Introduction
Lymphoepithelial carcinoma of the lung (pLELC) is a
rare lung cancer subtype caused by malignant hyperplasia of the
lymphoid tissue, now categorized as squamous carcinoma according to
the 2020 World Health Organization's Classification of Lung Tumors
(1). The pathogenesis of pLELC
remains elusive, though Epstein-Barr virus (EBV) is thought to be
the primary etiology (2). Although
not all pLELC patients test positive for EBV, EBV infection is
specific to Asian pLELC populations (3). Current pLELC treatment regimens are
consistent with those for squamous lung cancer. However, due to
persistent EBV infection, pLELC tumors always involve a strong
infiltration of lymphocytes in the tumor microenvironment and high
expression of programmed cell death ligand 1 (PD-L1) in tumor
tissue, providing a rationale for immunotherapy. Pang et al
(4) found that patients who
receive chemoimmunotherapy (the current first-line treatment for
advanced pLELC) gain significantly more survival benefits compared
with those receiving chemotherapy.
While immunotherapy provides new options,
immune-related adverse events (irAEs) have been widely reported.
Among these, immune checkpoint inhibitor-associated pneumonitis
(CIP) is rare but potentially fatal (5). CIP is the development of dyspnea
and/or other respiratory symptoms and the presence of new
infiltrative shadows on chest images, excluding clinical contexts
such as lung infection or tumor progression. In clinical practice,
the incidence of CIP is as high as 13-19%, and, when occurring
during grades 3-4, is frequently life-threatening (6,7).
Corticosteroids are the standard of care. While the pathogenesis of
CIP remains elusive, possible mechanisms include increased T-cell
function and autoimmunity due to elevated antibodies and
inflammatory factors (7). Among
the links between CIP and viruses, a possible potential association
with cytomegalovirus (CMV) has also been reported (8).
It has remained elusive whether the development of
CIP in patients with lymphoepithelial carcinoma is associated with
EBV. The present study reported the first case of a presumed
association between EBV and CIP, with reasonable speculation
regarding potential mechanisms of CIP development.
Case report
A 57-year-old male patient was admitted to another
hospital in May 2019 after his annual checkup prompted computed
tomography (CT) evaluation, which revealed a distinct right lung
mass. Undifferentiated pLELC of pathologic stage T3N1M1c was
diagnosed by needle biopsy. The patient was started on a course of
combined gemcitabine 1,730 mg and carboplatin 664 mg chemotherapy,
which had poor efficacy and was later changed to four cycles of
nivolumab 200 mg combined with bevacizumab anti-vascular therapy 90
mg. At this time, the patient had occasional cough with little
sputum, no chills or fever, and no shortness of breath. Lymph nodes
and multiple bone metastases were detected by positron emission
tomography (PET) conducted during the treatment phase to evaluate
disease progression in October 2020. The patient was treated with
multiple radiotherapy sessions and eventually received maintenance
therapy of bleomycin 20 mg plus toripalimab 200 mg.
Following the third course of chemoimmunotherapy, in
April 2021, the patient was immediately transferred to the First
Affiliated Hospital of Guangzhou Medical University after one month
of shortness of breath, which had become aggravated during the
preceding three days. The patient presented with cough with
expectoration, dyspnea, persistent pyrexia and a maximum body
temperature of 40.5˚C. CT indicated a right middle lung tumor
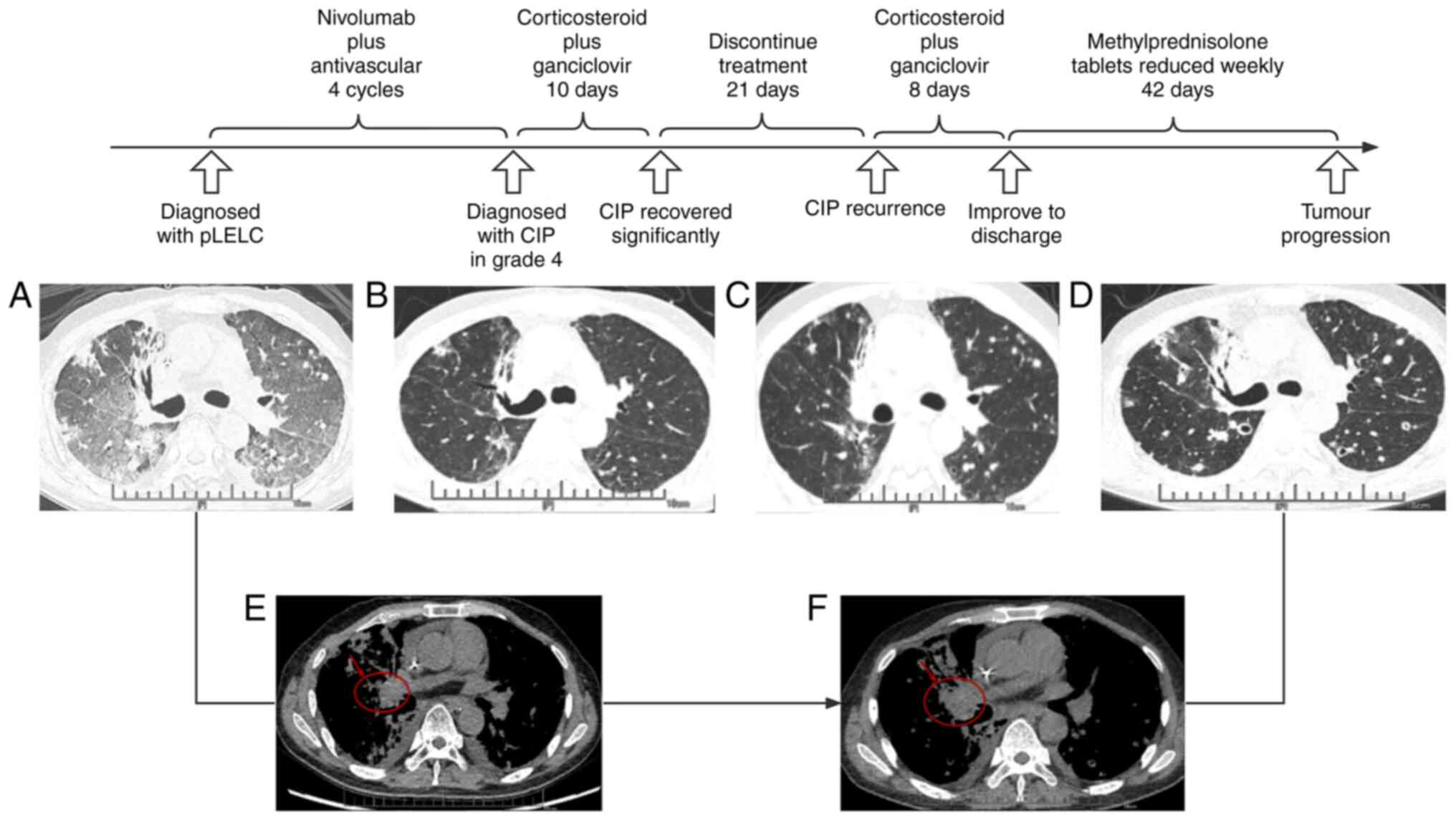
measuring ~2.3x1.8 cm, with interstitial inflammation in both lungs
(Fig. 1) and involvement of the
whole lung. It had been six months since the last radiotherapy and
imaging did not indicate any shadows or streak-like changes
consistent with the irradiated field or area, and radiation
pneumonitis was thus excluded.
Laboratory tests revealed a concomitant
gram-positive coccus infection. Based on medication history,
clinical presentation and imaging, the diagnosis of severe CIP at
the highest grade (IV) with concomitant pulmonary infection was
considered. The patient was in a critical, life-threatening
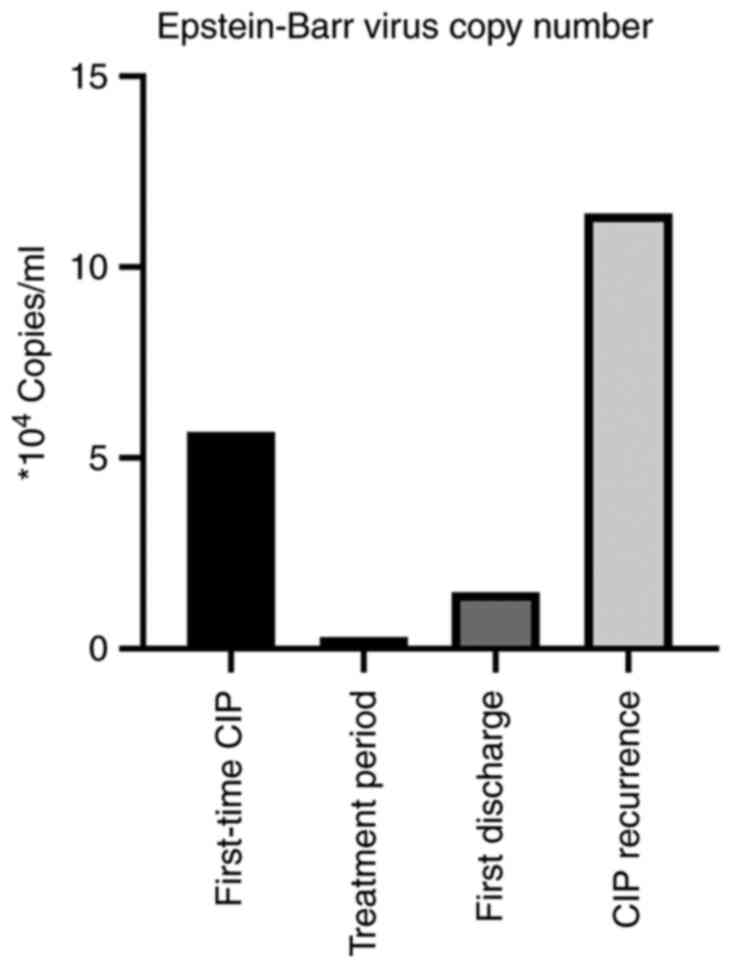
condition with plasma EBV 5.68x104 copies/ml as
determined by fluorometric PCR at the pathology department
(Fig. 2). Noninvasive assisted
ventilation was quickly initiated combined with conventional
intravenous corticosteroids 80 mg for anti-inflammation and
meropenem for anti-infection, with simultaneous ganciclovir
antiviral therapy for EBV. Following this, EBV dropped to
2.98x103 copies/ml on follow-up during antiviral
treatment and corticosteroid was reduced to 40 mg intravenously
(Fig. 2). Of note, the patient
improved significantly within 10 days, at which time CT indicated
that inflammation in both lungs was significantly reduced and other
clinical signs were significantly improved (Fig. 1). The patient was discharged with
only methylprednisolone tablets, despite final EBV
1.48x104 copies/ml, exceeding the normal level
(5x102 copies/ml) (Fig.
2).
After three weeks, the patient returned with
shortness of breath and cough with a small amount of white mucous
sputum. Chest CT indicated no significant tumor changes and both
lungs contained ground glass opacities (Fig. 1). Laboratory examination suggested
no other pulmonary infection. Medical history review revealed that
the patient had stopped corticosteroids without authorization after
discharge and it was determined that the CIP had recurred. At this
point, EBV had returned to a high level (1.14x105
copies/ml) (Fig. 2). The patient
was treated with a conventional protocol of corticosteroid 40 mg
combined with antiviral ganciclovir 0.25 g. The patient was stable
eight days later, when he was administered chemotherapy combined
with anti-vascular therapy to control the tumor, without
immunotherapy rechallenge.
The patient returned to the hospital every three
weeks for regular antineoplastic treatment. CT review six weeks
after resumption of antineoplastic treatment revealed that the
right lung tumor (4.5x3.1 cm) and lymph node metastases had all
increased in size. In order to better display the changes, the
mediastinal window on chest imaging is also provided (Fig. 1).
Discussion
While immunotherapy advances provide novel treatment
options for patients with advanced pLELC and increase overall
survival (9), unpredictable irAEs
may occur. An association between EBV and irAEs was previously
reported. Saikawa et al (10) reported a case of acute cerebellar
ataxia caused by EBV under immunotherapy and Pugh et al
(11) reported EBV-positive skin
ulcers complicated by colitis under immunotherapy. CIP after
immunotherapy is rare and potentially fatal. While the mechanisms
remain to be fully elucidated, studies have reported possible
associations with excessive T-cell activation, imbalanced
inflammatory factors and increased autoantibodies (12). A potential link between EBV and
immune pneumonia has not heretofore been reported, to the best of
our knowledge.
CIP was previously categorized into three types
based on clinical factors: Pure type, induced type (IT) and mixed
type pneumonitis. IT includes different etiologies, such as
radiotherapy, CMV or EBV reactivation, producing specific antigens
that lead to specific immune cell activation, which in turn leads
to CIP, with no evidence of viral or radiotherapy-induced organ
damage (13). As pLELC is closely
related to EBV virus, considering the CIP type of the patient was
IT pneumonitis, the patient underwent treatment with conventional
corticosteroids combined with ganciclovir antiviral therapy and
rapidly recovered from severe CIP (grade IV) within just 10 days,
which is not a common situation. This circumstance led to
speculations regarding a possible connection of EBV with the
pathogenesis of CIP.
The CIP of the patient of the present study was
hypothesized to be EBV-induced IT pneumonitis, leading us to
explore and consider alternative mechanisms of CIP. As immune
checkpoint inhibitors are immunotherapeutic agents with specific
targets, off-target toxicity is a specific mechanism by which
targeted therapies may cause negative effects (14). The possible mechanisms of CIP in
previous studies are stimulation of antigen cross-presentation
against self-peptides or shared epitopes between it and the tumor,
ultimately triggering an autoimmune response (15). Thus, it may be hypothesized that a
specific T-cell type, EBV-specific T cells, may be produced. These
are formed following specific antigen presentation, expressed by
infected cells following EBV infection, which may be functionally
defective in oncology patients and unable to develop effective
cellular immunity. After immunotherapy, a large number of T cells
are activated, including EBV-specific T cells, allowing them to
mediate cellular immunity, attack EBV-infected lung tissue and
cause lung injury (8,16,17).
The association between viruses and irAEs is not
unprecedented and an association between CIP and CMV was previously
reported by our group (8). CIP may
be an immune reconstitution syndrome associated with CMV infection,
which may occur as an immune response to infection manifested by a
cascade of T-cell responses to CMV-specific antigens due to the
blockage of the programmed cell death 1 (PD-1) pathway. A related
study also supports this hypothesis; a recent report of
immunotherapy for metastatic prostate cancer, in which clonal
expansion of CD8+ T cells preceded the development of grade 2-3
irAEs, suggested a potential role of antigen-specific cytotoxic T
cells in the pathogenesis of CIP (16). The authors of another case report
of PD-1 inhibitor treatment leading to fatal encephalitis
speculated similarly regarding the mechanism of immune
encephalitis. In their report, spatial and multivariate analyses of
immunotherapy-associated encephalitis revealed that memory CD4+ T
cells were highly aggregated at the encephalitis site, and T cell
receptor (TCR) clones found in inflamed brain tissue were mainly
those recognizing viral proteins rather than autoimmune correlates.
The TCR clone sequences shared the same TCRβ complementary
determinant region 3 sequence as those known to be EBV-specific,
identifying a highly oligoclonal T-cell receptor library localized
to activated memory cytotoxic CD4+ cells (expression of granzyme B,
CD45RO and Ki67) (17).
This cumulative evidence is consistent with our
suspicion that EBV-specific T cells may induce CIP. When the
patient of the present study recovered from CIP and the antiviral
drugs were discontinued, the EBV copy number exhibited a marked
increase. This also supports the notion that EBV in patients with
pLELC enables the formation of EBV-specific T cells to mediate the
onset of cellular immunity.
The treatment of immune-related pneumonitis is based
on systemic corticosteroids. Steroid-insensitive CIP may require
second-line immunosuppression, though often with poor prognosis
(6). The present case of pLELC had
a virus-associated tumor type and, to the best of our knowledge,
the present study was the first to report on CIP caused by EBV
during immunotherapy. The novel concept that EBV-specific T cells
are associated with the incidence of CIP was also proposed. The
present study may thus assist clinicians who, in similar cases,
should attend closely to virus detection, consider combination
therapy and note that a virus may be a factor of irAE etiology.
In conclusion, this is the first reported case
indicating possible CIP caused by EBV during immunotherapy. This
case and the existing literature suggest that EBV-specific T
cell-mediated cellular immunity may contribute to the occurrence of
CIP. This cumulative evidence indicates that patients with CIP
should be monitored for viral copy numbers in the face of
virus-associated tumors and emphasizes the importance of
combination therapy in such cases. However, the scenario of this
single case report must be confirmed and further explored with
larger samples.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from the State Key
Laboratory of Respiratory Disease-The Independent project (grant
no. SKLRD-Z-202206) and Wu Jieping Medical Foundation (grant no.
320.6750.2020-19-8).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JD and XL treated the patient and wrote the
manuscript. HD, YY, XX and WG treated the patient. CZ contributed
to PET/CT response evaluation. All authors contributed to the
article and have read and approved the final manuscript. JD and XL
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of any potentially identifiable images
and data included in this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nicholson AG, Tsao MS, Beasley MB, Borczuk
AC, Brambilla E, Cooper WA, Dacic S, Jain D, Kerr KM, Lantuejoul S,
et al: The 2021 WHO classification of lung tumors: Impact of
advances since 2015. J Thorac Oncol. 17:362–387. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Min BH, Tae CH, Ahn SM, Kang SY, Woo SY,
Kim S and Kim KM: Epstein-Barr virus infection serves as an
independent predictor of survival in patients with
lymphoepithelioma-like gastric carcinoma. Gastric Cancer.
19:852–859. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Qiu ZX, Zhou P and Wang K: Primary
pulmonary lymphoepithelioma-like carcinoma response favorably to
nivolumab: A case report. Onco Targets Ther. 12:8595–8600.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pang LL, Liao J, Huang YH, Gan JD, Zhuang
WT, Lv Y, Liang WT, Zhang L and Fang WF: Exploration of
immunotherapy in advanced pulmonary lymphoepithelioma-like
carcinoma. Int J Cancer: Jan 11, 2023 (Epub ahead of print).
|
|
5
|
Hao Y, Zhang X and Yu L: Immune checkpoint
inhibitor-related pneumonitis in non-small cell lung cancer: A
review. Front Oncol. 12(911906)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Suresh K, Naidoo J, Lin CT and Danoff S:
Immune checkpoint immunotherapy for non-small cell lung cancer:
Benefits and pulmonary Toxicities. Chest. 154:1416–1423.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Reuss JE, Suresh K and Naidoo J:
Checkpoint inhibitor pneumonitis: Mechanisms, characteristics,
management strategies, and beyond. Curr Oncol Rep.
22(56)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lin X, Lu T, Li S, Xie X, Chen X, Jiang J,
Qin Y, Xie Z, Liu M, Ouyang M, et al: Cytomegalovirus infection as
an underestimated trigger for checkpoint inhibitor-related
pneumonitis in lung cancer: A retrospective study. Clin Transl
Oncol. 23:389–396. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tan PS, Aguiar P Jr, Haaland B and Lopes
G: Comparative effectiveness of immune-checkpoint inhibitors for
previously treated advanced non-small cell lung cancer-A systematic
review and network meta-analysis of 3024 participants. Lung Cancer.
115:84–88. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Saikawa H, Nagashima H, Maeda T and
Maemondo M: Acute cerebellar ataxia due to Epstein-Barr virus under
administration of an immune checkpoint inhibitor. BMJ Case Rep.
12(e231520)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pugh MR, Leopold GD, Morgan M, Christian
AD, Hewett R, Durai D, Wagstaff J, Harris D and Dojcinov SD:
Epstein-Barr virus-positive mucocutaneous ulcers complicate colitis
caused by immune checkpoint regulator therapy and associate with
colon perforation. Clin Gastroenterol Hepatol. 18:1785–1795 e3.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yin J, Wu Y, Yang X, Gan L and Xue J:
Checkpoint inhibitor pneumonitis induced by Anti-PD-1/PD-L1 therapy
in non-small-cell lung cancer: Occurrence and Mechanism. Front
Immunol. 13(830631)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lin X, Deng H, Chen L, Wu D, Chen X, Yang
Y, Chen T, Xie X, Xie Z, Liu M, et al: Clinical types of checkpoint
inhibitor-related pneumonitis in lung cancer patients: A
multicenter experience. Transl Lung Cancer Res. 10:415–429.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fedorov VD, Themeli M and Sadelain M:
PD-1- and CTLA-4-based inhibitory chimeric antigen receptors
(iCARs) divert off-target immunotherapy responses. Sci Transl Med.
5:172ra–215ra. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mcgranahan N, Furness AJ, Rosenthal R,
Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak
NJ, Hiley CT, et al: Clonal neoantigens elicit T cell
immunoreactivity and sensitivity to immune checkpoint blockade.
Science. 351:1463–1469. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Subudhi SK, Aparicio A, Gao J, Zurita AJ,
Araujo JC, Logothetis CJ, Tahir SA, Korivi BR, Slack RS, Vence L,
et al: Clonal expansion of CD8 T cells in the systemic circulation
precedes development of ipilimumab-induced toxicities. Proc Natl
Acad Sci USA. 113:11919–11924. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Johnson DB, Mcdonnell WJ,
Gonzalez-Ericsson PI, Al-Rohil RN, Mobley BC, Salem JE, Wang DY,
Sanchez V, Wang Y, Chastain CA, et al: A case report of clonal
EBV-like memory CD4(+) T cell activation in fatal checkpoint
inhibitor-induced encephalitis. Nat Med. 25:1243–1250.
2019.PubMed/NCBI View Article : Google Scholar
|