Introduction
Prostate cancer (PCa) is a common cancer and the
second cause of cancer-related mortality in men (1,2).
Nonetheless, PCa prevalence at the histological level is higher
than the clinically detected disease rates. During autopsy studies,
prostatic adenocarcinoma has been histologically detected in
>30% of men older than 50 years. These tumors are usually small
and clinically indolent, with the ability to exist for several
years before presenting any change, such as accelerated cell
proliferation, tumor metastasis and clinical detection. More
importantly, accumulating evidence has shown that, in patients
affected by primary bladder cancer (BC) undergoing radical
cystoprostatectomy (RCP), there is a higher incidence of PCa
(3,4). RCP specimens from patients affected
by diseases other than PCa can be a random sample from the
prostates of asymptomatic men, offering a unique opportunity to
study the incidence and morphological features of these incidental
prostatic tumors. In terms of randomness, this cohort shows
similarities to that of the autopsy studies, but differs in the
reported higher PCa incidence in men with BC (3,4).
According to the European Association of Urology guidelines, for
patients affected by muscle-invasive bladder cancer (MIBC) or any
high-risk, recurrent and non-invasive BC, the RCP procedure with
bilateral pelvic lymphadenectomy and various types of urinary
diversion is the gold standard of therapy (5). The standard RCP in men is based on
the removal of the bladder along with prostate, seminal vesicles, a
part of the vasa deferentia and distal ureter, including regional
lymphadenectomy (in order to provide an effective local treatment
of the disease), which can have a high incidence of sexual
complications and urinary incontinence. Whereas alternative
techniques can be considered in highly selected cases in which it
is desired to preserve potency, fertility and urinary function. In
the modern era of orthotopic bladder substitution after RCP for BC,
sparing the entire prostate or a portion of it has become
controversial in recent years. However, these techniques, in an
effort to maintain sexual and urinary functions, have raised
concerns regarding the oncological outcomes due to two potential
risks: urothelial cancer local invasion of the prostate and a
probable association with incidental PCa (6). PCa is complex: on one hand, numerous
patients with PCa receive unnecessary treatment as their disease
will never become clinically significant or result in death. On the
other hand, some prostatic tumors require immediate treatment,
which are known as clinically detected PCa. For this reason,
incidentally identified PCas are divided in two groups: clinically
significant and clinically insignificant. The aim of the present
single-center retrospective study was to: i) assess incidence,
histopathological features and clinical significance of
incidentally identified prostatic tumors in RCP specimens obtained
from patients affected by bladder cancer, but with clinically
normal prostates; ii) examine patients' age, preoperative rectal
examination findings and prostate specific antigen (PSA) values, in
order to evaluate whether such features can help with the
prediction and treatment of significant PCas; iii) establish
whether prostate sparing-cystectomy could represent a feasible
option for these patients.
Materials and methods
The data of 303 male patients who underwent RCP with
bilateral pelvic lymphadenectomy and different urinary diversion
for BC at our Department of Urology were retrospectively reviewed.
Data from the pre-operative digital rectal exam (DRE) and PSA
assays were analyzed in patients diagnosed with incidental PCa, for
a total of 69/303 (22.7%) patients. Treatment and prognosis of
muscle invasive bladder cancer (MIBC) are determined by tumor stage
and grade. So, before any curative treatment, it is essential to
evaluate the presence of distant metastases. For this reason, all
patients enrolled in the current study underwent CT of the chest,
abdomen and pelvis, as well as MRI of the abdomen and pelvis. This
staging showed that none of the patients had distant metastases or
neoplastic disease of the prostate. The selection criteria were as
follows: i) no previous history of PCa; ii) no previous history of
chemotherapy or radiotherapy; iii) no evidence of PCa in the
imaging evaluation; and iv) age ≥40 years old. Routine pathological
examination was performed as by routine on bio-specimens. Beyond
evaluation of the bladder, it was considered i) the presence of
PCa; the stage of any detected prostatic adenocarcinoma following
the 2002 TNM classification (7)
and its Gleason score according to the World Health Organization
system (8) and ii) the surgical
margin status (a positive surgical margin was recorded upon
detection of tumor cells at the stained margin of the specimens).
Prostate involvement in bladder cancer was also assessed. The
intact RCP specimens were immersed in 10% buffered formalin
solution. Then, the prostate including seminal vesicles and vas
deferens, was cut out from bladder, weighed and stained with Indian
Ink. Sectioning was performed by cutting at 5-mm interval sections
transverse to the long axis, which were then embedded in paraffin
for H&E staining and examination. PCa was defined as clinically
significant when any of the following criteria was met: Gleason
Score ≥4, stage ≥pT3, extracapsular extension (ECE), lymph node
metastasis (LNM) or positive surgical margins (SM).
Results
In order to undergo surgery, all patients enrolled
in our study underwent DRE and MRI of the abdomen and pelvis to
specifically evaluate the prostate both clinically and
instrumentally. Both examinations did not reveal any prostate
abnormalities such as to require a prostate biopsy. Of the 303 RCP
specimens, incidental PCa was detected in 69 patients (22.7%), with
a median age of 71.6 years (age range, 54-89 years). We performed
orthotopic bladder substitution in 29 (42%) patients, ileal conduit
procedure in 14 patients (20.2%) and ureterocutaneostomy in 26
patients (37.7%). Bladder cancer features. Table I shows the histopathological
features of BC. All tumors were of high grade. PCa features.
The histopathological features of PCa identified within the RCP
specimens are reported in Table
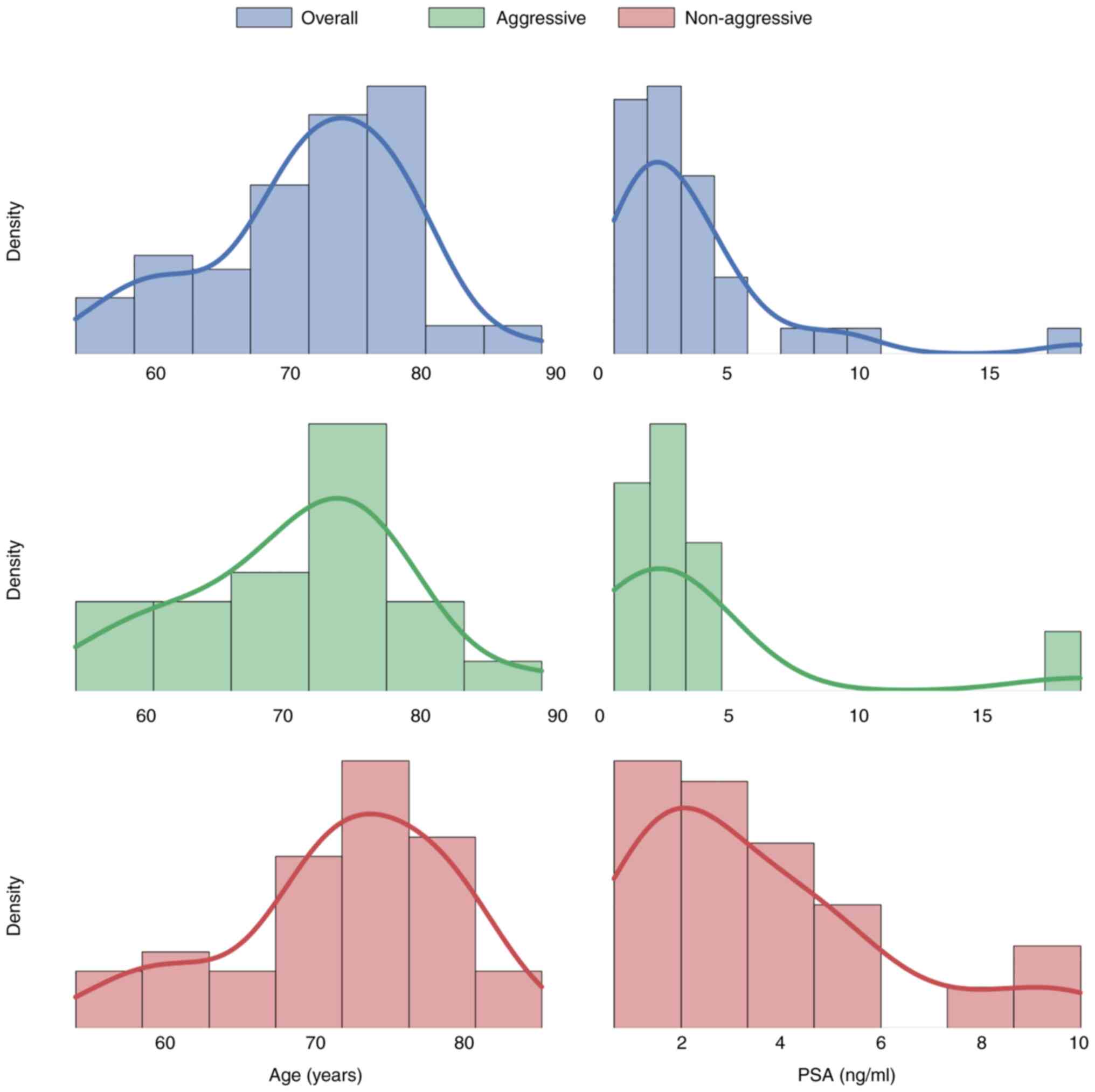
II. Whereas Fig. 1 reports the
preoperative PSA value and its distribution together with the age
distribution. In 69 patients with incidental PCa, 23 of these
cancers (33.33%) were regarded clinically significant. In this
group of patients, only seven (for a percentage equal to 10.1%)
were affected by locally advanced prostate cancer on
histopathological examination. From the retrospective analysis,
moreover, these patients presented a bladder tumor which invaded
the trigone and the bladder neck. For this reason, the differential
diagnosis between primary prostate tumor and bladder infiltration
of the prostate was very difficult. Incidence and
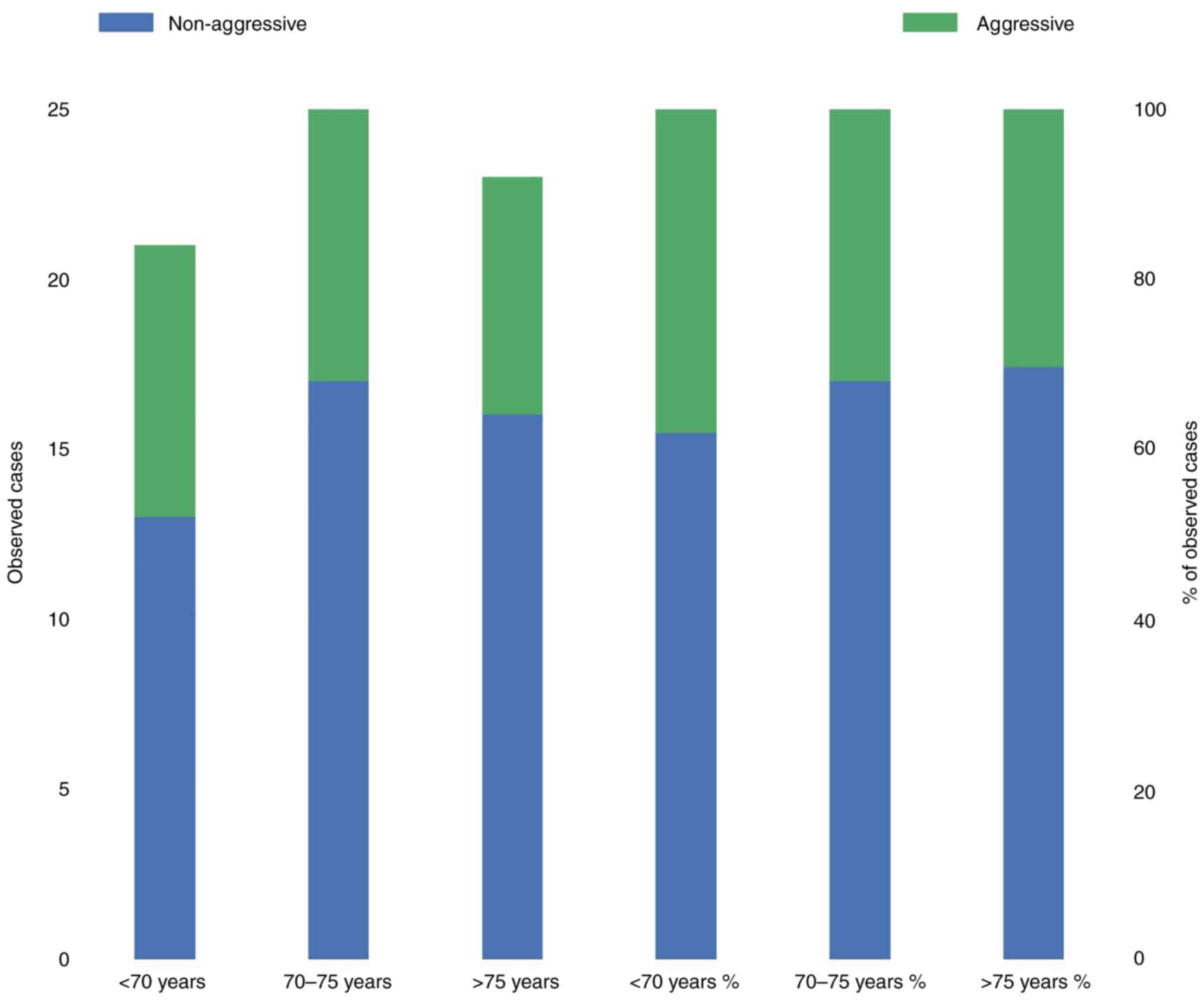
characteristics of PCa stratified according to age. Patients
were subdivided into three age groups based on the 33 and 66% age
quantiles (<70, between 70-75 and >75 years of age) and then
evaluated. For both TNM stage and Gleason score no significant
difference in the mean value of the respective parameter between
the three age categories was identified. Aggressiveness of
PCa. In total, 46 (66.66%) of 69 patients presented a
‘non-aggressive’ PCa. The incidence of aggressive tumor categorized
by age group is shown in Fig. 2.
None of the pre-operative factors, namely PSA level and age, were
predictive factors for non-aggressive PCa. Comparisons of the mean
values and rank order for age and PSA level between the patients
with aggressive PCa and the patients who had non-aggressive tumor
by means of unpaired t-tests and Mann-Whitney U tests did not
result in any significant difference as can be seen in Table III.
 | Table IClinicopathological characteristics of
bladder tumors in patients with incidentally diagnosed prostate
cancer. |
Table I
Clinicopathological characteristics of
bladder tumors in patients with incidentally diagnosed prostate
cancer.
| Variables | Value (n=303) |
|---|
| Age (years), median
(min-max) | 71.6 (54-89) |
| Histological type (n,
%) | |
|
Urothelial
(TCC) | 275 (90.8%) |
|
Other | 28 (9.2%) |
| pT stage (n, %) | |
|
Organ-confined
(<pT3) | 184 (60.7%) |
|
Locally-advanced
(≥pT3) | 119 (39.3%) |
| pN status (n, %) | |
|
N0 | 206 (68%) |
|
N+ | 97 (32%) |
| M status (n, %) | |
|
M0 | 299 (98.7%) |
|
M+ | 4 (1.3%) |
| Surgical margins (n,
%) | |
|
R0 | 288 (95%) |
|
R+ | 15 (5%) |
| Intraprostatic
urothelial proliferation (n, %) | |
|
Yes | 0 |
|
No | 303 (100%) |
 | Table IIClinicopathological characteristics of
prostate tumors from radical cystoprostatectomy specimens. |
Table II
Clinicopathological characteristics of
prostate tumors from radical cystoprostatectomy specimens.
| Variables | Value (n=69) |
|---|
| Age, years, median
(range) | |
|
Clinically
significant PCa group | 72 (55-89) |
|
Clinically
insignificant PCa group | 72.5 (54-85) |
| Preoperative PSA,
ng/ml, median (range) | |
|
Clinically
significant PCa group | 3.83 (0.68-18.6) |
|
Clinically
insignificant PCa group | 3.56 (0.68-9.9) |
| pT stage (n, %) | |
|
Organ-confined
disease (≤pT2) | 62 (89.9%) |
|
Locally
advanced disease (≥pT3) | 7 (10.1%) |
| pN status (n, %) | |
|
N0 | 69 (100%) |
|
N+ | 0 (0%) |
| Gleason score (n,
%) | |
|
<6 | 24 (34.8%) |
|
7 (3+4) | 26 (37.7%) |
|
7 (4+3) | 14 (20.3%) |
|
>7 | 5 (7.25%) |
| Surgical margins (n,
%) | |
|
R0 | 60 (87.0%) |
|
R+ | 9 (13.0%) |
 | Table IIIParametric and non-parametric
difference in the mean values and rank order of age and PSA level
between the clinically significant PCa group and the clinically
insignificant PCa group. |
Table III
Parametric and non-parametric
difference in the mean values and rank order of age and PSA level
between the clinically significant PCa group and the clinically
insignificant PCa group.
| A, Age |
|---|
| Test | Result |
|---|
| Welch two sample
t-test | |
|
t | -0.298 |
|
df | 40.629 |
|
p | 0.768 |
|
95%
confidence interval | -4.6,3.4 |
| Mann-Whitney U
test | |
|
W | 496 |
|
p | 0.679 |
| B, PSA |
| Test | Result |
| Welch two sample
t-test | |
|
t | -0.255 |
|
df | 28.317 |
|
p | 0.801 |
| Mann-Whitney U
test | |
|
W | 481 |
|
p | 0.545 |
Discussion
Incidental PCas identified in RCP samples, derived
from patients who underwent BC surgery but had no preoperative
evidence of prostatic disease, show histological and morphological
features similar to those of latent tumors identified in several
autopsies (9-11).
According to the literature, the frequency variability of
incidentally discovered PCa in cystoprostatectomy specimens is
extremely high, ranging from 17-70% (12,13),
owing to various factors. The first of these is the different
definition of clinically significant cancer in published studies
(14). Over the past two decades,
the emerging concept of ‘insignificant’ PCa has progressed to
indicate low-grade, small-volume and organ-confined prostatic
tumors that are likely slowly progressing, and these, although
might not need urgent therapeutic treatment, are eligible for
active surveillance (3).
Currently, the pathological assessment of the lesion indicates
further patient management (15).
Generally, PCa is diagnosed as ‘insignificant’ when all these
criteria are met: i) the disease has a Gleason score <7 (without
a Gleason pattern of 4 or 5); ii) it is confined to the organ
(stage pT2); and iii) the tumor mass has a <0.5 cm3
volume. Here, only tumor stage and grade could be taken into
account to cancer aggressiveness as tumor volume was not available
on the pathological report. Our results showed that 46 (66.66%) of
the incidentally diagnosed PCas were considered as ‘non-aggressive’
as they were organ-confined or with a Gleason score of <7 (4+3).
Then, an association between BC and PCa was suggested by several
previous studies (16,17). A previous study on a Japanese
cohort showed that the relative risk to develop PCas was 9x higher
in patients with BC (18).
Moreover, Kantor and McLaughlin (19) reported a 3-fold excess risk of PCa
within a year after the diagnosis of BC. Mersheimer et al
(20) also observed that the
combination of BC and PCa was common, being the second in frequency
after skin and colon cancers co-occurrence. However, the
association between BC and PCa can be explained as a possible
detection bias, associated with more detailed clinical assessment
and thorough pathological examination. For example, once a
diagnosis of BC has been made, a complete investigation of the
entire genitourinary system is likely to occur (21). Indeed, BC patients in the clinical
practice are more actively screened for prostatic tumors compared
with the general population. Kurokawa et al (22) examined a case cohort of 106
patients for BC (case cohort) in comparison with a 1,060
age-matched control cohort of men who were subjected to PCa
screening. They found a PCa rate of 12.3% in the cohort of BC
patients vs. 1.5% in the control cohorts, thereby confirming that
the risk of developing PCa in patients affected by BC could be
higher. In this regard, however, it is important to note that the
prognosis of patients bearing both PCa and BC is not considered to
be worse than the prognosis of patients bearing only one of these
two cancer types; rather, it is the stage of BC that impacts the
prognosis. The risk of death by the more aggressive tumor type is
not altered by the presence of other tumors in patients undergoing
radical pelvic surgery.
The different detection rate of PCa in RCP specimens
may be influenced by the thickness of the prostate histological
slices, because pathologists might focus more to the bladder.
Indeed, Kouriefs and colleagues (23) reasoned that the lower PCa incidence
observed in their study (18%) was possibly caused by thick gland
sections, indicating that thinner sectioning is recommended (≤10
mm). Consistently, Abbas et al (24) found a 45% incidence rate using
2-3-mm-thick slices and Moutzouris et al (25) a 27% of PCa using 5-mm slices. The
current study used 5-mm slices and the observed 22.7% incidence
rate of PCa supported the aforementioned hypothesis, indicating
that thin tissue sectioning should be used to optimize cancer
detection. Finally, genetic and environmental factors may influence
the variability of the findings from different countries. In the
present study, the majority of prostatic tumors were well
differentiated. Our data are consistent with what reported in other
studies in which most of detected tumors were not clinically
significant, with only few patients requiring therapeutic treatment
(10,11,24).
The preservation of continence and erectile function, as well as
guaranteeing excellent oncological results, remain the primary
goals of the treatment of BC with RCP. Various techniques can help
to preserve postoperative continence and erectile function, such as
leaving the apex or the entire tissue of the prostate; however, the
potential risk of not removing the synchronous PCa can be
problematic. By contrast, the probability that patients undergoing
RCP and have PCa will not die from prostatic disease is high.
Determining whether patients are suitable for prostate-sparing
surgery can be difficult due to the wide variability of both cancer
rates. In this regard, the RCP findings obtained in a study by
Moutzouris et al (25)
raise further concerns, showing apical involvement by PCa in the
31% of cases and the presence of multifocal PCa in the 31% of
patients (25). Moutzouris et
al (25) claimed that apical
involvement by PCa indicates the need of a complete prostate
resection. Indeed, a patient within their cohort bearing PCa in the
apex had recurrent prostatic disease in the urethro-ileal
anastomosis of an orthotopic bladder substitute. Similarly, Revelo
et al (26) reported a 25%
of patients with apical PCa, of which about 2/3 were clinically
significant. They found apical involvement of the prostate with BC
in 16% of patients. Overall, they suggested that prostatic apex
preservation was a feasible method to improve continence, but it
was associated with the risk of incomplete cancer resection. In the
attempt to overcome this risk, Revelo et al (26) suggested to perform a pre-operative
prostate biopsy and freeze intraoperative sections. However, due to
possible sampling error, a negative biopsy may not completely
exclude apical involvement of PCa in subjects elected for apical
sparing surgery. Hautmann et al (27) performed sextant biopsies of the
prostate upon removal of RCP specimens and detected through this
method PCa in only 5% of cases, showing that the biopsy detection
rate was 1 out of 9 tumors. Therefore, while sextant biopsies seem
not adequate to exclude clinically significant PCa, the optimal
prostatic biopsy procedure still needs to be defined. So, routine
biopsy has a certain degree of uncertainty regarding the ability to
identify clinically significant PCa with high sensitivity when
attempting to select patients for prostate-sparing cystectomy. For
a successful radical cancer removal it remains crucial not to leave
PCa in the apical prostatic margin or residual tissue of PCa, which
might be clinically significant. According to Pettus and colleagues
(28), only age was a predictive
factor for PCa (odds ratio=1.3, P=0.046). However, the present data
suggest that patients' age was not a preoperative factor associated
with a significant status of PCa. Likewise, the preoperative PSA
level seems not significantly associated with the ability to
incidentally discover PCa (3). In
the present study, PSA values and DRE findings were available for
all patients, but their results were not indicators for cancer.
This finding suggests that preoperative PSA screening and DRE in
RCP candidates provide no advantages in this setting, which was
consistent with results of previous studies (24). Identifying that there was not a
significant difference in PSA levels in men with clinically
significant PCa and those with clinically insignificant PCa at the
preoperative stage indicates that PSA is a weak predictor of
significant disease, and there is no reliable PSA threshold having
a 100% negative predictive value. Consistently, Gakis and
colleagues (29) showed that no
preoperative clinical value could formally exclude PCa in an RCP
specimen. Overall, these studies indicate that it is currently not
possible to adequately determine which patients can safely be
selected for prostate-sparing cystectomy and that the mainstay of
treatment, in cases of MIBC, remains RCP and the current study
agrees with these findings.
In conclusion, the present study demonstrated that
incidentally diagnosed PCa in specimens from RCP for BC was
frequently found, resulting in a rate of ~23% of the current RCP
specimens. As in other studies, also in the current report the
majority of these prostatic tumors were not clinically significant,
not requiring therapeutic treatment. This has increased the desire
to preserve the continence and erectile function in patient
undergoing RCP for bladder cancer; however, the risk of not
removing the synchronous PCa should be considered. In effect, in
our cohort, 33,3% of patients was affected by clinically
significant prostate cancer. It was suggested that the differences
in the incidence and behavior of prostatic disease were associated
with the patient's age. However, in this study, no preoperative
predictive factors (patient's age, PSA or DRE) were identified that
were able to determine ‘non-aggressive’ PCa status, resulting in
the inability to adequately determine which patients can be safely
selected for prostate-sparing surgery. So, the present results
demonstrate the need for a careful and complete prostate removal
during RCP. Nevertheless, since organ-sparing surgeries are widely
performed in young population, due to the impossibility of
predicting aggressive prostate cancer and considering the 33,3% of
clinically significant prostate cancer in our cohort, these
patients require close monitoring through lifelong PSA
surveillance, particularly focusing on the possible relapse of PCa
after RCP. Finally, in our study the technique for cutting the
prostate at 5-mm interval sections transverse to the long axis,
allowing the detection of nearly 23% of PCA, supports the
hypothesis that thin tissue sectioning should be used to optimize
cancer detection (regardless of prostate volume which traditionally
affects the number of biopsies to be taken).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RB made substantial contributions to conception and
design and contributed to writing the manuscript. RB and GM made
substantial contributions to acquisition of data and confirm the
authenticity of all the raw data. CC, UM, OI, UP, CD, AC, FP, RS,
RB and GM analyzed and interpreted the patient data regarding the
urological disease. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Signed informed consent was obtained from all the
patients for publication of this study and for processing their
medical data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Devesa SS, Blot WJ, Stone BJ, Miller BA,
Tarone RE and Franmeit JF Jr: Recent cancer trends in the United
States. J Natl Cancer Inst. 87:175–182. 1995.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wingo PA, Tong T and Bolden S: Cancer
statistics 1995. CA Cancer J Clin. 45:8–30. 1995.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Winkler MH, Livni N, Mannion EM, Hrouda D
and Christmas T: Characteristics of incidental prostatic
adenocarcinoma in contemporary radical cystoprostatectomy
specimens. BJU Int. 99:554–558. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Konski A, Rubin P, Disantagnese PA, Mayer
E, Keys H, Cockett A, Frank I, Davis R and Lush C: Simultaneous
presentation of adenocarcinoma of prostate and transitional cell
carcinoma of bladder. Urology. 37:202–206. 1991.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Witjes JA, Compérat E, Cowan NC, De Santis
M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG and Sherif A:
European Association of Urology. EAU guidelines on muscle-invasive
and metastatic bladder cancer: summary of the 2013 guidelines. Eur
Urol. 65:778–792. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nieuwenhuijzen JA, Meinhardt W and
Horenblas S: Clinical outcomes after sexuality preserving
cystectomy and neobladder (prostate sparing cystectomy) in 44
patients. J Urol. 173:1314–1317. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Heidenreich A, Aus G, Bolla M, Joniau S,
Matveev VB, Schmid HP and Zattoni F: European Association of
Urology. EAU guidelines on prostate cancer. Eur Urol. 53:68–80.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lopez-Beltran A, Mikuz G, Luque RJ,
Mazzucchelli R and Montironi R: Current practice of Gleason grading
of prostate carcinoma. Virchows Arch. 448:111–118. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Scott R Jr, Matchnik DH, Laskowski TZ and
Schmalforst WR: Carcinoma of the prostate in elderly men:
Incidence, growth characteristics and clinical significance. J
Urol. 101:602–607. 1969.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cabalin JN, McNeal JF, Price HM, Freiha FS
and Stamey TA: Unsuspected adenocarcinoma of the prostate in
patients undergoing cystoprostatectomy for other causes: Incidence,
histology and morphometric observations. J Urol. 141:1091–1094.
1989.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Montie JE, Wood DR Jr, Pontes E, Boyett JM
and Levin HS: Adenocarcinoma of the prostate in cystoprostatectomy
specimens removed for bladder cancer. Cancer. 63:381–385.
1989.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chun TY: Coincidence of bladder and
prostate cancer. J Urol. 157:65–67. 1997.PubMed/NCBI
|
|
13
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
et al: Radical cystectomy in the treatment of invasive bladder
cancer: Long-term results in 1,054 patients. J Clin Oncol.
19:666–675. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Damiano R, Di Lorenzo G, Cantiello F, De
Sio M, Perdonà S, D'Armiento M and Autorino R: Clinicopathologic
features of prostate adenocarcinoma incidentally discovered at the
time of radical cystectomy: An evidence-based analysis. Eur Urol.
52:648–657. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ploussard G, Epstein JI, Montironi R,
Carroll PR, Wirth M, Grimm MO, Bjartell AS, Montorsi F, Freedland
SJ, Erbersdobler A and van der Kwast TH: The contemporary concept
of significant versus insignificant prostate cancer. Eur Urol.
60:291–303. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Greenberg RS, Rustin ED and Clark S: Risk
of genitourinary malignancies after cancer of the prostate. Cancer.
61:396–401. 1988.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liskow AS, Neugut AI, Benson M, Olsson CA,
Birkhoff J and Chang GH: Multiple primary neoplasms in association
with prostate cancer in black and white patients. Cancer.
59:380–384. 1987.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kotake T and Kiyohama H: Multiple primary
cancers (MPC) associated with bladder cancer: An analysis of the
clinical and autopsy cases in Japan. Jpn J Clin Oncol. 15 (Suppl
1):S201–S210. 1985.PubMed/NCBI
|
|
19
|
Kantor AF and McLaughlin JK: Second cancer
following cancer of the urinary system in Connecticut, 1935-82.
Natl Cancer Inst Monogr. 68:149–159. 1985.PubMed/NCBI
|
|
20
|
Mersheimer WL, Ringel A and Eisenberg H:
Some characteristics of multiple primary cancers. Ann NY Acad Sci.
114:896–921. 1964.PubMed/NCBI
|
|
21
|
Barbisan F, Mazzucchelli R, Scarpelli M,
Lopez-Beltran A, Cheng L, Kirkali Z and Montironi R: Urothelial and
incidental prostate carcinoma in prostates from
cystoprostatectomies for bladder cancer: Is there a relationship
between urothelial and prostate cancer? BJU Int. 103:1058–1063.
2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kurokawa K, Ito K, Yamamoto T, Takechi H,
Miyamoto S, Suzuki K and Yamanaka H: Comparative study on the
prevalence of clinically detectable prostate cancer in patients
with and without bladder cancer. Urology. 63:268–272.
2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kouriefs C, Fazili T, Masood S, Naseem MS
and Mufti GR: Incidentally detected prostate cancer in
cystoprostatectomy specimens. Urol Int. 75:213–216. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Abbas F, Hochberg D, Givantos F and
Soloway M: Incidental prostatic adenocarcinoma in patients
under-going radical cystoprostatectomy for bladder cancer. Eur
Urol. 30:322–326. 1996.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Moutzouris G, Barbatis C, Plastiras D,
Mertziotis N, Katsifotis C, Presvelos V and Theodorou C: Incidence
and histological findings of unsuspected prostatic adenocarcinoma
in radical cystoprostatectomy for transitional cell carcinoma of
the bladder. Scand J Urol Nephrol. 33:27–30. 1999.PubMed/NCBI
|
|
26
|
Revelo MP, Cookson MS, Chang SS, Shook MF,
Smith JA Jr and Shappell SB: Incidence and location of prostate and
urothelial carcinoma in prostates from cystoprostatectomies:
Implications for possible apical sparing surgery. J Urol.
171:646–651. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hautmann SH, Conrad S, Henke RP,
Erbersdobler A, Simon J, Straub M, Graefen M, Hautmann RE and
Huland H: Detection rate of histologically insignificant prostate
cancer with systematic sextant biopsies and fine needle aspiration
cytology. J Urol. 163:1734–1738. 2000.PubMed/NCBI
|
|
28
|
Pettus JA, Al-Ahmadie H, Barocas DA,
Koppie TM, Herr H, Donat SM, Dalbagni G, Reuter VE, Olgac S and
Bochner BH: Risk assessment of prostatic pathology in patients
undergoing radical cystoprostatectomy. Eur Urol. 53:370–375.
2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gakis G, Schilling D, Bedke J, Sievert KD
and Stenzl A: Incidental prostate cancer at radical
cystoprostatectomy: Implications for apex-sparing surgery. BJU Int.
105:468–471. 2010.PubMed/NCBI View Article : Google Scholar
|