Introduction
Bitter melon (Momordica charantia) is a
monoecious plant of the genus Momordica in the
Cucurbitaceae family that is commonly utilized as both a
medicine for lowering blood sugar, treating diabetes and alleviate
the feeling of heat (1). It has
been eaten as fresh fruit for thousands of years, although it can
also be consumed sweetened with sugar. Although Momordica
charantia naturally tastes bitter, it has both a high
nutritional and medicinal value, because it has been documented to
contain proteins, carbonic dioxide hydrate, phenolic acids,
alkaloids, flavonoids, quinine, amino acids, saponin and fatty
acids (1). Momordica
charantia is also used as a herbal medicine in numerous
countries such as Japan, India and countries in the southeast Asia,
where the whole plant has been shown to possess potent
pharmacological properties, especially within its seeds and fruits
(1-3).
In previous years, the functional components and health-associated
benefits of Momordica charantia is gradually becoming
understood, which may have wide-ranging prospects in terms of its
clinical application. The active components of the plant are mainly
comprised of the water extract, alcoholic extract, polysaccharide,
saponin and Momordica charantia juice, protein and
polypeptide (which may or may not be separated (1). In addition to exerting hypoglycaemic
and lipid-lowering effects (2,3),
they have been shown to possess antioxidant, bacteriostatic and
antitumour properties, which may assist in preventing obesity and
improve metabolic syndromes, such as diabetes (4).
As a result of rapid developments in polysaccharide
research, increasing focus is being placed on Momordica
charantia polysaccharide (MCP) (1-4).
Numerous biological functions and activities of MCP (such as
antitumor, antidiabetic and antioxidant activities,
immunomodulation and radioprotection) are beginning to garner the
attention of the scientific community (5,6).
Different components of MCP (1-7)
have been previously extracted, separated and purified to examine
the potential hypoglycaemic and antioxidant activities of MCP
(1-7).
Previous studies have shown that MCP can exert numerous
physiological effects, such as improving immune function (5), antiviral (6) and antioxidant effects (7). In addition, it has been demonstrated
to reduce blood sugar levels (8)
and can act as an antitumor agent, for example in liver cancer, and
overcome resistance mutations. Therefore, furthering the
understanding of the functions and properties of MCP may have
far-reaching implications in terms of its potential use in the
medicinal and health care fields. However, although MCP is a
bioactive substance with development value, several problems
remain, such as discovering the target genes of MCP, unravelling
the regulation of signaling pathways or conducting functional
research in preclinical trials, and form the focus of the present
study. since its underlying mechanism of action remains
unclear.
Therefore, the present study aimed to investigate
the mode of regulation and mechanism of MCP in immunosuppressed
mice, in addition to identifying the potential role of MCP in terms
of its application in health care. It is hoped that the present
results provide valuable points of reference to further the
research on MCP, given its wide applicability in food and
medicine.
Materials and methods
Chemicals and reagents
Purified MCP (purity ≥90%) was obtained from
Chenguang Biotech Group Co., Ltd. Methyl thiazole blue (MTT),
trypan blue, RPMI-1640 medium, DMSO, trimethylol aminomethane, PBS
and chloroform were obtained from Beijing Solarbio Science &
Technology Co., Ltd. PrimerScript™ RT reagent Kit and TB Green™
Premix Ex Taq™ II (Tli RNase H Plus) kits were purchased from
Takara Biotechnology Co., Ltd.
Mice
In total, 60 Specific pathogen-free BALB/c male mice
(weighing 20±2 g; 6-8-week-old) were brought from the Academy of
Life Sciences, Jinzhou Medical University (Jinzhou, China). Housed
at a constant temperature of 25±2˚C and relative humidity of
50-70%, the mice were fed with food and water, and were permitted
to feed themselves in a standard environment with a 12-h light/dark
cycle. They were subjected to adaptive feeding for 1 week. These
experiments were approved by the Ethics Committee of Jinzhou
Medical University [Production License SCXY (Liao) 2019-0003;
experimental animal ethics approval no. 2019014].
Animal experiments
A total of six groups of animals were prepared by
randomly choosing 10 healthy mice for each group, namely the normal
(NG), the model (MG), the positive (PG), the MCP low-dose (MLG),
the MCP medium-dose (MMG) and the MCP high-dose (MHG) groups.
The animal protocols in the present study were
identical to those performed in previous studies (9-19).
Briefly, each group of mice was given an intragastric
administration via gavage once a day for 21 days according to the
following experimental design: From days 1 to 5, the mice all
groups except for NG were intraperitoneally injected with 80 mg/kg
cyclophosphamide (CY; by Jiangsu Hengrui Pharmaceutical Co., Ltd.),
whereas NG and MG mice were given distilled water by
intraperitoneal injection. From day 1, PG mice were given 200 mg/kg
levamisole whereas the MLG, MMG and MHG mice were given MCP at
different doses (100, 200 and 300 mg/kg, respectively; 0.1 ml/10 g
per day). During the feeding process, the activity of the mice was
observed daily. The body weight of the mice was also measured every
day and the doses of drugs administered were adjusted according to
changes in body weight.
Induction of delayed-type
hypersensitivity (DTH) by dinitrofluorobenzene (DNFB)
In the procedure used to determine the DTH, a
sensitizer, such as DNFB, is injected into the abdominal cavity of
mice before the ears of the mice are insulted with DNFB.
Subsequently, the degree of swelling of the ears is measured. A
total of five mice from each of the six different groups were
treated for 7 days as follows: Before administration on the first
day, the abdominal regions of mice in each group, except for those
in the NG group, were depilated and 1% DNFB (Shanghai Jizhi
Biochemical Technology Co., Ltd.)/acetone/olive oil solution was
applied to the depilated region for sensitization. The next day,
intensive application of the same solution was performed again On
day 6 of the sensitization test, 20 µl DNFB/acetone/olive oil
solution was used to sensitize the mice behind and in front of the
right ear. After 24 h, the mice were sacrificed by dislocation of
the cervical vertebrae before two ears of each mouse were removed
using a puncher and weighed. DTH was expressed as the weight
difference between the right and left ears.
Measurement of immune organ
indices
After the mice had been sacrificed by cervical
dislocation, their thymus and spleen were also dissected and rinsed
in 0.9% normal saline. The surfaces of the thymus and spleen were
dried with absorbent paper, after which their weights were measured
using an analytical balance. The thymus index (TI) and spleen index
(SI) were calculated (9) according
to the following equations: TI=mean weight of thymus in the
group/mean body weight in the group x10; SI=mean weight of spleen
in the group/mean body weight in the group x10.
Histomorphological observations of
thymus and spleen
The spleens and thymuses of the mice were dissected,
washed with normal saline, fixed with neutral formalin fixative
before paraffin sections were prepared. Tissues were then
dehydrated, made transparent and wax-soaked. Before sealing, slices
were embedded, made sticky and then baked, dewaxed, immersed in
alcohol, dyed and dehydrated. Pathological sections were prepared
and structural changes in each tissue were observed as previously
reported (10). Specific details
were as follows: Tissues (≤3 mm) were fixed in 10% neutral formalin
for 24 h, embedded in paraffin, and sliced at 3-5 µm. Subsequently,
the sections were dewaxed in xylene, rehydrated using a series of
ethanol (100, 95 and 80% for 10 min each), followed by washing in
tap water and distilled water (for 1 min each). Staining was
performed with hematoxylin for 4 min followed by washing with tap
water for 2 min, differentiation with 1% hydrochloric acid ethanol
for 20 sec, washing with tap water for 2 min, incubation with 6.1%
diluted ammonia water for 30 sec, within with tap water 2 min and
distilled water for 1 min. Eosin staining was subsequently
performed for 90 sec, followed by dehydration with 80, 95 and 100%
ethanol. The sections were washed with xylene and sealed with
neutral gum. After staining, the pathological changes of the tissue
were observed under a light microscope.
Isolation and culture of mice
peritoneal macrophages
After the mice were sacrificed by dislocation of the
cervical vertebrae, they were soaked in 75% ethanol at room
temperature for 3 min for disinfection. A total of 5 ml pre-cooled
PBS was then injected into the abdominal cavities before they were
gently massaged for ~2 min. After allowing the abdomen to rest for
5 min, the peritoneal fluid was extracted, centrifuged at ~670.8 x
g and 4˚C for 5 min before the supernatant was discarded. The
pellet was subsequently diluted in erythrocyte lysate (Red Blood
Cell Lysis Buffer; Beijing Solarbio Science & Technology Co.,
Ltd.), allowed to stand and then centrifuged at ~670.8 x g and 4˚C
for 5 min, before the supernatant was again discarded. The cells
were suspended in RPMI-1640 complete culture medium [with 10% FBS
(Zhejiang Tianhang Biotechnology Co., Ltd.)] for counting before
adjusting to the required concentration of 5x106
cells/ml. Subsequently, the cells were incubated in an incubator at
37˚C with 5% CO2 for 18 h until complete adherence had
occurred. The supernatant was subsequently removed and discarded,
where non-adherent cells were removed. Fresh RPMI-1640 complete
medium was added to obtain the purified peritoneal macrophages
(11,12).
Lymphocyte suspension preparation
After the mice had been sacrificed by cervical
dislocation, the mice were clamped with tweezers and soaked in 75%
alcohol for 1-2 min for disinfection purposes. The spleens were
extracted on a sterile clean bench, placed into a Petri dish with
PBS for 1-2 min at 4˚C, transferred to RPMI-1640 medium and ground
through a 200-mesh screen with the end of a syringe pull rod. The
filtrate was then sucked into a centrifuge tube before being
centrifuged at ~377.32 x g and 4˚C for 5 min, after which the
supernatant was discarded and 3 ml erythrocyte lysis solution (Red
Blood Cell Lysis Buffer) was added. The mixture was allowed to
stand for 5 min at 4˚C, after which it was re-centrifuged at
~377.32 x g at 4˚C for 5 min and the supernatant was discarded
again. This step was repeated until the erythrocytes were
completely lysed. The precipitated cells were resuspended in
RPMI-1640 complete culture medium (with 10% FBS) and filtered using
a 70-µm cell strainer into another centrifuge tube. After trypan
blue staining, cell viability was adjusted to >95% and RPMI-1640
complete culture medium was added until the cell density reached
5x106 cells/ml, thereby forming the lymphocyte
suspension (13). After the
preparation of lymphocyte suspension, the cells were cultured for
48 h and were observed to be in good condition.
Lymphocyte proliferation assay
Lymphocyte suspension (200 µl; ~100-300 cells) was
inoculated into a 96-well cell culture plate, cultured at 37˚C with
5% CO2 for 48 h and subsequently 20 µl MTT (5 mg/ml)
solution was added in each well. After 4 h (37˚C) of continuous
culture, the supernatant was discarded and 150 µl DMSO was added to
each well. Finally, the absorbance at 570 nm (Abs570)
was measured using a microplate reader and the proliferation rate
of the mouse spleen lymphocytes was calculated (14,15)
according to the following equation: Spleen lymphocyte
proliferation rate (%)=(Abs570 experimental
group)/(Abs570 control group) x100.
Determination of cell phagocytosis of
peritoneal macrophages
Mice peritoneal macrophages were collected before
the cell concentration was adjusted to 2x106 cells/ml in
RPMI-1640 complete medium to ensure that the concentration of the
macrophage suspension from each mouse was consistent. The
macrophage suspension was then added into 96-well plates and the
plates were cultured in an incubator at 37˚C with 5% CO2
for 3 h. After observing the adherent cells under a microscope, the
culture medium was removed and the cells were washed twice with PBS
pre-heated to 37˚C to remove the nonadherent cells. Subsequently,
200 µl 0.1% neutral red dye (cat. no. G1310; Beijing Solarbio
Science & Technology Co., Ltd.) was added to each well and the
culture was allowed to continue for 3 h (13-15).
After washing the cells three times with preheated PBS to remove
excess neutral red, 100 µl cell lysis solution [comprising absolute
ethanol/glacial acetic acid, 1:1 (v/v)] was added to each well
followed by mixing. The plates were incubated overnight at 4˚C
before absorbance at 540 nm was read using a microplate reader.
Cytokine assay
The lymphocyte suspension was inoculated into
24-well plates and 2 ml lymphocyte suspension (~1x104
cells) was used for each well. After 48 h of culture at 37˚C with
5% CO2, the cells were centrifuged at ~377.32 x g and
4˚C for 10 min and the supernatant was collected. The
concentrations of IFN-γ (cat. no. ml002277), IL-6 (cat. no.
ml063159) and IL-12 (cat. no. ml037868;) secreted by the
lymphocytes in the supernatant were measured using ELISA. These
procedures were performed following the protocols of the mouse
cytokine kit (all from Shanghai Enzyme-linked Biotechnology Co.
Ltd.). The absorbance values were measured at a wavelength of 450
nm using a microplate reader and a standard calibration curve was
generated to calculate the concentrations of the secreted compounds
in pg/ml (16).
Reverse transcription-quantitative PCR
(RT-qPCR)
The lymphocyte suspension was inoculated on a
24-well cell culture plate and cultured at 37˚C with 5%
CO2 for 48 h. Subsequently, the cells were collected,
centrifuged at 377.32 x g at 4˚C for 5 min and the supernatant was
discarded, before washing the cells twice with PBS and
TRIzol® (Thermo Fisher Scientific, Inc.) was added to
completely lyse the cells. Total RNA was extracted according to the
protocols of the TRIzol kit and the ratio between the optical
density measured at 260 and 280 nm was used to assess the RNA
purity (17).
Following precisely the protocols of the
PrimerScript™ RT reagent Kit with gDNA Eraser (cat. no. RR047A;
Takara Bio, Inc.), RNA was reverse-transcribed into cDNA using the
temperature protocols of 37˚C for 15 min and 85˚C for 5 sec. qPCR
was subsequently performed using the TB Green™ Premix Ex Taq™ II
kit, using the following thermocycling conditions: Initial
denaturation at 95˚C for 30 sec, followed by 40 cycles of 95˚C for
5 sec and 60˚C for 30 sec. The primer pairs used for qPCR (Table I) were designed the mRNA levels
were quantified using the 2-ΔΔCq method and normalized
to the internal reference gene β-actin (18).
 | Table IForward and reverse primers used for
reverse transcription-quantitative PCR. |
Table I
Forward and reverse primers used for
reverse transcription-quantitative PCR.
| Primer | Sequence | Accession no. |
|---|
| IFN-γF |
5'-CGGCACAGTCATTGAAAGCCTA-3' | K00083 |
| IFN-γR |
5'GTTGCTGATGGCCTGATTGTC-3' | |
| IL-6F |
5'-CCACTTCACAAGTCGGAGGCTTA-3' | X54542 |
| IL-6R |
5'-CCAGTTTGGTAGCATCCATCATTTC-3' | |
| IL-12F |
5'-TTCATAAGAGTCAGGTGGTCTTGG-3' | NM_008351 |
| IL-12R |
5'-CCTTTGGGGAGATGAGATGTG-3' | |
| β-actin F |
5'-CATCCGTAAAGACCTCTATGCCAAC-3' | NM_007393 |
| β-actin R |
5'-ATGGAGCCACCGATCCACA-3' | |
Reverse transcription-PCR
Using a 24-well cell culture plate, 1 ml lymphocyte
suspension (~2x104 cells) was added into each well
incubated at 37˚C for 48 h. Total RNA of lymphocytes was extracted
using the TRIzol method according to the manufacturer's protocol. A
protein/nucleic acid analyzer (NanoDrop One; Thermo Fisher
Scientific, Inc.) was used to detect the RNA concentration and
purity, before the total RNA concentration was adjusted to 400-600
ng/µl range. Reverse transcription was performed according to the
instructions of PrimeScript™ RT Reagent Kit with gDNA Eraser (cat.
no. RR047A; Takara Bio, Inc.), which was 37˚C for 15 min followed
by 85˚C 5 sec. The products of qPCR (described in the previous
paragraph) were used for 2% agarose gel electrophoresis. The
following reagents were used: DL 1000DNA Marker Takara Japan (cat.
no. 3591Q; Takara Bio, Inc.); gel stain 10,000X (Beijing TransGen
Biotech Co., Ltd.).
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software (IBM Corp.) and the experimental data are shown as the
mean ± standard deviation. Statistical comparisons were performed
using one-way ANOVA followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect of MCP on organ indices in
mice
Compared with those in the NG mice, the thymus and
spleen indices of mice in the MG group were significantly decreased
(P<0.05; Table II), suggesting
that the immune function of the mice was significantly inhibited.
Compared with those MG mice, the TI was significantly increased in
the PG, MHG, MMG and MLG groups (P<0.05; Table II), whereas the SI was increased
significantly in the PG, MMG and MHG groups (P<0.05; Table II). The SI of mice in MLG was also
significantly increased compared with that in the MG group
(P<0.05). These data suggest that the immune organ indices of
the immunosuppressed mice increased in response to MCP in a
dose-dependent manner, although it remained lower compared with
that of the NG mice.
 | Table IIEffects of MCP on immune organ index
of immunosuppressed mice. |
Table II
Effects of MCP on immune organ index
of immunosuppressed mice.
| Group | Thymus index,
mg/g | Spleen index,
mg/g |
|---|
| Normal saline | 1.58±0.01 | 5.70±0.27 |
| Model |
0.67±0.14a |
0.85±0.01a |
| Positive |
1.49±0.11a |
3.90±0.34a |
| MCP low-dose |
0.90±0.14b | 1.39±0.34 |
| MCP
medium-dose |
1.14±0.20c |
2.35±0.78b |
| MCP high-dose |
1.31±0.14d,e |
4.73±0.30b |
Histomorphological observation of the
thymus and spleen
The spleen tissue of MG mice was found to be
markedly smaller compared with that of NG mice, with lower density,
uneven distribution of lymphocytes and unclear boundaries between
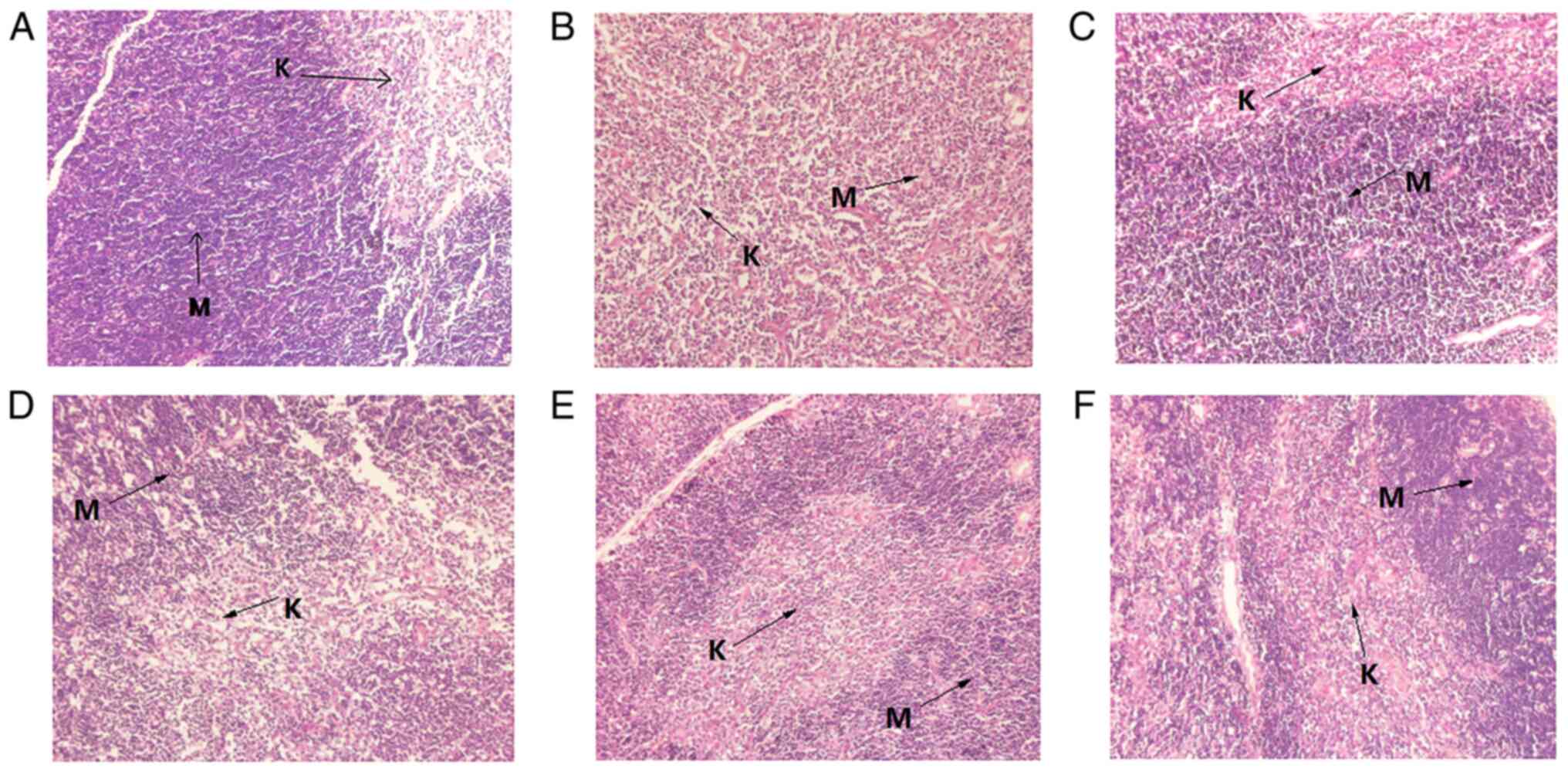
the red and the white medulla (Fig.
1B). Lymphocytes in the spleen tissue of mice in the MHG and PG
groups were densely but unevenly distributed, with deep staining
(Fig. 1C and F). The distribution of lymphocytes in the
red medulla region of the spleen tissue from the MLG and MMG mice
were loose with no evident damage found in the spleen tissues of
mice in either group (Fig. 1D and
E). These observations suggest
that MCP can promote the development of spleen in mice with
CY-induced hypoimmunity, thereby improving the immunity of mice
(Fig. 1).
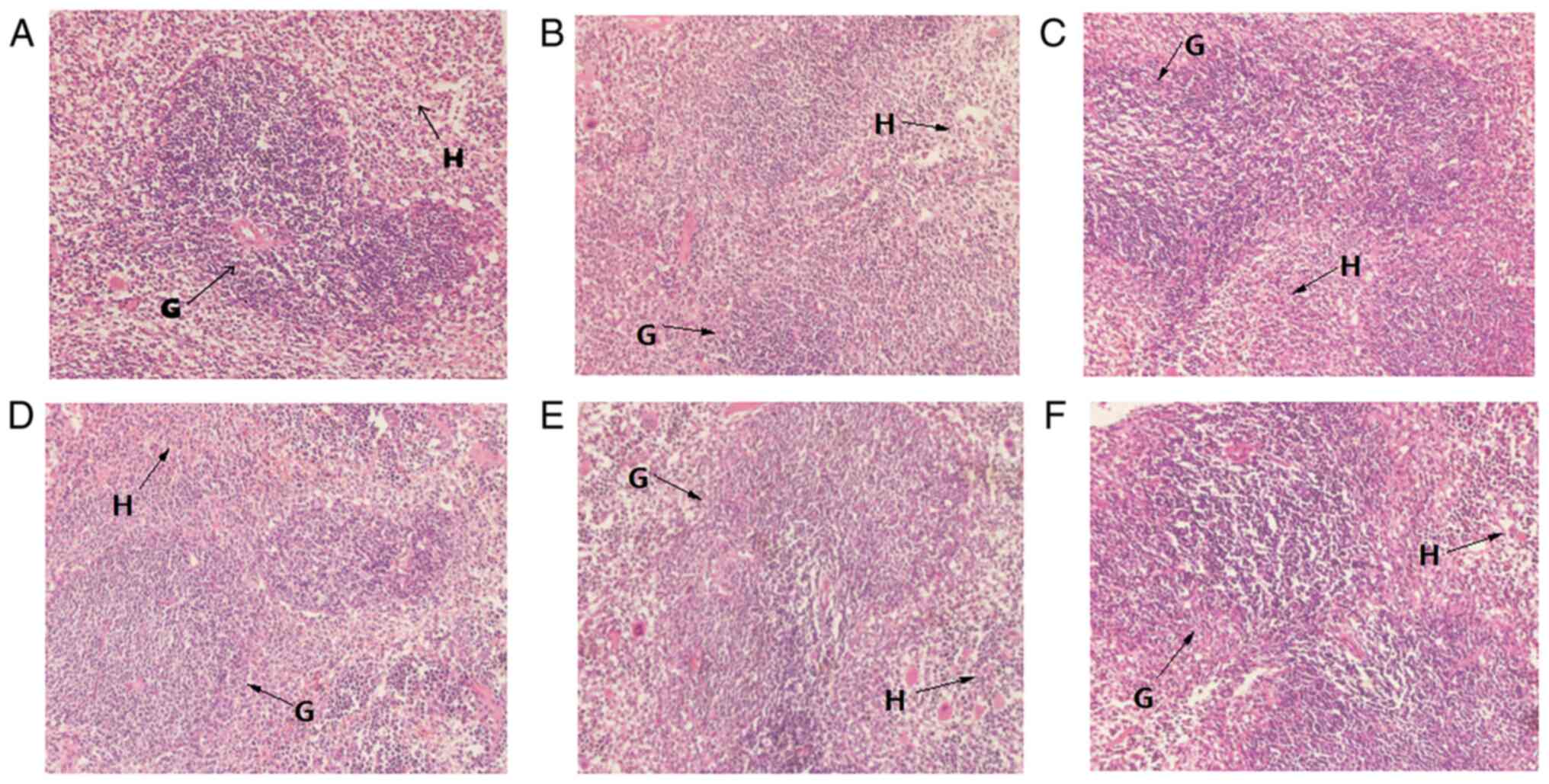
The number of lymphocytes in the thymus tissue of MG
mice was decreased compared with that of NG mice, where the
boundary between the medulla and cortex was not clear (Fig. 2B). In the PG, MMG and MHG mice, the
cortical lymphocytes were dense, evenly distributed and deeply
stained, where medullary lymphocytes were loosely distributed, and
the blood vessels and reticular cells were clear (Fig. 2C, E and F).
These observations suggest that MCP can enhance the development of
lymphocytes. The lymphocyte distribution in the tissues of MLG mice
was loose and uneven, which indicated that MCP was able to repair
injuries sustained to the thymus of immunocompromised mice
(Fig. 2D).
Effect of MCP on DTH in mice
The degree of DTH (Table III) in the MG mice was
significantly decreased compared with that in the NG mice
(P<0.05). The DTH in PG mice and those administered with MCP at
concentrations of 200 or 300 mg/kg (MMG and MHG) was significantly
increased compared with that in MG mice (P<0.05). The difference
in DTH resulting from treatment with MCP at a concentration of 100
mg/kg (MLG) was smaller compared with that in the MMG and MHG
groups, but remained significant compared with that in the MG group
(P<0.05).
 | Table IIIEffect of MPC on splenic lymphocyte
proliferation, DTH and phagocytosis of macrophages in
immunosuppressive mice. |
Table III
Effect of MPC on splenic lymphocyte
proliferation, DTH and phagocytosis of macrophages in
immunosuppressive mice.
| Group | DTH, mg | Lymphocyte
proliferation index | Phagocytic capacity
of macrophages |
|---|
| Normal saline | 5.35±0.09 | 0.87±0.06 | 0.73±0.04 |
| Model |
2.19±0.65a |
0.44±0.02a |
0.42±0.01a |
| Positive |
4.15±0.27b |
0.79±0.06b |
0.65±0.02b |
| MCP low-dose | 2.85±0.35 |
0.49±0.04c |
0.47±0.04d |
| MCP
medium-dose |
3.54±0.17b |
0.57±0.05b,d |
0.56±0.08e |
| MCP high-dose |
4.76±0.33b |
0.69±0.06e |
0.61±0.07b |
Effect of MCP on splenic lymphocyte
proliferation in immunosuppressive mice
The effect of MCP on the proliferation of splenic
lymphocytes show that CY treatment in MG mice significantly reduced
lymphocyte proliferation compared with that in NG mice (P<0.05;
Table III). The proliferation of
splenic lymphocytes in the MCP treatment groups (MLG, MMG and MHG)
appeared to be dose-dependent (Table
III). The proliferation of splenic lymphocytes in the PG, MMG
and MHG mice was significantly increased compared with that in the
MG mice (P<0.05; Table III).
These data suggest that MCP can effectively promote the
proliferation of spleen lymphocytes in immunosuppressive mice.
Effect of MCP on cell phagocytosis of
mice macrophages
As shown in Table
III, the phagocytic activity of peritoneal macrophages in MG
mice was significantly decreased compared with that in NG mice
(P<0.05), suggesting that CY treatment reduced the activity of
phagocytotic macrophages in mice. The phagocytic activity of
peritoneal macrophages in the PG, MMG and MHG mice was
significantly higher compared with that in the MG mice (P<0.05;
the activity of phagocytotic macrophages). By contrast, cell
phagocytosis of mice in the MLG group was also markedly enhanced
compared with that of MG mice, although these changes were found to
be not significant.
Effect of MCP on the lymphokine
content in splenic lymphocytes of mice
The effects of MCP on the levels of the cytokines
IFN-γ, IL-6 and IL-12 in immunosuppressed mice are shown in
Table IV. In MG mice, the levels
of the helper T (Th) cell type 1 cytokines IL-12 and IFN-γ and the
Th2 cytokine IL-6 were significantly lower compared with those in
the NG mice (P<0.05). Compared with those in the MG mice, the
levels of IL-12 and IFN-γ in the PG, MLG, MMG and MHG mice were
significantly higher (P<0.05; Table IV). The levels of IL-6 in MMG and
MHG mice were significantly increased (P<0.05), whereas those in
MLG mice was also significantly increased but to a lesser extent
(P<0.05; Table IV), compared
with those in the MG group. As the MCP dose increased, the levels
of secreted IFN-γ, IL-6 and IL-12 were also increased.
 | Table IVEffect of MPC on the secretion of
IFN-γ, IL-6 and IL-12 by lymphocytes. |
Table IV
Effect of MPC on the secretion of
IFN-γ, IL-6 and IL-12 by lymphocytes.
| Group | IFN-γ, pg/ml | IL-6, pg/ml | IL-12, pg/ml |
|---|
| Normal saline | 693.81±0.02 | 112.13±0.03 | 104.96±1.51 |
| Model |
506.88±0.03a |
77.31±0.03a |
78.66±0.03a |
| Positive |
615.15±0.04b |
104.42±0.05c |
99.24±0.02c |
| MCP low-dose |
559.87±0.00d |
84.40±0.04d |
86.22±0.05e |
| MCP
medium-dose |
585.83±0.03e |
92.53±0.02e |
92.88±0.06b |
| MCP high-dose |
647.08±0.00c |
99.23±0.03b |
97.08±0.01c |
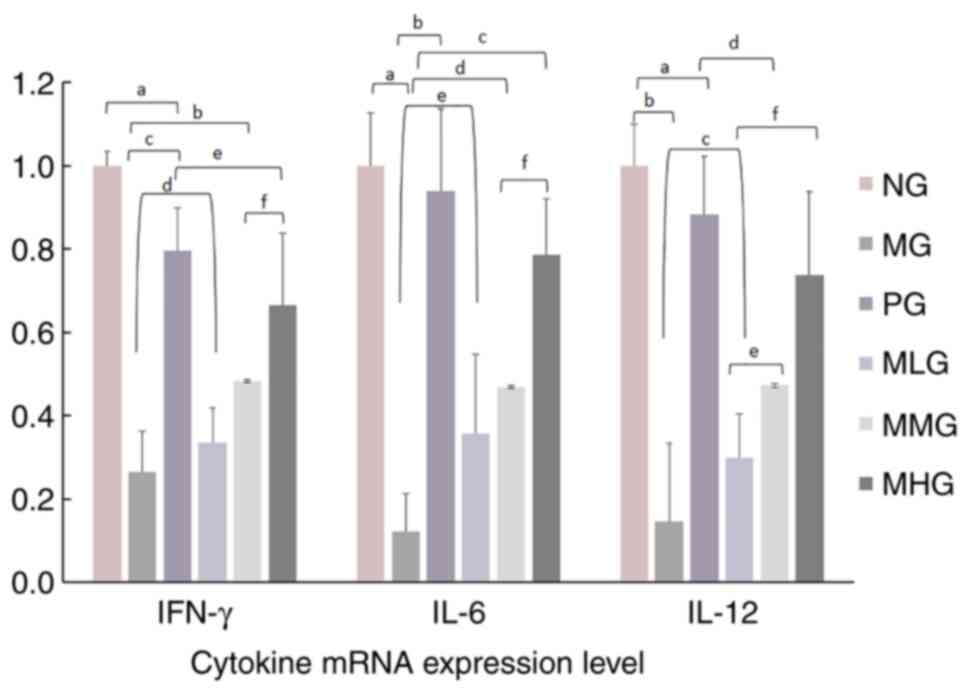
Effects of MCP on the mRNA expression
levels of cytokines in splenic lymphocytes
The present study next examined the mRNA expression
levels of IFN-γ, IL-6 and IL-12 after MCP was administered at
different concentrations (Fig. 3).
The mRNA expression levels of IFN-γ, IL-6 and IL-12 in the
spleen lymphocytes of MG mice were significantly lower compared
with those in the spleen lymphocytes of NG mice (P<0.05).
Fig. 4 also shows that the
expression levels of the cytokines IFN-γ, IL-6 and IL-12 in the
spleens of MG mice were markedly lower compared with those in the
NG group. After treatment with different concentrations of MCP, the
mRNA expression levels of IFN-γ, IL-6 and IL-12 were observed to
increase by varying degrees. The mRNA expression levels of IFN-γ,
IL-6 and IL-12 in the PG and MCP dosage groups were significantly
higher compared with those in MG mice (P<0.05; Fig. 3). In addition, the mRNA expression
levels of IFN-γ, IL-6 and IL-12 showed a certain dose-effect
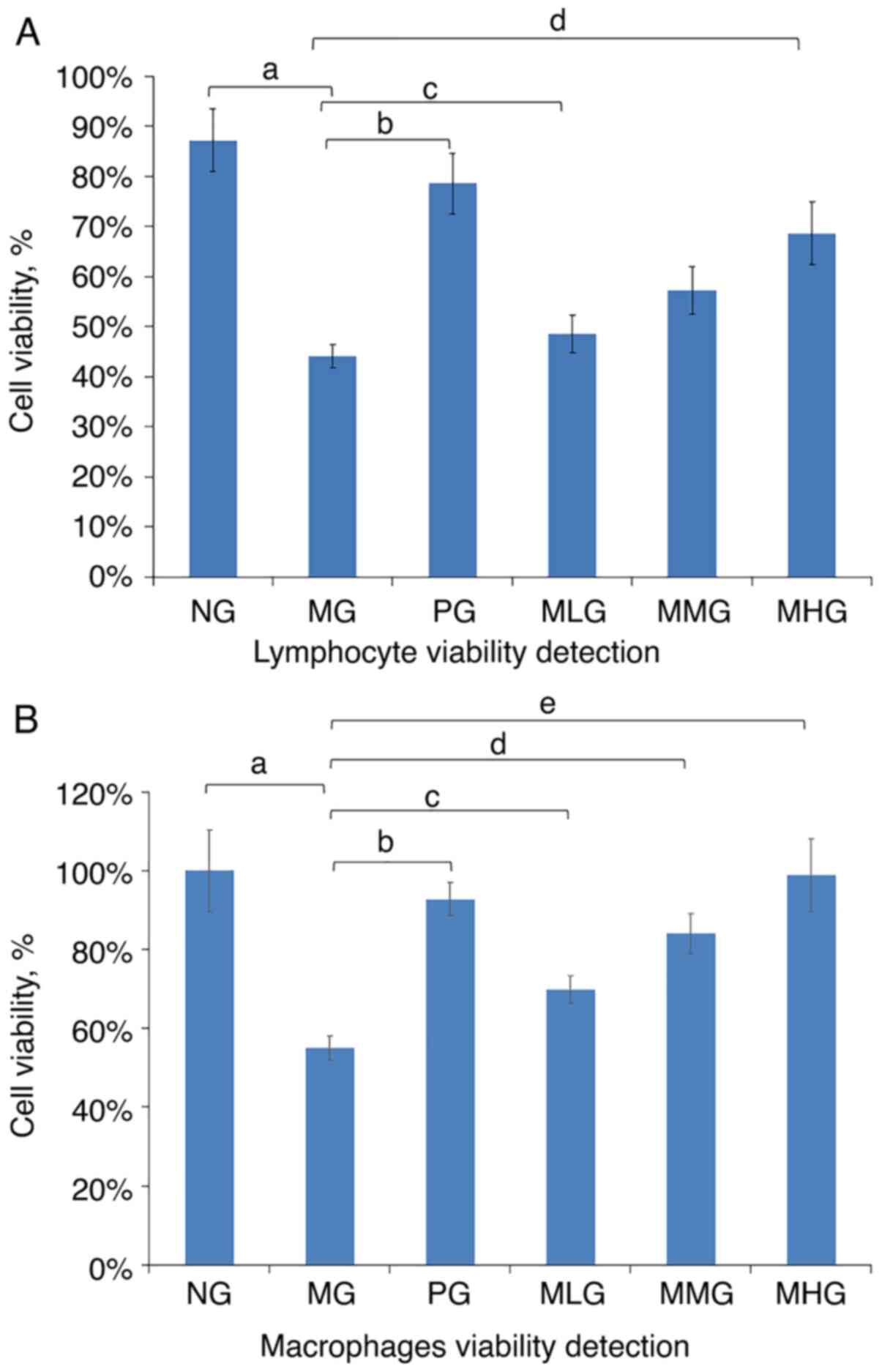
relationship with the dose of MCP used. Fig. 5 shows the results of the cell
viability test measured with lymphocytes and macrophages at 570 nm.
Although the results obtained with the MHG and PG mice were
comparable, they did not fully return to normal levels. Finally,
the cell viability of CY-immunosuppressed mice was found to be
increased in the MLG mice. Compared with that in the NG mice, the
lymphocyte viability of mice in the MG group was significantly
decreased (P<0.05; Table II).
Compared with the MG mice, the lymphocyte viability was
significantly increased in the PG, MHG, MMG and MLG groups
(P<0.05; Table II). These data
suggested that the lymphocyte viability of the immunosuppressed
mice increased in response to MCP in a dose-dependent manner,
although it remained lower compared with that of the NG mice
(Fig. 5A). Compared with that in
the NG group, the macrophage viability of mice in the MG group was
significantly decreased (P<0.05; Table II). Compared with the MG mice, the
macrophage viability was significantly increased in the PG, MHG,
MMG and MLG groups (P<0.05; Table
II). These data suggested that the macrophage viability of the
immunosuppressed mice increased in response to MCP in a
dose-dependent manner (Fig.
5B).
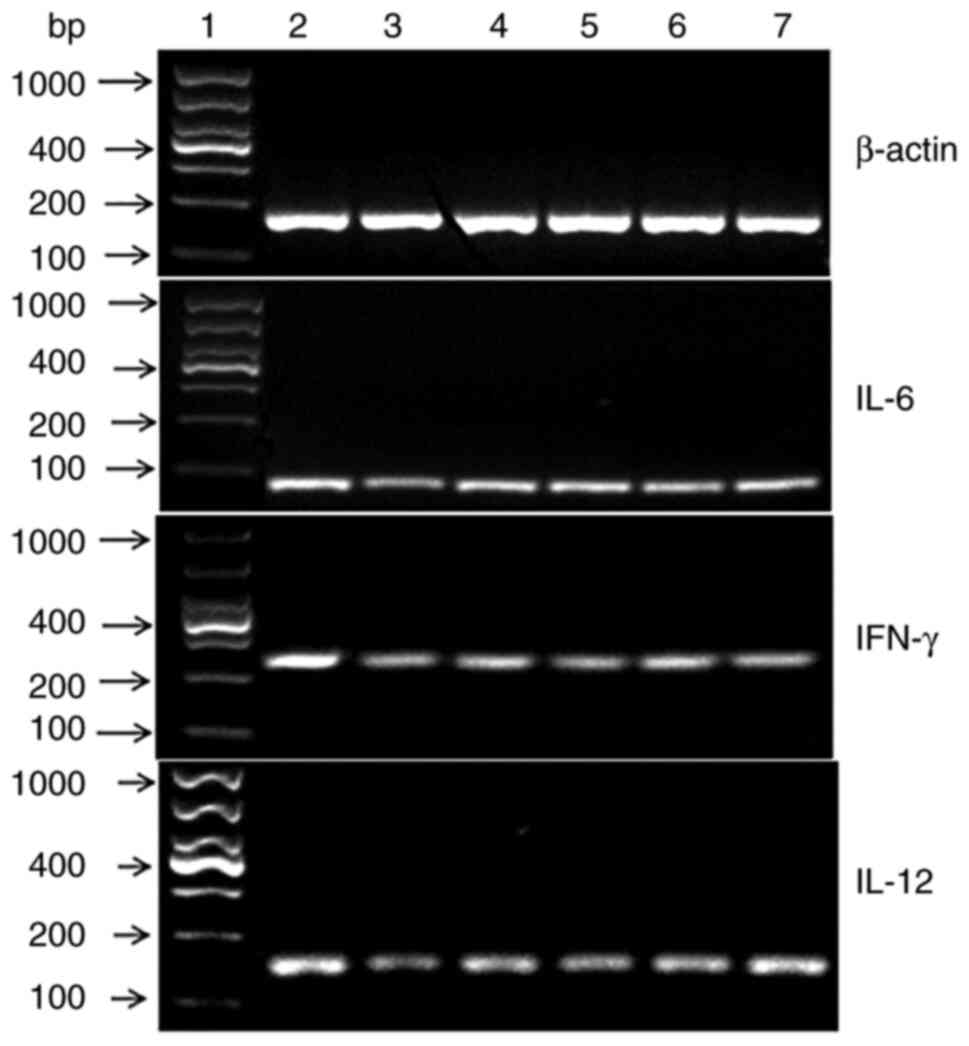 | Figure 4Results of RT-PCR reaction for the
expression of IFN-γ, IL-6 and IL-12 mRNA. A representative image of
a gel electrophoresis used to visualize the results of RT-PCR
measuring the expression of IFN-γ, IL-6 and IL-12 mRNA in the six
experimental groups. MCP, Momordica charantia
polysaccharide; NG, normal saline group; MG, model group; PG,
positive group; MLG, MCP low-dose group; MMG, MCP medium-dose
group; MHG, MCP high-dose group; RT, reverse transcription; 1,
Marker; 2, NG; 3, MG; 4, PG; 5, MLG; 6, MMG; 7, MHG. |
Discussion
CY is a potent alkylating agent that is widely used
in clinical chemotherapy (19,20).
It has been previously shown that it can inhibit humoral and
cellular immune responses of animals, resulting in
immunosuppression (19). The dose
and time of CY administration are key factors that can determine
whether the model of hypoimmunity can be successfully established
(20). In the present study,
normal mice were injected intraperitoneally with CY at a
concentration of 80 mg/kg from days 1 to 5, rendering this a
suitable method for establishing a long-term immunosuppressive
model.
The main function of the immune system is to
identify non-self substances for removal from the body (21,22).
All cells that perform this function belong to the immune system
(21,22). Immune organs include bone marrow,
thymus, spleen and lymph nodes, whereas immune cells include
lymphocytes, monocytes, macrophages and natural killer (NK) cells
(21-23).
In addition, immune molecules include antibodies, complement
proteins and cytokines (23).
The spleen and thymus are crucial immune organs of
the body, since they form sites where immune cells proliferate
(24). Activity in the immune
organs can directly regulate downstream immune function and the
ability to resist various diseases, which can be measured using
indices of immune organs. It has been shown that polysaccharides
can significantly increase the index of immune organs and inhibit
their atrophy in mice (9,24-26).
In the present study, the organ indices of MG mice was
significantly lowered compared with that of NG mice, suggesting
that CY can significantly reduce the weight of the thymus and
spleen of mice. This also suggests that the immunosuppressive mice
model was successfully established. Compared with that in the MG
mice, the organ indices of mice in each dose group of MCP (MLG, MMG
and MHG) was significantly higher, suggesting that MCP treatment
could correct atrophy of the thymus and spleen caused by CY.
However, compared with NG mice, differences remain in the MCP
treatment groups, where the detrimental effects of CY could not be
completely reversed. This finding was consistent with the results
obtained by Chao et al (25), who studied the immunological
effects of Cheonggukjang polysaccharides and those of Mei
et al (9), who studied the
protective effects of chitosan oligosaccharide on CY-induced
immunosuppression in mice.
The measurement of DTH reflects the strength of
cellular immunity (26).
Therefore, the degree of DHT can be used as an indicator of the
health of cellular immune function in mice. A previous study
reported that treatment with Chaenomeles speciose
polysaccharides (CSP) can support a possible role of CSP in
assisting the cell-mediated immune response in the spleen (27). The present results showed that the
degree of DTH in the immunosuppressed mice was improved following
MCP treatment, suggesting that MCP was able to effectively enhance
DTH.
Immune function can be divided into specific
immunity and non-specific immunity (28,29).
Lymphocytes can reflect the state of specific immunity, whereas the
ability of phagocytosis by macrophages may be applied to reflect
the strength of non-specific immunity (28). Peritoneal macrophages have a potent
capacity to engulf foreign bodies and fulfil an important role in
immune surveillance (28). By
contrast, lymphocytes form the core components of the immune system
and serve as one of the most important immune cell types in the
body (28). The proliferation and
differentiation of lymphocytes are the most important stage of the
immune response (28). Since the
spleen contains a large number of lymphocytes, splenic lymphocyte
proliferation tests provide an important method to test the
vitality of lymphocytes (28). A
previous study on the effects of Rehmannia glutinosa
polysaccharide (RGP) on the immune function in mice showed that the
administration of RGP increased the population of sheep red blood
cells, induced DTH and led to an increase in the spleen and thymus
indices (29). In addition,
treatment with RGP led to increases in both splenic lymphocyte
proliferation and in the level of peritoneal macrophage
phagocytosis in a dose-dependent manner. These results indicated
that RGP can enhance the cellular immune response in mice (29). The present study also demonstrated
that the proliferation rate of spleen lymphocytes in
immunosuppressed MG mice was significantly decreased. Compared with
that in the MG mice, the proliferation index of spleen lymphocytes
in mice treated with different concentrations of MCP was increased
significantly, suggesting that MCP could effectively stimulate the
proliferation of spleen lymphocytes in mice whilst enhancing the
specific immune function of immunosuppressed mice.
Another previously published study (30) evaluated the possible immune
activity of Cordyceps militaris polysaccharides (CMP) in
vivo and found that the administration of CMP was able to
overcome CY-induced immunosuppression, led to an increase in the
spleen and thymus indices and enhanced both the spleen lymphocyte
activity and macrophage function. In addition, Chen et al
(31) previously evaluated the
regulatory effects of Potentilla anserina polysaccharide
(PAP) by using mice peritoneal macrophages and CY induction as an
immunosuppressive model. They demonstrated that PAP could
upregulate phagocytosis by phagocytes and also cause spleen cell
proliferation, leading to increases in both the TI, SI, serum IL-10
and IFN-γ levels of immunosuppressive mice, thereby exerting
an immunoregulatory role (31). In
the present study, the level of phagocytosis by macrophages in MG
mice was shown to be significantly reduced, whereas the
phagocytosis index of immunosuppressed mice was significantly
increased after treatment with MCP (MLG, MMG and MHG) and in the PG
mice. Therefore, these observations suggest that MCP can
effectively enhance the level of phagocytosis by macrophages and
the non-specific immune function of immunosuppressed mice.
Cytokines are proteins or small-molecule
polypeptides synthesized by stimulating immune cells (such as
mononuclear phagocytes, T, B and NK cells) and certain non-immune
cells (such as vascular endothelial cells, epidermal cells and
fibroblasts), which can transmit information among cells to
regulate immune regulation (32).
The profile of cytokines reflects the state of both the immune
system and the nutritional metabolic status of the body (32,33).
Th cells can be mainly divided into the Th1 and Th2 cell subsets
according to their different cytokine production types and
biological functions (32,33). Th1 cell subsets mainly secrete
inflammatory cytokines, such as IL-2, IFN-γ and IL-12, which
mediate cytotoxicity and delay hypersensitivity inflammation,
participate in the cellular immune response and have an important
role in resisting intracellular pathogen infections (32,33).
By contrast, Th2 cells mainly secrete anti-inflammatory cytokines,
such as IL-4, IL-5, IL-6 and IL-10, which can promote B cells into
differentiating into plasma cells and produce antibodies (32,33).
Th2 responses are typically associated with humoral immunity
(32,33). It was previously reported that
Astragalus polysaccharide (APS) liposome (APSL) could
significantly promote lymphocyte proliferation, enhance the
antibody titer and promote the secretion of IFN-γ, IL-2, IL-4 and
IL-10 (32,33). In addition, medium dosage was shown
to possess optimal efficacy, suggesting that APSL could
significantly improve the adjuvanticity and drug action of APS
(34,35).
In the present study, it was found that the levels
of the Th1-type cytokines, IL-12 and IFN-γ, in addition to the Th2
cytokine IL-6 secreted by spleen lymphocytes, were decreased
significantly in MG mice. By contrast, the levels of IFN-γ, IL-6
and IL-12 secreted in the MCP treatment groups were increased in a
dose-dependent manner. These results suggest that MCP can promote
lymphocyte proliferation, increase cytokine levels and enhance
cellular immunity in immunosuppressed mice, implying that MCP
exerts an immunomodulatory role.
Interleukins comprise a group of cytokines that can
transmit information among immune cells. IL-6 is a pleiotropic
cytokine, which can participate in the immune defence of the body
(35,36). IL-6 can induce lymphocyte
proliferation and the production of IL-12 and TFN-α, thereby
mediating antiviral effects (36).
IL-4 is a cytokine that is produced by mononuclear macrophages and
endothelial cells (36,37). IL-4 has been reported to be
involved in a series of processes, such as inflammation, the immune
response and stress (36). In
addition, it can participate in the occurrence and development of
diseases, such as breast cancer and asthma (37). IFN-γ is a highly active and
multifunctional cytokine that is secreted by Th1 cells, which can
enhance both the activity of Th1 cells, the overall cellular immune
function and antiviral effects (38). Furthermore, it can inhibit the
proliferation of Th2 cells, thereby inhibiting humoral immune
function (38). IL-12 is another
type of cytokine and mainly targets T-cells, NK cells and bone
marrow progenitor cells (39,40).
IL-12 can promote the proliferation of activated T and NK cells,
enhance their cytotoxicity and promote the production of IFN-γ and
TNF-β (39,40). IL12 serves an important role in the
balance of the Th1/Th2 response (39,40).
IL-12 can induce Th0 cells into differentiating into Th1 cells,
thereby exerting an important role in cell-mediated immunity
(39,40). A previous study showed that
polysaccharides from Physalis alkekengi can improve the
state of immunity by stimulating the expression of IL-4 and IFN-γ
mRNA in chicken spleen lymphocytes (41). Wang et al (42) demonstrated that polysaccharide
isolated from Kadasura marmorata can improve the immune
activity of the body by promoting the secretion of IL-2, IFN-γ and
TNF-α by spleen lymphocytes in vitro.
In the present study, RT-qPCR was used to determine
the relative expression levels of IFN-γ, IL-6 and IL-12 in the
mouse spleen cells. The results showed that the mRNA expression
levels of IFN-γ, IL-6 and IL-12 in the spleen lymphocytes of MG
mice were significantly decreased. Upon treating the cells with
different concentrations of MCP, the mRNA expression levels of
IFN-γ, IL-6 and IL-12 were all significantly increased in a
dose-dependent manner. Based on these results, it is possible that
MCP can promote the secretion of both Th1 and Th2 cytokines by mice
spleen lymphocytes and increase their mRNA expression levels,
thereby fulfilling a dual immunomodulatory role.
In conclusion, to investigate the role of MCP on the
immunological activity of mice, six groups of samples were
organized by assigning 10 male BALB/c mice aged 6-8-week-old into
each group. An immunosuppressive model was established by the
intraperitoneal injection of CY in all groups, except for NG. The
different groups of mice were then administered distilled water,
levamisole (200 mg/kg) and different doses of MCP (100, 200 or 300
mg/kg). The organ index was measured, the tissue sections of immune
organs were imaged, the degree of mouse ear swelling model was
established, the delayed allergic reaction was measured, the
phagocytic function of the peritoneal macrophages was measured
using the neutral red phagocytosis test, lymphocyte proliferation
was measured using the MTT method, the spleen lymphocyte factors of
IFN-γ, IL-6 and IL-12 were measured by the ELISA method and the
mRNA expression levels of cytokineassociated genes were analysed
using RT-qPCR.
Collectively, the present results showed that MCP
could enhance immune function and improve the TI and SI in
immunosuppressed mice. MCP could also repair the damage caused by
CY to the immune organs. In summary, MCP was shown to enhance the
DTH and cell phagocytosis by abdominal macrophages, in addition to
stimulating the proliferation of splenic lymphocytes, induce the
secretion of the cytokines IL-6, IFN-γ and IL-12 and upregulate
their mRNA expression levels. The present study demonstrated,
through the establishment of an immunosuppressed mice model, that
the daily intake of MCP can lead to a reversal of the decline of
immune function in mice treated with CY. This may provide a basis
for the further research and development of MCP as an effective
immunopotentiator.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Liaoning Province, China (grant no. 20170540369).
This work is also supported by the fundamental research program of
higher school education from Education Department of Liaoning
Province (grant no. LJKZZ20220094).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AA and YY conceived and designed the experiments. AA
performed the animal experiments. AB, YY and XJ performed the
experiments. AA, YY and JSL analyzed the data. AA and YY confirm
the authenticity of all the raw data. AA wrote the first
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Jinzhou
Medical University Ethics Committee [production license SCXY(Liao)
2019-0003; approval no. 2019014].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Joseph B and Jini D: Antidiabetic effects
of Momordica charantia (bitter melon) and its medicinal
potency. Asian Pac J Trop Dis. 3:93–102. 2013.
|
|
2
|
Rajasekhar MD, Badri KR, Vinay Kumar K,
Babu KR, Fatima SS, Sampath Kumar MT and Appa Rao C: Isolation and
characterization of a novel antihyperglycemic protein from the
fruits of Momordica cymbalaria. J Ethnopharmacol. 128:58–62.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lucas EA, Dumancas GG, Smith BJ, Clarke SL
and Arjmandi BH: Health benefits of bitter melon (Momordica
charantia). Bioact Foods Promot Health. 35:525–549. 2010.
|
|
4
|
Zhang F, Lin L and Xie J: Mini-review of
chemical and biological properties of polysaccharides from
Momordica charantia. Int J Biol Macromol. 92:246–253.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Spreafico F, Malfiore C, Moras ML,
Marmonti L, Filippeschi S, Barbieri L, Perocco P and Stripe F: The
immunomodulatory activity of the plant proteins Momordica
charantia inhibitor and Pokeweed antiviral protein. Int J
Immunopharmacol. 5:335–343. 1983.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Puri M, Kaur I, Kanwar RK, Gupta R,
Chauhan A and Kanwar J: Ribosome inactivating proteins (RIPs) from
Momordica charantia for anti viral therapy. Curr Mol Med.
9:1080–1094. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Panda BC, Mondal S, Devi KS, Maiti TK,
Khatua S, Acharya K and Islam SS: Pectic polysaccharide from the
green fruits of Momordica charantia (Karela): Structural
characterization and study of immunoenhancing and antioxidant
properties. Carbohydr Res. 401:24–31. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Elangovan A, Subramanian A, Durairaj S,
Ramachandran J, Lakshmanan DK, Ravichandran G, Nambirajan G and
Thilagar S: Antidiabetic and hypolipidemic efficacy of skin and
seed extracts of Momordica cymbalaria on alloxan induced diabetic
model in rats. J Ethnopharmacol. 24(111989)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mei YX, Chen HX, Zhang J, Zhang XD and
Liang YX: Protective effect of chitooligosaccharides agaist
cyclophosphamide-induced immunosuppression in mice. Int J Biol
Macromol. 62:330–335. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yu JH, Cong L, Wang CM, Li H, Zhang C,
Guan X, Liu P, Xie Y, Chen J and Sun J: Immunomodulatory effect of
Schisandra polysaccharides in cyclophosphamide-induced
immunocompromised mice. Exp Ther Med. 15:4755–4762. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nishi C, Toda S, Segawa K and Nagata S:
Tim4-and Mer TK-mediated engulfment of apoptotic Cells by mouse
resident peritoneal macrophages. Mol Cell Biol. 34:1512–1520.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu Z, Xing J, Huang Y, Bo R, Zheng S, Luo
L, Niu Y, Zhang Y, Hu Y, Liu J, et al: Activation effect of
Ganoderma lucidum polysaccharides liposomes on murine
peritoneal macrophages. Int J Biol Macromol. 82:973–978.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Khan D, Dai R, Karpuzoglu E and Ahmed SA:
Estrogen increase, whereas IL-27 and IFN-γ decrease, splenocyte
IL-17 production in WT mice. Eur J Immunol. 40:2459–2556.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang P, Ni Z, Wang B, Ma B, Duan H, Li X,
Ma X, Wei Q, Ji X, Liu Q, et al: Acutetoxicity, twenty-eight days
repeated dose toxicity and genotoxicity of vanadyl trehalose in
kunming mice. Regul Toxicol Pharmacol. 85:86–97. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang C, Jin E, Deng J, Pei Y, Ren M, Hu Q,
Gu Y and Li S: GRP30 mediated effects of boron on ret spleen
lymphocyte proliferation, apoptosis and immune function. Food and
Chemical Toxicol. 146(111838)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bharshiv CK, Grag CK and Bhatia AK:
Immunomodulatory activity of aqucous extract of Nyctanthes
arbor-tristis flowers with particular reference to splenocytes
proliferation an cytokine induction. Indian J Pharmacol.
48:412–417. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamada S, Zaima N, YoshimuraY Inaba S,
Fujimori T, Sogon T and Moriyama T: Vasualization of the
distribution of anthocyanin species in mice eyeball by
matrix-assisted laser desorption/ionization masss pectrometry
imaging. Rapid Commun Mass Spectrom. 32:380–384. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ahlmanm M and Hempel G: The effect of
cyclophosphamide on the immune system: Implications for clinical
cencer therapy. Cancer Chemother Pharmacol. 78:661–671.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yamazaki M and Iwasawa H: Anti-oxidant and
immunoactivating effects of vegetables and fruits. Food Sci.
47:73–77. 2005.
|
|
21
|
Ueda H and Yamazaki M: Anti-inflammatory
effect of orally administration of xanthine derivatives.
Inflammation Res. 46(s220)1997.
|
|
22
|
Zhang R, He C, Fan Y, Si H, Wang Y, Shi Z,
Zhao X, Zheng Y, Liu Q and Zhang H: Immune-enhancing activity of
polysaccharides from Cyrtomium macrophyllum. Int J Biol Macromol.
70:590–595. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lan MB, Zhang YH, Zheng Y, Yuan HH and
Zhao HL: Antioxidant and immunomodulatory activities of
polysaccharides from moxa (artemisia argyi) leaf. Food Sci
Biotechnol. 19:1463–1469. 2010.
|
|
24
|
Oh MJ, Choi HD, Ha SK, Choi I and Park HY:
Immunomodulatory effects of polysaccharide fraction isolated from
Fagopyrum esculentum on innate immune system. Biochem Biophys Res
Commun. 49:1210–1216. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chao CW, Han CJ, Rhee YK, Lee YC, Shin KS,
Shin JS, Lee KT and Hong HD: Cheonggukjang polysaccharides
enhance immune activities and prevent cyclophosphamide-induced
immunosuppression. Int J Biol Macromol. 72:519–525. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dai Z, Zhang H, Zhang Y and Wang H:
Chemical properties and immunostimulatory activity of a
water-soluble polysaccharide from the clam of Hyriopsis
cumingii Lea. Carbohydr Polym. 77:365–369. 2009.
|
|
27
|
Xie X, Zou G and Li C: Antitumor and
immunomodulatory activities of a water-soluble polysaccharide from
Chaenomeles speciose. Carbohydr Polym. 132:323–329.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nakano K and Matsushita S: The
immunomodulatory effect of dopamine. Allergy. 56:679–684.
2007.PubMed/NCBI(In Japanese).
|
|
29
|
Li H, Hong T, Jinag H, Liu S and Di L: The
effects of Rehmannia glutinosa polysaccharide on immune
function of mice. International Conference on Informaton Technology
in Medicine and Education. 63:286–288. 2015.
|
|
30
|
Wang M, Meng XY, Yang RL, Qin T, Wang XY,
Zhang KY, Fei CZ, Li Y, Hu YL and Xue FQ: Cordyceps
militaris polysaccharides can enhance the immunity and
antioxidation activity in immunosuppressed mice. Carbohydr Polym.
89:461–466. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen JR, Yang ZQ, Hu TJ, Yan ZT, Niu TX,
Wang L, Cui DA and Wang M: Immunomodulatory activity in vitro and
in vivo of polysaccharide from Potentilla anserine. Fitoterapia.
81:1117–1124. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sattler A, Wagner U, Rossol M, Sieper J,
Wu P, Krause A, Schmidt WA, Radmer S, Kohler S, Romagnani C and
Thiel A: Cytokine-induced human IFN-gamma-secreting effector-memory
Th cells in chronic autoimmune inflammation. Blood. 113:1948–1956.
2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mosmann TR and Coffman RL: Heterogeneity
of cytokine secretion patterns and functions of helper T cells. Adv
Immunol. 46:111–147. 1989.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fan Y, Hu Y, Wang D, Liu J, Zhang J, Zhao
X, Liu X, Liu C, Yuan J and Ruan S: Effects of Astragalus
polysaccharide liposome on lymphocyte proliferation in vitro and
adjuvanticity in vivo. Carbohydr Polym. 88:68–74. 2012.
|
|
35
|
Huang Y, Jiang C, Hu Y, Zhao X, Shi C, Yu
Y, Liu C, Tao Y, Pan H, Feng Y, et al: Immunoenhancement effect of
Rehmannia glutinosa polysaccharide on lymphocyte
proliferation and dendritic cell. Carbohydr Polym. 96:516–521.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nakahara H, Song J, Sugimoto M, Hagihara
K, Kishimoto T, Yoshizaki K and Nishimoto N: Anti-interleukin-6
receptor antibody therapy reduces vascular endothelial growth
factor production in rheumatoid arthritis. Arthritis Rheum.
48:1521–1529. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hennekes H, Gerhard D and Klein HU:
Detection of interleukin-4-induced tyrosine phosphorylation of the
IL-4 receptor by an enzyme linked immuno sorbent assay. J Dermatol
Sci. 16(s168)1998.
|
|
38
|
Habijanic J, Berovic M, Boh B, Plankl M
and Wraber B: Submerged cultivation of Ganoderma lucidum and
the effects of its polysaccharides on the production of human
cytokines TFN-α, IL-12, IFN-γ, IL-2, IL-4, IL-10 and IL-17. N
Biotechnol. 32:85–95. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Trinchier G: Interleukin-12:A cytokine
produced byantigenpresenting cells with immunoregulatory
functionsin the generation of T-helper cells type I and
cytotoxiclymphocytes. Blood. 84:4008–4027. 1994.PubMed/NCBI
|
|
40
|
Kataoka TR, Komazawa N, Oboki K, Morii E
and Nakano T: Reduced expression of IL-12 receptor beta and IL-18
receptor alpha genes in natural killer cells and macrophages
derived from B6-mi/mi mice. Lab Invest. 105:146–153.
2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Han J, Jiang X and Zhang L: Optimisisation
of extraction conditions for polysaccharides from the roots of
Isatis tinctoria L. by response surface methodology and their in
vitro free radicals scavenging activities and effects on IL-4 AND
IFN-gamma mRNA expression in chicken lymphocytes. Carbohydr Polym.
86:1320–1326. 2011.
|
|
42
|
Wang H, Deng X, Zhou T, Wang C, Hou Y,
Jiang H and Liu G: The in vitro immunomodulatory activity of a
polysaccharides isolated from Kadsura marmorata. Carbohydr Polym.
97:710–715. 2013.PubMed/NCBI View Article : Google Scholar
|