
attenuation of sound waves in tumour tissue. Thus, when magnetic
NPs selectively penetrate the tumour tissues through the EPR
effect, a lower HIFU dose is needed to achieve tumour destruction
efficacy, with decreased collateral damage to surrounding normal
tissues at these lower HIFU irradiation doses (72). Sadeghi-Goughari et al
(36,73) discovered that viscous and thermal
reaction with medium at the surface of magnetic particles is the
primary mechanism that aids conversion of acoustic energy into
heat, achieving greater temperature rises with directed HIFU
ablation. Additionally, a numerical model was established that
could accurately predict and analyse HIFU ablation process when NPs
were used, thus providing a novel tool to uncover the detailed
mechanism by which magnetic NPs affect HIFU ablation, which is
beneficial for future magnetic NP development and potential
clinical application.
Other NPs, including paclitaxel-loaded thiolated
human serum albumin NP-conjugated microbubble complexes (86), heat shock-targeted
N-(2-hydroxypropyl) methacrylamide copolymer-docetaxel conjugates
(87), Cy5.5-labelled glycol
chitosan, nitroxide free radical-generating (88,89),
1,1,2-trichlorotrifluoroethane incorporating pullulan-DOX (90) and phospholipid hydrophobic
mesoporous silica NPs (91,92),
enhance the efficacy of HIFU ablation with decreased side effects,
although additional studies on similar types of NP are required to
confirm their effectiveness. The clinical transition of NPs lack
systemic examination regarding the safety profile including
biocompatibility, biodegradation, tumour accumulation and stability
in animals other than mice. Furthermore, establishment of scalable,
economical and reproducible synthesis methods for these NPs is also
needed before they can be used clinically (93).
Cancer theranostics (cancer therapy and diagnosis
through the packaging of various therapeutic drugs and diagnostic
contrast agents) is a relatively new concept in the field of
precision medicine. In addition to incorporating therapeutic agents
to enhance HIFU ablation efficacy to induce selective therapeutic
drug release, NPs can be used to encapsulate diagnostic agents that
can enhance the imaging contrast of tumourigenic sites (94-96).
This can allow accurate targeting of HIFU ablation, real-time
imaging monitoring of ablation procedure and evaluation of the
therapeutic response without the need for extra medical tests
(Fig. 1C) (97), as well as adjustments of the
treatment to maximize the therapeutic efficacy and minimize the
collateral damage to the surrounding normal tissue.
HIFU is a type of ultrasound-based treatment method,
thus the specific therapeutics used for HIFU have acoustic
properties, which makes ultrasounds one of the first choices for
monitoring of HIFU. However, given that diagnostic and therapeutic
ultrasound have different frequencies (98,99),
the acoustic properties of the therapeutics may respond to only one
type of ultrasound. Therefore, it is important to design
therapeutics that are responsive to both diagnostic and therapeutic
ultrasound. Blum et al (100) constructed a polyethylene glycol
(PEG)-lipid-shelled microbubble that creates microbubble NPs in the
presence of fluorocarbon interiors (C4F10,
C5F12 and C6F14) and
ultrasound pulses. These microbubble NPs are not detectable by
ultrasound, but under HIFU irradiation, the integrated image
brightness of NPs on the cadence contrast pulse sequencing mode
increases, making them visible on ultrasound scans. The addition of
fluorocarbons to this nanosystem also allows enhancement of HIFU
efficacy, which was confirmed by complete detachment of breast
cancer cells in vitro under HIFU irradiation in presence of
these NPs. However, further in vivo studies are required to
examine the theranostic efficacy and biosafety profiles of these
NPs. Zhu et al (101)
examined the in vivo efficacy of nano-theranostics for HIFU
ablation. They synthesized a pH-sensitive poly(ethylene glycol)
that produced O2 from endogenous
H2O2. The generated O2 not only
served as a contrast agent for diagnostic ultrasound imaging but
served as a synergist agent to enhance HIFU ablation efficacy.
Furthermore, they also discovered that this nano-theranostic
induced normoxic conditions in the tumour tissues to enhance the
chemotherapeutic efficacy of DOX, allowing both theranostics and
combination therapy of HIFU and chemotherapy. Li et al
(102) designed
pentafluoropentane/C9F17-PAsp-ss-camptothecin
(CPT) nanodroplets that allowed ultrasound imaging and combination
therapy of not only HIFU ablation and chemotherapy but also
immunotherapy. The nanodroplets demonstrated an HIFU/glutathione
(GSH)-dual responsive drug release profile and successfully
delivered the loaded chemotherapeutic CPT into tumour tissue upon
HIFU irradiation. These nanodroplets can also generate immunogenic
debris following HIFU irradiation and induce maturation of
dendritic cells (DCs) via exposure of damage-associated molecular
patterns, effectively increasing the infiltration of effector T
cells into tumour tissue and thus enhancing the efficacy of tumour
immunotherapy. The incorporation of PFP also allows ultrasound
monitoring of the whole procedure, making these nanodroplets
another promising HIFU-based theranostic candidate. On the other
hand, Chen et al (35)
focused on HIFU and synthesized PFP-loaded polymer NPs (PFP@Polymer
NPs) that were responsive to a dual-frequency HIFU pattern.
Compared with single-frequency HIIFU, PFP@Polymer NPs under the
irradiation of dual-frequency HIFU (1.1 and 5.0 MHz) were reported
to significantly decrease the acoustic intensity threshold needed
for ablation from 216.86 to 62.38 W/cm2, thus
effectively decreasing collateral damage. Furthermore, these
polymer NPs combined with dual-frequency HIFU also demonstrated
improved tumour inhibition rates at half the irradiation time of
single-frequency HIFU and improved ultrasound contrast-generating
quality compared with traditional PFP@BSA nanodroplets. Whether
these NPs responsive to dual-frequency HIFU can also encapsulate
other therapeutics such as chemotherapeutics or immunotherapeutics
to achieve a synergistic theranostic effect remains to be examined;
combination therapies are desirable due to potentially improved
treatment outcomes and decreased side effects (103,104).
Although ultrasound is often utilized as an imaging
tool for HIFU theranostics, MRI is considered an improved imaging
tool given its non-invasive nature and high spatial and anatomical
resolution (105). Although the
majority of HIFU synergists do not have paramagnetic properties
that can be seen using an MR scan, certain MR sequences such as MRI
thermometry allow for real-time quantification of the local
temperature in the tumour tissues (106,107), thus allowing HIFU ablation. Given
that magnetic NPs may disrupt the magnetic field when applied for
MRI (70), gold NPs are an
alternative for MRI-guided HIFU ablation. By using MRI thermometry
to evaluate the tissue temperature, Devarakonda et al
(108) discovered that the
addition of gold NPs significantly enhances the increase in
temperature to increase lesion volume compared with HIFU ablation
alone. This enhancing effect of gold NPs was also confirmed in
vivo (109), although a
localized direct injection of NPs into the superficial tumour
tissue was used, which is not a method used in clinical practice.
Thus, further intravenous injection studies are required to assess
the theranostic efficacy of gold NPs and their impact on efficacy
of HIFU ablation. Although MRI thermometry can be utilized to
evaluate response to treatments, this method of evaluation is
indirect (through the measurement of local temperature) and
non-selective with unsatisfactory imaging precision due to the lack
of involvement of MRI contrast agents (37). Studies have used NPs to combine
both MRI contrast and HIFU synergetic agents to achieve MRI-guided
HIFU theranostics. Tang et al (39) constructed a temperature-responsive
nanoplatform [PFH/DOX@PLGA/Fe3O4-folate (FA)]
that achieved HIFU theranostics. The encapsulation of
Fe3O4 allowed T2-weighted imaging
of the tumour once particles had accumulated into the hepatoma
tissue through the EPR effect and active targeting induced by the
attached FA. The encapsulation of PFH also permits
contrast-enhanced ultrasound imaging of tumour tissue, allowing for
a multi-modal imaging profile. In addition, the incorporation of
PFH and DOX significantly improved the efficacy of HIFU ablation
and allowed enhanced chemotherapeutic efficacy, respectively,
evidenced by the strongest in vivo tumour inhibition rate
and greatest reduction in tumour volumes among all experimental and
control groups. Thus, this nanoplatform could achieve not only
multi-modal cancer imaging but also multi-modal treatment. Kuai
et al (110) designed a
type of perfluorooctyl bromide (PFOB) nanoemulsion that contained
MnO2 NPs to allow a combination of computed tomography
(CT) and MRI for multi-modal imaging and combination of HIFU
ablation and immunotherapy for multi-modal treatment. The use of
PFOB not only allowed CT imaging of tumour tissues as it is a
desirable CT contrast agent (111,112), but also transformed into
microbubbles under HIFU irradiation and enhanced the cavitation
effect for stronger HIFU ablation efficacy. The encapsulation of
MnO2 also allowed T1-weighted enhanced
imaging of tumour tissues instead of T2-weighted
enhanced imaging, which is preferable due to difficulties of
detecting small negative-contrast lesions on T2-weighted
enhanced imaging (113). In
addition to the stronger HIFU ablation efficacy, which allowed
lower HIFU exposure doses and administration times and thus less
collateral damage to the normal tissue, these NPs were also
reported to deplete GSH as a result of MnO2-mediated
disruption of the antioxidant defence system of tumour tissue and
to promote strong immunogenic cell death by inducing maturation of
DCs and enhancing activation of CD4+ and CD8+
cells, significantly inhibiting growth of the primary tumour and
lung metastasis through combination therapy (114).
Photoacoustic imaging is a promising biomedical
imaging technology that can overcome certain limitations of current
ultrasound with its high optical contrast, relatively low cost and
portability (115). It can be
used to visualize both endogenous and exogenous chromophores with a
high spatial resolution (116,117), penetrate >5 cm biological
tissue for imaging (118) and is
not associated with the potential side effects caused by ionizing
radiation. Studies have indicated that photoacoustic imaging can be
utilized to image small molecules, including those that are readily
extravasated and are present on the cell membrane or
intracellularly (119,120). Thus, studies have adopted
photoacoustic imaging as the imaging tool for HIFU cancer
theranostics. Feng et al (121) constructed an ammonium
bicarbonate-containing liposome (Lip-ABC) that could generate
microbubbles under HIFU irradiation (122). Through photoacoustic imaging,
these liposomes were shown to accumulate in the tumour interstitial
space where they generated bubbles to increase cavitation and
energy deposition, resulting in higher HIFU ablation rate in a
theranostic manner. Gao et al (123) on the other hand designed
HMME-loaded CaCO3 NPs (Ca@H) (108). Ca@H NPs responded to the acid
tumour microenvironment to produce CO2 and release HMME.
These agents may serve as a photoacoustic imaging enhancer for
guidance and monitoring of the entire therapeutic process, allowing
combination therapy using HIFU ablation and sonodynamic therapy to
promote near-complete removal of residual tumour tissue. Although
photoacoustic imaging has its diagnostic advantages, its clinical
applications are still limited currently (123-125).
Thus, several studies have attempted to combine this novel imaging
technology with other clinical imaging methods to allow multi-modal
imaging of HIFU cancer theranostics. Yan et al (126) and Zhang et al (127) designed NPs that allowed a
combination of ultrasound and photoacoustic monitoring. Zhang et
al (127) encapsulated the
chemotherapeutic DOX in NPs to achieve synergetic therapy of both
HIFU ablation and chemotherapy, allowing multi-modal imaging and
treatment of cancer theranostics. Both ultrasound and photoacoustic
imaging are based on acoustic characteristics of NPs and tumour
tissue; this could simplify the design of nanomedicines but risks
missing information on the tumour when imaging (128). Thus, studies have combined
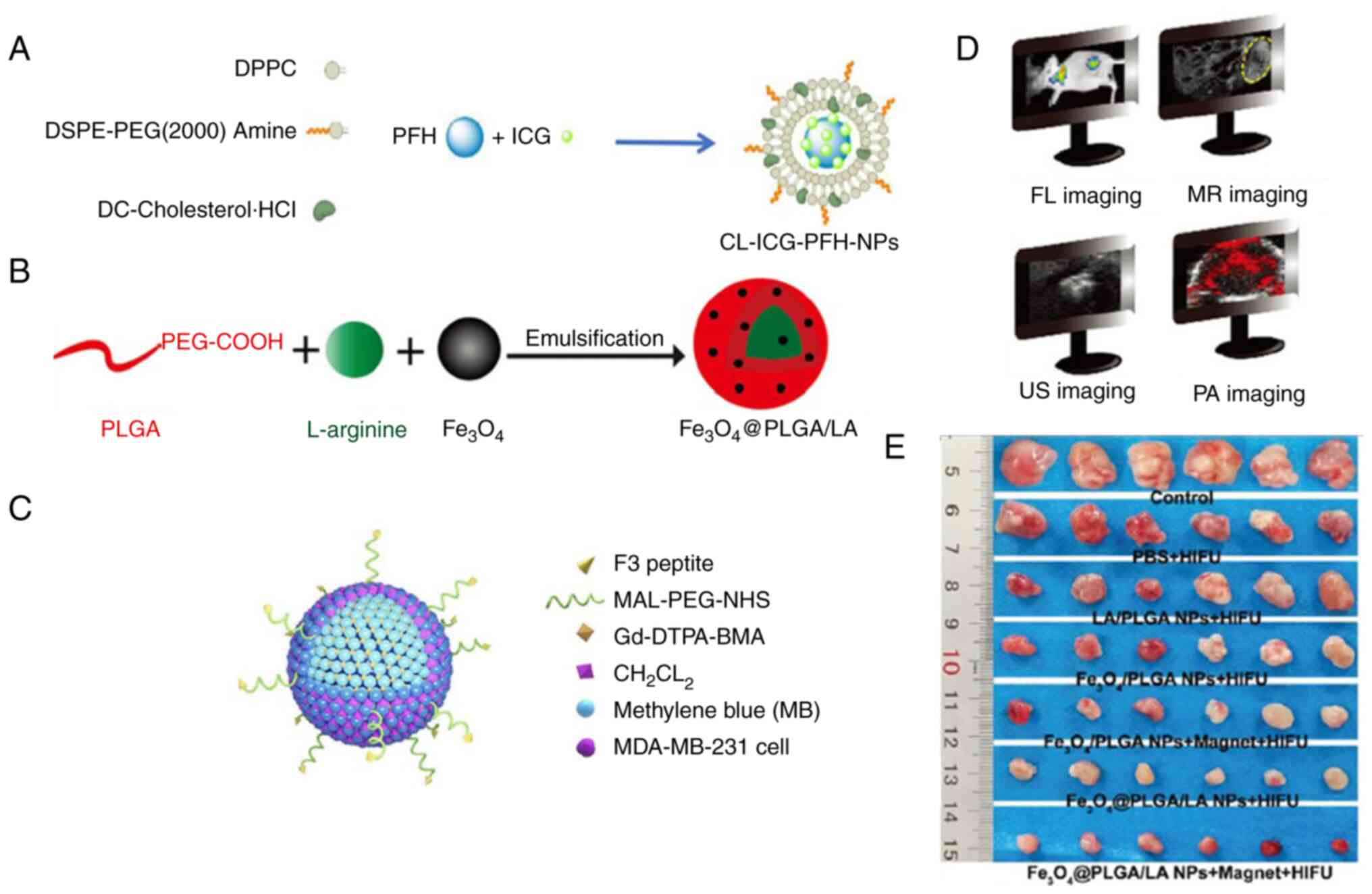
photoacoustic with other imaging methods. For example, Li et
al (129) prepared an F3
(penetrating peptide)-PLGA nanoplatform that could co-deliver
sonosensitizer methylene blue and the magnetic resonance contrast
agent gadolinium
2-[bis[2-(carboxylatomethyl-(methylcarbamoylmethyl)amino)ethyl]amino]acetate
to allow photoacoustic imaging and MRI. This F3-PLGA@MB/Gd platform
could further induce a synergistic therapeutic effect via tumour
cell apoptosis triggered by HIFU and sonodynamic ultrasound
(Fig. 3C-D). Yang et al
(130) designed a
Fe3O4-shelled and L-arginine-encapsulated
PLGA NP that could allow for tri-model imaging (ultrasound, MRI and
photoacoustic imaging). These NPs also release nitric oxide as an
antitumour gas therapy agent and change the acoustic properties of
the tumour tissue to augment HIFU ablation efficacy, realizing
synergetic cancer theranostics (Fig.
3B, D and E). Although promising, further clinical
trials are required on these nano-based HIFU theranostic methods
before they can be translated into clinical practice to benefit
patients with cancer.
Overall, the combination of nanotechnologies with
non-invasive HIFU cancer ablation-based therapies may prove to be a
beneficial future treatment. These nanomedicines increase the local
HIFU ablation efficacy by enhancing cavitation and changing the
acoustic properties of tumour tissue, decrease incidence of
collateral damage by allowing for lower HIFU exposure doses and
shorter exposure times, achieve a synergetic therapeutic effect by
allowing for the concomitant delivery of other therapeutics such as
chemotherapeutics, photothermal therapeutics or immunotherapeutics
and enable theranostic disease management by allowing monitoring of
treatment using single- or multi-modal imaging.
Although progress has been made in this field,
challenges remain regarding these HIFU-appliable nanomedicines
before they can be used clinically. Although most of these
nanomedicines have been reported to exhibit low toxicity in
vivo, a degree of hepatotoxicity is observed, often as hepatic
fibrosis, particularly in patients with hepatoma (131,132). Thus, it is important to assess
and minimise the toxicity and side effects of these nanomedicines.
Furthermore, as the majority of the aforementioned nano-based HIFU
cancer treatment studies were conducted on small animals, whether
the same HIFU dosages used in mice to stimulate these nanomedicines
also apply in humans remains to be determined. Additionally,
whether the higher HIFU dosages used in clinical practice may
hamper therapeutic effects of these nanomedicines and trigger other
undesired side effects remain to be assessed (133,134). These issues should be addressed
in future studies to improve the value of HIFU-appliable
nanomedicines and thus promote their clinical transition.
Not applicable.
Funding: The present study was funded by the Chongqing Science
and Technology Bureau ‘Doctor through train’ Scientific Research
Project (grant no. CSTB2022BSXM-JCX0082) and the China Postdoctoral
Science Foundation (grant no. 2020TQ0212).
Not applicable.
QZ conceived, wrote and reviewed the manuscript. BX
wrote the manuscript. BX and JL performed the literature review and
constructed figures. SZ, XH and XL reviewed the manuscript and
agreed to be accountable for all aspects of the work. SZ and XL
acquired the funding. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Patel D, Shah Y, Thakkar N, Shah K and
Shah M: Implementation of artificial intelligence techniques for
cancer detection. Augment Hum Res. 5:1–10. 2020.
|
|
2
|
Burugu S, Dancsok AR and Nielsen TO:
Emerging targets in cancer immunotherapy. Semin Cancer Biol.
52:39–52. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu S, Zhou X, Yao Y, Shi K, Yu M and Ji
F: Resection of the gastric submucosal tumor (G-SMT) originating
from the muscularis propria layer: Comparison of efficacy,
patients' tolerability, and clinical outcomes between endoscopic
full-thickness resection and surgical resection. Surg Endosc.
34:4053–4064. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pirisinu M, Pham TC, Zhang DX, Hong TN,
Nguyen LT and Le MT: Extracellular vesicles as natural therapeutic
agents and innate drug delivery systems for cancer treatment:
Recent advances, current obstacles, and challenges for clinical
translation. Semin Cancer Biol. 80:340–355. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Westhoff N, Ernst R, Kowalewski KF, Derigs
F, Neuberger M, Nörenberg D, Popovic ZV, Ritter M, Stephan Michel M
and von Hardenberg J: Medium-term oncological efficacy and
patient-reported outcomes after focal high-intensity focused
ultrasound: The FOXPRO trial. Eur Urol Focus. 22:S2405–S4569.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li Y, Wang S, Chen L, Feng Y, Shen Z, Chen
X, Huang G and Ni Y: Sequential administrations of a
vascular-disrupting agent, high-intensity focused ultrasound, and a
radioactively labeled necrosis avid compound for eradicating solid
malignancies. Technol Cancer Res Treat.
21(15330338221136716)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhong Q, Tang F, Ni T, Chen Y, Liu Y, Wu
J, Zhou W, Feng Z, Lu X, Tan S and Zhang Y: Salvage high intensity
focused ultrasound for residual or recurrent cervical cancer after
definitive chemoradiotherapy. Front Immunol.
13(995930)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lindstrom PA: Prefrontal ultrasonic
irradiation-a substitute for lobotomy. AMA Arch Neurol Psychiatry.
72:399–425. 1954.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fry WJ, Barnard JW, Fry EJ, Krumins RF and
Brennan JF: Ultrasonic lesions in the mammalian central nervous
system. Science. 122:517–518. 1955.PubMed/NCBI
|
|
10
|
ter Haar G: Intervention and therapy.
Ultrasound Med Biol 26 Suppl. 1:S51–S54. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu L, Wang T and Lei B: High-intensity
focused ultrasound (HIFU) ablation versus surgical interventions
for the treatment of symptomatic uterine fibroids: A meta-analysis.
Eur Radiol. 32:1195–1204. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang C, Li Z and Bai J: Bubble-assisted
HIFU ablation enabled by calcium peroxide. J Mater Chem B.
10:4442–4451. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chaussy CG and Thüroff S: High-Intensity
focused ultrasound for the treatment of prostate cancer: A review.
J Endourol. 31(S1):S30–S37. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Napoli A, Alfieri G, Scipione R, Leonardi
A, Fierro D, Panebianco V, De Nunzio C, Leonardo C and Catalano C:
High-intensity focused ultrasound for prostate cancer. Expert Rev
Med Devices. 17:427–433. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fishman PS and Fischell JM: Focused
ultrasound mediated opening of the blood-brain barrier for
neurodegenerative diseases. Front Neurol. 12(749047)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Martínez-Fernández R, Máñez-Miró JU,
Rodríguez-Rojas R, Del Álamo M, Shah BB, Hernández-Fernández F,
Pineda-Pardo JA, Monje MHG, Fernández-Rodríguez B, Sperling SA, et
al: Randomized trial of focused ultrasound subthalamotomy for
Parkinson's Disease. N Engl J Med. 383:2501–2513. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Moosa S, Martínez-Fernández R, Elias WJ,
Del Alamo M, Eisenberg HM and Fishman PS: The role of
high-intensity focused ultrasound as a symptomatic treatment for
Parkinson's disease. Mov Disord. 34:1243–1251. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Giordano M, Caccavella VM, Zaed I, Foglia
Manzillo L, Montano N, Olivi A and Polli FM: Comparison between
deep brain stimulation and magnetic resonance-guided focused
ultrasound in the treatment of essential tremor: A systematic
review and pooled analysis of functional outcomes. J Neurol
Neurosurg Psychiatry. 91:1270–1278. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Abe K, Horisawa S, Yamaguchi T, Hori H,
Yamada K, Kondo K, Furukawa H, Kamada H, Kishima H, Oshino S, et
al: Focused ultrasound thalamotomy for refractory essential tremor:
A Japanese multicenter single-arm study. Neurosurgery. 88:751–757.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jeng CJ, Ou KY, Long CY, Chuang L and Ker
CR: 500 cases of high-intensity focused ultrasound (HIFU) ablated
uterine fibroids and adenomyosis. Taiwan J Obstet Gynecol.
59:865–871. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sequeiros RB, Joronen K, Komar G and
Koskinen SK: High intensity focused ultrasound (HIFU) in tumor
therapy. Duodecim. 133:143–149. 2017.PubMed/NCBI
|
|
22
|
Marinova M, Wilhelm-Buchstab T and Strunk
H: Advanced pancreatic cancer: High-Intensity Focused Ultrasound
(HIFU) and other local ablative therapies. RoFo. 191:216–227.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhou YF: High intensity focused ultrasound
in clinical tumor ablation. World J Clin Oncol. 2:8–27.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bachu VS, Kedda J, Suk I, Green JJ and
Tyler B: High-Intensity Focused Ultrasound: A Review of Mechanisms
and Clinical Applications. Ann Biomed Eng. 49:1975–1991.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Izadifar Z, Izadifar Z, Chapman D and
Babyn P: An introduction to high intensity focused ultrasound:
Systematic review on principles, devices, and clinical
applications. J Clin Med. 9(460)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Miller DL, Smith NB, Bailey MR, Czarnota
GJ, Hynynen K and Makin IR: Bioeffects Committee of the American
Institute of Ultrasound in Medicine. Overview of therapeutic
ultrasound applications and safety considerations. J Ultrasound
Med. 31:623–634. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Awad NS, Paul V, AlSawaftah NM, Ter Haar
G, Allen TM, Pitt WG and Husseini GA: Ultrasound-responsive
nanocarriers in cancer treatment: A review. ACS Pharmacol Transl
Sci. 4:589–612. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang Q, Dai X, Zhang H, Zeng Y, Luo K and
Li W: Recent advances in development of nanomedicines for multiple
sclerosis diagnosis. Biomed Mater. 16(024101)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zeng Y, Li Z, Zhu H, Gu Z, Zhang H and Luo
K: Recent advances in nanomedicines for multiple sclerosis therapy.
ACS Appl Bio Mater. 3:6571–6597. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou Y, Wang Z, Chen Y, Shen H, Luo Z, Li
A, Wang Q, Ran H, Li P, Song W, et al: Microbubbles from
gas-generating perfluorohexane nanoemulsions for targeted
temperature-sensitive ultrasonography and synergistic HIFU ablation
of tumors. Adv Mater. 25:4123–4130. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Du Y, Lin L, Zhang Z, Tang Y, Ou X, Wang Y
and Zou J: Drug-loaded nanoparticles conjugated with genetically
engineered bacteria for cancer therapy. Biochem Biophys Res Commun.
606:29–34. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Manthe RL, Foy SP, Krishnamurthy N, Sharma
B and Labhasetwar V: Tumor ablation and nanotechnology. Mol Pharm.
7:1880–1898. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen D and Wu J: An in vitro feasibility
study of controlled drug release from encapsulated nanometer
liposomes using high intensity focused ultrasound. Ultrasonics.
50:744–749. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
O'Neill BE, Vo H, Angstadt M, Li KP, Quinn
T and Frenkel V: Pulsed high intensity focused ultrasound mediated
nanoparticle delivery: Mechanisms and efficacy in murine muscle.
Ultrasound Med Biol. 35:416–424. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen J, Nan Z, Zhao Y, Zhang L, Zhu H, Wu
D, Zong Y, Lu M, Ilovitsh T, Wan M, et al: Enhanced HIFU
Theranostics with dual-frequency-ring focused ultrasound and
activatable perfluoropentane-loaded polymer nanoparticles.
Micromachines (Basel). 12(1324)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sadeghi-Goughari M, Jeon S and Kwon HJ:
Analytical and numerical model of high intensity focused ultrasound
enhanced with nanoparticles. IEEE Trans Biomed Eng. 67:3083–3093.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen Y, Chen H and Shi J:
Nanobiotechnology promotes noninvasive high-intensity focused
ultrasound cancer surgery. Adv Healthc Mater. 4:158–165.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Poh S, Chelvam V and Low PS: Comparison of
nanoparticle penetration into solid tumors and sites of
inflammation: Studies using targeted and nontargeted liposomes.
Nanomedicine (Lond). 10:1439–1449. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tang H, Guo Y, Peng L, Fang H, Wang Z,
Zheng Y, Ran H and Chen Y: In Vivo targeted, responsive, and
synergistic cancer nanotheranostics by magnetic resonance
imaging-guided synergistic high-intensity focused ultrasound
ablation and chemotherapy. ACS Appl Mater Interfaces.
10:15428–15441. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tharkar P, Varanasi R, Wong WSF, Jin CT
and Chrzanowski W: Nano-Enhanced drug delivery and therapeutic
ultrasound for cancer treatment and beyond. Front Bioeng
Biotechnol. 7(324)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang X, Yan F, Liu X, Wang P, Shao S, Sun
Y, Sheng Z, Liu Q, Lovell JF and Zheng H: Enhanced drug delivery
using sonoactivatable liposomes with membrane-embedded porphyrins.
J Control Release. 286:358–368. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chaudhuri A, Kumar DN, Shaik RA, Eid BG,
Abdel-Naim AB, Md S, Ahmad A and Agrawal AK: Lipid-Based
nanoparticles as a pivotal delivery approach in triple negative
breast cancer (TNBC) therapy. Int J Mol Sci.
23(10068)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yudina A and Moonen C: Ultrasound-induced
cell permeabilisation and hyperthermia: Strategies for local
delivery of compounds with intracellular mode of action. Int J
Hyperthermia. 28:311–319. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cha JM, You DG, Choi EJ, Park SJ, Um W,
Jeon J, Kim K, Kwon IC, Park JC, Kim HR and Park JH: Improvement of
Antitumor Efficacy by Combination of Thermosensitive Liposome with
High-Intensity Focused Ultrasound. J Biomed Nanotechnol.
12:1724–1733. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Deng Z, Xiao Y, Pan M, Li F, Duan W, Meng
L, Liu X, Yan F and Zheng H: Hyperthermia-triggered drug delivery
from iRGD-modified temperature-sensitive liposomes enhances the
anti-tumor efficacy using high intensity focused ultrasound. J
Control Release. 243:333–341. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yang Q, Zhou Y, Chen J, Huang N, Wang Z
and Cheng Y: Gene therapy for drug-resistant glioblastoma via
lipid-polymer hybrid nanoparticles combined with focused
ultrasound. Int J Nanomedicine. 16:185–199. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Arsiwala TA, Sprowls SA, Blethen KE,
Adkins CE, Saralkar PA, Fladeland RA, Pentz W, Gabriele A,
Kielkowski B, Mehta RI, et al: Ultrasound-mediated disruption of
the blood tumor barrier for improved therapeutic delivery.
Neoplasia. 23:676–691. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Luo Z, Jin K, Pang Q, Shen S, Yan Z, Jiang
T, Zhu X, Yu L, Pang Z and Jiang X: On-Demand drug release from
dual-targeting small nanoparticles triggered by high-intensity
focused ultrasound enhanced glioblastoma-targeting therapy. ACS
Appl Mater Interfaces. 9:31612–31625. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sokka S, King R and Hynynen K: MRI-guided
gas bubble enhanced ultrasound heating in in vivo rabbit thigh.
Phys Med Biol. 48:223–241. 2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Clark A, Bonilla S, Suo D, Shapira Y and
Averkiou M: Microbubble-Enhanced Heating: Exploring the effect of
microbubble concentration and pressure amplitude on high-intensity
focused ultrasound treatments. Ultrasound Med Biol. 47:2296–2309.
2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Xin Y, Zhang A, Xu LX and Fowlkes JB:
Numerical study of bubble cloud and thermal lesion evolution during
acoustic droplet vaporization enhanced HIFU treatment. J Biomech
Eng. 144(031007)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Okita K, Sugiyama K, Takagi S and Matsumto
Y: Microbubble behavior in an ultrasound field for high intensity
focused ultrasound therapy enhancement. J Acoust Soc Am.
134:1576–1585. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hamano N, Negishi Y, Takatori K,
Endo-Takahashi Y, Suzuki R, Maruyama K, Niidome T and Aramaki Y:
Combination of bubble liposomes and high-intensity focused
ultrasound (HIFU) enhanced antitumor effect by tumor ablation. Biol
Pharm Bull. 37:174–177. 2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
VanOsdol J, Ektate K, Ramasamy S, Maples
D, Collins W, Malayer J and Ranjan A: Sequential HIFU heating and
nanobubble encapsulation provide efficient drug penetration from
stealth and temperature sensitive liposomes in colon cancer. J
Control Release. 247:55–63. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhou LQ, Li P, Cui XW and Dietrich CF:
Ultrasound nanotheranostics in fighting cancer: Advances and
prospects. Cancer Lett. 470:204–219. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Li K, Liu Y, Zhang S, Xu Y, Jiang J, Yin
F, Hu Y, Han B, Ge S, Zhang L and Wang Y: Folate receptor-targeted
ultrasonic PFOB nanoparticles: Synthesis, characterization and
application in tumor-targeted imaging. Int J Mol Med. 39:1505–1515.
2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sheeran PS, Matsunaga TO and Dayton PA:
Phase change events of volatile liquid perfluorocarbon contrast
agents produce unique acoustic signatures. Phys Med Biol.
59:379–401. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Picheth G, Houvenagel S, Dejean C, Couture
O, Alves de Freitas R, Moine L and Tsapis N: Echogenicity
enhancement by end-fluorinated polylactide perfluorohexane
nanocapsules: Towards ultrasound-activable nanosystems. Acta
Biomater. 64:313–322. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ashida R, Kawabata K, Maruoka T, Asami R,
Yoshikawa H, Takakura R, Ioka T, Katayama K and Tanaka S: New
approach for local cancer treatment using pulsed high-intensity
focused ultrasound and phase-change nanodroplets. J Med Ultrason
(2001). 42:457–466. 2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Xu T, Cui Z, Li D, Cao F, Xu J, Zong Y,
Wang S, Bouakaz A, Wan M and Zhang S: Cavitation characteristics of
flowing low and high boiling-point perfluorocarbon phase-shift
nanodroplets during focused ultrasound exposures. Ultrason
Sonochem. 65(105060)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Rapoport N: Phase-shift,
stimuli-responsive perfluorocarbon nanodroplets for drug delivery
to cancer. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 4:492–510.
2012.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Sheeran PS and Dayton PA: Phase-change
contrast agents for imaging and therapy. Curr Pharm Des.
18:2152–2165. 2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kwizera EA, Stewart S, Mahmud MM and He X:
Magnetic nanoparticle-mediated heating for biomedical applications.
J Heat Transfer. 144(030801)2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zhang Y, Yong L, Luo Y, Ding X, Xu D, Gao
X, Yan S, Wang Q, Luo J, Pu D and Zou J: Enhancement of HIFU
ablation by sonosensitizer-loading liquid fluorocarbon
nanoparticles with pre-targeting in a mouse model. Sci Rep.
9(6982)2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Sun Y, Zheng Y, Ran H, Zhou Y, Shen H,
Chen Y, Chen H, Krupka TM, Li A, Li P, et al: Superparamagnetic
PLGA-iron oxide microcapsules for dual-modality US/MR imaging and
high intensity focused US breast cancer ablation. Biomaterials.
33:5854–5864. 2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Sun Y, Zheng Y, Li P, Wang D, Niu C, Gong
Y, Huang R, Wang Z, Wang Z and Ran H: Evaluation of
superparamagnetic iron oxide-polymer composite microcapsules for
magnetic resonance-guided high-intensity focused ultrasound cancer
surgery. BMC Cancer. 14(800)2014.PubMed/NCBI View Article : Google Scholar
|
|
67
|
You Y, Wang Z, Ran H, Zheng Y, Wang D, Xu
J, Wang Z, Chen Y and Li P: Nanoparticle-enhanced synergistic HIFU
ablation and transarterial chemoembolization for efficient cancer
therapy. Nanoscale. 8:4324–4339. 2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Ho VH, Smith MJ and Slater NK: Effect of
magnetite nanoparticle agglomerates on the destruction of tumor
spheroids using high intensity focused ultrasound. Ultrasound Med
Biol. 37:169–175. 2011.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Dibaji SAR, Al-Rjoub MF, Myers MR and
Banerjee RK: Enhanced heat transfer and thermal dose using magnetic
nanoparticles during HIFU thermal ablation-an in-vitro study. J
Nanotechnol Eng Med. 4(040902)2014.
|
|
70
|
Devarakonda SB, Myers MR, Giridhar D,
Dibaji SA and Banerjee RK: Enhanced thermal effect using magnetic
nano-particles during high-intensity focused ultrasound. PLoS One.
12(e0175093)2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Devarakonda SB, Myers MR and Banerjee RK:
Comparison of heat transfer enhancement between magnetic and gold
nanoparticles during HIFU sonication. J Biomech Eng.
140:2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Kaczmarek K, Hornowski T, Kubovčíková M,
Timko M, Koralewski M and Józefczak A: Heating induced by
therapeutic ultrasound in the presence of magnetic nanoparticles.
ACS Appl Mater Interfaces. 10:11554–11564. 2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Sadeghi-Goughari M, Jeon S and Kwon HJ:
Magnetic nanoparticles-enhanced focused ultrasound heating: Size
effect, mechanism, and performance analysis. Nanotechnology.
31(245101)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Kimura NT, Taniguchi S, Aoki K and Baba T:
Selective localization and growth of Bifidobacterium bifidum in
mouse tumors following intravenous administration. Cancer Res.
40:2061–2068. 1980.PubMed/NCBI
|
|
75
|
Li X, Fu GF, Fan YR, Liu WH, Liu XJ, Wang
JJ and Xu GX: Bifidobacterium adolescentis as a delivery system of
endostatin for cancer gene therapy: Selective inhibitor of
angiogenesis and hypoxic tumor growth. Cancer Gene Ther.
10:105–111. 2003.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Luo CH, Huang CT, Su CH and Yeh CS:
Bacteria-Mediated hypoxia-specific delivery of nanoparticles for
tumors imaging and therapy. Nano Lett. 16:3493–3499.
2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Xu D, Zou W, Luo Y, Gao X, Jiang B, Wang
Y, Jiang F, Xiong J, Chen C, Tang Y, et al: Feasibility between
bifidobacteria targeting and changes in the acoustic environment of
tumor tissue for synergistic HIFU. Sci Rep. 10(7772)2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Jiang BL, Gao X, Xiong J, Zhu PY, Luo Y,
Xu D, Tang Y, Wang YT, Chen C, Yang HY, et al: Experimental study
on synergistic effect of HIFU treatment of tumors using
Bifidobacterium bound with cationic phase-change nanoparticles. Eur
Rev Med Pharmacol Sci. 24:5714–5725. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Zhu C, Ji Z, Ma J, Ding Z, Shen J and Wang
QW: Recent advances of nanotechnology-facilitated bacteria-based
drug and gene delivery systems for cancer treatment. Pharmaceutics.
13(940)2021.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Yin T, Diao Z, Blum NT, Qiu L, Ma A and
Huang P: Engineering bacteria and bionic bacterial derivatives with
nanoparticles for cancer therapy. Small.
18(e2104643)2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Kalia VC, Patel SKS, Cho BK, Wood TK and
Lee JK: Emerging applications of bacteria as antitumor agents.
Semin Cancer Biol. 86(Pt 2):1014–1025. 2022.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Tang Y, Chen C, Jiang B, Wang L, Jiang F,
Wang D, Wang Y, Yang H, Ou X, Du Y, et al: Bifidobacterium
bifidum-Mediated specific delivery of nanoparticles for tumor
therapy. Int J Nanomedicine. 16:4643–4659. 2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Wang D, Jiang F, Wang L, Tang Y, Zhang Z,
Du Y and Zou J: Polyethylenimine (PEI)-modified poly
(lactic-co-glycolic) acid (PLGA) nanoparticles conjugated with
tumor-homing bacteria facilitate high intensity focused
ultrasound-mediated tumor ablation. Biochem Biophys Res Commun.
571:104–109. 2021.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Tee JK, Yip LX, Tan ES, Santitewagun S,
Prasath A, Ke PC, Ho HK and Leong DT: Nanoparticles' interactions
with vasculature in diseases. Chem Soc Rev. 48:5381–5407.
2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Wang Y, Chen C, Luo Y, Xiong J, Tang Y,
Yang H, Wang L, Jiang F, Gao X, Xu D, et al: Experimental study of
tumor therapy mediated by multimodal imaging based on a biological
targeting synergistic agent. Int J Nanomedicine. 15:1871–1888.
2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Han H, Lee H, Kim K and Kim H: Effect of
high intensity focused ultrasound (HIFU) in conjunction with a
nanomedicines-microbubble complex for enhanced drug delivery. J
Control Release. 266:75–86. 2017.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Frazier N, Payne A, Dillon C, Subrahmanyam
N and Ghandehari H: Enhanced efficacy of combination heat shock
targeted polymer therapeutics with high intensity focused
ultrasound. Nanomedicine. 13:1235–1243. 2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Li Q, Zhang J, Li J, Ye H, Li M, Hou W, Li
H and Wang Z: Glutathione-Activated NO-/ROS-Generation
nanoparticles to modulate the tumor hypoxic microenvironment for
enhancing the effect of HIFU-Combined Chemotherapy. ACS Appl Mater
Interfaces. 13:26808–26823. 2021.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Kang Y, Kim J, Park J, Lee YM,
Saravanakumar G, Park KM, Choi W, Kim K, Lee E, Kim C and Kim WJ:
Tumor vasodilation by N-Heterocyclic carbene-based nitric oxide
delivery triggered by high-intensity focused ultrasound and
enhanced drug homing to tumor sites for anti-cancer therapy.
Biomaterials. 217(119297)2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Li H, Yu C, Zhang J, Li Q, Qiao H, Wang Z
and Zeng D: pH-sensitive pullulan-doxorubicin nanoparticles loaded
with 1,1,2-trichlorotrifluoroethane as a novel synergist for high
intensity focused ultrasound mediated tumor ablation. Int J Pharm.
556:226–235. 2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Yildirim A, Shi D, Roy S, Blum NT,
Chattaraj R, Cha JN and Goodwin AP: Nanoparticle-Mediated acoustic
cavitation enables high intensity focused ultrasound ablation
without tissue heating. ACS Appl Mater Interfaces. 10:36786–36795.
2018.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Yildirim A, Chattaraj R, Blum NT, Shi D,
Kumar K and Goodwin AP: Phospholipid capped mesoporous
nanoparticles for targeted high intensity focused ultrasound
ablation. Adv Healthc Mater. 6:2017.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Yildirim A, Blum NT and Goodwin AP:
Colloids, nanoparticles, and materials for imaging, delivery,
ablation, and theranostics by focused ultrasound (FUS).
Theranostics. 9:2572–2594. 2019.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Jain K and Zhong J: Theranostic
applications of nanomaterials. Curr Pharm Des.
28(77)2022.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Hartl D, de Luca V, Kostikova A, Laramie
J, Kennedy S, Ferrero E, Siegel R, Fink M, Ahmed S, Millholland J,
et al: Translational precision medicine: An industry perspective. J
Transl Med. 19(245)2021.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Sisodiya SM: Precision medicine and
therapies of the future. Epilepsia. 62 (Suppl 2):S90–S105.
2021.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Li H, Zeng Y, Zhang H, Gu Z, Gong Q and
Luo K: Functional gadolinium-based nanoscale systems for cancer
theranostics. J Control Release. 329:482–512. 2021.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Kavros SJ and Coronado R: Diagnostic and
therapeutic ultrasound on venous and arterial ulcers: A focused
review. Adv Skin Wound Care. 31:55–65. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Meng Y, Pople CB, Budiansky D, Li D,
Suppiah S, Lim-Fat MJ, Perry J, Sahgal A and Lipsman N: Current
state of therapeutic focused ultrasound applications in
neuro-oncology. J Neurooncol. 156:49–59. 2022.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Blum NT, Yildirim A, Chattaraj R and
Goodwin AP: Nanoparticles formed by acoustic destruction of
microbubbles and their utilization for imaging and effects on
therapy by high intensity focused ultrasound. Theranostics.
7:694–702. 2017.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Zhu J, Li Z, Zhang C, Lin L, Cao S, Che H,
Shi X, Wang H and van Hest JCM: Single enzyme loaded nanoparticles
for combinational ultrasound-guided focused ultrasound ablation and
hypoxia-relieved chemotherapy. Theranostics. 9:8048–8060.
2019.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Li C, Lu Y, Cheng L, Zhang X, Yue J and
Liu J: Combining mechanical high-intensity focused ultrasound
ablation with chemotherapy for augmentation of anticancer immune
responses. Mol Pharm. 18:2091–2103. 2021.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Zhu S, Zhang T, Zheng L, Liu H, Song W,
Liu D, Li Z and Pan CX: Combination strategies to maximize the
benefits of cancer immunotherapy. J Hematol Oncol.
14(156)2021.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Chen J, Tan Q, Yang Z and Jin Y:
Engineered extracellular vesicles: Potentials in cancer combination
therapy. J Nanobiotechnology. 20(132)2022.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Barisano G, Sepehrband F, Ma S, Jann K,
Cabeen R, Wang DJ, Toga AW and Law M: Clinical 7 T MRI: Are we
there yet? A review about magnetic resonance imaging at ultra-high
field. Br J Radiol. 92(20180492)2019.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Lutz NW and Bernard M: Contactless
Thermometry by MRI and MRS: Advanced methods for thermotherapy and
biomaterials. iScience. 23(101561)2020.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Sparacia G and Sakai K: Temperature
measurement by diffusion-weighted imaging. Magn Reson Imaging Clin
N Am. 29:253–261. 2021.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Devarakonda SB, Myers MR, Lanier M,
Dumoulin C and Banerjee RK: Assessment of gold
nanoparticle-mediated-enhanced hyperthermia using MR-Guided
high-intensity focused ultrasound ablation procedure. Nano Lett.
17:2532–2538. 2017.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Devarakonda SB, Stringer K, Rao M, Myers M
and Banerjee R: Assessment of enhanced thermal effect due to gold
nanoparticles during MR-Guided high-intensity focused ultrasound
(HIFU) procedures using a mouse-tumor model. ACS Biomater Sci Eng.
5:4102–4111. 2019.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Kuai X, Zhu Y, Yuan Z, Wang S, Lin L, Ye
X, Lu Y, Luo Y, Pang Z, Geng D and Yin B: Perfluorooctyl bromide
nanoemulsions holding MnO2 nanoparticles with
dual-modality imaging and glutathione depletion enhanced
HIFU-eliciting tumor immunogenic cell death. Acta Pharm Sin B.
12:967–981. 2022.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Mattrey RF: Perfluorooctylbromide: A new
contrast agent for CT, sonography, and MR imaging. AJR Am J
Roentgenol. 152:247–252. 1989.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Li X, Sui Z, Li X, Xu W, Guo Q, Sun J and
Jing F: Perfluorooctylbromide nanoparticles for ultrasound imaging
and drug delivery. Int J Nanomedicine. 13:3053–3067.
2018.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Pellico J, Ellis CM and Davis JJ:
Nanoparticle-based paramagnetic contrast agents for magnetic
resonance imaging. Contrast Media Mol Imaging.
2019(1845637)2019.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Cai X, Zhu Q, Zeng Y, Zeng Q, Chen X and
Zhan Y: Manganese oxide nanoparticles As MRI contrast agents in
tumor multimodal imaging and therapy. Int J Nanomedicine.
14:8321–8344. 2019.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Das D, Sharma A, Rajendran P and Pramanik
M: Another decade of photoacoustic imaging. Phys Med Biol.
66(5)2021.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Jacques SL: Optical properties of
biological tissues: A review. Phys Med Biol. 58:R37–R61.
2013.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Wu D, Huang L, Jiang MS and Jiang H:
Contrast agents for photoacoustic and thermoacoustic imaging: A
review. Int J Mol Sci. 15:23616–23639. 2014.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Palma-Chavez J, Pfefer TJ, Agrawal A,
Jokerst JV and Vogt WC: Review of consensus test methods in medical
imaging and current practices in photoacoustic image quality
assessment. J Biomed Opt. 26(090901)2021.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Steinberg I, Huland DM, Vermesh O, Frostig
HE, Tummers WS and Gambhir SS: Photoacoustic clinical imaging.
Photoacoustics. 14:77–98. 2019.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Brunker J, Yao JJ, Laufer J and Bohndiek
SE: Photoacoustic imaging using genetically encoded reporters: A
review. J Biomed Opt. 22:2017.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Feng G, Hao L, Xu C, Ran H, Zheng Y, Li P,
Cao Y, Wang Q, Xia J and Wang Z: High-intensity focused
ultrasound-triggered nanoscale bubble-generating liposomes for
efficient and safe tumor ablation under photoacoustic imaging
monitoring. Int J Nanomedicine. 12:4647–4659. 2017.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Attia ABE, Balasundaram G, Moothanchery M,
Dinish US, Bi R, Ntziachristos V and Olivo M: A review of clinical
photoacoustic imaging: Current and future trends. Photoacoustics.
16(100144)2019.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Gao H, Wang Z, Tan M, Liu W, Zhang L,
Huang J, Cao Y, Li P, Wang Z, Wen J, et al: pH-Responsive
nanoparticles for enhanced antitumor activity by high-intensity
focused ultrasound therapy combined with sonodynamic therapy. Int J
Nanomedicine. 17:333–350. 2022.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Chen Q, Qin W, Qi W and Xi L: Progress of
clinical translation of handheld and semi-handheld photoacoustic
imaging. Photoacoustics. 22(100264)2021.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Neprokin A, Broadway C, Myllylä T, Bykov A
and Meglinski I: Photoacoustic Imaging in Biomedicine and Life
Sciences. Life (Basel). 12(588)2022.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Yan S, Lu M, Ding X, Chen F, He X, Xu C,
Zhou H, Wang Q, Hao L and Zou J: HematoPorphyrin Monomethyl Ether
polymer contrast agent for ultrasound/photoacoustic dual-modality
imaging-guided synergistic high intensity focused ultrasound (HIFU)
therapy. Sci Rep. 6(31833)2016.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Zhang N, Cai X, Gao W, Wang R, Xu C, Yao
Y, Hao L, Sheng D, Chen H, Wang Z and Zheng Y: A multifunctional
theranostic nanoagent for dual-mode image-guided
HIFU/Chemo-Synergistic cancer therapy. Theranostics. 6:404–417.
2016.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Park EY, Lee H, Han S, Kim C and Kim J:
Photoacoustic imaging systems based on clinical ultrasound
platform. Exp Biol Med (Maywood). 247:551–560. 2022.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Li Y, Hao L, Liu F, Yin L, Yan S, Zhao H,
Ding X, Guo Y, Cao Y, Li P, et al: Cell penetrating
peptide-modified nanoparticles for tumor targeted imaging and
synergistic effect of sonodynamic/HIFU therapy. Int J Nanomedicine.
14:5875–5894. 2019.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Yang H, Jiang F, Zhang L, Wang L, Luo Y,
Li N, Guo Y, Wang Q and Zou J: Multifunctional l-arginine-based
magnetic nanoparticles for multiple-synergistic tumor therapy.
Biomater Sci. 9:2230–2243. 2021.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Isoda K, Nagata R, Hasegawa T, Taira Y,
Taira I, Shimizu Y, Isama K, Nishimura T and Ishida I:
Hepatotoxicity and drug/chemical interaction toxicity of nanoclay
particles in mice. Nanoscale Res Lett. 12(199)2017.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Dai X, Zeng Y, Zhang H, Gu Z, Gong Q and
Luo K: Advances on Nanomedicines for Diagnosis and Theranostics of
Hepatic Fibrosis. Adv Biomed Res. 1(2000091)2021.
|
|
133
|
Federau C, Goubran M, Rosenberg J,
Henderson J, Halpern CH, Santini V, Wintermark M, Butts Pauly K and
Ghanouni P: Transcranial MRI-guided high-intensity focused
ultrasound for treatment of essential tremor: A pilot study on the
correlation between lesion size, lesion location, thermal dose, and
clinical outcome. J Magn Reson Imaging. 48:58–65. 2018.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Huber PM, Afzal N, Arya M, Boxler S,
Dudderidge T, Emberton M, Guillaumier S, Hindley RG, Hosking-Jervis
F, Leemann L, et al: An exploratory study of dose escalation vs
standard focal high-intensity focused ultrasound for treating
nonmetastatic prostate cancer. J Endourol. 34:641–646.
2020.PubMed/NCBI View Article : Google Scholar
|