Introduction
Bacterial ventriculitis or meningitis (BVM) is a
comparatively unusual, yet life-threatening complication that often
follows the treatment of acute hydrocephalus with external
ventricular drainage (EVD) (1).
The prevalence of BVM ranges between 1 and 18% (2-5),
and the diagnosis is frequently difficult to establish due to its
often insidious onset and uncommon symptoms (1).
BVM may be caused by Gram-negative (GRn) or
Gram-positive (GRp) bacteria. Still, there is a predominance in
more recent years of GRn infections (6,7), and
considering Gram-negative multi-drug resistant (GRn-MDR) bacteria,
BVM is associated with severe underlying disease and a worse
prognosis (8).
The mortality rate among patients with GRn bacteria
ventriculitis or meningitis (GRn-BVM) has been 8-70% (1-8).
Furthermore, the most commonly identified microbes (98%) of GRn are
Pseudomonas aeruginosa and Acinetobacter baumannii
MDR, which demonstrate resistance in antibiotic classes and are
considered to be selective mediators for central nervous system
(CNS) infections, such as carbapenems. On this basis, older
therapeutic agents, such as colistin are being researched again as
a potential treatment (9).
Due to the poor infiltration of colistin in the CNS,
with ~10% of its concentration in serum even when meninges are
contaminated, the intrathecal (ITH) use of colistin has been
proposed as an alternative therapy to the intravenous (IV)
administration for disease management (10,11).
In the present meta-analysis, the authors aimed to
provide further evidence on the management of BVM compared to the
efficacy of IV or IV plus IT (IV/ITH) treatment with colistin.
Data and methods
Literature search strategy
The study searched the comparative articles
involving IV and IV/ITH colistin treatment in patients with
meningitis or ventriculitis through electronic databases, including
the Cochrane Library, Medline (1983-August, 2020), PubMed
(1983-August, 2020), and EMBASE (1983-August, 2020). Preferred
reporting items for systematic reviews and meta-analyses (PRISMA)
were applied for establishing protocol and manuscript design
(12). The key words ‘meningitis’,
‘intravenous intraventricular antibiotics’ and ‘intrathecal
colistin’ were used in the MeSH list.
Procedures
The intrathecal infusion of colistimethate sodium
was administered in a standard manner through an EVD system at a
mean dose of 170,000±400 IU (range, 50,000-250,000 IU) or
(13.6±0.03 mg), which was then closed for 1 h.
Inclusion and exclusion criteria
The literature was included in the present
meta-analysis if the article met the following criteria, as
determined by PICOS: i) Population: Limited to patients with
meningitis or ventriculitis; ii) intervention: For meningitis and
ventriculitis, the IV-strict and IV/ITH-colistin treatments were
used; iii) comparison: The outcomes were compared; iv) outcome
measures: One of the primary outcomes, such as the Glasgow Coma
Scale (GCS) score upon admission, treatment duration, Acute
Physiological and Chronic Health Evaluation II (APACHE II), the
length of intensive unit (ICU) stay, treatment efficacy and
mortality, were all included. To avoid publication bias, the final
aim was to collect a homogenous pool of manuscripts, including only
articles that compare only two modalities. The articles that were
excluded were editorials, reviews, case reports, articles focusing
on the pediatric population, comorbidities, unrelated outcomes,
experimental techniques, or one of the two treatment modalities,
and all those that demonstrated mixed or unclear results, being
separated between IV and IV/ITH treatment.
Data extraction and outcome
definition
Two of the authors (GF and VEG) independently
extracted data from the included articles, following the guidelines
of the epidemiology of meta-analysis. The following essential
information was captured: The main authors, year of publication,
total case number in the IV and IV/ITH groups, study type, outcome
indicator, etc. The extracted data were input into a designed,
standardized table according to the Cochrane Handbook (https://training.cochrane.org/handbook).
When there was disagreement, another author with authority made the
final decision.
Data regarding one of the primary outcomes,
including treatment efficacy [patients discharged from the ICU who
had three negative cerebrospinal fluid (CSF) CSF or blood
cultures]; length of ICU stay, mortality, treatment duration, the
GCS score upon admission and the severity of the clinical condition
according to the APACHE II scoring system were retrieved from
patients' records and documented. The APACHE II score is a
severity-of-disease classification system that is applied within 24
h of a patient's admission: An integer score from 0 to 71 is
calculated based on numerous measurements; higher scores correspond
to more severe disease and a higher risk of mortality (6). Post-operative outcomes mentioned in
the included articles were evaluated at least 6 months following
treatment (IV or IV/ITH). Additionally, in order to decrease the
risk of bias in the articles, a quality assessment tool (the
Newcastle-Ottawa Scale) was utilized Table I (13). Additionally, the patients were
divided into two groups as follows: Those receiving therapy with IV
colistin (IV group) and those receiving therapy with IV/ITH
colistin (IV/ITH group).
 | Table INewcastle-Ottawa scale quality
assessment of the final article pool. |
Table I
Newcastle-Ottawa scale quality
assessment of the final article pool.
| | Newcastle-Ottawa
scale |
|---|
| Trial, year | Study design | Selection | Comparability | Exposure | Total scores | (Refs.) |
|---|
| Chusri et
al, 2018 | Retrospective | 3 | 3 | 3 | 9 | (14) |
| Fotakopoulos et
al, 2016 | Retrospective | 3 | 3 | 3 | 6 | (11) |
| Shoftly et
al, 2015 | Retrospective | 3 | 3 | 3 | 9 | (15) |
| De Bonis et
al, 2015 | Retrospective | 3 | 2 | 2 | 7 | (16) |
| Moon et al,
2013 | Retrospective | 3 | 2 | 2 | 7 | (17) |
| Wang et al,
2012 | Retrospective | 3 | 3 | 3 | 9 | (18) |
| Tangden et
al, 2011 | Retrospective | 3 | 3 | 3 | 9 | (19) |
Statistical analysis
All analyses were carried out using STATA, version
16 (Stata Corporation, College Station, TX, USA). Heterogeneity
across trials was assessed using I2 statistics;
considering I2 >50% as high heterogeneity, a
meta-analysis was performed using a random-effect model according
to the Cochrane Handbook for Systematic Reviews of Interventions
(version 5.1.0). Otherwise, the fixed-effect model was performed.
The continuous outcomes (GCS of admission, APACHE II) were
expressed as a weighted mean difference with 95% confidence
intervals (CIs). For discontinuous variables (treatment duration,
ICU stay, cure rate and mortality), odds ratios (ORs) with 95% CIs
were applied for the assessment. A P-value <0.05 was considered
to indicate a statistically significant difference.
Results
Studies in the final pool
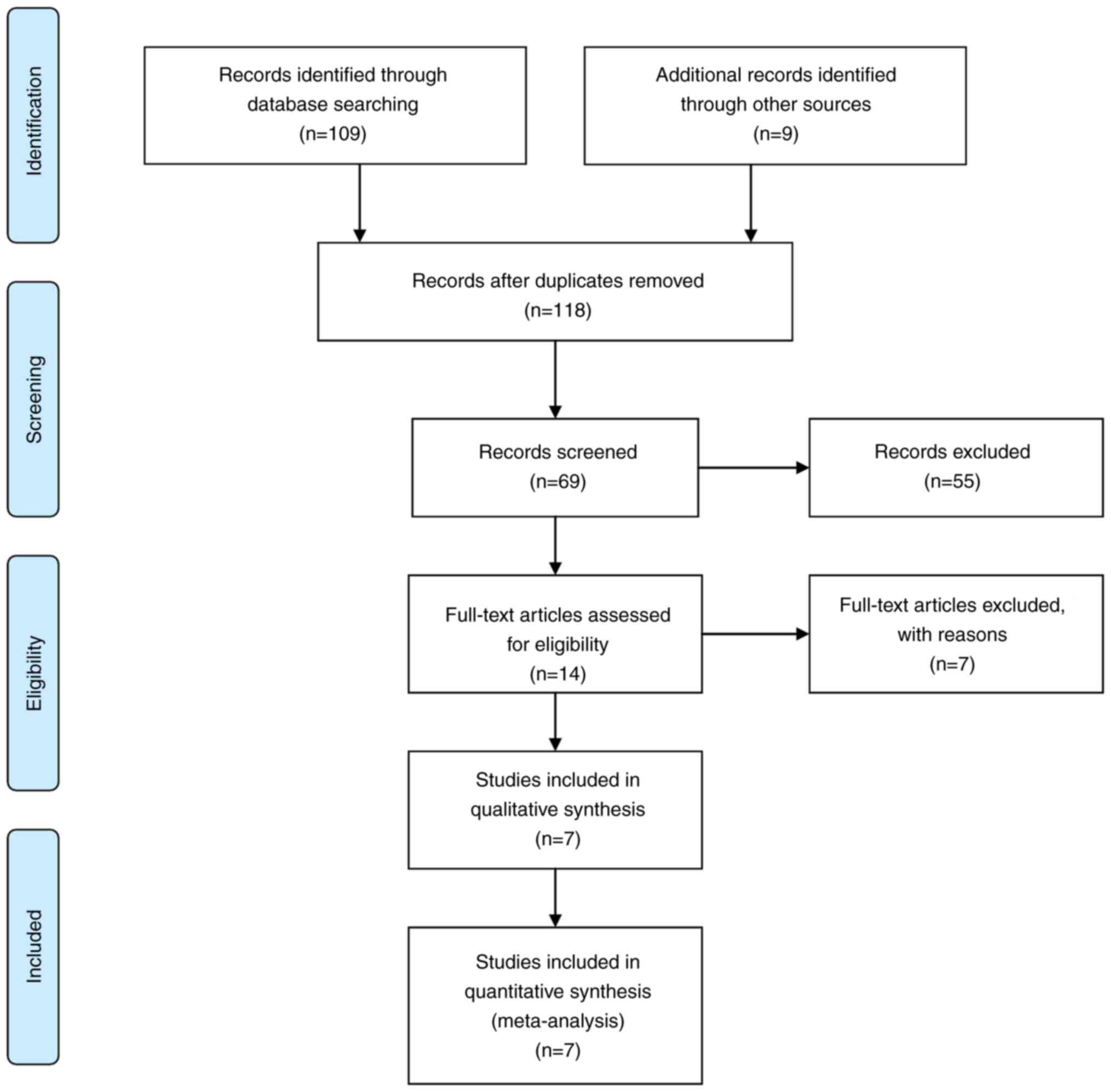
Following the initial search, 14 articles were
eligible for further analysis. Applying all exclusion and inclusion
criteria, seven articles remained in the final article pool
(Fig. 1) (11,14-19).
The detailed data on these articles are presented in Table IΙ. The total number of patients
included in these seven articles was 293 (186 in the IV group and
107 in the IV/ITH group).
GCS score at admission
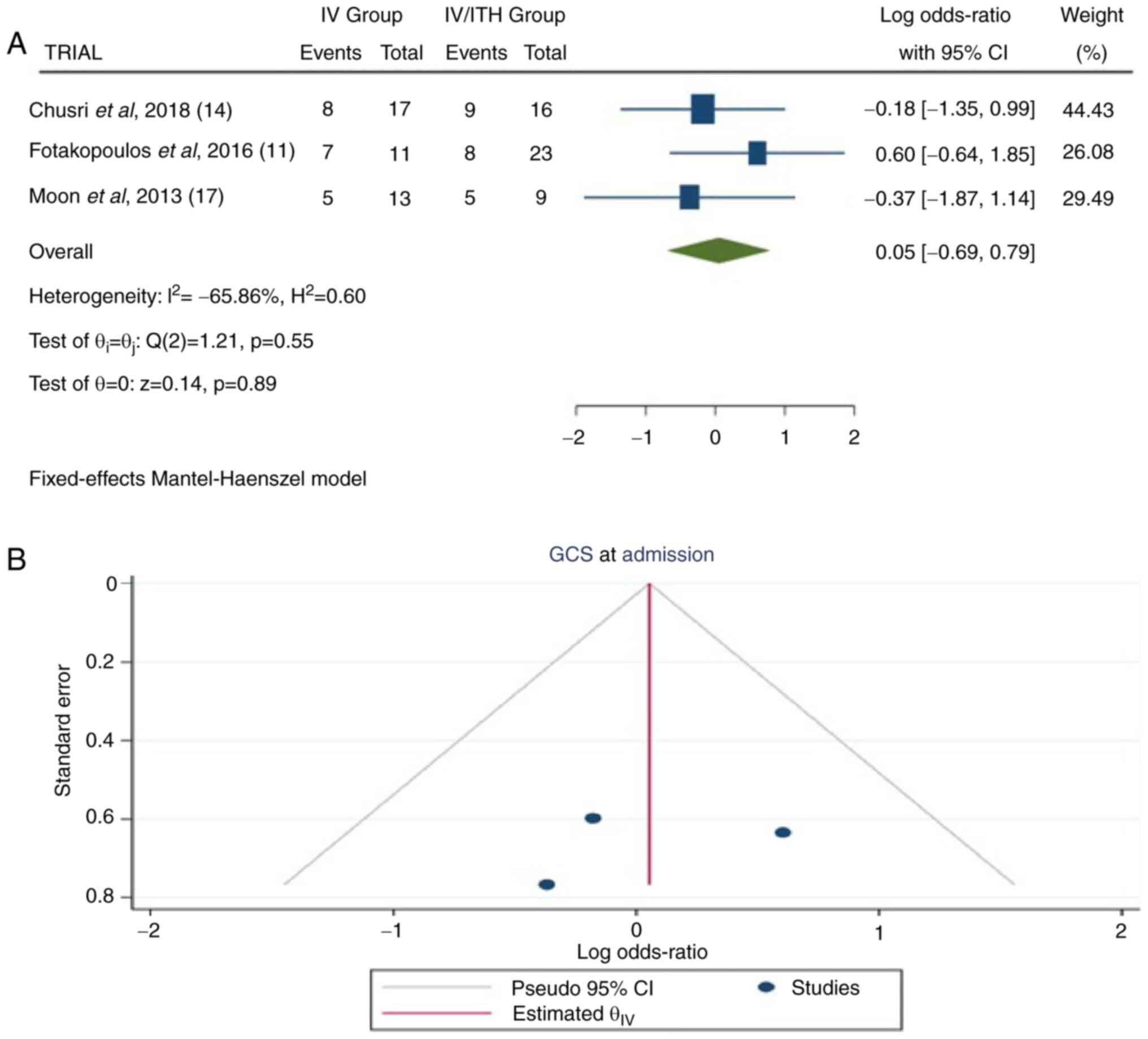
In total, three articles (11,14,17)
provided information on GCS at the time of admission. In the total
group of patients, there were 89 patients: 41 in the IV group and
48 in the IV/ITH group. The pooled results demonstrated no
statistically significant difference between the IV and IV/ITH
groups [OR, 0.05; 95% CI, -0.69 to 0.79; P=0.89] with a low
heterogeneity (P=0.55 and I2=-65.86%) (Fig. 2).
Treatment duration
Information regarding treatment duration was
available in five articles (11,15,16,18,19).
There were 238 patients (156 in the IV group and 82 in the IV/ITH
group), and there was no statistically significant difference
between treatments (OR, -0.30; 95% CI, -0.76 to 0.15; P=0.19);
however, there was heterogeneity (P=0.05 and I2=74.51%)
(Fig. 3A). While evaluating the
sensitivity, one study was removed at a time. After removing the
article by Wang et al (2012) (18), there was additionally no
statistically significant superiority over the groups (OR, 0.17;
95% CI, -0.37 to 0.70; P=0.55), with no heterogeneity (P=0.60 and
I2=-62.32%) (Fig. 3B).
When examining the funnel plot of the same parameter, it was
observed that the study results without the study by Wang et
al (2012) (18) exhibited
better dispersion, with no publication bias, in contrast to the
same analysis including this article (Fig. 3C and D). This was expected as the patients in
the study by Wang et al (18) represented 45.7% (109/238) of the
patients in the included articles.
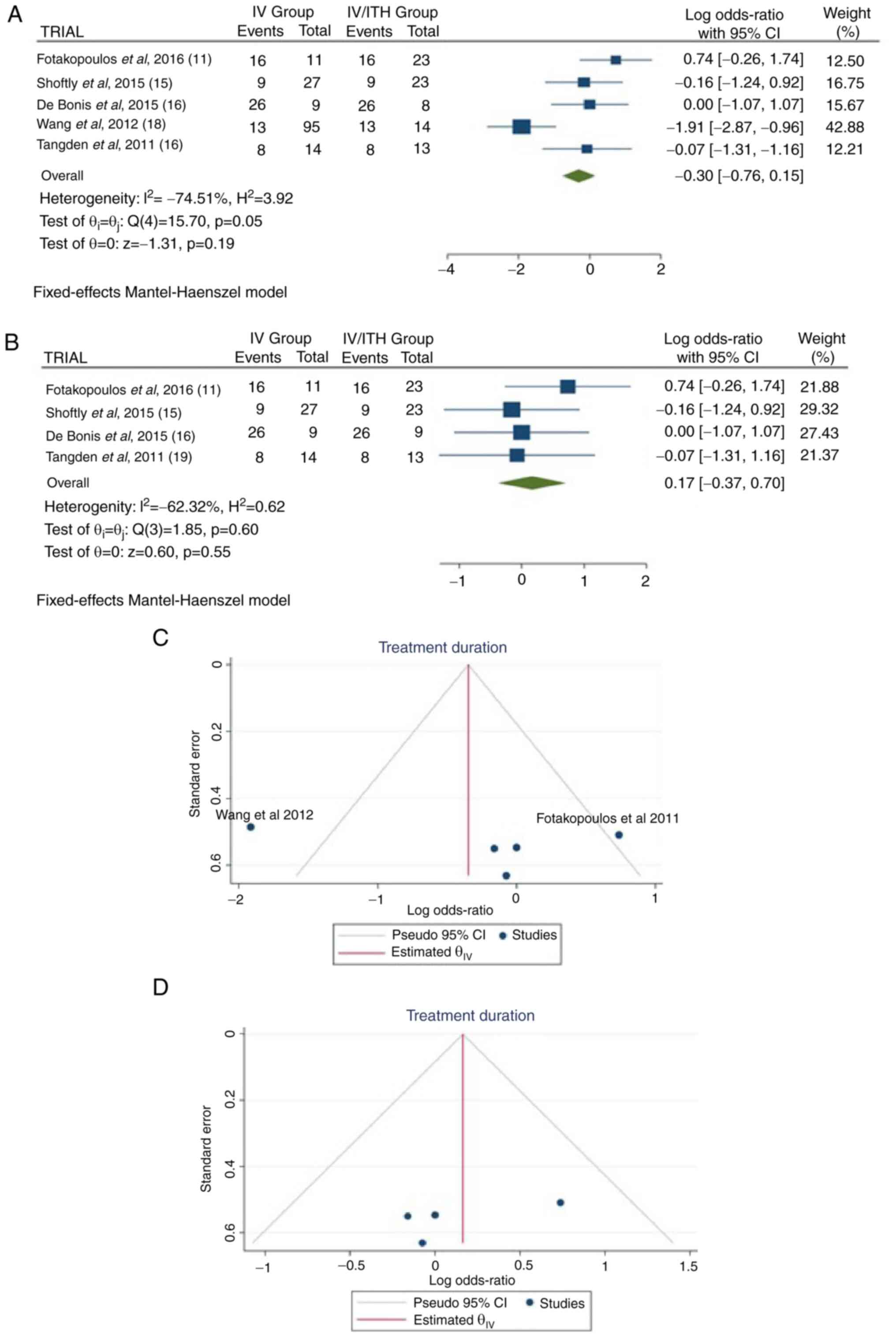 | Figure 3(A) Forest plot for treatment
duration. The results demonstrated no statistically significant
difference between the two groups (OR, -0.30; 95% CI, -0.76 to
0.15; P=0.19), but with heterogeneity (P=0.05 and
I2=74.51%). (B) Forest plot for treatment duration
without the study by Wang et al (2012) (18). The results demonstrated an
additionally no statistically significant difference between the
two groups (OR, 0.17; 95% CI, -0.37 to 0.70; P=0.55). (C and D)
Funnel plots for treatment duration in the groups, with (left) or
without (right) the study by Wang et al (2012) (18), and with a high (left) heterogeneity
(P=0.01 and I2=74.51%) or with no (right) heterogeneity
(P=0.60 and I2=-62.32%). IV, intravenous treatment
group; IV/ITH, intravenous combined with intrathecal treatment
group; I2, percentage of total variation across studies
that is due to heterogeneity rather than chance; CI, confidence
interval. |
APACHE II score
Information regarding APACHE II scores was available
in three articles (11,14,18).
There were 176 patients (123 in the IV group and 53 in the IV/ITH
group), with no statistically significant difference between
treatments (OR, -0.42; 95% CI, -0.96 to 0.12; P=0.13); however,
there was heterogeneity (P=0.05 and I2=86.23%) (Fig. 4A). While evaluating the
sensitivity, one study was removed at a time. After removing the
study by Wang et al (2012) (18), there was no statistically
significant superiority over the groups (OR, 0.30; 95% CI, -0.40 to
1.00; P=0.40), with no heterogeneity (P=0.31 and
I2=3.73%) (Fig. 4B).
When examining the funnel plot of the same parameter, it was
observed that the results without the study by Wang et al
(2012) (18) exhibited better
dispersion with no publication bias, in contrast with the same
analysis including this article (Fig.
4C and D). This was expected,
given that in the study by Wang et al (18), the patients accounted for 61.9%
(109/176) of all patients.
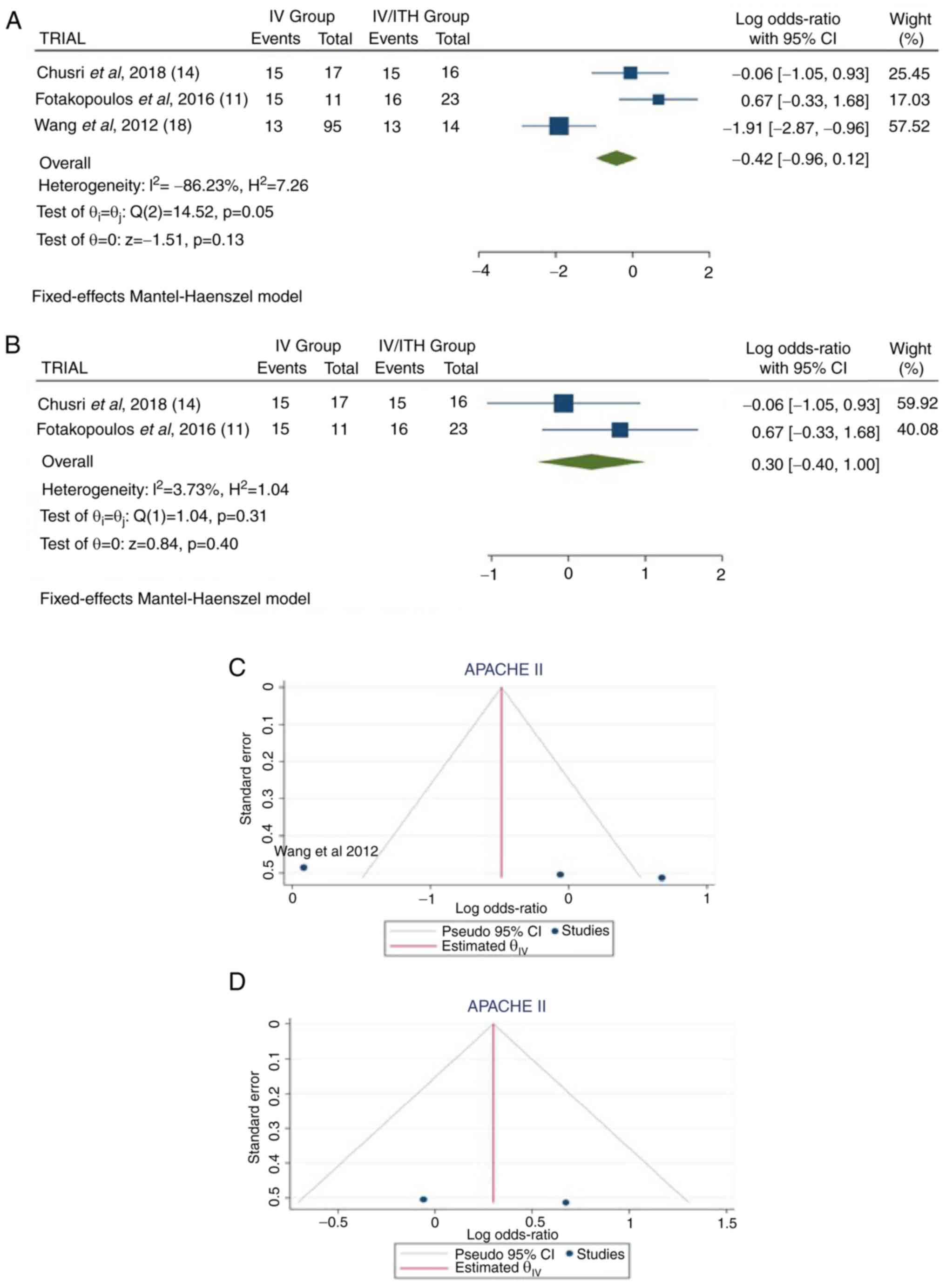 | Figure 4(A) Forest plot for APACHE II. The
results demonstrated no statistically significant difference
between the two groups (OR, -0.42; 95% CI, -0.96 to 0.12; P=0.13),
but with heterogeneity (P=0.05 and I2=86.23%). (B)
Forest plot for APACHE II without the study by Wang et al
(2012) (18). The results
demonstrated an additionally no statistically significant
difference between the two groups (OR, 0.30; 95% CI, -0.40 to 1.00;
P=0.40); (C and D) Funnel plots for APACHE II in the groups, with
(left) or without (right) the study by Wang et al (2012)
(18), and with high (left)
heterogeneity (P=0.05 and I2=86.23%) or with no (right)
heterogeneity (P=0.31 and I2=3.73%). IV, intravenous
treatment group; IV/ITH, intravenous combined with intrathecal
treatment group; I2, the percentage of total variation
across studies that is due to heterogeneity rather than chance; CI,
confidence interval; APACHE II, Acute Physiological and Chronic
Health Evaluation II. |
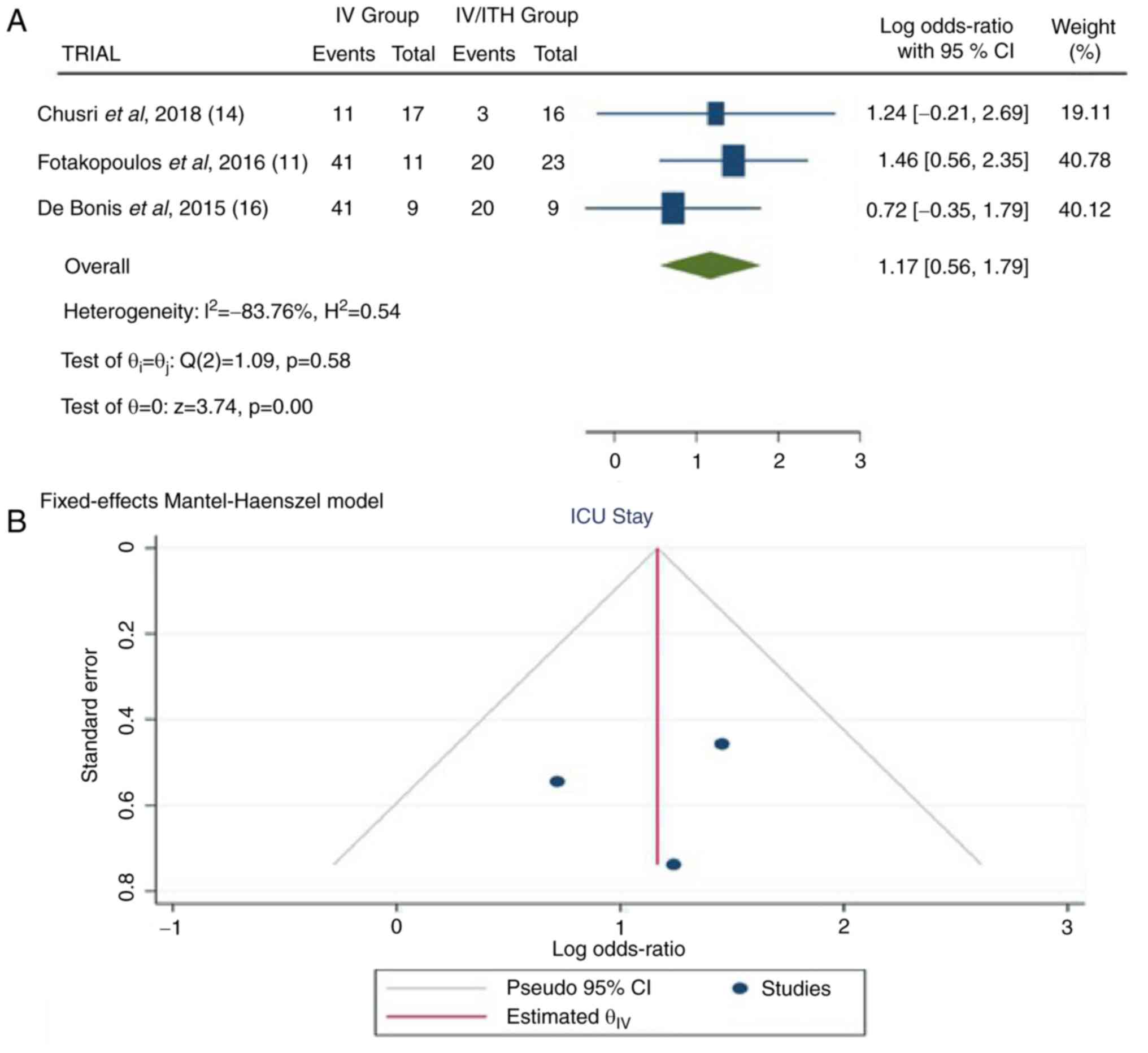
Length/duration of stay in ICU
Information regarding the duration of ICU stay was
available in three articles (11,14,16).
In the total group of patients, there were 95 patients, 37 in the
IV group and 48 in the IV/ITH group. The pooled results
demonstrated a statistically significant difference between the IV
and IV/ITH groups (OR, 1.17; 95% CI, 0.56 to 1.79; P<0.05] with
no heterogeneity (P=0.58 and I2=-83.76%) (Fig. 5).
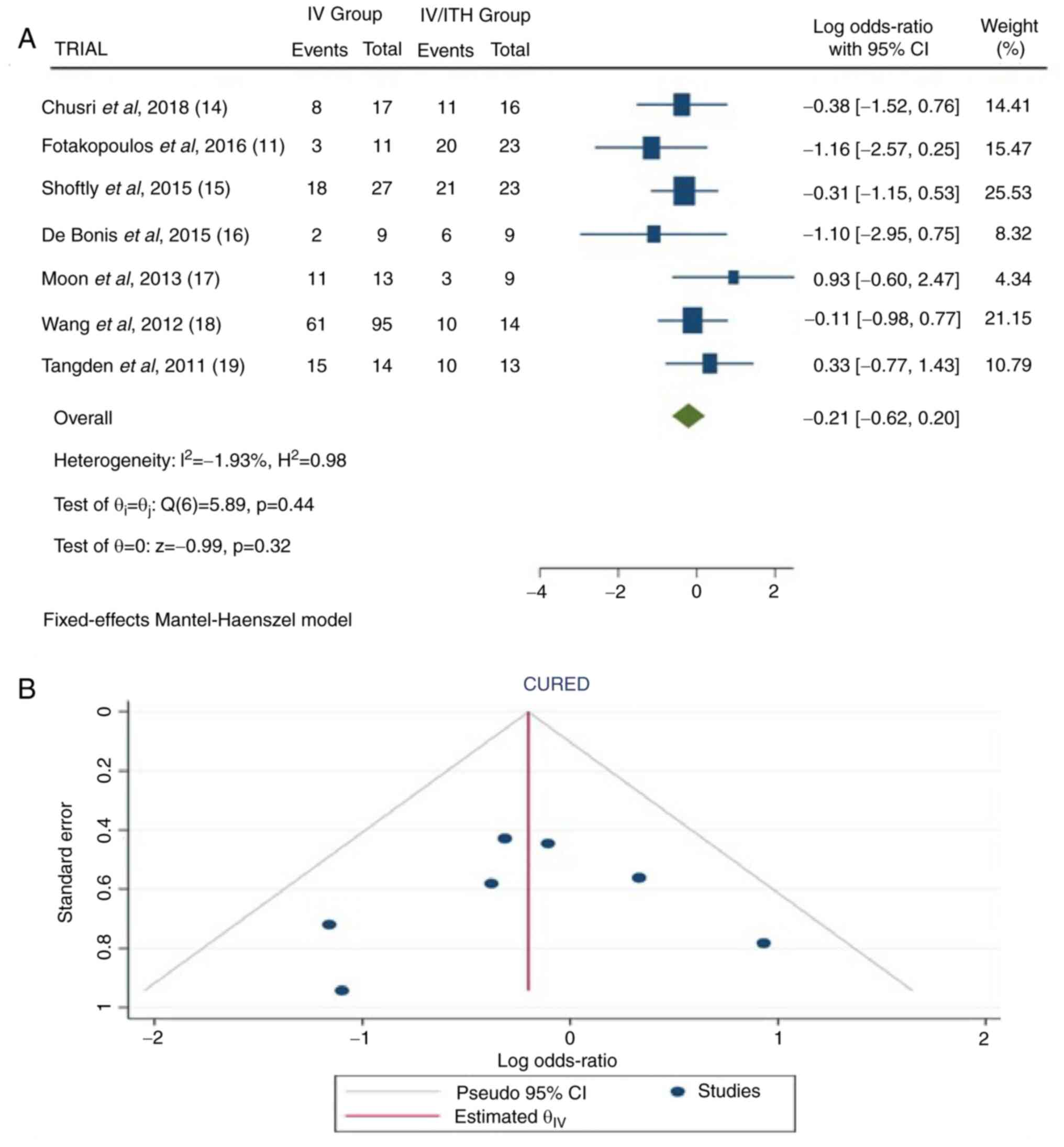
Cure rate
Information on cure rate was available in seven
articles (11,14-19).
In the total group of patients, there were 293 patients: 186 in the
IV group and 107 in the IV/ITH group. The combined results revealed
no statistically significant difference between the IV and IV/ITH
groups (OR, -0.21; 95% CI, -0.62 to 0.20; and P=0.32), as well as
no heterogeneity (P=0.44 and I2=-1.93%) (Fig. 6).
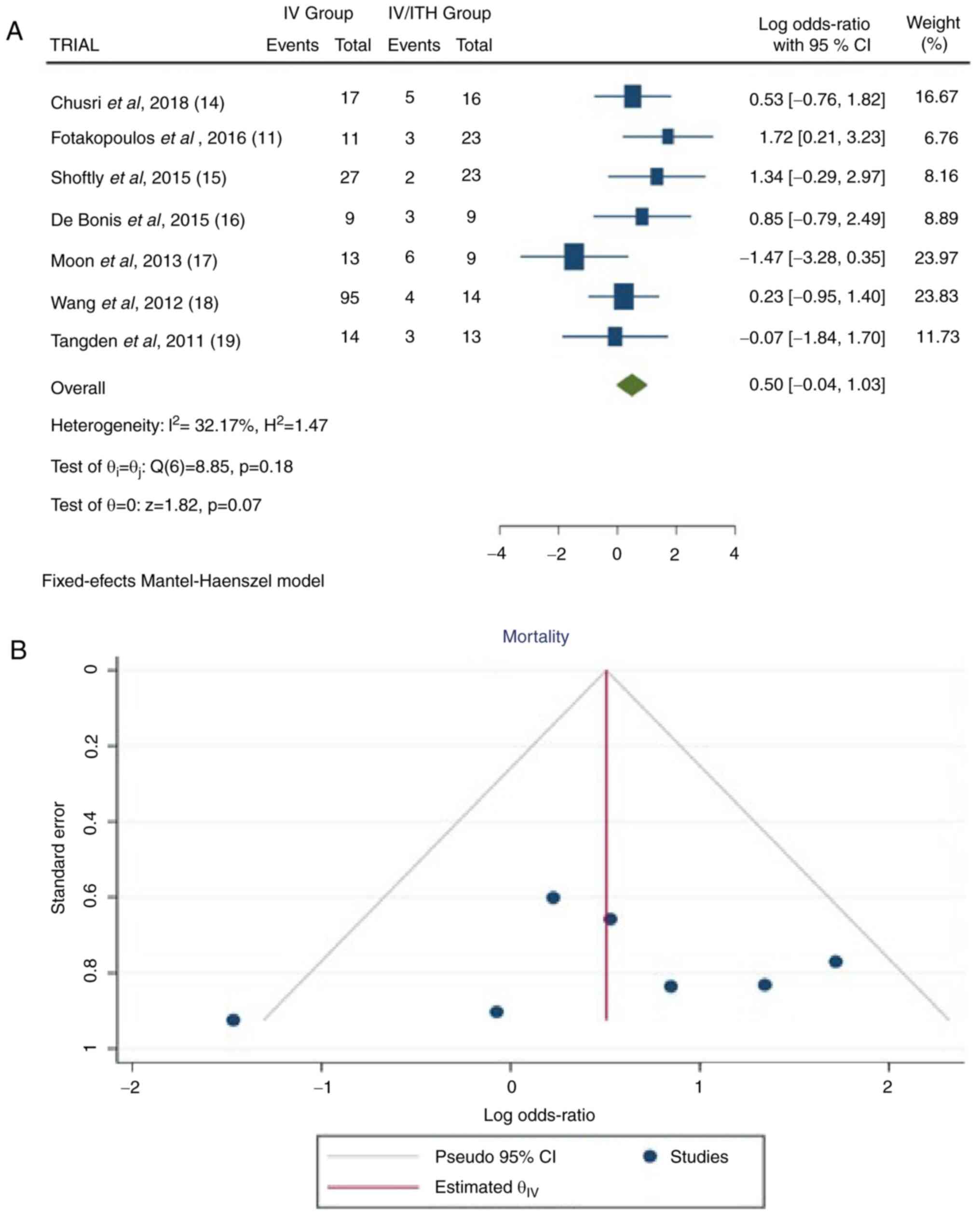
Mortality rate
Information was available in seven articles
(11,14-19).
In the total group of patients, there were 293 patients, 186 from
the IV group and 107 from the IV/ITH group, showing a statistically
significant difference between the IV and IV/ITH groups (OR, 0.50;
95% CI, -1.04 to 1.03; P=0.05) with very low heterogeneity (P=0.18
and I2=32.17% (Fig.
7).
A summary of the results of the present
meta-analysis is presented in Table
III.
 | Table IIIA summary of the results of the
present meta-analysis. |
Table III
A summary of the results of the
present meta-analysis.
| | Groups | Overall effect | Heterogeneity |
|---|
| Outcomes | Trial, n=7 | IV | IV/ITH | Effect
estimate | 95% CI | P-value | I2
(%) | P-value |
|---|
| GCS at
admission | 3 | 41 | 48 | 0.50 | -0.69 to 0.79 | 0.89 | -65.86 | 0.55 |
| Treatment duration
(days) | 5 | 203 | 82 | 0.17 | -0.37 to 0.70 | 0.55 | -62.32 | 0.60 |
| APACHE II | 3 | 123 | 53 | 0.30 | -0.40 to 1.00 | 0.40 | 3.73 | 0.31 |
| Duration of ICU
stay (days) | 3 | 37 | 48 | 1.17 | 0.56 to 1.79 | 0.01 | -83.76 | 0.58 |
| Cured | 7 | 186 | 107 | -0.21 | -0.62 to 0.20 | 0.32 | -1.93 | 0.44 |
| Mortality | 7 | 186 | 107 | 0.50 | -0.04 to 1.03 | 0.05 | 32.17 | 0.18 |
Discussion
The present meta-analysis suggests that IV/ITH
colistin administration is associated with the increased survival
of patients with BVM caused by GRn-MDR bacteria compared to those
treated with IV colistin alone. More precisely, the length of stay
in the ICU was a statistically significant parameter in patients
with BVM treated with colistin, exhibiting the superiority of
IV/ITH over IV colistin administration. In addition, mortality was
also another statistically significant factor, demonstrating the
advantage of IV/ITH colistin management more than the IV treatment
alone. The findings of the present meta-analysis indicate that this
treatment may benefit the management of GRn-MDR CNS infections.
BVM is one of the most severe complications in
patients with EVD, accounting for 8% of cases, with MDR bacteria,
such as Acinetobacter baumannii in the majority (11). ITH treatment with colistin is used
as the band of activity of colistin consists of GRn bacteria, and
particularly as it is effective against Acinetobacter
baumannii, which has appeared over the past few years as an
endo-nosocomial infection agent. However, colistin has a reduced
infiltration through the blood-brain barrier, and of note, with
intravenous monotherapy, it can reach the CSF at only 5-10% of the
levels in the blood (20). The
present meta-analysis revealed an improved clinical outcome in
cases with BVM caused by GRn-MDR bacteria using combined IV/ITH
colistin therapy.
ITH management with colistin is a well-established
treatment modality; however, the treatment conditions for this are
not yet concrete due to some aversion to its use (11). In addition, there have been
mentions of fears concerning the efficiency of ITH colistin, the
threat of secondary infections or chemical meningitis/ventriculitis
due to the manipulations during the colistin placement via the EVD,
as well as the length of its treatment (11). In the present meta-analysis, the
length of ICU stay and mortality rates yielded statistically
significant results, exhibiting the privilege of IV/ITH use over IV
colistin administration. However, there were no data regarding
brain magnetic resonance imaging that could provide an indication
of a possible unfavorable outcome of ITH treatment in the
epithelium of the brain ventricle, as well as the risk of chemical
meningitis.
Clinical studies have mentioned an increased risk of
nephrotoxicity associated with colistin (21,22),
mostly by its IV management (23).
However, no severe nephrotoxic effect was mentioned in the eligible
articles of the present meta-analysis by the ITH colistin
placement, most likely due to an adequately managed
fluid-electrolyte balance and other potential risks for renal
damage during treatment (11).
Although colistin has been proposed as an alternative for treating
neurosurgical meningitis, further data are required in order to be
able to draw firm conclusions about the role of intrathecal
antibiotic therapy, apart from pharmacokinetic data demonstrating
that colistin reaches bactericidal concentrations in CSF (20).
In conclusion, considering the low number of
studies, the results of the present meta-analysis support the
addition of ITH colistin to its IV administration, which is an
effective treatment for BVM caused by GRn-MDR bacteria.
Furthermore, colistin is a viable and safe option for the systemic
antimicrobial treatment of these severe neurointensive care
infections.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GF and VEG conceptualized the study. VEG, DAS, IT,
PP, AG, EA, AAF, GF and NT analyzed the data, and wrote and
prepared the draft of the manuscript. DAS and GF provided critical
revisions. All authors contributed to manuscript revision and have
read and approved the final version of the manuscript. GF and VEG
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Berk SL and McCabe WR: Meningitis caused
by gram-negative bacilli. Ann Intern Med. 93:253–260.
1980.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Camacho EF, Boszczowski I, Basso M, Jeng
BC, Freire MP, Guimarães T, Teixeira MJ and Costa SF: Infection
rate and risk factors associated with infections related to
external ventricular drain. Infection. 39:47–51. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cascio A, Conti A, Sinardi L, Iaria C,
Angileri FF, Stassi G, David T, Versaci A, Iaria M and David A:
Post-neurosurgical multidrug-resistant Acinetobacter baumannii
meningitis successfully treated with intrathecal colistin. A new
case and a systematic review of the literature. Int J Infect Dis.
14:e572–e579. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Faillace WJ: A no-touch technique protocol
to diminish cerebrospinal fluid shunt infection. Surg Neurol.
43:344–350. 1995.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tsolaki V, Karvouniaris M, Manoulakas E,
Kotlia P, Karadontas V, Fotakopoulos G, Zakynthinos E and Makris D:
Intraventricular CNS treatment with colistin-tigecycline
combination: A case series. J Crit Care. 47:338–341.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Laxmi S and Tunkel AR:
Healthcare-associated bacterial meningitis. Curr Infect Dis Rep.
13:367–373. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Stenehjem E and Armstrong WS: Central
nervous system device infections. Infect Dis Clin North Am.
26:89–110. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Parodi S, Lechner A, Osih R, Vespa P and
Pegues D: Nosocomial enterobacter meningitis: Risk factors,
management, and treatment outcomes. Clin Infect Dis. 37:159–166.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Falagas ME and Kasiakou SK: Toxicity of
polymyxins: A systematic review of the evidence from old and recent
studies. Crit Care. 10(R27)2006.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Baiocchi M, Catena V, Zago S, Badolati L
and Baccarin M: Intrathecal colistin for treatment of multidrug
resistant (MDR) Pseudomonas aeruginosa after neurosurgical
ventriculitis. Infez Med. 18:182–186. 2010.PubMed/NCBI
|
|
11
|
Fotakopoulos G, Makris D, Chatzi M,
Tsimitrea E, Zakynthinos E and Fountas K: Outcomes in
meningitis/ventriculitis treated with intravenous or
intraventricular plus intravenous colistin. Acta Neurochir (Wien).
158:603–610; discussion 610. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Foster RL: Reporting guidelines: CONSORT,
PRISMA, and SQUIRE. J Spec Pediatr Nurs. 17:1–2. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bae JM: A suggestion for quality
assessment in systematic reviews of observational studies in
nutritional epidemiology. Epidemiol Health.
38(e2016014)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chusri S, Sakarunchai I, Kositpantawong N,
Panthuwong S, Santimaleeworagun W, Pattharachayakul S, Singkhamanan
K and Doi Y: Outcomes of adjunctive therapy with intrathecal or
intraventricular administration of colistin for post-neurosurgical
meningitis and ventriculitis due to carbapenem-resistant
acinetobacter baumannii. Int J Antimicrob Agents. 51:646–650.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shofty B, Neuberger A, Naffaa ME, Binawi
T, Babitch T, Rappaport ZH, Zaaroor M, Sviri G and Paul M:
Intrathecal or intraventricular therapy for post-neurosurgical
gram-negative meningitis: Matched cohort study. Clin Microbiol
Infect. 22:66–70. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
De Bonis P, Lofrese G, Scoppettuolo G,
Spanu T, Cultrera R, Labonia M, Cavallo MA, Mangiola A, Anile C and
Pompucci A: Intraventricular versus intravenous colistin for the
treatment of extensively drug resistant acinetobacter baumannii
meningitis. Eur J Neurol. 23:68–75. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Moon C, Kwak YG, Kim BN, Kim ES and Lee
CS: Implications of postneurosurgical meningitis caused by
carbapenem-resistant Acinetobacter baumannii. J Infect Chemother.
19:916–919. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang JH, Lin PC, Chou CH, Ho CM, Lin KH,
Tsai CT, Wang JH, Chi CY and Ho MW: Intraventricular antimicrobial
therapy in postneurosurgical gram-negative bacillary meningitis or
ventriculitis: A hospital-based retrospective study. J Microbiol
Immunol Infect. 47:204–210. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tängdén T, Enblad P, Ullberg M and Sjölin
J: Neurosurgical gram-negative bacillary ventriculitis and
meningitis: A retrospective study evaluating the efficacy of
intraventricular gentamicin therapy in 31 consecutive cases. Clin
Infect Dis. 52:1310–1316. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ziaka M, Markantonis SL, Fousteri M,
Zygoulis P, Panidis D, Karvouniaris M, Makris D and Zakynthinos E:
Combined intravenous and intraventricular administration of
colistin methanesulfonate in critically ill patients with central
nervous system infection. Antimicrob Agents Chemother.
57:1938–1940. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Koch-Weser J, Sidel VW, Federman EB,
Kanarek P, Finer DC and Eaton AE: Adverse effects of sodium
colistimethate. Manifestations and specific reaction rates during
317 courses of therapy. Ann Intern Med. 72:857–868. 1970.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li J, Nation RL, Turnidge JD, Milne RW,
Coulthard K, Rayner CR and Paterson DL: Colistin: The re-emerging
antibiotic for multidrug-resistant gram-negative bacterial
infections. Lancet Infect Dis. 6:589–601. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vicari G, Bauer SR, Neuner EA and Lam SW:
Association between colistin dose and microbiologic outcomes in
patients with multidrug-resistant gram-negative bacteremia. Clin
Infect Dis. 56:398–404. 2013.PubMed/NCBI View Article : Google Scholar
|