Introduction
Pandemics such as COVID-19 have drawn attention to
human immunity. The immune system protects the human body from
disease by detecting invasion and removing foreign pathogens
(1). Thus, maintenance of a
well-functioning immune system is essential for human survival
(1). The immune system employs
both innate and adaptive immunity to fight foreign pathogens
(2). Innate immunity is the first
rapid defense response against these pathogens, whereas adaptive
immunity is an antigen-specific immune response that is important
when these pathogens are not eliminated by the innate immune system
(3,4). Strong interactions between the innate
and adaptive immune systems are crucial for an effective response
to foreign pathogens and defects in either immune response result
in various diseases (2). The most
effective way to strengthen these immune systems is to activate the
functions of various immune cells that constitute the immune
system.
Obesity increases infection and mortality rates in
infectious diseases such as COVID-19(5). There exists a close connection
between obesity and infectious diseases (6). Obesity increases susceptibility to
infectious diseases by weakening the immune response of innate
immune cells when they are confronted with invading foreign
pathogens (6). In addition,
obesity leads to impairment of the adaptive immune system (7). Therefore, obesity management is
considered a good strategy for strengthening the body's immune
system.
Adenocaulon himalaicum Edgew. (A.
himalaicum) is a plant distributed across Korea, China, Japan,
Russia and Southeast Asia. In Korea, the leaves of A.
himalaicum have long been used as a wild vegetable (8). A. himalaicum has been
traditionally used as an herbal medicine to treat abscesses,
bleeding and inflammation (9).
A. himalaicum has been reported to have antioxidant
(8), anticancer (10) and skin damage-protection activities
(8). However, other
pharmacological activities of A. himalaicum remain to be
elucidated. Specifically, there is currently no background on the
immunostimulatory and anti-obesity activity of A.
himalaicum. Thus, the present study investigated the
immunostimulatory activity of A. himalaicum leaf extracts in
RAW264.7 cells and the anti-obesity activity of A.
himalaicum leaf extracts in 3T3-L1 cells.
Materials and methods
Chemical reagents
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), Griess reagent and Neutral Red were purchased from
MilliporeSigma. Inhibitors, such as C29 [Toll-like receptor (TLR)2
inhibitor], TAK-242 (TLR4 inhibitor), PD98059 (ERK1/2 inhibitor),
SB203580 (p38 inhibitor) and SP600125 (JNK inhibitor) were
purchased from MilliporeSigma. The primers for inducible nitric
oxide synthase (iNOS), IL-1β, IL-6 and GAPDH were synthesized by
Invitrogen (Thermo Fisher Scientific, Inc.). The primary antibodies
individually raised against phosphorylated (p-)ERK1/2 (cat. no.
4377), ERK1/2 (cat. no. 9102), p-p38 (cat. no. 4511), p38 (cat. no.
9212), p-JNK (cat. no. 9251), JNK (cat. no. 9252), CCAAT enhancer
binding protein α (CEBPα; cat. no. 8178), proliferator-activated
receptor γ (PPARγ; cat. no. 2430), adipose triglyceride lipase
(ATGL; cat. no. 2138), p-hormone-sensitive lipase (HSL; cat. no.
4137), HSL (cat. no. 4107), perilipin-1 (cat. no. 9349),
p-adenosine monophosphate activated protein kinase (AMPK; cat. no.
2535), AMPK (AMPK, cat. no. 5831), uncoupling protein 1 (UCP-1;
cat. no. 14670) and β-actin (cat. no. 5125) were purchased from
Cell Signaling Technology, Inc. Other primary antibodies, such as
anti-peroxisome proliferator-activated receptor-γ coactivator 1α
(PGC-1α; cat. no. sc-518025) and anti-PR domain containing 16
(PRDM16; cat. no. ab106410), were purchased from Santa Cruz
Biotechnology, Inc. and Abcam. Secondary antibodies, such as
anti-rabbit IgG horseradish peroxidase (HRP)-linked antibody (cat.
no. 7074) and anti-mouse IgG HRP-linked antibody (cat. no. 7076),
were purchased from Cell Signaling Technology, Inc.
Sample preparation
A. himalaicum leaves (AHL) used in this study
were provided as dry powder by the National Institute of Forest
Science, Korea. A. himalaicum is not listed in the IUCN Red
List of Threatened Species, so it is neither an endangered nor a
protected species. Water extraction from A. himalaicum
leaves was performed by adding 20 times the weight of distilled
water to 10 g of A. himalaicum leaves and extracting them in
a water bath at 40˚C for 24 h. After 24 h, the extract was
lyophilized, dissolved in distilled water and added to the
cells.
Cell culture
The murine macrophage cell line RAW264.7 and mouse
pre-adipocyte 3T3-L1 cells used in this study were purchased from
the American Type Culture Collection. RAW264.7 cells were cultured
in a CO2 incubator (37˚C, 5% CO2) using a
DMEM/F-12 medium (Thermo Fisher Scientific, Inc.) containing 10%
fetal bovine serum (FBS) and penicillin/streptomycin (100 unit/100
µg/ml). Subculture of RAW264.7 cells was performed once every 2
days. 3T3-L1 cells were cultured in a CO2 incubator
(37˚C; 5% CO2) using DMEM/F-12 medium containing 10%
bovine calf serum and penicillin/streptomycin (100 unit/100 µg/ml).
The 3T3-L1 cells were subcultured once every 3 days.
Measurement of cell viability in
RAW264.7 cells
Toxicity to cells was measured using an MTT assay.
RAW264.7 cells were treated with AHL and cultured for 24 h at 37˚C
in a 96-well plate. MTT solution (1 mg/ml) was then added and
incubated for an additional 4 h. After 4 h, the DMSO solution was
added and incubated for 10 min. Absorbance was measured at 570 nm
using a UV/visible spectrophotometer (Human Cop., Xma-3000PC,
Seoul, Korea).
Treatment of RAW264.7 cells with the
chemical inhibitors
The chemical inhibitors, such as C29, TAK-242,
PD98059, SB203580 and SP600125, were used to pre-treat RAW264.7
cells for 2 h at 37˚C, 2 h prior to AHL treatment.
Measurement of NO in RAW264.7
cells
RAW264.7 cells were cultured in a 96-well plate
using DMEM/F-12 medium supplemented with 10% FBS and treated with
AHL for 24 h. To measure the amount of NO, the cell culture medium
and Griess solution were mixed 1:1 and allowed to react for 15 min.
The absorbance was then measured at 540 nm using a UV/Visible
spectrophotometer (Humancorp; Xma-3000PC). Values were calculated
using NaNO2 as a standard.
Measurement of phagocytotic activity
in RAW264.7 cells
Phagocytosis of RAW264.7 cells was performed using
Neutral Red assay (11). RAW264.7
cells were cultured in a 96-well plate using DMEM/F-12 medium
supplemented with 10% FBS and treated with AHL for 24 h. To measure
the phagocytosis in RAW264.7 cell line, cells were stained with
0.01% Neutral Red solution for 2 h and then Neutral Red was eluted
with lysis buffer containing 50% ethanol and 1% acetic acid at a
ratio of 1:1. The absorbance of the eluted Neutral Red was measured
at 540 nm using a UV/visible spectrophotometer (Humancorp;
Xma-3000PC).
Differentiation from preadipocytes to
mature adipocytes in 3T3-L1 cells
Two days (D0) after culturing 3T3-L1 cells in a
6-well plate to 100% confluency, the 3T3-L1 cells were
differentiated for 2 days in a DMEM/F-12 medium containing DMI
(0.05 mM IBMX + 1 µM dexamethasone + 10 µg/ml insulin) (D2). Then,
the 3T3-L1 cells were cultured for 2 days in a DMEM/F-12 medium
containing 10 µg/ml insulin (D4). 3T3-L1 cells were further
cultured for 4 days, with the DMEM/F-12 medium replaced once every
2 days (D6-D8). AHL were administered from D0 to D8 and
experimental analysis was performed on D8.
Oil-Red O (ORO) staining in 3T3-L1
cells
Lipid accumulation in 3T3-L1 cells was confirmed
using ORO staining. The 3T3-L1 cells were washed with PBS, fixed
with 10% formalin and stained with the ORO staining reagent for 20
min at room temperature. After staining, the staining solution was
removed, the cells were washed with distilled water and the degree
of lipid accumulation was observed under a light microscope
(Olympus Corporation). After microscopic observation, the stained
ORO was eluted with 100% isopropanol and the absorbance was
measured at 500 nm using a UV/visible spectrophotometer (Humancorp;
Xma-3000PC). Lipid accumulation in ORO staining was calculated by
measuring the absorbance of released ORO dye in cells.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
To obtain RNA from RAW264.7 cells, total RNA was
extracted from RAW264.7 cells using a RNeasy Mini kit (Qiagen
GmbH). The extracted RNA was synthesized into cDNA using 1 µg of
total RNA through quantitative analysis and Verso cDNA kit (Thermo
Fisher Scientific, Inc.). RT-PCR of iNOS, IL-1β, IL-6, TNF-α and
GAPDH genes was performed using the synthesized cDNA and PCR Master
Mix kit (Promega Corporation). The primer sequences used in this
study are listed in Table I. PCR
reaction (PCR Thermal Cycler Dice; Takara Bio, Inc.) was performed
for 30 cycles under the following conditions: Denaturation at 94˚C
for 30 sec, annealing at 55˚C for 1 min and extension at 72˚C for 1
min. After the PCR, the products were electrophoresed on a 1%
agarose gel at 100 V for 15 min. The results presented are not
mRNA, but rather DNA. Therefore, the brightness of the expressed
DNA bands was quantified using UN-SCAN-IT (Silk Scientific,
Inc.).
 | Table ISequences of primers used in the
amplification of cDNA. |
Table I
Sequences of primers used in the
amplification of cDNA.
| Primers | Sequences |
|---|
| iNOS | Forward
5'-ttgtgcatcgacctaggctggaa-3' |
| | Reverse
5'-gacctttcgcattagcatggaagc-3', |
| IL-1β | Forward
5'-ggcaggcagtatcactcatt-3' |
| | Reverse
5'-cccaaggccacaggtattt-3' |
| IL-6 | Forward
5'-gaggataccactcccaacagacc-3' |
| | Reverse
5'-aagtgcatcatcgttgttcataca-3' |
| TNF-α | Forward
5'-tggaactggcagaagaggca-3' |
| | Reverse
5'-tgctcctccacttggtggtt-3' |
| GAPDH | Forward
5'-ggactgtggtcatgagcccttcca-3' |
| | Reverse
5'-actcacggcaaattcaacggcac-3' |
Western blot analysis
After washing the cells with PBS, RIPA buffer was
added to extract proteins, the recovered cells were centrifuged at
25,200 x g for 30 min at 4˚C and the protein concentration was
quantified using a bicinchoninic acid (BCA) protein assay kit
(Thermo Fisher Scientific, Inc.). The quantified proteins (25
µg/well) were separated using SDS-PAGE (10% polyacrylamide gel) and
transferred onto a nitrocellulose blotting membrane (Thermo Fisher
Scientific, Inc.). The transferred membranes were blocked with 5%
skimmed milk for 1 h at room temperature. For the primary antibody
reaction, the membrane was incubated at 4˚C overnight with a 5% BSA
solution containing the primary antibodies (1:1,000). Then, a 5%
BSA solution containing secondary antibodies (1:1,000) was added to
the membrane and allowed to react at room temperature for 1 h. To
activate the horseradish peroxidase molecules bound to the
secondary antibody, western blotting substrate was added to the
membrane (Cytiva) and protein expression was confirmed using a
LI-COR C-DiGit Blot Scanner (LI-COR Biosciences). Protein bands
were quantified using UN-SCAN-IT gel software version 5.1 (Silk
Scientific, Inc.). Actin was used as a loading control for
normalization in western blot analysis.
Statistical analysis
All experiments were repeated at least three times.
Statistical analyses were verified using GraphPad Prism version 5.0
(Dotmatics) and data are presented as mean ± standard deviation.
Each data point was analyzed using one-way analysis of variance and
the data was analyzed using the Bonferroni post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
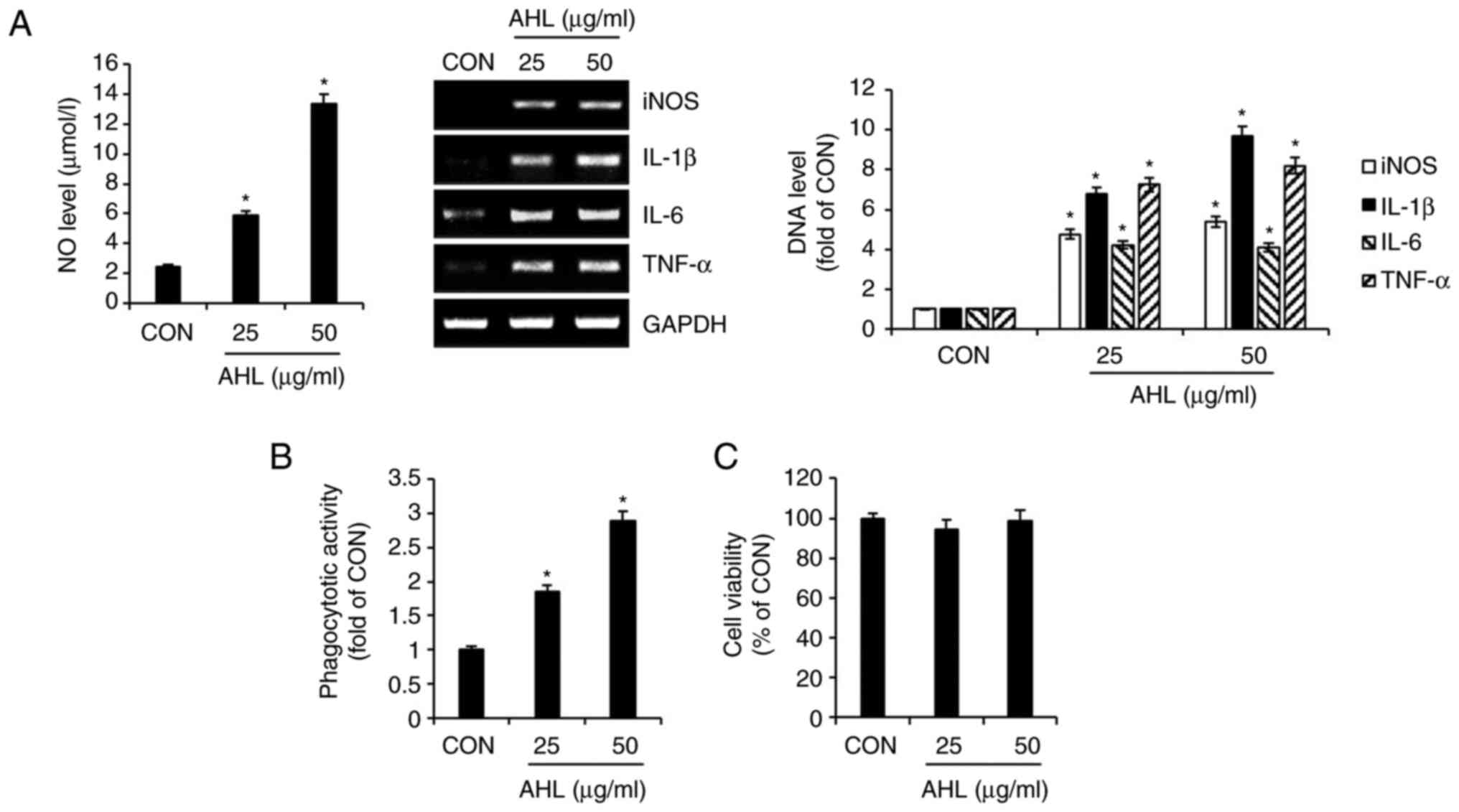
Effect of AHL on macrophage activation
in RAW264.7 cells
To evaluate the effect of AHL on macrophage
activation, the production of immunostimulatory factors and the
activation of phagocytosis were analyzed in AHL-treated RAW264.7
cells. The production of NO was increased in AHL-treated RAW264.7
cells. DNA levels of immunostimulatory factors such as iNOS, IL-1β,
IL-6 and TNF-α were increased in AHL-treated RAW264.7 cells
(Fig. 1A). In addition, AHL
increased the phagocytosis of RAW264.7 in a concentration-dependent
manner (Fig. 1B). However, AHL had
no effect on the viability of RAW264.7, indicating that AHL is not
cytotoxic to RAW264.7 cells (Fig.
1C). Taken together, these results suggested that AHL activates
macrophages.
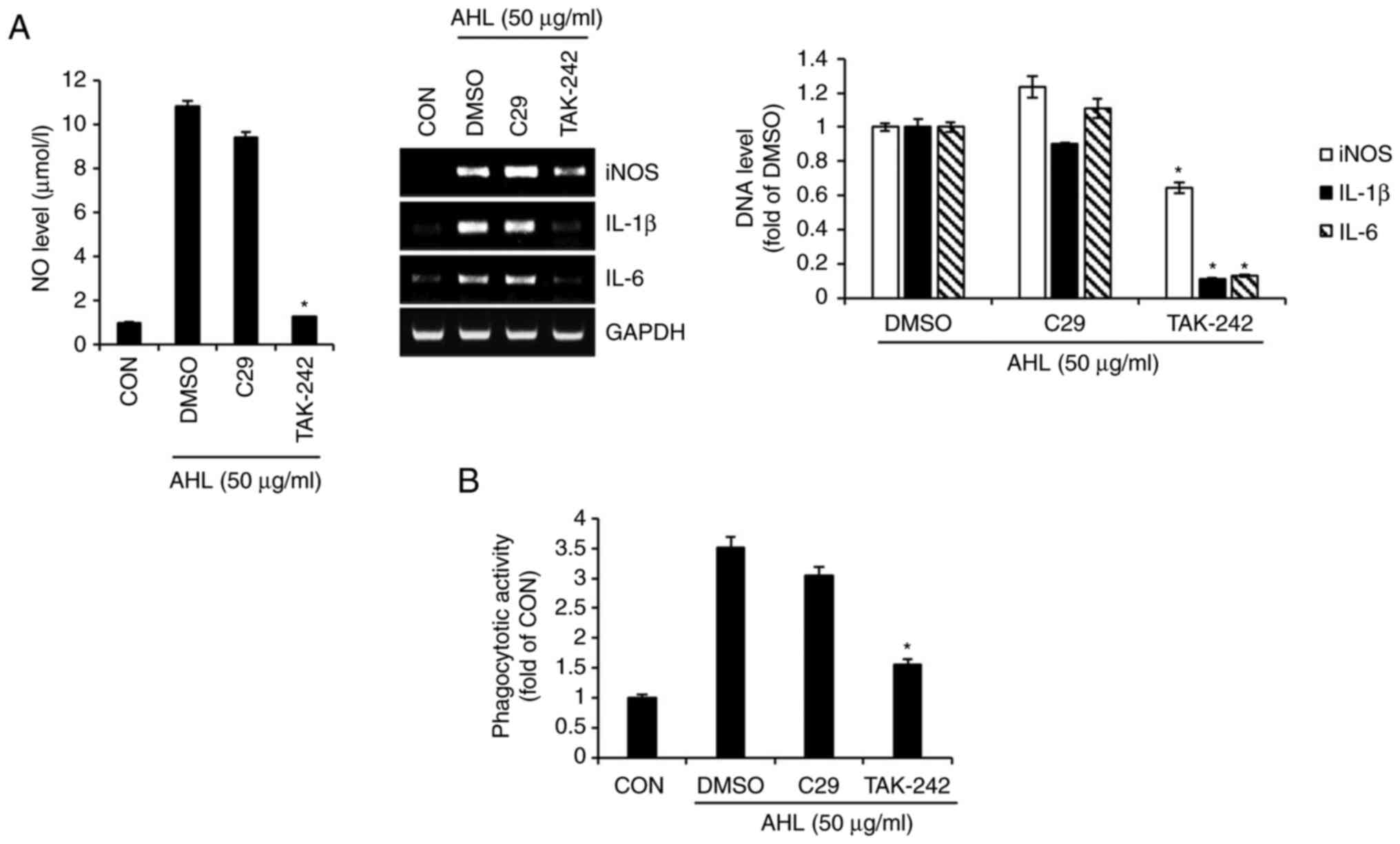
Effects of TLR2/4 on AHL-mediated
activation of RAW264.7 cells
To investigate the involvement of TLR2 and TLR4 in
AHL-mediated macrophage activation, the production of
immunostimulatory factors and phagocytosis was measured in
AHL-treated RAW264.7 cells in the presence of C29 (TLR2 inhibitor)
and TAK-242 (TLR4 inhibitor). The NO level and DNA level for iNOS,
IL-1β and IL-6 were increased in both AHL treatment alone and AHL
treatment in the presence of C29 with little difference (Fig. 2A). However, inhibition of TLR4 by
TAK-242 markedly reduced the AHL-mediated increase in The NO level
and DNA level for iNOS, IL-1β and IL-6 (Fig. 2A). Furthermore, inhibition of TLR2
by C29 did not reduce AHL-mediated phagocytosis of RAW264.7 cells,
whereas inhibition of TLR4 by TAK-242 significantly blocked
AHL-mediated phagocytosis (Fig.
2B). Taken together, these results indicated that AHL-mediated
activation of macrophages may be dependent on TLR4.
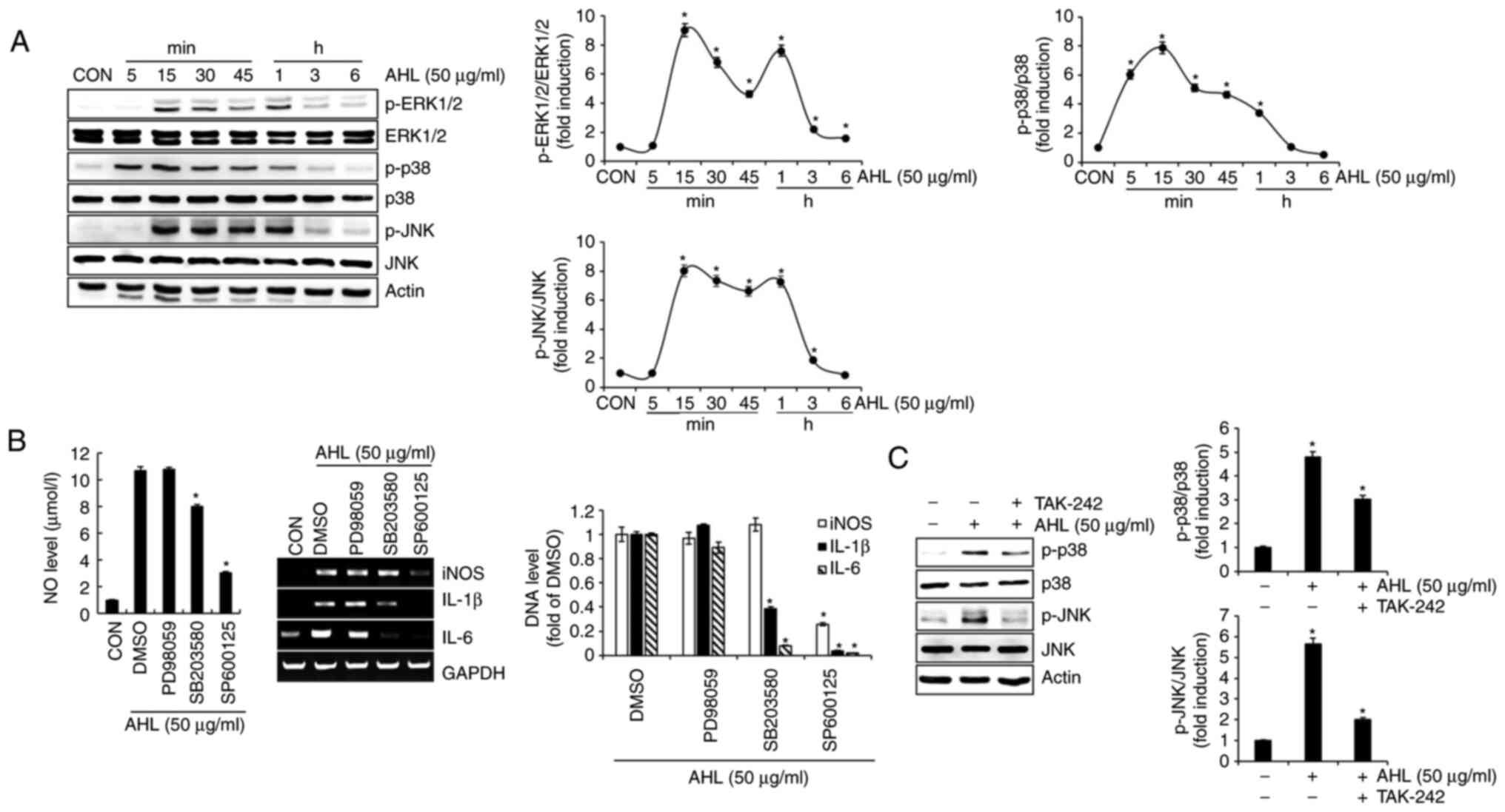
Effects of MAPK signaling pathway on
AHL-mediated activation of RAW264.7 cells
To analyze the effect of AHL on MAPK activation, the
phosphorylation of extracellular signal-regulated protein kinase
1/2 (ERK1/2), p38 and c-JNK were investigated in AHL-treated
RAW264.7 cells. AHL increased the phosphorylation of ERK1/2, p38
and JNK, indicating that AHL can activate ERK1/2, p38 and JNK
signaling (Fig. 3A). To analyze
the effect of ERK1/2, p38 and JNK signaling on AHL-mediated
activation of RAW264.7 cells, the present study investigated the
level of immunostimulatory factors in AHL-treated RAW264.7 cells in
the presence of specific ERK1/2, p38 and JNK signaling inhibitors.
Inhibition of p38 by SB203580 and inhibition of JNK by SP600125
significantly reduced AHL-mediated increase in the NO level and DNA
level for iNOS, IL-1β and IL-6 (Fig.
3B). However, inhibition of ERK1/2 by PD98059 did not reduce
AHL-mediated increase in the NO level and DNA level for iNOS, IL-1β
and IL-6. These results indicate that the activation of p38 and JNK
signaling may be important for AHL activation in macrophages.
Finally, the effect of TLR4 on AHL-mediated activation of p38 and
JNK signaling was investigated. Inhibition of TLR4 by TAK-242
reduced the phosphorylation of p38 and JNK by AHL (Fig. 3C), indicating that the activation
of p38 and JNK signaling by AHL may depend on TLR4. Taken together,
these results suggested that AHL activates macrophages by
activating p38 and JNK signaling in a TLR4-dependent manner.
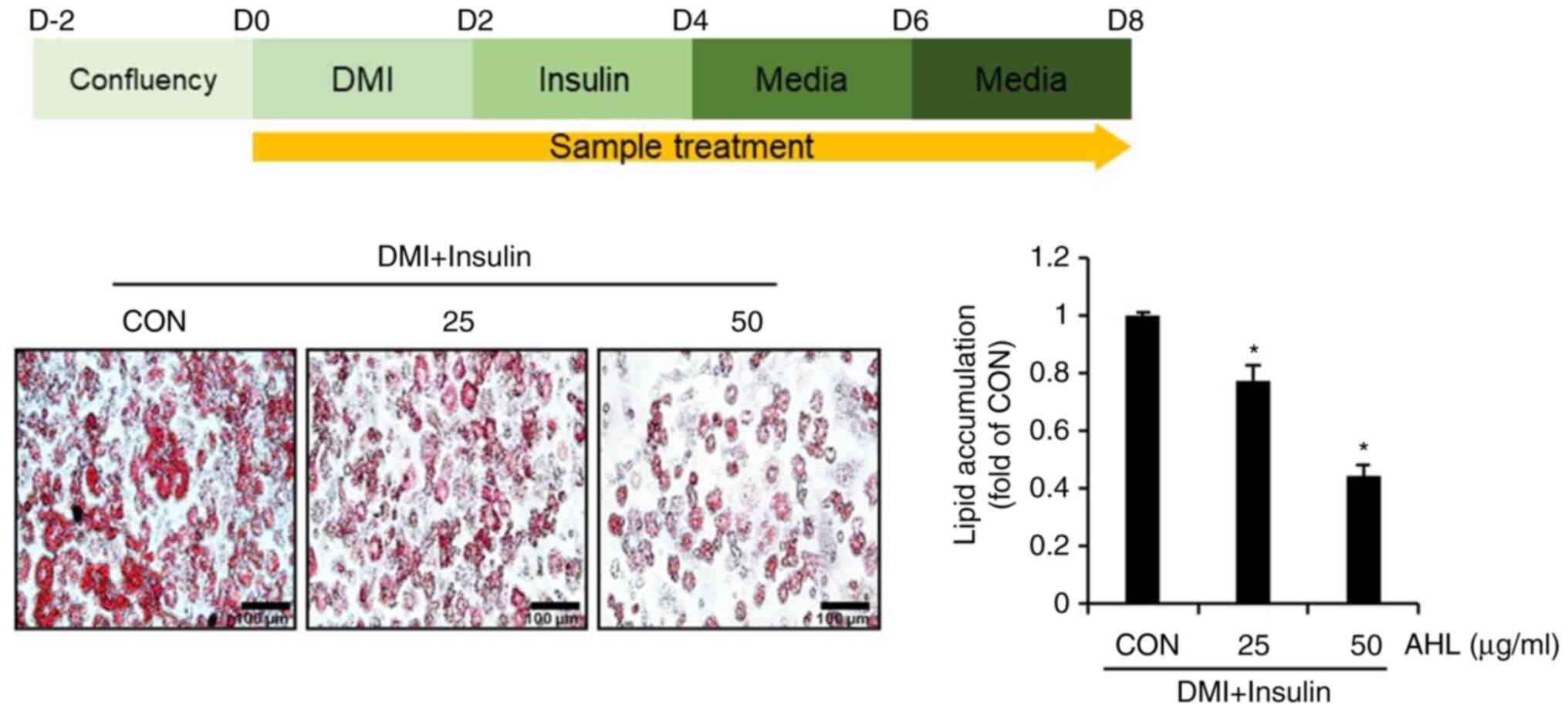
Effect of AHL on lipid accumulation in
3T3-L1 cells
To analyze whether AHL has anti-obesity activity,
3T3-L1 cells were treated with AHL during the differentiation of
3T3-L1 cells with DMI and insulin and the degree of lipid
accumulation was examined by ORO staining. Intracellular lipid
accumulation was reduced in AHL-treated cells compared to that in
untreated cells (Fig. 4). These
results suggested that AHL may have anti-obesity effects.
Effect of AHL on the level of protein
related to lipid accumulation in 3T3-L1 cells
The present study investigated whether the
inhibitory effect of AHL on lipid accumulation could be attributed
to the regulation of proteins related to adipogenesis, lipolysis
and browning of white adipocytes. AHL reduced the protein levels of
CEBPα and PPARγ, which induce adipogenesis (Fig. 5). By contrast, AHL increased ATGL
protein and the phosphorylation level of HSL and decreased
perilipin-1 protein, which is essential for lipolysis.
Additionally, AHL increased the phosphorylation level of AMPK and
increased UCP-1, PGC-1α and PRDM16 proteins, which induce browning
of white adipocytes. Taken together, these results suggested that
AHL inhibits lipid accumulation in mature adipocytes by inhibiting
adipogenesis and inducing lipolysis and browning of white
adipocytes.
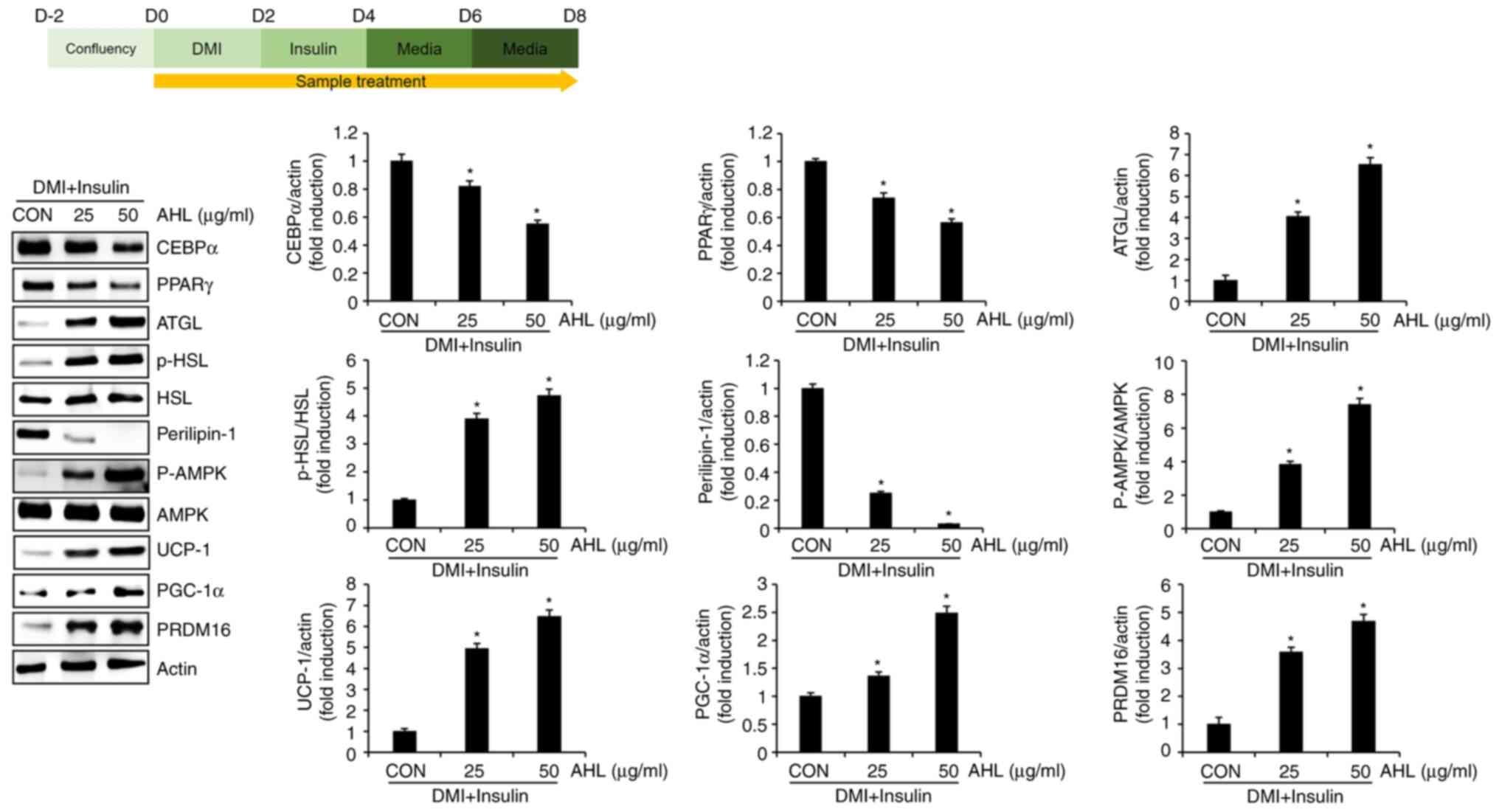 | Figure 5Effect of AHL on the protein levels
associated with excessive accumulation of lipid droplets in 3T3-L1
cells. *P<0.05 vs. CON group. AHL, Adenocaulon
himalaicum leaf extracts; DMI, mixture of 0.05 mM IBMX, 1 µM
dexamethasone and 10 µg/ml insulin; CON, control group without AHL
treatment; CEBPα, CCAAT enhancer binding protein α; PPARγ,
proliferator-activated receptor γ; ATGL, adipose triglyceride
lipase; p-, phosphorylated; HSL, hormone-sensitive lipase; AMPK,
adenosine monophosphate activated protein kinase; UCP-1, uncoupling
protein 1; PGC-1α, peroxisome proliferator-activated receptor-γ
coactivator 1α; PRDM16, PR domain containing 16; p-,
phosphorylated. |
Discussion
The innate immune response is triggered by immune
cells, such as monocytes, macrophages, neutrophils and dendritic
cells (12). Macrophages eliminate
invading foreign pathogens by phagocytosis and secreting various
immunostimulatory factors (13).
Macrophages present information regarding antigens to T cells,
which are adaptive immune cells and induce T cell differentiation
for cell-mediated immunity (13).
Thus, because macrophages are involved in both innate and adaptive
immunity, their activation is important for maintaining homeostasis
of the human body against foreign pathogens and for strengthening
the immune system of the human body (13). The present study showed that AHL
increases the NO levels and DNA level of iNOS, IL-1β, IL-6 and
TNF-α and activates phagocytosis in RAW264.7 cells. Although the
lack of improved quantitative RT-PCR data for assessing gene
expression levels is a limitation of the present study, the results
indicated that AHL may have immunostimulatory activity and that AHL
can be used as a potential agent to strengthen the immune system of
the human body. Foreign pathogens possess pathogen-associated
molecular patterns (PAMPs) that initiate an immune response by
recognizing PAMPs via pattern recognition receptors (PRRs)
(14). Among the PRRs of
macrophages, TLR2 and TLR4 are the most specialized receptors that
recognize foreign pathogens and are essential for innate and
adaptive immune responses (15-17).
The present study observed that AHL increased the levels of
immunostimulatory factors and activated phagocytosis in a
TLR4-dependent manner in RAW264.7 cells. These findings suggested
that AHL activates macrophages by stimulating TLR4 expression.
However, as various PRRs exist in macrophages, additional PRRs
related to macrophage activation by AHL need to be identified. In
addition, the present study confirmed that inhibition of p38 and
JNK downregulated the AHL-mediated production of immunostimulatory
factors and inhibition of TLR4 blocked AHL-mediated phosphorylation
of p38 and JNK in RAW264.7 cells. MAPK signaling, involving p38,
ERK1/2 and JNK, serves an important role in strengthening the human
immune system by contributing to TLR4-mediated macrophage
activation (18-21).
Thus, the results suggested that AHL may activate macrophages
through the TLR4-dependent activation of p38 and JNK signaling. AHL
may have immunostimulatory activity and can be used as a potential
agent to strengthen the immune system of the human body. However,
to confirm that the immunostimulation caused by AHL can strengthen
the immune system without causing excessive inflammatory reaction
it is necessary for in vivo studies to verify that this
immune activation is indeed beneficial to human body.
An imbalance between energy intake and consumption
leads to an excessive accumulation of fat in the body, eventually
leading to obesity. As it causes various metabolic diseases,
obesity has been considered as a disease that must be controlled.
Moreover, as obesity reduces immunity, obesity treatment is
urgently needed during the COVID-19 pandemic (5-7).
In the present study, AHL inhibited excessive lipid accumulation in
3T3-L1 cells, indicating that AHL could be used as a potential
agent for obesity treatment. Obesity can be prevented by regulating
the various molecules involved in lipid accumulation in adipocytes.
The first treatment for obesity is the inhibition of adipogenesis,
which is the process by which preadipocytes differentiate into
mature adipocytes (22). A number
of anti-obesity agents that inhibit adipogenesis have been
developed (22). The present study
confirmed that AHL effectively reduced the protein levels of CEBPα
and PPARγ in 3T3-L1 cells. CEBPα and PPARγ are major factors
involved in adipogenesis and inhibition of CEBPα and PPARγ reduces
lipid accumulation in the body in animal models (23,24).
Based on these preliminary reports, AHL appears to be capable of
inhibiting adipogenesis through the reduction of CEBPα and PPARγ in
adipocytes. In addition, although not staining the nuclei with
hematoxylin was a limitation of this study, the number of cells
decreased in the AHL-treated group. As adipogenesis is accompanied
by an increase in the number of adipocytes, the decrease in cell
number in AHL-treated cells suggested that AHL inhibited
adipogenesis. Obesity can be treated by inducing the lysis of
lipids accumulated in adipocytes (25). Lipolysis is the process by which
triacylglycerol (TAG) in adipose tissue is converted to
diacylglycerol and monoacylglycerol and finally decomposes into
fatty acids and glycerol (26,27).
The present study found that ATGL protein levels and HSL
phosphorylation increased and perilipin-1 protein levels decreased
in AHL-treated 3T3-L1 cells. ATGL and HSL are enzymes that
participate in the hydrolysis of TAG and a deficiency of these
enzymes inhibits lipolysis in adipose tissue, resulting in
excessive lipid accumulation (27). There is evidence that a decrease in
perilipin-1, which surrounds lipid droplets in adipocytes, promotes
the hydrolysis of TAG (26). As
the increase of ATGL and HSL and the decrease of perilipin-1 imply
the induction of lipolysis induction (26,27),
AHL is presumed to induce lipolysis by increasing ATGL and HSL and
decreasing perilipin-1. Finally, the browning of white adipose
tissue is also involved in suppressing excessive lipid accumulation
(28). Brown adipose tissue plays
a role in dissipating the energy stored as TAG in white adipose
tissue as heat (29). The present
study observed that AHL increased the phosphorylation of AMPK and
the levels of UCP-1, PGC-1α and PRDM16 in 3T3-L1 cells. AMPK
promotes fatty acid oxidation and induces the browning of white
adipose tissue (30). UCP-1 and
PGC-1α plays a role in releasing the energy stored in white adipose
tissue as heat (29,31). PRDM16, a major factor in the
development of brown adipose tissue, increases energy consumption
by promoting the browning of white adipose tissue (32). Therefore, considering the results
on AMPK phosphorylation, as well as the increase of UCP-1, PGC-1
and PRDM16 by AHL, along with previous research, it is hypothesized
that AHL may induce browning of adipocyte. The overall anti-obesity
results of AHL derived from the present study indicated that AHL
may block excessive lipid accumulation by inhibiting adipogenesis
and inducing lipolysis and browning of white adipose tissue.
However, as the present study was purely performed in vitro,
a limitation is that in order to validate the clinical
applicability of AHL, in vivo studies using animal models
are necessary to investigate the anti-obesity effects of AHL.
The concentration of AHL used in the present study
was 25-50 µg/ml, which showed immunostimulatory and anti-obesity
activity in vitro. However, it is necessary to determine the
optimal concentration for in vivo studies using animal
models. It is difficult to determine how much AHL should be
administered in mouse models to reach the concentrations used in
the present study in the serum and tissues at the current state.
Thus, it is necessary to conduct in vivo studies using
various concentrations within the range permitted in animal models
to determine the optimal concentration that exhibits no toxicity
and shows immunostimulatory and anti-obesity activity. If such
studies are conducted, they will serve as a basis for the clinical
application of AHL.
A. himalaicum contains various functional
substances, such as neochlorogenic acid, chlorogenic acid,
3,4-dicaffeoylquinic acid, 3,5-dicaffeoyl-epi-quinic acid,
isoquercitrin and 4,5-dicaffeoylquinic acid (8,10).
Functional ingredients, such as chlorogenic acid,
3,5-dicaffeoyl-epi-quinic acid and isoquercitrin, inhibit lipid
accumulation in 3T3-L1 cells (33-35).
Ingredients such as chlorogenic acid, 3,5-dicaffeoyl-epi-quinic
acid and isoquercitrin may potentially contribute to the
AHL-mediated inhibition of lipid accumulation. However, the
possibility that other components of AHL may also be involved in
the AHL-mediated inhibition of lipid accumulation cannot be
excluded. However, among the components of A. himalaicum
identified to date, none exhibit immunostimulatory activity. In
fact. most of component of A. himalaicum are polyphenols
that inhibit excessive immune responses (8,10).
However, polysaccharides present in plants have been reported to
exhibit immunostimulatory activity in macrophages (36,37).
The AHL used in the present study was extracted with water. Solvent
extraction, such as with ethanol or methanol, is commonly used to
extract compounds such as flavonoids or polyphenols, while water
extraction is primarily used to extract components such as
polysaccharides, although flavonoids and polyphenols are also
extracted (38). Therefore, the
present study hypothesized that the polysaccharides in AHL exhibit
immunostimulatory activity in RAW264.7 cells. However, since the
component analysis of AHL is a limitation of the present study, it
is necessary to investigate which components of AHL have
immunostimulatory activity and anti-obesity activity.
The present study verified that AHL activated
macrophages through TLR4-mediated activation of p38 and JNK and
suppressed excessive lipid accumulation by inhibiting adipogenesis
and inducing lipolysis and browning of white adipocytes. These
results implied that AHL exerted immunostimulatory and anti-obesity
effects. Given that both macrophage activation and anti-obesity
activity can have a positive effect on strengthening the body's
immune system, AHL can be used as a potential food and drug agent
to enhance this. However, since the lack of in vivo research
using animal models and precise component analysis in the present
study is a limitation, these limitations for immunostimulatory
activity and anti-obesity activity will be addressed in future
research.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Basic Science
Research Program of the National Research Foundation of Korea (NRF)
and funded by the Ministry of Education (grant nos.
NRF-2018R1A6A1A03024862 and NRF-2022R1I1A3055428).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HJC and JWC wrote the manuscript, performed the
experiments and analyzed the data. SWI performed the experiments
and drafted the manuscript. JBJ designed the experiments and wrote
and edited the manuscript. HJC, JWC, SWI and JBJ confirm the
authenticity of all the raw data. All the authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Childs CE, Calder PC and Miles EA: Diet
and immune function. Nutrients. 11(1933)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marshall JS, Warrington R, Watson W and
Kim HL: An introduction to immunology and immunopathology. Allergy
Asthma Clin Immunol. 14 (Suppl 2)(S49)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bonilla FA and Oettgen HC: Adaptive
immunity. J Allergy Clin Immunol. 125 (2 Suppl 2):S33–S40.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Turvey SE and Broide DH: Innate immunity.
J Allergy Clin Immunol. 125 (2 Suppl 2):S24–S32. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
de Frel DL, Atsma DE, Pijl H, Seidell JC,
Leenen PJM, Dik WA and van Rossum EFC: The impact of obesity and
lifestyle on the immune system and susceptibility to infections
such as COVID-19. Front Nutr. 7(597600)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Muscogiuri G, Pugliese G, Laudisio D,
Castellucci B, Barrea L, Savastano S and Colao A: The impact of
obesity on immune response to infection: Plausible mechanisms and
outcomes. Obes Rev. 22(e13216)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Martí A, Marcos A and Martínez JA: Obesity
and immune function relationships. Obes Rev. 2:131–140.
2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ahn HS, Kim HJ, Na C, Jang DS, Shin YK and
Lee SH: The protective effect of Adenocaulon himalaicum
Edgew. and its bioactive compound neochlorogenic acid against
UVB-induced skin damage in human dermal fibroblasts and epidermal
keratinocytes. Plants (Basel). 10(1669)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kwon HC and Lee KR: An acetylene and a
monoterpene glycoside from Adenocaulon himalaicum. Planta
Med. 67:482–484. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yun JH, Lee SB, Kang K, Lee EH, Lee HJ,
Jung SH and Nho CW: Bifunctional chemopreventive effects of
Adenocaulon himalaicum through induction of detoxification
enzymes and apoptosis. J Med Food. 16:701–710. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Teng L, Fu H, Wang M, Deng C and Chen J:
Stimulation of RAW264.7 macrophages by sulfated Escherichia coli K5
capsular polysaccharide in vitro. Mol Med Rep. 12:5545–5553.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Blaszczak AM, Jalilvand A and Hsueh WA:
Adipocytes, innate immunity and obesity: A mini-review. Front
Immunol. 12(650768)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hirayama D, Iida T and Nakase H: The
phagocytic function of macrophage-enforcing innate immunity and
tissue homeostasis. Int J Mol Sci. 19(92)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mukherjee S, Karmakar S and Babu SPS: TLR2
and TLR4 mediated host immune responses in major infectious
diseases: A review. Braz J Infect Dis. 20:193–204. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Iwasaki A and Medzhitov R: Toll-like
receptor control of the adaptive immune responses. Nat Immunol.
5:987–995. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Kawai T and Akira S: Toll-like receptors
and their crosstalk with other innate receptors in infection and
immunity. Immunity. 34:637–650. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Park BS and Lee JO: Recognition of
lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med.
45(e66)2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee JC, Laydon JT, McDonnell PC, Gallagher
TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter
SW, et al: A protein kinase involved in the regulation of
inflammatory cytokine biosynthesis. Nature. 372:739–746.
1994.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Li Y, Meng T, Hao N, Tao H, Zou S, Li M,
Ming P, Ding H, Dong J, Feng S, et al: Immune regulation mechanism
of Astragaloside IV on RAW264.7 cells through activating the
NF-κB/MAPK signaling pathway. Int Immunopharmacol. 49:38–49.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nick JA, Avdi NJ, Gerwins P, Johnson GL
and Worthen GS: Activation of a p38 mitogen-activated protein
kinase in human neutrophils by lipopolysaccharide. J Immunol.
156:4867–4875. 1996.PubMed/NCBI
|
|
21
|
Xu Z, Lin R, Hou X, Wu J, Zhao W, Ma H,
Fan Z, Li S, Zhu Y and Zhang D: Immunomodulatory mechanism of a
purified polysaccharide isolated from Isaria cicadae Miquel on
RAW264.7 cells via activating TLR4-MAPK-NF-κB signaling pathway.
Int J Biol Macromol. 164:4329–4338. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang L, Jia X, Fang D, Cheng Y, Zhai Z,
Deng W, Du B, Lu T, Wang L, Yang C and Gao Y: Metformin inhibits
lipid droplets fusion and growth via reduction in cidec and its
regulatory factors in rat adipose-derived stem cells. Int J Mol
Sci. 23(5986)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guo L, Kang JS, Kang NJ, Je BI, Lee YJ,
Park YH and Choi YW: Pelargonidin suppresses adipogenesis in 3T3-L1
cells through inhibition of PPAR-γ signaling pathway. Arch Biochem
Biophys. 686(108365)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Millward CA, Heaney JD, Sinasac DS, Chu
EC, Bederman IR, Gilge DA, Previs SF and Croniger CM: Mice with a
deletion in the gene for CCAAT/enhancer-binding protein beta are
protected against diet-induced obesity. Diabetes. 56:161–167.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Langin D: Adipose tissue lipolysis as a
metabolic pathway to define pharmacological strategies against
obesity and the metabolic syndrome. Pharmacol Res. 53:482–491.
2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Duncan RE, Ahmadian M, Jaworski K,
Sarkadi-Nagy E and Sul HS: Regulation of lipolysis in adipocytes.
Annu Rev Nutr. 27:79–101. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lass A, Zimmermann RZ, Oberer M and
Zechner R: Lipolysis-a highly regulated multi-enzyme complex
mediates the catabolism of cellular fat stores. Prog Lipid Res.
50:14–27. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kuryłowicz A and Puzianowska-Kuźnicka M:
Induction of adipose tissue browning as a strategy to combat
obesity. Int J Mol Sci. 21(6241)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lo KA and Sun L: Turning WAT into BAT: A
review on regulators controlling the browning of white adipocytes.
Biosci Rep. 33(e00065)2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
van der Vaart JI, Boon MR and Houtkooper
RH: The role of AMPK signaling in brown adipose tissue activation.
Cells. 10(1122)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cannon B and Nedergaard J: Brown adipose
tissue: Function and physiological significance. Physiol Rev.
84:277–359. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Seale P, Kajimura S, Yang W, Chin S, Rohas
LM, Uldry M, Tavernier G, Langin D and Spiegelman BM:
Transcriptional control of brown fat determination by PRDM16. Cell
Metab. 6:38–54. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kong L, Xu M, Qiu Y, Liao M, Zhang Q, Yang
L and Zheng G: Chlorogenic acid and caffeine combination attenuates
adipogenesis by regulating fat metabolism and inhibiting adipocyte
differentiation in 3T3-L1 cells. J Food Biochem.
45(e13795)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Oh JH, Lee JI, Karadeniz F, Seo Y and Kong
CS: 3,5-Dicaffeoyl-epi-quinic acid isolated from edible halophyte
Atriplex gmelinii inhibits adipogenesis via AMPK/MAPK pathway in
3T3-L1 adipocytes. Evid Based Complement Alternat Med.
2018(8572571)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim JH, Lee S and Cho EJ: Flavonoids from
Acer okamotoanum inhibit adipocyte differentiation and promote
lipolysis in the 3T3-L1 cells. Molecules. 25(1920)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou R, Teng L, Zhu Y, Zhang C, Yang Y and
Chen Y: Preparation of Amomum longiligulare polysaccharides 1-PLGA
nanoparticle and its immune enhancement ability on RAW264.7 cells.
Int Immunopharmacol. 99(108053)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shen CY, Zhang WL and Jiang JG:
Immune-enhancing activity of polysaccharides from Hibiscus
sabdariffa Linn. via MAPK and NF-kB signaling pathways in
RAW264.7 cells. J Funct Foods. 34:118–129. 2017.
|
|
38
|
Lin S, Li HY, Wang ZY, Liu X, Yang Y, Cao
ZW, Du G, Zhao L, Zhang Q, Wu DT and Qin W: Analysis of methanolic
extracts and crude polysaccharides from the leaves of
Chuanminshen violaceum and their antioxidant activities.
Antioxidants (Basel). 8(266)2019.PubMed/NCBI View Article : Google Scholar
|