Introduction
According to the World Health Organization data,
stroke is the second leading cause of death worldwide, and the
third leading cause of death and disability combined. In recent
decades, the incidence and prevalence of stroke has increased by
>70% (1). In Europe only,
stroke affects >1 million people every year with a mortality
rate of 40% (2,3), and the prognosis is even worse in the
context of population aging and exposure to risk factors, such as
hypertension or other cardiovascular diseases, diabetes, low
physical activity, obesity, unhealthy diet, smoking and abusive
alcohol consumption (4,5). In addition, the forecast of the
cost-related burden of stroke has severely increased, with an
estimated increase by 27% by 2050(6).
It is widely known that there are two major
categories of stroke: Ischaemic and haemorrhagic. Ischaemic strokes
are generated by interruption of the blood supply to a certain
cerebral region, which leads to a sudden loss of function.
Haemorrhagic strokes, also called intracerebral haemorrhages, have
their origin in a burst blood vessel and the subsequent bleeding
into and around the brain (7).
Worldwide, ischaemic strokes are more common than haemorrhagic
strokes, and account for ~80% of the total number of strokes;
however, these data vary depending on the country and population
(4).
Starting with symptoms that include trouble
speaking, confusion, paralysis or paresis of the face and upper or
lower limbs, visual impairment, headache, vomiting, dizziness, loss
of coordination or altered state of consciousness, stroke can lead,
in the majority of cases, to long-term effects (8). These long-lasting consequences are
usually localized in the neurological (post-stroke seizures,
hydrocephalus, spasticity), psychiatric (depression, anxiety,
anger, frustration, personality change, post-stroke pain
syndromes), cognitive (impairment in the field of communication,
dysarthria, aphasia, spatial awareness, difficulties with memory,
concentration, executive function and praxis, vascular dementia)
and vascular (recurrent stroke, coronary artery disease, peripheral
vascular disease) fields (9).
One of the most important sequelae of stroke is
chronic motor deficits in the upper or lower limbs, which can lead
to a severe impairment of the activities of daily living (ADL) and
thus a decrease in the quality of life. Therefore, this disability
represents an important target in the post-stroke rehabilitation
process (10).
The assessment of motor status for stroke survivors
is performed in both the acute and chronic phases through
electromyography (EMG), which is the most often used and accurate
study of muscle electrical activity. EMG is employed to localize
the lesion and appreciate its degree of severity, to determine the
nerve pathophysiology, and to evaluate the evolutionary timeline
and rate of recovery (11). EMG
studies have the advantage of customization in order to
specifically localize the nerve lesion and offer to the clinician a
clear image of the physiological status and the pathological
processes that underlie the muscle disease or neuromuscular
dysfunction. Based on these advantages, EMG is a useful tool for
narrowing down differential diagnoses.
The recovery process after a stroke is strongly
related to the evolutionary timeline, and currently, the most
common categories of stroke stages are those proposed by the Stroke
Roundtable Consortium: The first 24 h, hyperacute phase; the first
7 days, acute phase; the first 3 months, early sub-acute phase;
months 4-6, late sub-acute phase; and from 6 months on, chronic
phase (12). Related to this
timeline, it has been reported that the most favourable period for
recovery interventions is the early sub-acute phase, when the most
significant improvements have been shown to occur (13). The subsequent stages are often
characterized by a relative plateau in the amplitude of the
recovery process, whereas the chronic phase is dominated by a
deficit of rehabilitation (14).
However, even though these phases are well-defined regarding their
duration, in reality recovery is substantially different between
affected individuals and the processes supporting the recovery for
a given phase are still being studied (15). It has been reported that in the 6th
month after a stroke, only 60% of survivors with hemiparesis who
received rehabilitation procedures in hospital became independent
in simple ADL (16).
Post-stroke rehabilitation involves a
multidisciplinary approach, which needs to start in stable patients
at least 48 h after the initial symptoms. The outcomes of the
rehabilitation can lead to significant advantages for the patients,
their families or caregivers and even for the community in which
they live, in terms of reducing social and economic burden. As
aforementioned, multidisciplinary effort is needed in order to
solve the sequalae of strokes, and must include physicians
(neurologists, neurosurgeons, rehabilitation medicine specialists)
and physical, occupational and speech therapists, as well as
psychological services, social support and medical equipment
providers (17).
One of the main goals of the neurobiological
rehabilitation strategies is represented by the restitution of
neuronal activity, which can be achieved through pharmacological
approaches and physical techniques. The physical techniques used
include kinesiotherapy, cryotherapy, thermotherapy, electrotherapy
and massage; all of these methods are currently used during the
post-stroke rehabilitation process (18). The present study aimed to evaluate
and to compare the efficiency of different approaches of physical
rehabilitation in patients in the acute and early sub-acute stages
following a stroke.
Materials and methods
Patients
For the aim of the present study, which was carried
out over a period of 2 years (January 1, 2019-December 31, 2021),
68 hemiparetic stroke survivors were recruited, who were admitted
to the Rehabilitation Clinic, Emergency County Hospital of Craiova
(Craiova, Romania), with which the University of Medicine and
Pharmacy Craiova has a clinical agreement for medical services. All
subjects had their diagnosis confirmed by a neurologist following a
specialized examination, where the haemorrhagic aetiology of the
brain injury was ruled out by a skull CT scan. At the time of
recruitment, all subjects were clinically stable and within 48-72 h
after the onset of a stroke.
The inclusion criteria were as follows: i)
Post-stroke duration between 48 and 72 h after the onset of
symptoms; ii) unilateral movement impairment of the upper limbs;
iii) clinically stable; iv) without recent injury; v) without being
under medication that can impact neuromuscular function; vi)
without a positive history for recurrent vascular episodes. The
exclusion criteria were as follows: i) Patients with aphasia, as
they were not able to provide informed consent; ii) patients with
cardiac implantable electronic devices where the electrotherapy
could interfere with their functionality; iii) patients who did not
agree to participate.
All patients were clinically evaluated by the
medical recovery specialist at the beginning of the rehabilitation
process by measuring the force of the brachial biceps and triceps
muscles, as well as grading the spasticity of the upper limbs using
the Modified Ashworth Scale (19).
This assessment procedure was repeated every 2 weeks during the
entire period of the study.
The motor function of the upper limbs was assessed
using the Fugl-Meyer Assessment for Upper Extremity (FMA-UE)
subscale, which consists of 33 items (domains: A, upper extremity;
B, wrist; C, hand; D, coordination/speed), each item being scored
on a 3-point ordinal scale (0, 1 or 2), with 0 generally
corresponding to no function, 1 to partial function and 2 to
perfect function. The general score was represented by the sum of
the scores, with a maximum score (no impairment) of 66 points
(20). The functional ability of
the subjects was evaluated using the Barthel Index for ADL (0-100)
(21), ADL (0-6) (22) and iADL (0-8) (23), scoring different activities that
evaluate the patient's ability to act in activities of daily life
using data related to occurrence of urinary or faecal incontinence,
quantifying the ability of the patient to perform hair grooming,
dressing, feeding, walking, climbing stairs, using the toilet and
bathing independently. These four evaluation tools were used at the
beginning and at the end of the rehabilitation program (study
period). Demographic (age, sex and residency) and clinically
relevant (comorbidities, health-risk behaviours and laboratory
results) data were also collected.
All subjects were included in the present study on a
voluntary basis and provided written informed consent. The present
study was approved by the Ethics Committee of the University of
Medicine and Pharmacy of Craiova (approval no. 167/12.06.2017;
Craiova, Romania) and was in line with The Declaration of
Helsinki.
Recovery strategies
All patients underwent complex physical therapy,
consisting of kinesiotherapy, cryotherapy, thermotherapy,
electrostimulation and massage, for 12 weeks. The patients were
divided into two study groups that received an identical program of
recovery therapies in terms of the procedures; however, the
frequency of the rehabilitation sessions was different between the
two groups: A total of 48 patients received continuous physical
therapy, weekly for the whole study period, whereas the remaining
20 patients received intermittent rounds of physical therapy every
2 weeks (weeks 2, 4, 6, 8, 10 and 12). The two study groups were
created based on the therapeutic approach decided on by the
specialists from the Rehabilitation Clinic, Emergency County
Hospital of Craiova.
Every patient underwent the same type of
rehabilitation program as follows (Table I): i) Kinesiotherapy, consisting of
passive and active-passive mobilizations of the affected shoulder,
elbow and wrist joints, was performed twice a day for 15 min at a
time, every morning and afternoon; ii) Cryotherapy was applied to
the biceps brachii and triceps muscles, and consisted of ice pack
applications for 10 min on each of the muscles, only if spasticity
was present on the affected limb. This procedure was done daily
from the onset of spasticity until its remission; iii)
Thermotherapy was performed using paraffin wraps at a temperature
of 55˚C placed on the affected arm, for 20 min, encompassing both
the shoulder and elbow joints. This procedure was performed daily
before the first kinesiotherapy session of the day. It increases
ligament laxity, and thus could increase the passive and active
range of motion of the affected arm; iv) A total of 10 min of
upward massage of the affected upper limb was performed, targeting
the reduction of swelling by stimulating fluid circulation in the
arm. Massage was performed two times a week; v) Electrotherapy
consisting of transcutaneous electrical nerve stimulation (TENS)
and galvanization. The electrodes were applied with the negative
pole placed on the proximal third of the stimulated muscle and the
positive pole placed on the distal tendon of the respective muscle.
Electrostimulation began with 10 min of galvanization, then TENS
was applied followed by another 10 min of galvanization. TENS
amplitude was raised until a 6-10 sec sustained contraction
appeared, followed by a 1-min pause. The number of contractions per
session was set at 20, thus accounting for ~25 min of TENS and a
total of 45 min of electrotherapy. The biceps brachii and triceps
were stimulated on a daily alternative basis, for example: Monday,
biceps; Tuesday, triceps; Wednesday, biceps; Thursday, triceps, and
so forth.
 | Table IRecovery therapies applied in the
present study. |
Table I
Recovery therapies applied in the
present study.
| Procedure | Monday | Tuesday | Wednesday | Thursday | Friday |
|---|
| Kinesiotherapy | 30 min | 30 min | 30 min | 30 min | 30 min |
| Cryotherapy | 20 min | 20 min | 20 min | 20 min | 20 min |
| Thermotherapy | 20 min | 20 min | 20 min | 20 min | 20 min |
| Electrotherapy | 45 min | 45 min | 45 min | 45 min | 45 min |
| Massage | Not applicable | 10 min | Not applicable | 10 min | Not applicable |
Experimental setup and EMG
recording
For EMG recording, Participants were seated in an
upright position with their arm comfortably supported. The forearm
was immobilized by the examiner during the examination and was
placed in full supination at 90˚ with the arm. Subjects were
instructed to voluntarily produce hand-to-forearm or forearm-to-arm
flexion. EMG recordings were done over the biceps brachii or flexor
carpi ulnaris using Neuropack M1 MEB-9100 EMG/EP/IOM System (Nihon
Kohden Corporation). In order to ensure appropriate electric
contact and low baseline noise, the surface of the electrodes was
placed over the skin only after it was cleaned with alcohol. The
signal sampled every other 100 µsec was amplified and filtered with
a bandwidth of 10 Hz to 10 kHz. Each subject was asked to perform
the same protocol at the time of admission (week 1) and every 2
weeks until the end of the recovery program (weeks 2, 4, 6, 8, 10
and 12). When the setup was completed, patients were asked to
perform maximal contraction of the recorded muscles. Discharge
firing rate (DFR) was considered the main objective data that could
provide an indication of how the rehabilitation sessions should be
targeted.
Statistical analysis
All clinical and non-clinical items, and the results
of the working instruments were recorded in a Microsoft Excel file
(Microsoft Excel 2016; Microsoft Corporation). The descriptive
analysis of the samples was realized using absolute and relative
frequencies (%) or median and interquartile range (IQR). After
testing, some of the data were found not to be parametrical. More
complex analysis of the possible associations between recorded
variables was performed using Prism 9.3.0 (GraphPad Software;
Dotmatics) and SPSS version 20 (IBM Corp.). The following
statistical tests were performed: Shapiro-Wilk's test for data
normality analysis; Wilcoxon's rank-sum or Wilcoxon signed-rank
tests followed by Bonferroni's corrections, or Friedman followed by
Nemenyi's test, and mixed ANOVA followed by Sidak's test. Where the
statistical tests could not be carried out due to the small number
of subjects, this was noted in the tables of results as not
applicable. P<0.05 was considered to indicate a statistically
significant difference.
Results
The socio-demographic data of the study samples
revealed that the two samples had a comparable average age of the
subjects, whereas in the continuous therapy group, the sex ratio
was 2:1 male-to-female, while the intermittent group had a 1:1
ratio. Regarding where the patients lived, the distribution was
also similar between the two groups (approximately two-thirds of
the patients lived in a rural area) (Table II).
 | Table IISociodemographic data of the two study
groups. |
Table II
Sociodemographic data of the two study
groups.
| Characteristic | Continuous therapy
group, n=48 (%) | Intermittent therapy
group, n=20 (%) |
|---|
| Mean age, years | 57.83±1.73 | 58.05±1.64 |
| Sex | | |
|
Male | 32 (66.67) | 10 (50.00) |
|
Female | 16 (33.33) | 10 (50,00) |
| Environment | | |
|
Urban | 20 (41.67) | 8 (40.00) |
|
Rural | 28 (58.33) | 12 (60.00) |
According to the study methodology, clinical and
laboratory data considered to have an influence on the onset and
evolution of stroke were collected. It was revealed that almost
two-thirds of both groups consisted of smokers and >75% of
patients had one or multiple associated medical conditions
(diabetes mellitus and/or high blood pressure). Laboratory data
revealed that both total cholesterol and triglyceride levels were
high, which is characteristic for dyslipidaemia (Table III). All these collected data
suggested that almost all patients were exposed to severe risk
factors for the development of stroke.
 | Table IIIClinical data of the two
study-samples. |
Table III
Clinical data of the two
study-samples.
| Characteristic | Continuous therapy
group, n=48 (%) | Intermittent
therapy group, n=20 (%) |
|---|
| Smokers | 31 (64.58) | 14 (70.00) |
| Comorbidities | | |
|
Diabetes
mellitus | 4 (8.33) | 1 (5.00) |
|
High blood
pressure | 25 (52.08) | 11 (55.00) |
|
Diabetes
mellitus + Hypertension | 10 (20.83) | 3 (15.00) |
| Laboratory
data | | |
|
Total
cholesterol, mg/dl | 229.66±46.55 | 252.00±52.47 |
|
Triglycerides,
mg/dl | 208.60±57.94 | 222.40±54.50 |
The number of brain lesions was revealed by a CT
scan. In the continuous therapy group, 33 patients (68.75%)
presented with a left hemisphere injury resulting in an impairment
of the right hemibody, and the remaining 15 patients (31.25%) had a
lesion localized in the right hemisphere resulting in an impairment
of the left hemisphere. In the intermittent therapy group, the
lesion was situated on the left side for 14 subjects (70.00%),
whereas it was situated on the right side for the remaining 6
subjects (30.00%).
According to the obtained results, the intermittent
recovery appeared to be similar in effectiveness compared with
continuous recovery for acute and sub-acute stroke. Clinical
monitoring of the patients based on the scoring scales developed
for patients with stroke revealed no differences between the
patients enrolled in a continuous form of recovery program compared
with those enrolled in an intermittent regimen (Fig. 1). The scores of the FMA-UE
exhibited an improvement between weeks 1 and 12 for both continuous
(n=48; P<0.0001) and intermittent (n=20; P<0.0001) groups.
This improvement was observed in all domains of the FMA-UE: Upper
extremity (Fig. 1A), wrist
(Fig. 1B), hand (Fig. 1C) and coordination/speed (Fig. 1D). The total motor score of the two
tested groups was similar (P>0.05) after 12 weeks of functional
recovery (Fig. 1E). The aim of the
present study was to determine if the intermittent recovery
strategy differed from the continuous strategy. However, patients
were recruited in both acute (<24 h post-stroke) and early
sub-acute phases (within the first 72 h post-stroke), making the
direct comparison difficult due to the different elapsed time from
the stroke. Therefore, the present study, first investigated if
there was any improvement within patients recruited in the
continuous and intermittent groups. This was quantified as the
percentage of improvement between the beginning and endpoint. There
was no overall impact on the rehabilitation, as both acute and
early sub-acute patients had similar improvements (P>0.05;
Fig. S1).
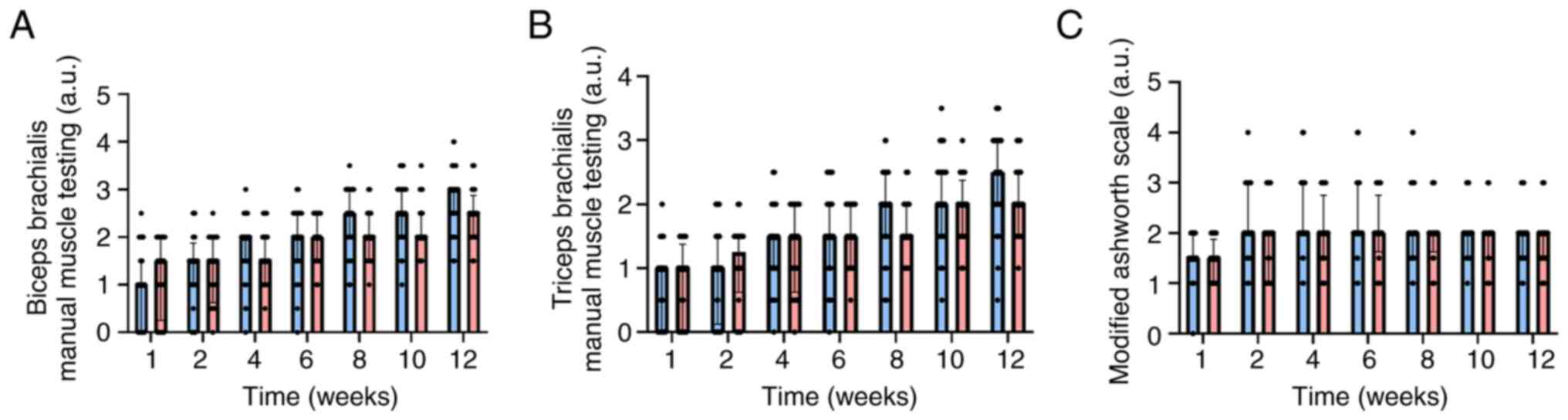
Manual muscle testing (MMT) by EMG recordings
detected no significant difference between the study groups. When
MMT was measured for the biceps brachii (P=0.2718; Fig. 2A) and the triceps brachii muscle
(P=0.3692; Fig. 2B), no
significant difference was noted between the groups for the whole
study period. The improvement in recovery between the beginning and
end of the rehabilitation program in the continuous therapy group
was significant, the biceps MMT score increased from 1.03±0.74 to
2.78±0.63 (P<0.0001) and the triceps MMT score increased from
0.79±0.63 to 2.39±0.69 (P<0.0001). A similar increase was
observed in the intermittent therapy group for MMT [the biceps MMT
score increased from 1.18±0.78 to 2.43±0.57 (P<0.0001) and the
triceps MMT score increased from 0.78±0.60 to 2.08±0.57
(P<0.0001)]. The degree of spasticity evaluated by the Modified
Ashworth Scale was did not differ significantly (P>0.05) during
the study period (Fig. 2C).
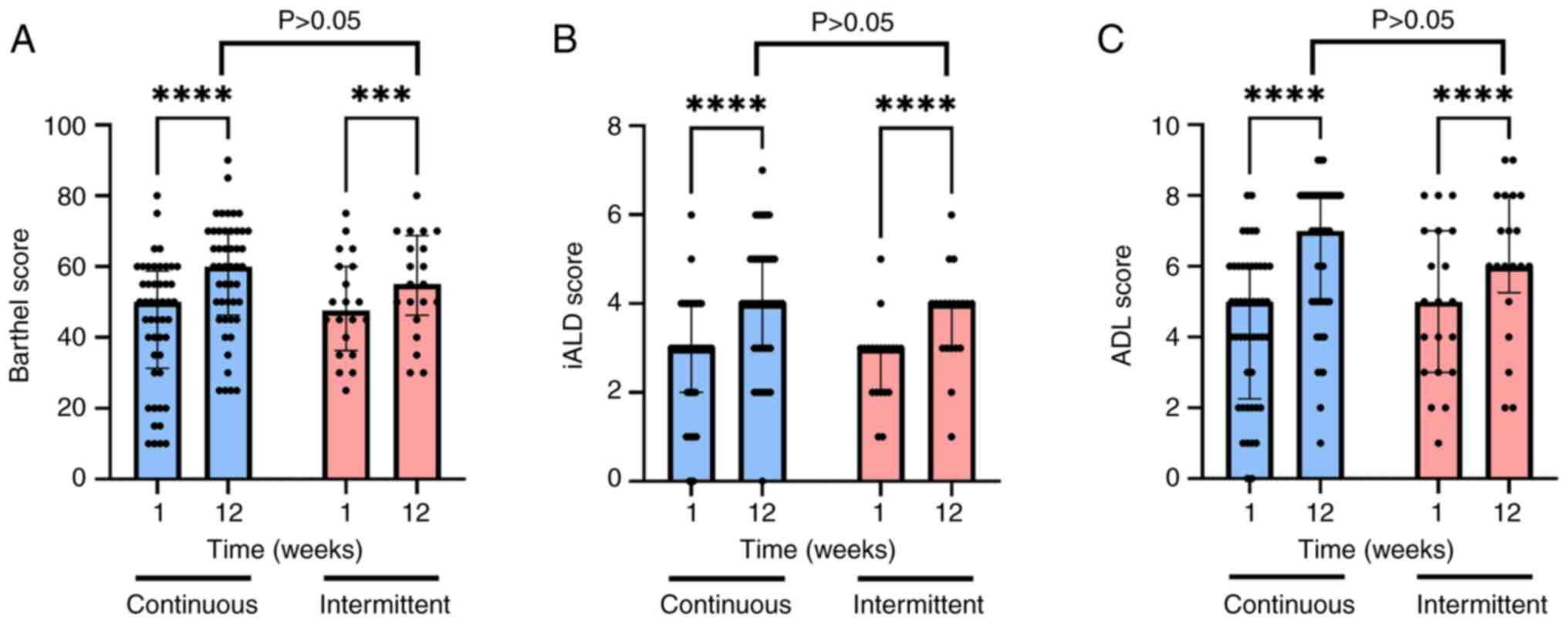
Clinical monitoring of the patients based on the
scoring scales developed for patients with stroke revealed no
differences between the patients enrolled in the continues recovery
program compared with those enrolled in the intermittent regimen
(Fig. 3). The Barthel score was
significantly improved between weeks 1 and 12 for both the
continuous (n=48; 43.95±18.1 vs. 57.39±15.97; P<0.0001) and
intermittent (n=20; 48.75±14.03 vs. 55±14.23; P=0.0003) groups
(Fig. 3A). The same effects were
observed for both iADL and ADL scores; after 12 weeks of stroke
recovery procedures, both the continuous (n=48) and intermittent
(n=20) groups improved in terms of iADL (P<0.0001) and ADL
(P<0.0001) scores. Notably, no significant difference was
recorded between the two groups at the final assessment on week 12
(iADL: 4.00±1.41 vs. 3.70±1.08, P=0.6075; ADL: 6.35±2 vs.
6.15±2.08, P=0.9171) (Fig. 3B and
C).
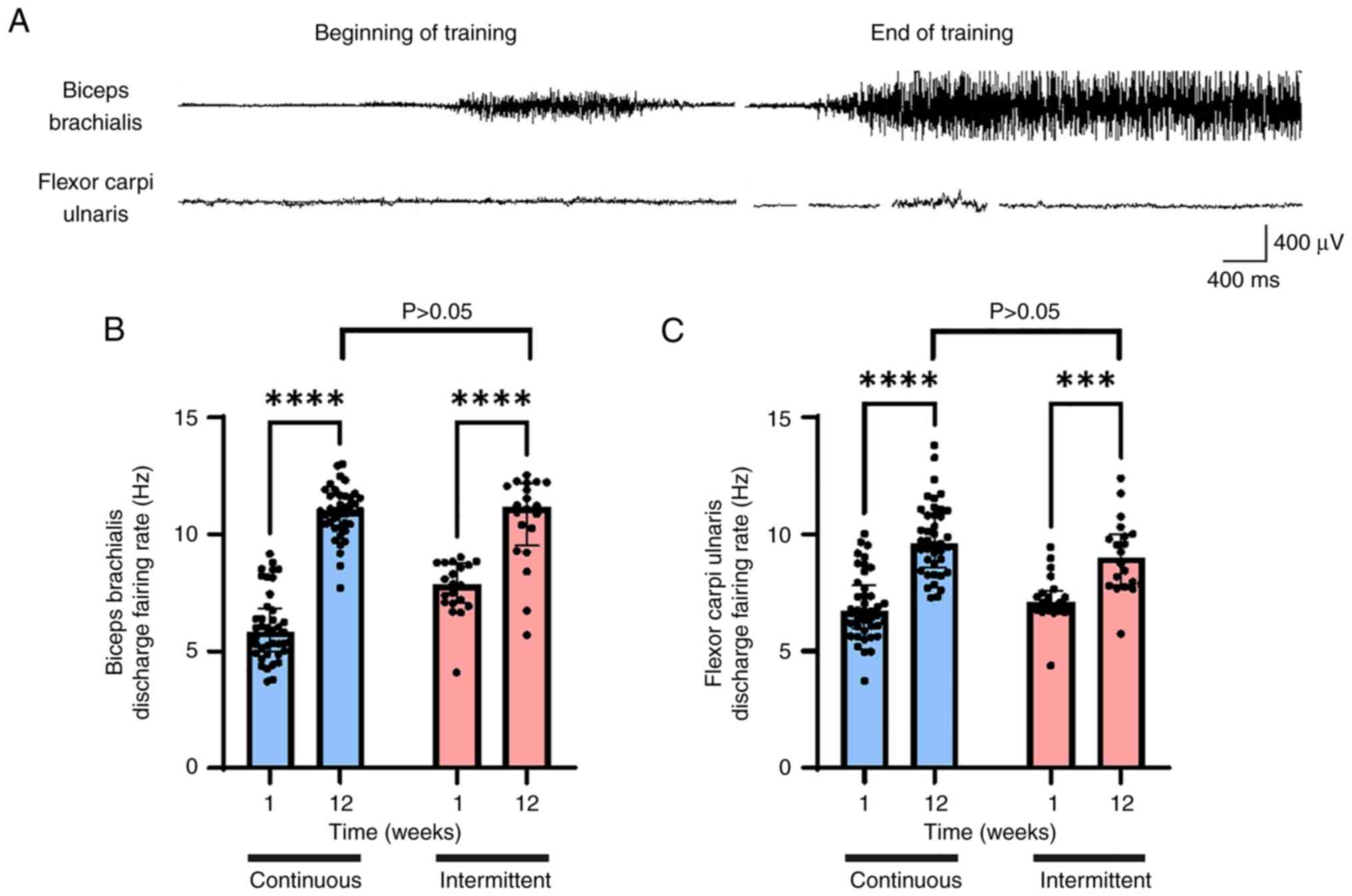
As an objective measuring tool, the DFR was compared
between the two groups, using the most basic clinical EMG tests. No
differences were observed among the two groups. The DFR was similar
between the groups measured at week 12: Biceps brachii, 10.934±1.08
Hz in the continuous group vs. 10.62±1.89 Hz in the intermittent
group; flexor carpi ulnaris, 9.81±1.54 Hz in the continuous group
compared with 9.06±1.58 Hz (Fig.
4).
Analysing the relationship between FMA-UE scores on
all four domains and socio-demographic data, it was revealed that
for both study samples the evolution of recovery in terms of
improvement during the 12 weeks of therapeutic process was
statistically significant independent of sex and residence
(P<0.01; Table IV).
 | Table IVAssociation between FMA-UE scores and
demographic data during the recovery process. |
Table IV
Association between FMA-UE scores and
demographic data during the recovery process.
| | Continuous therapy
group, n=48 | Intermittent
therapy group, n=20 |
|---|
| | FMA-UE scores | FMA-UE scores |
|---|
| Patients | Week 1 | Week 12 | P-value | Week 1 | Week 12 | P-value |
|---|
| Male patients | | | | | | |
|
A-upper
extremity | 17.00
(13.00-20.00) | 30.00
(28.00-31.00) | <0.001 | 16.00
(13.50-18.75) | 29.00
(22,00-30.75) | <0.010 |
|
B-wrist | 4.00
(3.00-5.00) | 7.00
(7.00-8.00) | <0.001 | 4.00
(3.25-5.00) | 8.00
(6.25-8.00) | <0.010 |
|
C-hand | 6.00
(4.75-6.00) | 10.00
(9.00-11.00) | <0.001 | 6.00
(5.00-6.00) | 10.00
(7.25-10.75) | <0.010 |
|
D-coordination/speed | 0.50
(0.00-1.25) | 3.00
(2.00-4.00) | <0.001 | 0.00
(0.00-0.75) | 3.00
(2.25-4.00) | <0.010 |
| Female
patients | | | | | | |
|
A-upper
extremity | 17.50
(15.00-20.25) | 28.50
(27.75-32.25) | <0.001 | 18.50
(14.50-22.00) | 30.50
(27.25-32.75) | <0.010 |
|
B-wrist | 4.00
(3.00-5.00) | 6.50
(5.75-8.00) | <0.001 | 5.50
(4.00-6.00) | 7.50
(7.00-9.00) | <0.010 |
|
C-hand | 6.00
(5.00-6.25) | 9.50
(8.00-11.00) | <0.001 | 5.50
(5.00-7.00) | 10.00
(9.00-11.00) | <0.010 |
|
D-coordination/speed | 0.50
(0.00-1.25) | 3.00
(2.00-3.25) | <0.001 | 0.50
(0.00-1.75) | 3.00
(2.25-4.00) | <0.010 |
| Living in urban
areas | | | | | | |
|
A-upper
extremity | 15.50
(13.00-18.25) | 28.00
(24.50-31.00) | <0.001 | 16.50
(14.75-20.25) | 29.00
(27.75-31.25) | <0.010 |
|
B-wrist | 3.50
(3.00-4.00) | 7.00
(6.00-8.00) | <0.001 | 4.50
(4.00-6.00) | 8.00
(7.00-8.00) | <0.010 |
|
C-hand | 5,00
(4.00-6.00) | 9.50
(8.00-10.00) | <0.001 | 6.00
(5.00-6.00) | 10.00
(8.75-11.00) | <0.010 |
|
D-coordination/speed | 0.00
(0.00-1.00) | 3.00
(2.00-3.00) | <0.001 | 0.00
(0.00-1.00) | 3.00
(2.75-4.00) | <0.010 |
| Living in rural
areas | | | | | | |
|
A-upper
extremity | 18.50
(16.75-21.25) | 30.00
(28.75-32.25) | <0.001 | 17.50
(12.50-22.00) | 30.00
(24.00-33.00) | <0.010 |
|
B-wrist | 5.00
(4.00-5.00) | 7.50
(7.00-8.00) | <0.001 | 4.50
(2.75-6.25) | 7.50
(5.50-9.00) | <0.010 |
|
C-hand | 6.00
(5.00-7.00) | 10.00
(9.75-11.00) | <0.001 | 5.50
(4.50-7.00) | 9.50
(8.25-11.00) | <0.010 |
|
D-coordination/speed | 1.00
(0.00-2.00) | 4.00
(3.00-4.00) | <0.001 | 0.00
(0.00-2.00) | 3.50
(2.00-4.00) | <0.010 |
The present study examined the influence of
demographics (sex and environment) on the evolution of recovery
from week 1 to 12 in both groups. The improvement in the level of
functional ability for ADL, iADL and Barthel, as expressed by the
average scores of the working tools, was significant (P<0.05) in
the intermittent and highly significant (P<0.001) in the
continuous group, associated with both sex and residency (Table V).
 | Table VAssociation between demographic data
and evolution of the recovery process. |
Table V
Association between demographic data
and evolution of the recovery process.
| A, Continuous
therapy group, n=48 |
|---|
| | Barthel | iADL | ADL |
|---|
| Patients | Week 1 | Week 12 | P-value | Week 1 | Week 12 | P-value | Week 1 | Week 12 | P-value |
|---|
| Male | 50.00
(27.50-60.00) | 60.00
(48.75-70.00) | <0.0010 | 3.00
(2.00-3.25) | 4.00
(3.00-5.00) | <0.0010 | 5.00
(2.75-6.00) | 7.00
(5.00-8.00) | <0.0010 |
| Female | 45.00
(35.00-55.00) | 57.50
(48.75-70.00) | <0.0010 | 3.00
(2.00-4.00) | 4.00
(3.75-5.00) | <0.0010 | 4.50
(2.75-6.00) | 6.50
(5.00-8.00) | <0.0010 |
| Urban | 42.50
(20.00-51.25) | 55.00
(43.75-70.00) | <0.0010 | 3.00
(1.75-3.25) | 4.00
(2.75-4.25) | <0.0010 | 4.00
(2.00-6.00) | 6.00
(4.75-8.00) | <0.0010 |
| Rural | 55.00
(48.75-60.00) | 65.00
(57.50-70.00) | <0.0010 | 3.00
(3.00-4.00) | 4.50
(4.00-5.00) | <0.0010 | 5.00
(3.75-6.25) | 8.00
(6.50-8.00) | <0.0010 |
| B, Intermittent
therapy group, n=20 |
| | Barthel | iADL | ADL |
| Patients | Week 1 | Week 12 | P-value | Week 1 | Week 12 | P-value | Week 1 | Week 12 | P-value |
| Male | 47.50
(32.50-50.00) | 55.00
(37.50-60.00) | <0.0500 | 3.00
(2.25-3.00) | 4.00
(3.00-4.00) | 0.0110 | 4.50
(2.50-5.75) | 6.00
(3.75-7.00) | <0.0100 |
| Female | 52.50
(45.00-65.00) | 57.50
(50.00-70.00) | <0.0100 | 3.00
(2.00-3.00) | 4.00
(3.25-4.75) | 0.0015 | 5.50
(3.25-7.75) | 6.50
(6.00-8.00) | <0.0500 |
| Urban | 47.50
(43.75-51.25) | 55.00
(48.75-61.25) | <0.0100 | 3.00
(2.75-3.00) | 4.00
(3.00-4.00) | 0.0015 | 5.00
(3.75-6.00) | 6.00
(6.00-7.00) | <0.0100 |
| Rural | 52.50
(33.75-66.25) | 60.00
(45.00-70.00) | <0.0500 | 2.50
(1.75-3.25) | 4.00
(2.75-5.00) | 0.0133 | 5.50
(2.75-7.25) | 6.50
(3.50-8.25) | <0.0500 |
The association between risk behaviours (smoking)
and medical conditions (diabetes, hypertension and dyslipidaemia)
with recovery outcomes, as measured by mean FMA-EU scores, were
also statistically analysed. Notably, no differences were
identified in the recovery period for both groups. Thus, during the
12 weeks of the study period, all subjects showed a significant
improvement (P<0.005) in their health status where comorbidities
were recorded, even if the recovery procedures were applied
continuously or intermittently, according to the study methodology
(Table VI). The low number of
patients with diabetes mellitus and diabetes mellitus in
association with hypertension did not allow for statistical
analysis (n.a. in Table VI).
 | Table VIAssociation between clinical data and
the FMA-UE scores during the recovery process. |
Table VI
Association between clinical data and
the FMA-UE scores during the recovery process.
| | Continuous therapy
group, n=48 | Intermittent
therapy group, n=20 |
|---|
| | FMA-UE scores | FMA-UE scores |
|---|
| Risk
behaviour/comorbidities | Week 1 | Week 12 | P-value | Week 1 | Week 12 | P-value |
|---|
| Smokers | | | | | | |
|
A-upper
extremity | 18.00
(15.00-20.00) | 30.00
(28.00-31.00) | <0.001 | 16.00
(14.00-20.50) | 28.50
(26.25-31.00) | <0.001 |
|
B-wrist | 4.00
(3.00-5.00) | 7.00
(6.50-8.00) | <0.001 | 4.50
(3.25-5.75) | 7.50
(6.25-8.00) | <0.001 |
|
C-hand | 6.00
(5.00-6.00) | 10.00
(9.00-10.50) | <0.001 | 5.50
(5.00-6.00) | 10.00
(9.00-11.00) | <0.001 |
|
D-coordination/speed | 1.00
(0.00-1.00) | 3.00
(3.00-4.00) | <0.001 | 0.00
(0.00-0.75) | 3.00
(2.00-3.75) | <0.001 |
| Diabetes | | | | | | |
|
A-upper
extremity | 14.00
(11.00-18.00) | 28.00
(25.75-29.00) | <0.001 | 15.00
(13.25-18.00) | 25.00
(20.25-30.00) | n.a. |
|
B-wrist | 3.00
(3.00-4.00) | 7.00
(6.00-8.00) | <0.001 | 3.00
(2.75-4.00) | 6.00
(4.75-7.50) | n.a. |
|
C-hand | 5.00
(4.00-6.00) | 9.00
(8.25-10.00) | <0.001 | 4.50
(3.75-5.75) | 8.50
(6.75-10.50) | n.a. |
|
D-coordination/speed | 0.00
(0.00-1.00) | 3.00
(2.00-3.00) | <0.001 | 0.00
(0.00-0.5) | 2.50
(1.50-3.25) | n.a. |
| Hypertension | | | | | | |
|
A-upper
extremity | 17.00
(14.00-20.00) | 29.00
(28.00-31.50) | <0.001 | 17.50
(15.00-21.00) | 30.00
(27.25-32.00) | <0.001 |
|
B-wrist | 4.00
(3.00-5.00) | 7.00
(6.50-8.00) | <0.001 | 5.00
(3.25-6.00) | 8.00
(7.00-9.00) | <0.001 |
|
C-hand | 6.00
(5.00-6.50) | 10.00
(9.00-11.00) | <0.001 | 6.00
(5.00-6.75) | 10.00
(8.25-11.00) | <0.001 |
|
D-coordination/speed | 0.00
(0.00-1.00) | 3.00
(2.00-4.00) | <0.001 | 0.00
(0.00-1.00) | 4.00
(2.25-4.00) | <0.001 |
| Diabetes +
hypertension | | | | | | |
|
A-upper
extremity | 14.00
(10.25-17.25) | 28.00
(25.75-29.75) | <0.010 | 16.00
(13.50-20.00) | 29.00
(23.50-31.00) | n.a. |
|
B-wrist | 3.00
(3.00-4.00) | 7.00
(6.25-8.00) | <0.010 | 3.00
(2.50-5.00) | 7.00
(5.50-8.00) | n.a. |
|
C-hand | 5.00
(4.00-5.75) | 9.00
(8.25-10.00) | <0.010 | 5.00
(4.00-6.50) | 10.00
(8.00-11.00) | n.a. |
|
D-coordination/speed | 0.00
(0.00-0.75) | 2.50
(2.00-3.00) | <0.010 | 0.00
(0.00-1.00) | 3.00
(1.50-3.50) | n.a. |
| Dyslipidaemia | | | | | | |
|
A-upper
extremity | 17.00
(12.00-19.00) | 29.00
(27.5-31) | <0.001 | 16.00
(14.00-21.00) | 29.00
(26.50-32.00) | <0.001 |
|
B-wrist | 4.00
(3.00-5.00) | 7.00
(7.00-8.00) | <0.001 | 5.00
(3.50-6.00) | 8.00
(6.50-8.50) | <0.001 |
|
C-hand | 6.00
(5.00-6.00) | 10.00
(9.00-10.5) | <0.001 | 6.00
(5.00-6.50) | 10.00
(8.50-11.00) | <0.001 |
|
D-coordination/speed | 0.00
(0.00-1.00) | 3.00
(2.00-4.00) | <0.001 | 0.00
(0.00-1.00) | 3.00
(2.00-4.00) | <0.001 |
The association between risk behaviours (smoking)
and comorbidities (as identified through clinical assessment) with
positive outcomes of the rehabilitation program was also
statistically significant (P<0.05). Thus, the efficiency of the
recovery process was not impacted by these clinical aspects for the
subjects who followed the continuous rehabilitation program, as
well as for those who received intermittent procedures (Table VII). The low number of patients
with diabetes mellitus and diabetes mellitus in association with
hypertension did not allow for statistical analysis (n.a. in
Table VII).
 | Table VIIAssociation between clinical data and
evolution of the recovery process. |
Table VII
Association between clinical data and
evolution of the recovery process.
| A, Continuous
therapy group, n=48 |
|---|
| | Barthel | iADL | ADL |
|---|
| Risk
behaviour/Comorbidities | Week 1 | Week 12 | P-value | Week 1 | Week 12 | P-value | Week 1 | Week 12 | P-value |
|---|
| Smokers | 50.00
(40.00-57.50) | 60.00
(50.00-70.00) | <0.001 | 3.00
(2.50-4.00) | 4.00
(3.00-5.00) | <0.001 | 5.00
(3.50-6.00) | 7.00
(5.00-8.00) | <0.001 |
| Diabetes | 40.00
(22.50-45.00) | 47.50
(45.00-55.00) | n.a. | 2.50
(1.25-3.00) | 3.00
(3.00-4.00) | n.a. | 3.50
(2.00-4.75) | 5.00
(5.00-6.75) | n.a. |
| Hypertension | 50.00
(32.50-57.50) | 60.00
(45.00-70.00) | <0.001 | 3.00
(2.00-3.50) | 4.00
(3.00-4.50) | <0.001 | 5.00
(2.50-6.00) | 7.00
(5.00-8.00) | <0.001 |
| Diabetes +
hypertension | 35.00
(22.50-48.75) | 45.00
(37.50-65.00) | <0.010 | 2.50
(1.00-3.00) | 3.00
(2.25-4.00) | <0.050 | 3.50
(2.00-5.50) | 5.00
(4.25-6.75) | <0.010 |
| Dyslipidaemia | 50.00
(30.00-55.00) | 60.00
(45.00-67.50) | <0.001 | 3.00
(2.00-3.50) | 4.00
(3.00-5.00) | <0.001 | 4.00
(2.00-6.00) | 7.00
(5.00-8.00) | <0.001 |
| B, Intermittent
therapy group, n=20 |
| | Barthel | iADL | ADL |
|
Patients | Week 1 | Week 12 | P-value | Week 1 | Week 12 | P-value | Week 1 | Week 12 | P-value |
| Smokers | 45.00
(37.50-57.50) | 52.50
(50.00-63.75) | <0.010 | 3.00
(2.00-3.00) | 4.00
(3.00-4.00) | <0.010 | 4.50
(3.00-5.75) | 6.00
(5.25-7.00) | <0.001 |
| Diabetes | 40.00
(33.75-52.50) | 50.00
(45.00-57.50) | n.a. | 2.00
(1.75-2.50) | 3.50
(2.50-4.25) | n.a. | 3.00
(2.75-4.25) | 5.50
(4.25-6.75) | n.a. |
| Hypertension | 50.00
(37.50-60.00) | 57.50
(50.00-68.75) | <0.001 | 3.00
(2.25-3.00) | 4.00
(4.00-4.00) | <0.001 | 5.00
(3.25-7.00) | 6.00
(5.25-8.00) | <0.001 |
| Diabetes +
hypertension | 35.00
(32.50-55.00) | 50.00
(40.00-65.00) | n.a. | 2.00
(1.50-3.00) | 4.00
(2.50-4.50) | n.a. | 3.00
(2.50-5.50) | 5.00
(3.50-7.00) | n.a. |
| Dyslipidaemia | 45.00
(40.00-57.50) | 55.00
(50.00-65.00) | <0.001 | 3.00
(2.00-3.00) | 4.00
(3.00-4.00) | <0.001 | 5.00
(3.00-6.50) | 6.00
(5.50-7.50) | <0.010 |
Discussion
The present study investigated the benefits of the
rehabilitation programs for stroke survivors in its continuous and
intermittent variants. The clinical characteristics of the study
subjects from both groups were similar to those mentioned by the
specialized literature in terms of the presence of behavioural and
clinical risk factors, including modifiable (hypertension, diabetes
mellitus, high blood cholesterol, dyslipidaemia, smoking) (4) and nonmodifiable risk factors (age and
sex) (5,24).
For the patients in the acute and sub-acute phases
of stroke involved in the present study, the degree of functional
improvement after the initial recovery program was significant, and
could be considered a short-term recovery in functional status, as
mentioned in previous studies (25,26).
Thus, the initial approach of the rehabilitation process was the
correctly selected, according to the clinical status of the
subjects involved in the study. Based on the outcomes of the
therapeutic process, it became clear that for the best possible
recovery, the procedures should be initiated as soon as possible
(18). Moreover, after the first
series of rehabilitation procedures applied for 12 weeks, clinical
evaluation is necessary to provide a clear and complete picture of
the stroke survivor. The results of this evaluation will provide
the background for the subsequent monitoring and recovery
processes, in order to avoid a possible worsened evolution of the
functional abilities as described in long-term studies (26,27).
Even if the medical conditions identified
(hypertension, diabetes and dyslipidaemia) in the present subjects
were not significantly involved in the deterioration of the
evolution and prognosis during the post-stroke period, the presence
of cardiac and metabolic comorbidities are associated with reduced
survival after stroke, and this is another aspect that
necessitating a mandatory long-term monitoring and recovery program
(28).
Assessment in the acute phase using EMG has been
recognized as the most accurate, accessible and useful tool for
diagnosis, and has also shown its value as a long-term monitoring
tool. The EMG assessment provides data on both the temporal course
and rate of recovery of the lesion (11,29).
The evaluation method used in the present study was similar to that
of other studies, measuring the movement control of the upper
extremities in patients in the sub-acute and acute phases following
a stroke (30). Based on the
results obtained by the present study and another study (31), it is recommended that this type of
evaluation is used for the long-term monitoring process of chronic
stroke survivors.
Previous studies have highlighted the importance of
complex rehabilitation programs for patients following stroke in
both the acute and chronic phases, based on the individualized
evaluation of each patient, rather than that provided by guidelines
(32,33). The program proposed in the present
study (kinesiotherapy, cryotherapy, thermotherapy, electrotherapy
and massage) covered the entire spectrum of the patients' needs, as
reflected in the positive outcomes measured by the working tools.
Moreover, the way in which the therapeutic program was
administered, either continuous or intermittent, did not influence
the benefits of the rehabilitation. Although the number of subjects
undergoing the intermittent program (n=20) was not substantial
enough to impose this approach as a reliable and standardized one,
which could be considered one of the limitations of the present
study, the results after 12 weeks were promising. Another
limitation of the current study could be the lack of long-term
monitoring of the evolution of patients following the study
period.
In conclusion, the rehabilitation process of stroke
survivors represents one of the most important elements for their
future quality of life. Intermittent physical recovery could be
considered a valid approach for sub-acute and acute stroke
survivors following an individualized clinical evaluation. Future
studies are required to confirm the findings for the chronic stages
of this disease.
Supplementary Material
Improvement in recovery. Considering
the FMA-UE scores obtained by each patient as 100%, it was
evaluated how each score evolved after 12 weeks of medical
recovery. For each individual, the improvement of all domains of
the FMA-UE: (A) upper extremity, (B) wrist, (C) hand and (D)
coordination/speed and (E) total motor score of the two tested
groups was similar (P>0.05) was calculated after 12 weeks of
functional recovery. FMA-UE, Fugl-Meyer Assessment Upper
Extremity.
Acknowledgements
The authors gratefully acknowledge Dr Mihail
Cristian Pirlog (Department of Medical Sociology, University of
Medicine and Pharmacy Craiova, Craiova, Romania) for critical
discussion and valuable support during the realization of the
study, and Dr Bogdan Catalin (Department of Physiology, University
of Medicine and Pharmacy Craiova, Craiova, Romania) for technical
assistance with electromyography recording.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request and after the approval of the Ethics Committee of the
University of Medicine and Pharmacy of Craiova, Craiova,
Romania.
Authors' contributions
AMP, ATB, SCB and OCR conceptualized the present
study. AMP, GC, ATB, SCB and OCR were major contributors to writing
the manuscript and collected the clinical data and analyzed the
data. The methodology used was established by AMP, ATB, GC and OCR.
Software input was performed by AMP and GC. Data curation was
performed by GC. The original draft was prepared by AMP and GC. The
editing and rewriting of the manuscript was performed by ATB and
OCR. ATB and OCR supervised the project. AMP and GC confirm the
authenticity of all the raw data. All have authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the University of Medicine and Pharmacy of Craiova
(approval no. 167/12.06.2017; Craiova, Romania) and was in line
with The Declaration of Helsinki. Written informed consent was
obtained from all subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2019 Stroke Collaborators. Global,
regional, and national burden of stroke and its risk factors,
1990-2019: A systematic analysis for the global burden of disease
study 2019. Lancet. 20:795–820. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Béjot Y, Bailly H, Durier J and Giroud M:
Epidemiology of stroke in Europe and trends for the 21st century.
Presse Med. 45:e391–e398. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
OECD. Mortality from heart disease and
stroke. In: Health at a Glance: Europe 2016: State of Health in the
EU Cycle. OECD Publishing: Paris, France, 2016.
|
|
4
|
O'Donnell MJ, Xavier D, Liu L, Zhang H,
Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ,
et al: Risk factors for ischaemic and intracerebral haemorrhagic
stroke in 22 countries (the INTERSTROKE study): A case-control
study. Lancet. 376:112–123. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lopez AD, Mathers CD, Ezzati M, Jamison DT
and Murray CJ: Global and regional burden of disease and risk
factors, 2001: Systematic analysis of population health data.
Lancet. 367:1747–1757. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wafa HA, Wolfe CDA, Emmett E, Roth GA,
Johnson CO and Wang Y: Burden of stroke in Europe: Thirty-year
projections of incidence, prevalence, deaths, and
disability-adjusted life years. Stroke. 51:2418–2427.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bamford J, Sandercock P, Dennis M, Burn J
and Warlow C: Classification and natural history of clinically
identifiable subtypes of cerebral infarction. Lancet.
337:1521–1526. 1991.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yew KS and Cheng EM: Diagnosis of acute
stroke. Am Fam Physician. 91:528–536. 2015.PubMed/NCBI
|
|
9
|
Singh RJ, Chen S, Ganesh A and Hill MD:
Long-term neurological, vascular, and mortality outcomes after
stroke. Int J Stroke. 13:787–796. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pang MY, Harris JE and Eng JJ: A
community-based upper-extremity group exercise program improves
motor function and performance of functional activities in chronic
stroke: A randomized controlled trial. Arch Phys Med Rehabil.
87:1–9. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Preston DC, Shapiro BE and Katirji B:
Electromyography and neuromuscular disorders:
Clinical-electrophysiologic correlations. Butterworth-Heinemann:
Boston, MA, 1998.
|
|
12
|
Bernhardt J, Hayward KS, Kwakkel G, Ward
NS, Wolf SL, Borschmann K, Krakauer JW, Boyd LA, Carmichael ST,
Corbett D and Cramer SC: Agreed definitions and a shared vision for
new standards in stroke recovery research: The Stroke recovery and
rehabilitation roundtable taskforce. Neurorehabil Neural Repair.
31:793–799. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kwakkel G, Kollen BJ, van der Grond J and
Prevo AJ: Probability of regaining dexterity in the flaccid upper
limb: Impact of severity of paresis and time since onset in acute
stroke. Stroke. 34:2181–2186. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nishimura Y, Onoe H, Morichika Y,
Perfiliev S, Tsukada H and Isa T: Time-dependent central
compensatory mechanisms of finger dexterity after spinal cord
injury. Science. 318:1150–1155. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
van der Vliet R, Selles RW, Andrinopoulou
ER, Nijland R, Ribbers GM, Frens MA, Meskers C and Kwakkel G:
Predicting upper limb motor impairment recovery after stroke: A
mixture model. Ann Neurol. 87:383–393. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Patel AT, Duncan PW, Lai SM and Studenski
S: The relation between impairments and functional outcomes
poststroke. Arch Phys Med Rehabil. 81:1357–1363. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dobkin BH: Strategies for stroke
rehabilitation. Lancet Neurol. 3:528–536. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
LaClair BJ, Reker DM, Duncan PW, Horner RD
and Hoenig H: Stroke care: A method for measuring compliance with
AHCPR guidelines. Am J Phys Med Rehabil. 80:235–242.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bohannon RW and Smith MB: Interrater
reliability of a modified Ashworth scale of muscle spasticity.
Physical Ther. 67:206–207. 1987.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fugl-Meyer AR, Jääskö L, Leyman I, Olsson
S and Steglind S: The post-stroke hemiplegic patient. 1. A method
for evaluation of physical performance. Scand J Rehabil Med.
7:13–31. 1975.PubMed/NCBI
|
|
21
|
Mahoney FI and Barthel DW: Functional
evaluation: The barthel index. Md State Med J. 14:61–65.
1965.PubMed/NCBI
|
|
22
|
Katz S, Ford AB, Moskowitz RW, Jackson BA
and Jaffe MW: Studies of illness in the aged: The index of ADL-A
standardized measure of biological and psychosocial function. JAMA.
185:914–919. 1963.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lawton MP and Brody EM: Assessment of
older people: Self-maintaining and instrumental activities of daily
living. Gerontologist. 9:179–186. 1969.PubMed/NCBI
|
|
24
|
Abdi H: The Bonferonni and Šidák
Corrections for Multiple Comparisons. In: Salkind NJ (ed),
Encyclopedia of Measurement and Statistics. Thousand Oaks (CA):
Sage, pp103-107, 2007.
|
|
25
|
Kelly-Hayes M, Wolf AP, Kase SC, Gresham
GE, Kannel WB and D'Agostino RB: Time course of functional recovery
after stroke: The Framingham study. Neurorehabil Neural Repair.
3:65–70. 1989.
|
|
26
|
Meyer S, Verheyden G, Brinkmann N,
Dejaeger E, De Weerdt W, Feys H, Gantenbein AR, Jenni W, Laenen A,
Lincoln N, et al: Functional and motor outcome 5 years after stroke
is equivalent to outcome at 2 months: Follow-up of the
collaborative evaluation of rehabilitation in stroke across Europe.
Stroke. 46:1613–1619. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dhamoon MS, Moon YP, Paik MC, Sacco RL and
Elkind MS: Trajectory of functional decline before and after
ischemic stroke: The Northern Manhattan study. Stroke.
43:2180–2184. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Johansson S, Rosengren A, Young K and
Jennings E: Mortality and morbidity trends after the first year in
survivors of acute myocardial infarction: A systematic review. BMC
Cardiovasc Disord. 17(53)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wolf SL, Butler AJ, Alberts JL and Kim MW:
Contemporary linkages between EMG, kinetics and stroke
rehabilitation. J Electromyogr Kinesiol. 15:229–239.
2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wolf SL, Edwards DI and Shutter LA:
Concurrent assessment of muscle activity (CAMA). A procedural
approach to assess treatment goals. Phys Ther. 66:218–224.
1986.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wolf SL, LeCraw DE and Barton LA:
Comparison of motor copy and targeted biofeedback training
techniques for restitution of upper extremity function among
patients with neurologic disorders. Phys Ther. 69:719–735.
1989.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Torres Cruz A, de Oliveira Januário P,
Rodrigues de Paula Júnior A, Pupio F, Lima S, Oliveira Lima M, et
al: Effects of cryotherapy associated with kinesiotherapy and
electrical stimulation on spastic hemiparetic patients. Fisioter
Pesqui. 26:208–212. 2019.
|
|
33
|
Clarke DJ and Foster A: Improving
post-stroke recovery: The role of the multidisciplinary health care
team. J Multidiscip Healthc. 8:433–442. 2015.PubMed/NCBI View Article : Google Scholar
|