Introduction
Diabetic nephropathy (DN) is a common severe
microvascular complication of diabetes mellitus (DM) and is the
primary cause of end-stage renal disease (1). The incidence rate of DN in developed
and developing countries is increasing annually, and 20-40% of
diabetic patients are at risk of developing DN (2). In the early stages of DN, the number
of glomerular podocytes decreases, the filtration membrane is
destroyed and excessive proteinuria is observed. In addition, due
to adhesion between the glomerular basement membrane (GMB) and the
upper layer of the wall, focal segmental glomerular sclerosis is
formed (3,4). Therein, the podocyte is a type of
highly differentiated visceral epithelial cell located outside the
GBM. As a key part of the glomerular filtration barrier, the
podocyte is responsible for the filtration of proteins and renewal
of GBM components (5). A previous
study found that podocyte damage occurs in the early stages of DN
and pathological alterations such as compensatory hypertrophy and
degeneration, extensive fusion and the disappearance of foot
processes can be observed under an electron microscope (6). In addition, the programmed death of
high glucose (HG)-stimulated podocytes is a cause of glomerular
hyperfiltration in the early stages of DN (7).
Pyroptosis is a newly discovered form of programmed
cell death characterized by the activation of inflammasomes and
cysteine aspartate-specific protease 1 (caspases-1) (8). A previous study suggested that
inhibiting inflammatory factors suppresses pyroptosis and
attenuates DN (9). Therefore, the
inhibition of podocyte pyroptosis may contribute to the development
of novel treatment strategies for DN. According to a previous
study, increased mRNA levels of ADAM metallopeptidase domain 10
(ADAM10) in urine sediment of patients with type 2 DM indicates
that the activity of ADAM10 in podocytes is increased and its
expression is associated with dysfunction of proximal tubules in
patients with DN (10). Another
study revealed that ADAM10 knockdown decreases intrahepatic
inflammation in mice with acute liver injury and protects liver
function; however, ADAM10 overexpression aggravates inflammation
and liver damage (11). Moreover,
ADAM10 is associated with the expression of inflammatory cytokines
and negatively regulates the inflammatory response (12). In brief, ADAM10 is likely to be a
regulatory gene in podocytes and a potential therapeutic
target.
The present study used HG to stimulate podocytes to
examine the role of ADAM10 in podocyte damage. The current findings
may aid in the further understanding of the pathology of DN and
promote the development of novel treatment strategies for this
condition.
Materials and methods
Cells and cell culture
MPC5 mouse podocytes were obtained from the Shanghai
Institute of Cell Biology, Chinese Academy of Sciences. LM22B-10
(ERK1/2 agonist, 5 mM) (13) and
p79350 (p38 agonist, 50 µM) (14)
were purchased from MedChemExpress. Podocytes were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Invitrogen; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.). The podocytes were divided into
normal glucose (NG, 5 mM glucose), mannitol control (MA, 5 mM
glucose + 25 mM mannitol), HG (30 mM glucose) and transfection
groups. The podocytes were cultured in an incubator with 5%
CO2 at 37˚C.
Cell transfection
The podocytes were plated into a six-well plate at a
density of 6x105 cells/well and incubated at 37˚C for 24
h. The podocytes were subsequently transfected with 2 µg/ml pGPU6
short hairpin RNA vector targeting ADAM10 (shRNA-ADAM10) and
scrambled shRNA-negative control (NC) (Shanghai GenePharma Co.,
Ltd.) using Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) for 24 h at 37˚C. 24 h after the
end of transfection, the cells were utilized in subsequent
experiments. Target sequences for shRNA-ADAM10 and shRNA-NC were
5'-GCAAAGATGATAAGGAATTAT-3' and 5'-GACAAGATGATAAGGATATAT-3',
respectively.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from podocytes in a 6-well
plate at a density of 2x105 cells/well using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Total RNA was
reverse-transcribed into cDNA using the ReverTra Ace™ qPCR RT kit
(cat. no. FSQ-101; Toyobo Life Science) according to the
manufacturer's instructions. qPCR was performed using the
SYBR-Green qPCR Master Mix fit (MedChemExpress) using 2 µl cDNA as
a template. The following thermocycling conditions were used for
qPCR: Initial denaturation at 95˚C for 2 min, followed by 40 cycles
of 95˚C for 15 sec and 60˚C for 1 min. The RNA expression levels of
ADAM10 were quantified using the 2-∆∆Cq method (15) and normalized to the internal
reference gene GAPDH. The following primer pairs were used for
qPCR: ADAM10 forward, 5'-TCATCAAGACTCGTGGTGGC-3' and reverse,
5'-GCATGCTTCTCTGGATGTGC-3'; GAPDH forward,
5'-GGGTCCCAGCTTAGGTTCATC-3' and reverse,
5'-TACGGCCAAATCCGTTCACA-3'.
Western blot analysis
Total protein was extracted from the podocytes
(2x106) using RIPA lysis (Beyotime Institute of
Biotechnology). Total protein was quantified using a BCA assay kit
(Beyotime Institute of Biotechnology) and 30 µg protein/lane was
separated by SDS-PAGE on a 10% gel (Bio-Rad Laboratories, Inc.).
The separated proteins were transferred onto PVDF membranes
(MilliporeSigma) and blocked with 5% skimmed milk at room
temperature for 1 h. The membranes were incubated overnight at 4˚C
with the following primary antibodies: Anti-ADAM10 (1:1,000; cat.
no. ab124695; Abcam), anti-cyclooxygenase (COX)-2 (1:1,000; cat.
no. ab179800; Abcam), anti-inducible nitric oxide synthase (iNOS;
1:1,000; cat. no. ab178945; Abcam), anti-Bax (1:1,000; cat. no.
ab53154; Abcam), anti-Bcl-2 (1:1,000; cat. no. ab59348; Abcam),
anti-cleaved caspase-3 (1:1,000; cat. no. PA5-114687; Thermo Fisher
Scientific, Inc.), anti-cleaved caspase-9 (1:1,000; cat. no.
ABP50009; AmyJet Scientific, Inc.), anti-phosphorylated (p-)ERK1/2
(1:1,000; cat. no. ab176640; Abcam), anti-ERK1/2 (1:1,000; cat. no.
ab17942; Abcam), anti-p-p38 (1:1,000; cat. no. ab4822; Abcam),
anti-p38 (1:2,000; cat. no. ab170099; Abcam), anti-p-JNK (1:5,000;
cat. no. ab124956; Abcam), anti-JNK (1:500; cat. no. ab208035;
Abcam), anti-NLR family pyrin domain containing 3 (NLRP3; 1:1,000;
cat. no. ab263899; Abcam), anti-cleaved caspase-1 (1:1,000; cat.
no. sc-22166; Santa Cruz Biotechnology, Inc.),
anti-apoptosis-associated speck-like protein containing a CARD
(ASC; 1:1,000; cat. no. ARG41743; Arigo Biolaboratories Corp.),
anti-caspase-1 (1:1,000; cat. no. 3019-100; AmyJet Scientific,
Inc.), anti-cleaved N-terminal gasdermin-D (GSDMD-N; 1:1,000; cat.
no. DF13758; Affinity Biosciences, Ltd.), anti-podocin (1:1,000;
cat. no. 113216; NovoPro Bioscience, Inc.), anti-CD2-associated
protein (CD2AP; 1:1,000; cat. no. DF2298; Affinity Biosciences,
Ltd.), anti-nephrin (1:2,000; cat. no. DF7501; Affinity
Biosciences, Ltd.) and anti-GAPDH (1:2,000; cat. no. MA1-16757;
Thermo Fisher Scientific, Inc.). Following primary antibody
incubation, the membranes were incubated with a horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(1:2,000; cat. no. ab6721; Abcam) for 1 h at room temperature. The
membranes were visualized using Hypersensitive ECL kit (Hanbio
Biotechnology Co., Ltd.). Blots were quantified using ImageJ v1.52
software (National Institutes of Health).
ELISA
The podocytes seeded into a 24-well plate
(5x104 cells/well) were centrifuged at 500 x g for 5 min
at 4˚C and supernatant was collected. The levels of TNF-α (cat. no.
ZC-39024), IL-6 (cat. no. ZC-37988), IL-1β (cat. no. ZC-37974) and
IL-18 (cat. no. ZC-37973; all ZCIBIO Technology Co., Ltd.) in the
podocytes were measured using ELISA kits, according to the
manufacturer's instructions. A microplate reader (Molecular
Devices, LLC) was used to measure the optical density at 450
nm.
TUNEL assay
The podocytes were seeded into a 24-well plate at a
density of 5x105 cells/well. After reaching 70-80%
confluency, the podocytes were fixed with 4% paraformaldehyde for
30 min and treated with permeable fluid for a further 5 min, both
at room temperature. TUNEL staining was performed at 37˚C for 1 h
using the TUNEL assay kit (cat. no. C1086; Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. 1 mg/ml
DAPI (Beyotime Institute of Biotechnology) was used to counterstain
the nuclei for 10 min at room temperature. The results were
observed in five random fields of view under a fluorescence
microscope (magnification, x200; Olympus Corporation).
Statistical analysis
Graph Pad Prism 8.0 software (GraphPad Software;
Dotmatics) was utilized to analyze all the experimental data. Data
are presented as the mean ± standard deviation (n=3). One-way ANOVA
followed by Tukey's post hoc test was applied to compare
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
ADAM10 knockdown inhibits HG-induced
podocyte inflammation and apoptosis
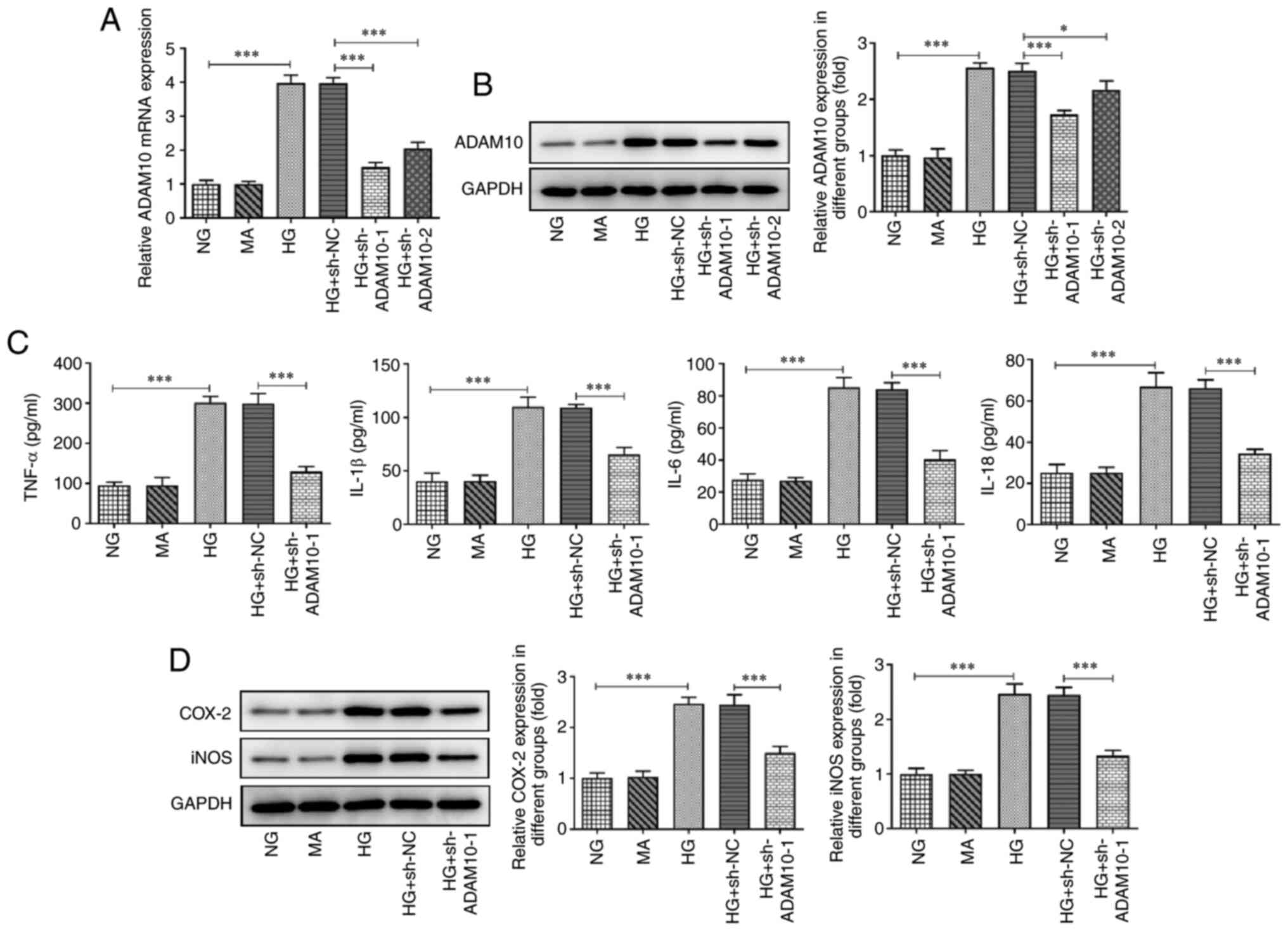
The expression levels of ADAM10 were determined
using RT-qPCR and western blot analysis (Fig. 1A and B). ADAM10 expression was significantly
upregulated in the HG group compared with that in the NG group,
while it was downregulated in the ADAM10 knockdown group compared
with that in the HG + sh-NC group. The HG + sh-ADAM10-1 group
showed a greater decrease in ADAM10-1 mRNA and protein levels;
therefore, this group of podocytes was used as the ADAM10-1
knockdown group in subsequent experiments. The levels of
inflammatory factors in podocytes were measured using ELISA kits
(Fig. 1C). The levels of TNF-α,
IL-1β, IL-6 and IL-18 in the HG group were upregulated compared
with those in the NG group, whereas the levels of these cytokines
were decreased in the HG + sh-ADAM10 group compared with those in
the HG + sh-NC group. In addition, protein expression levels of
iNOS and COX-2 were semi-quantified using western blot analysis
(Fig. 1D). Expression levels of
iNOS and COX-2 were increased in the HG group compared with those
in the NG group and decreased in the HG + sh-ADAM10 group compared
with those in the HG + sh-NC group. These results suggested that
ADAM10 knockdown inhibited HG-induced podocyte inflammation.
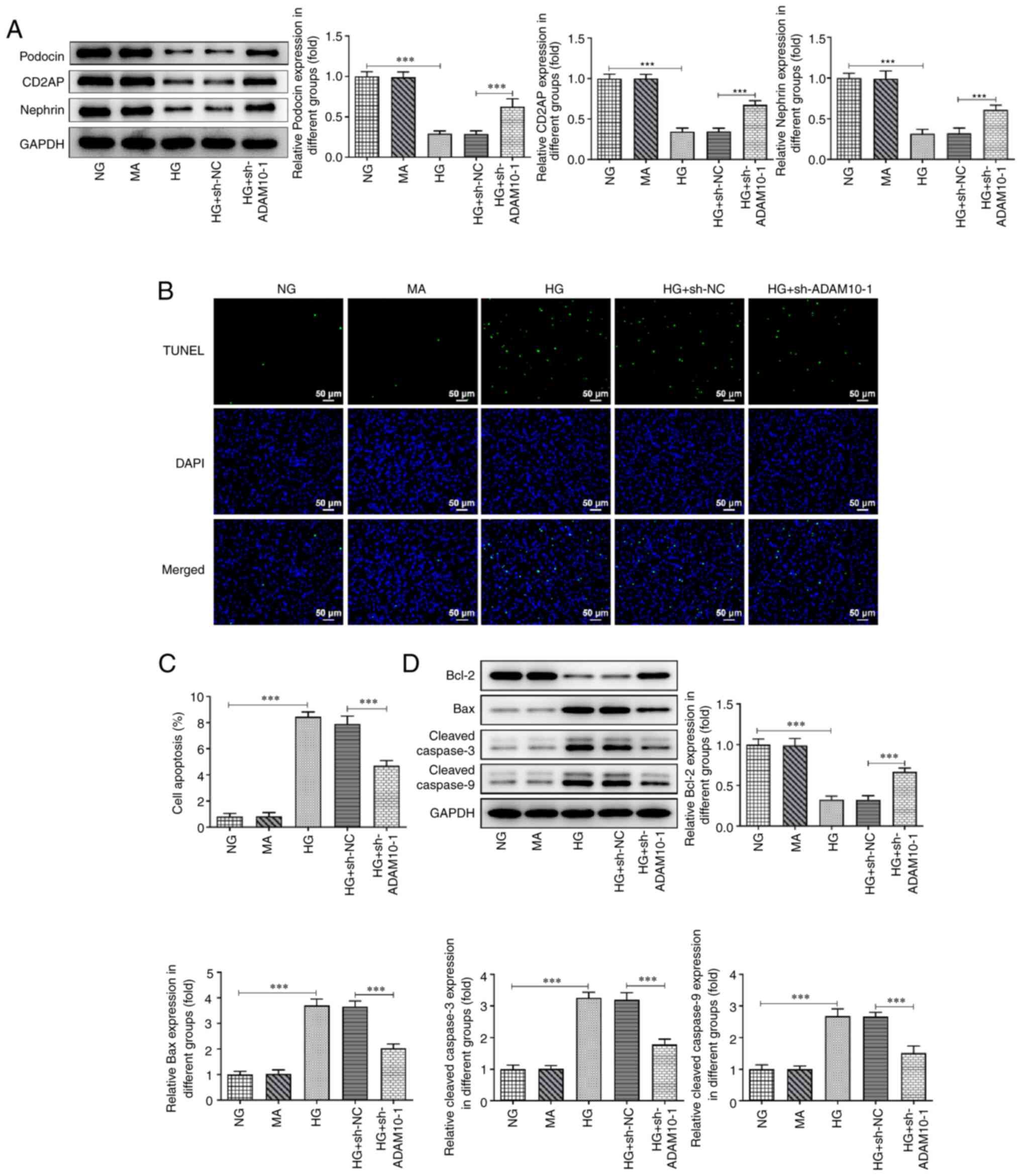
Moreover, levels of the podocyte markers podocin,
CD2AP and nephrin significantly decreased following HG treatment
compared with those in the NG group, whereas ADAM10 knockdown
increased the levels of these protein levels compared with those in
the HG + sh-NC group (Fig. 2A).
Podocyte apoptosis levels in each group were evaluated using the
TUNEL assay (Fig. 2B and C). The apoptotic podocytes in the HG
group emitted more fluorescence than the NG group, whereas the HG +
sh-ADAM10 group did not emit as much fluorescence. Subsequently,
expression levels of proteins related to apoptosis were assessed
using western blot analysis (Fig.
2D). The protein levels of Bax, cleaved caspase-3 and cleaved
caspase-9 were increased in the HG group, while ADAM10 knockdown
counteracted the effect of HG induction. However, the expression of
Bcl-2 was decreased in the HG group, whereas it increased following
ADAM10 knockdown. These results indicated that HG induced the
apoptosis of podocytes, whereas ADAM10 knockdown attenuated
apoptosis.
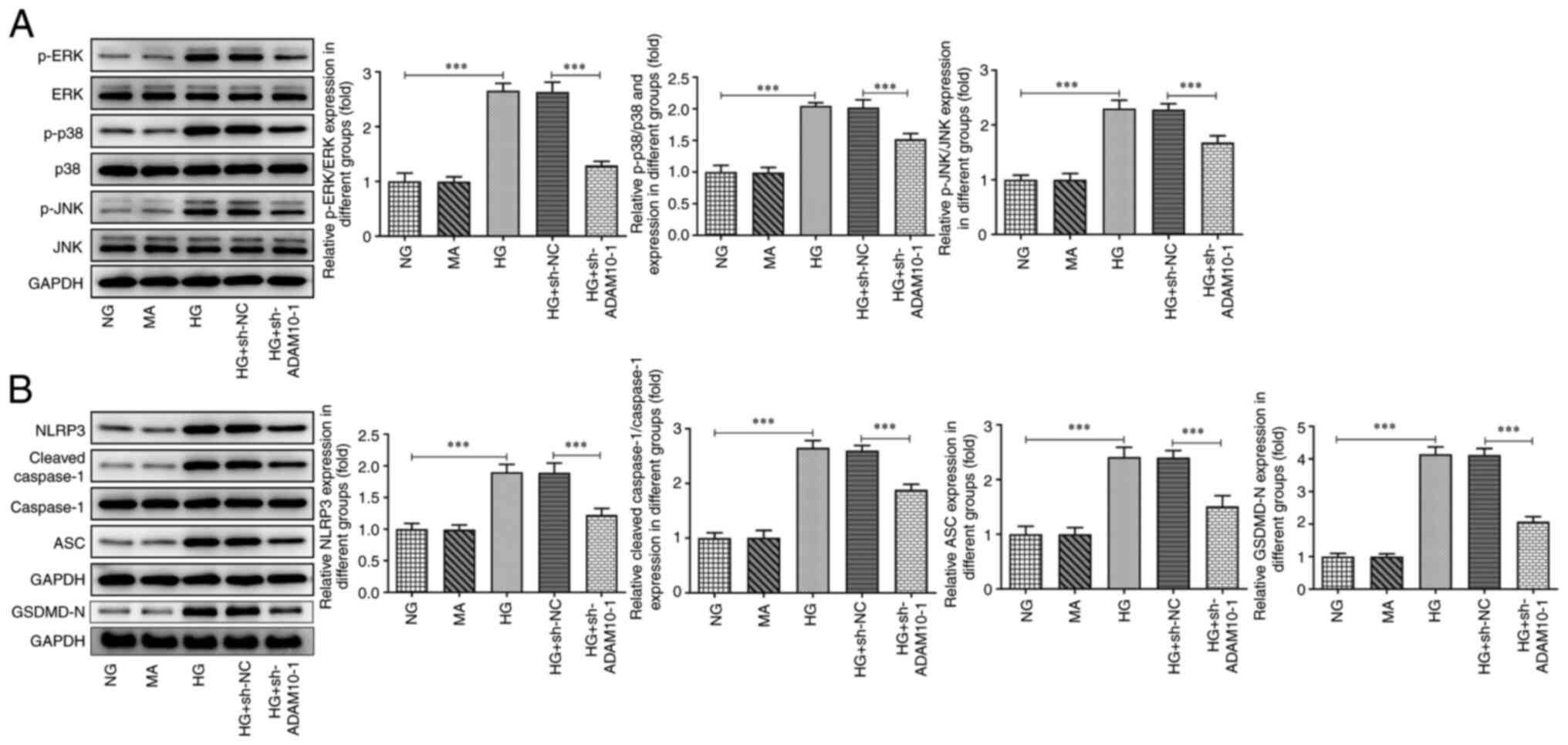
ADAM10 knockdown inhibits the MAPK
signaling pathway and pyroptosis
The expression levels of MAPK-associated proteins
were assessed using western blot analysis (Fig. 3A). The expression levels of p-ERK,
p-p38 and p-JNK were increased in the HG group compared with those
in the NG group. ADAM10 knockdown decreased these expression levels
compared with those in the HG + sh-NC group. Furthermore, the
expression levels of pyroptosis-associated proteins were assessed
using western blot analysis (Fig.
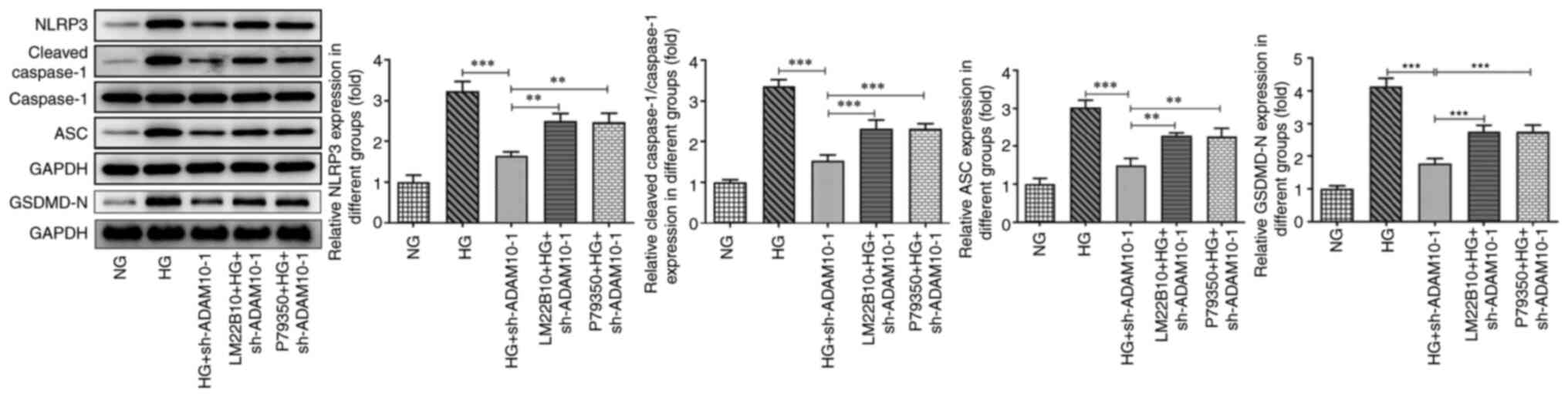
3B). The expression levels of NLRP3, cleaved caspase-1, ASC and
GSDMD-N were increased in the HG group, whereas ADAM10 knockdown
attenuated this effect. These results indicated that ADAM10 blocked
the MAPK signaling pathway and suppressed pyroptosis.
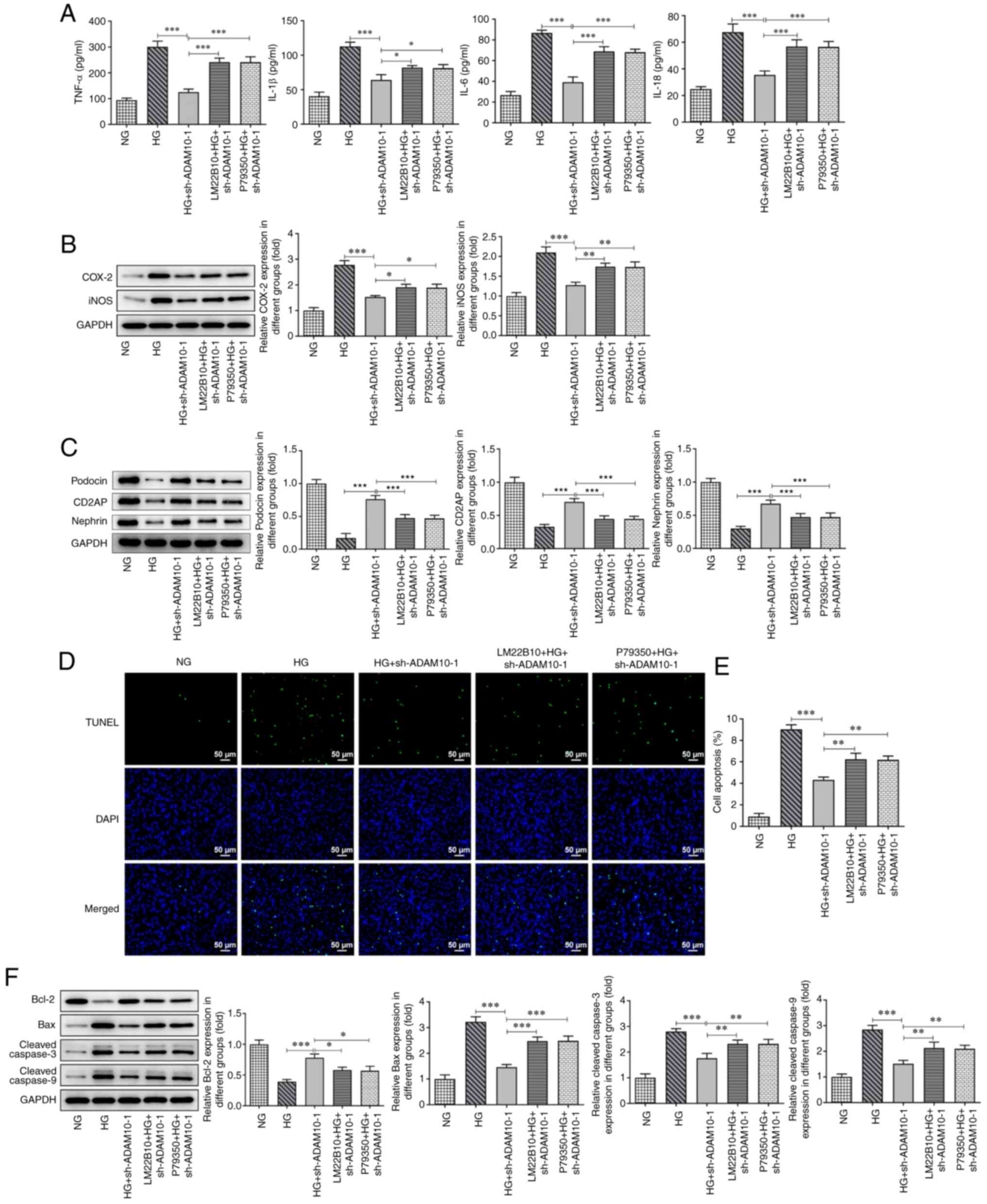
ADAM10 knockdown inhibits HG-induced
podocyte inflammation, apoptosis and pyroptosis by blocking the
MAPK signaling pathway
Podocytes were pretreated with LM22B-10 or p79350 to
investigate the role of the MAPK signaling pathway in the
regulatory effects of ADAM10. Subsequently, levels of inflammatory
factors and inflammation-related proteins in each group of
podocytes were measured using ELISA kits and western blot analysis,
respectively (Fig. 4A and B). The levels of inflammatory factors and
expression of COX-2 and iNOS in podocytes pretreated with agonists
increased, attenuating the inhibitory effects of ADAM10 knockdown
on podocyte inflammation. The levels of podocyte markers decreased
in response to LM22B-10 or p79350 pretreatment compared with those
in the HG + sh-ADAM10 group (Fig.
4C). The apoptosis of podocytes in each group was assessed
using TUNEL assay and western blot analysis (Fig. 4D-F). TUNEL assay demonstrated that
the apoptotic rates of podocytes pretreated with agonist increased
compared with those in the HG + sh-ADAM10 group, exhibiting a
higher fluorescence intensity. The protein levels of Bax, cleaved
caspase-3 and cleaved caspase-9 were increased in the pretreatment
groups, whereas the expression of Bcl-2 decreased. The addition of
the agonists also attenuated the inhibitory effects of AMAD10
knockdown on podocyte apoptosis. Finally, the effects of agonists
on the expression levels of pyroptosis-associated proteins were
evaluated using western blot analysis (Fig. 5). The levels of NLRP3, cleaved
caspase-1, ASC and GSDMD-N were increased in the pretreatment
groups as a consequence of the activation of the pathway. These
results suggested that activation of the MAPK signaling pathway
attenuated the suppressive effects of AMAD10 knockdown on podocyte
inflammation, apoptosis and pyroptosis.
Discussion
At present, the clinical treatment options for
patients with DN are limited and primarily include strict control
of blood glucose levels, a high-quality, low-protein diet and
administration of angiotensin II type 1 receptor antagonists and
angiotensin II converting enzyme inhibitors (16). However, there is a lack of
effective therapeutic drugs to protect cells from programmed death.
Although the pathogenesis of DN remains unclear (17), podocyte damage is involved in the
development of this disease (18),
which provides innovative directions for prevention and control of
DN. Some studies have demonstrated that inflammation is a key
factor that promotes DN renal podocyte damage and proteinuria
(19-21).
The secretion of inflammatory factors is unregulated in the early
stages of DN and the expression of IL-1, IL-6, TNF-α and other
inflammatory factors significantly increases (22). This is also identified in the
podocytes of mice cultured under HG conditions, where the secretion
of inflammatory factors is elevated (23). The present study demonstrated that
ADAM10 expression was upregulated in podocytes stimulated with HG
and that ADAM10 knockdown inhibited podocyte inflammation and
apoptosis. These findings indicated that the expression of ADAM10
could affect the state of podocytes; thus, monitoring the
expression of ADAM10 may prove to be useful for the diagnosis of
clinical diabetes.
Preliminary exploration of the signaling pathway
regulated by ADAM10 was performed in the present study. ADAM10 is a
sheddase capable of hydrolyzing >30 transmembrane proteins. The
effector secreted by enteropathogenic Escherichia coli III
stimulates the sheddase activity of ADAM10 and the signaling
cascade of ERK and p38 MAPK (24).
This suggests a certain connection between ADAM10 and the MAPK
signaling pathway. In addition, a previous study indicated that
ADAM10 regulates p38 MAPK-mediated NF-κBp65 activity to induce
astrocyte inflammation (25). In
the present study, HG led to an increase in expression levels of
proteins related to the MAPK signaling pathway, whereas ADAM10
knockdown attenuated this effect. Furthermore, ADAM10 knockdown
decreased the increase in the levels of pyroptosis-related proteins
induced by HG. In summary, the MAPK signaling pathway mediated the
regulatory effects of ADAM10 on podocyte pyroptosis. Notably, a
functional ginger extract decreases pyroptosis and the release of
mature IL-1β and IL-18 by preventing MAPK activation (26). Moreover, a sesquiterpene lactone
derivative decreases HG-induced podocyte damage by inhibiting the
NF-κB and MAPK signaling pathways (27). In addition to pyroptosis, in the
present study, ADAM10 knockdown regulated podocyte inflammation and
apoptosis by inhibiting the activation of the MAPK pathway. The
exploration of the regulatory effects of ADAM10 on podocyte damage
may lead to a more in-depth understanding of the pathogenesis or
progression of DN. However, the present study was limited to
investigation of the regulatory effects of ADAM10 on the MAPK
pathway; thus, further studies are required to investigate the
underlying mechanisms in more detail.
In conclusion, the present study demonstrated that
ADAM10 knockdown inhibited the MAPK signaling pathway, and thereby
inhibited pyroptosis, inflammation and apoptosis of HG-stimulated
podocytes. The findings of the present study may provide insight
into the pathological mechanisms of DN and a theoretical basis for
the development of clinical treatments for this condition.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CS and DZ designed the study, analyzed data and
wrote the manuscript. CS and DZ confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sagoo MK and Gnudi L: Diabetic
nephropathy: An overview. Methods Mol Biol. 2067:3–7.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gheith O, Farouk N, Nampoory N, Halim MA
and Al-Otaibi T: Diabetic kidney disease: World wide difference of
prevalence and risk factors. J Nephropharmacol. 5:49–56.
2016.PubMed/NCBI
|
|
3
|
Szrejder M and Piwkowska A: AMPK
signalling: Implications for podocyte biology in diabetic
nephropathy. Biol Cell. 111:109–120. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eftekhari A, Vahed SZ, Kavetskyy T,
Rameshrad M, Jafari S, Chodari L, Hosseiniyan SM, Derakhshankhah H,
Ahmadian E and Ardalan M: Cell junction proteins: Crossing the
glomerular filtration barrier in diabetic nephropathy. Int J Biol
Macromol. 148:475–482. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
An X, Zhang L, Yuan Y, Wang B, Yao Q, Li L
and Zhang J, He M and Zhang J: Hyperoside pre-treatment prevents
glomerular basement membrane damage in diabetic nephropathy by
inhibiting podocyte heparanase expression. Sci Rep.
7(6413)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dai H, Liu Q and Liu B: Research progress
on mechanism of podocyte depletion in diabetic nephropathy. J
Diabetes Res. 2017(2615286)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chagnac A, Zingerman B, Rozen-Zvi B and
Herman-Edelstein M: Consequences of glomerular hyperfiltration: The
role of physical forces in the pathogenesis of chronic kidney
disease in diabetes and obesity. Nephron. 143:38–42.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tsuchiya K: Inflammasome-associated cell
death: Pyroptosis, apoptosis, and physiological implications.
Microbiol Immunol. 64:252–269. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li X, Zeng L, Cao C, Lu C, Lian W, Han J,
Zhang X, Zhang J, Tang T and Li M: Long noncoding RNA MALAT1
regulates renal tubular epithelial pyroptosis by modulated miR-23c
targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res.
350:327–335. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Petrica L, Ursoniu S, Gadalean F, Vlad A,
Gluhovschi G, Dumitrascu V, Vlad D, Gluhovschi C, Velciov S, Bob F,
et al: Urinary podocyte-associated mRNA levels correlate with
proximal tubule dysfunction in early diabetic nephropathy of type 2
diabetes mellitus. Diabetol Metab Syndr. 9(31)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cheng W, Meng W and Gu Y: Metalloprotease
Adam10 inhibition mitigates acute liver injury via repression of
intrahepatic inflammation. Minerva Med. 113:506–512.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang W, Lu F, Xie Y, Lin Y, Zhao T, Tao
S, Lai Z, Wei N, Yang R, Shao Y and He J: miR-23b negatively
regulates sepsis-induced inflammatory responses by targeting ADAM10
in human THP-1 monocytes. Mediators Inflamm.
2019(5306541)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu Y, Ma Y, Liu XL and Gao SL: miR-133b
affects cell proliferation, invasion and chemosensitivity in renal
cell carcinoma by inhibiting the ERK signaling pathway. Mol Med
Rep. 22:67–76. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang QG, Xiong CF and Lv YX: Kin17
facilitates thyroid cancer cell proliferation, migration, and
invasion by activating p38 MAPK signaling pathway. Mol Cell
Biochem. 476:727–739. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Y, Wang C, Zhang X, Gu HF and Wu L:
Common drugs for stabilization of renal function in the progression
of diabetic nephropathy and their relations with hypertension
therapy. Curr Diabetes Rev. 14:149–161. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xiong Y and Zhou L: The signaling of
cellular senescence in diabetic nephropathy. Oxid Med Cell Longev.
2019(7495629)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tung CW, Hsu YC, Shih YH, Chang PJ and Lin
CL: Glomerular mesangial cell and podocyte injuries in diabetic
nephropathy. Nephrology (Carlton). 23 (Suppl 4):S32–S37.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Moreno JA, Gomez-Guerrero C, Mas S, Sanz
AB, Lorenzo O, Ruiz-Ortega M, Opazo L, Mezzano S and Egido J:
Targeting inflammation in diabetic nephropathy: A tale of hope.
Expert Opin Investig Drugs. 27:917–930. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
An X, Zhang Y, Cao Y, Chen J, Qin H and
Yang L: Punicalagin protects diabetic nephropathy by inhibiting
pyroptosis based on TXNIP/NLRP3 pathway. Nutrients.
12(1516)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Koka S, Xia M, Zhang C, Zhang Y, Li PL and
Boini KM: Podocyte NLRP3 inflammasome activation and formation by
Adipokine Visfatin. Cell Physiol Biochem. 53:355–365.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen Y, Liu Q, Shan Z, Zhao Y, Li M, Wang
B, Zheng X and Feng W: The protective effect and mechanism of
catalpol on high glucose-induced podocyte injury. BMC Complement
Altern Med. 19(244)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen L, Wang Y, Luan H, Ma G, Zhang H and
Chen G: DUSP6 protects murine podocytes from high glucose-induced
inflammation and apoptosis. Mol Med Rep. 22:2273–2282.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ramachandran RP, Spiegel C, Keren Y,
Danieli T, Melamed-Book N, Pal RR, Zlotkin-Rivkin E, Rosenshine I
and Aroeti B: Mitochondrial targeting of the enteropathogenic
escherichia coli map triggers calcium mobilization, ADAM10-MAP
kinase signaling, and host cell apoptosis. mBio. 11:e01397–e01320.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Park JH, Choi JY, Jo C and Koh YH:
Involvement of ADAM10 in acrolein-induced astrocytic inflammation.
Toxicol Lett. 318:44–49. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang FL, Zhou BW, Yan ZZ, Zhao J, Zhao
BC, Liu WF, Li C and Liu KX: 6-Gingerol attenuates macrophages
pyroptosis via the inhibition of MAPK signaling pathways and
predicts a good prognosis in sepsis. Cytokine.
125(154854)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen XW, Liu WT, Wang YX, Chen WJ, Li HY,
Chen YH, Du XY, Peng FF, Zhou WD, Xu ZZ and Long HB:
Cyclopropanyldehydrocostunolide LJ attenuates high glucose-induced
podocyte injury by suppressing RANKL/RANK-mediated NF-κB and MAPK
signaling pathways. J Diabetes Complications. 30:760–769.
2016.PubMed/NCBI View Article : Google Scholar
|