Introduction
Acute kidney injury (AKI) is a common complication
in patients undergoing coronary angiography (CAG) or percutaneous
coronary intervention (PCI), especially higher in acute myocardial
infarction (AMI), with a prevalence ranging from 7.1 to 18.69%
(1,2), and it raises the mortality risk of
AMI patients by a factor of 2 to 20 times (3-6).
Current prevention strategy for post-AMI AKI
patients mainly focused on minimizing the volume of iodinated
radiocontrast media and optimizing periprocedural intravenous
hydration. Indeed, previous research has indicated that post-AMI
AKI was related to various complicated pathophysiological changes,
which include contrast media inducing hypoxic injury in renal
tubular epithelial cells, oxidative stress inducing the increase of
oxygen free radicals, and vasoconstriction (7). Preclinical models of post-AMI AKI are
needed to better clarify the underlying mechanisms and may
therefore help to find therapeutic targets.
The majority of previous animal models, such as
cisplatin-induced AKI, bilateral nephrectomy or renal
ischemia-reperfusion injury induced AKI, and contrast-induced AKI
model, are insufficient to mimic the clinical characteristics of
post-AMI AKI because they primarily focused on one single factor
that causes kidney damage, ignoring the effect of myocardial injury
influence on kidney (8). Thus,
basis on previous models, we establish an animal model of post-AMI
AKI by combining IRI surgery with CM that closely resembles the
clinical situation. To investigate the reliability and stability of
the CM-IRI model, we also constructed single factor model of CM or
IRI surgery, compared the incidence rate of AKI in two definitions,
as well as assessed the role of oxidative stress and the extent of
kidney injury.
Materials and methods
Animals
Animal studies were carried out in accordance with
China's National Guidelines for The Care and Use of Laboratory
Animals and were approved by the Animal Ethics Committee of
Guangdong Provincial People's Hospital (Ethics No.
GDRECKY2020-266-01). Adult male Sprague-Dawley rats (200-250 g)
were purchased from Guangdong Laboratory Animals Monitoring
Institute [License No SYXK (Yue) 2021-0122] and were given at least
a week to acclimate before the study began. The rats were
maintained at 2 or 3 rats/cage, given unrestricted standard diet
and sterile water, housed at a temperature at 25˚C as well as a
12:12 light-dark cycle. Meanwhile, the pad of rats was observed and
replaced daily.
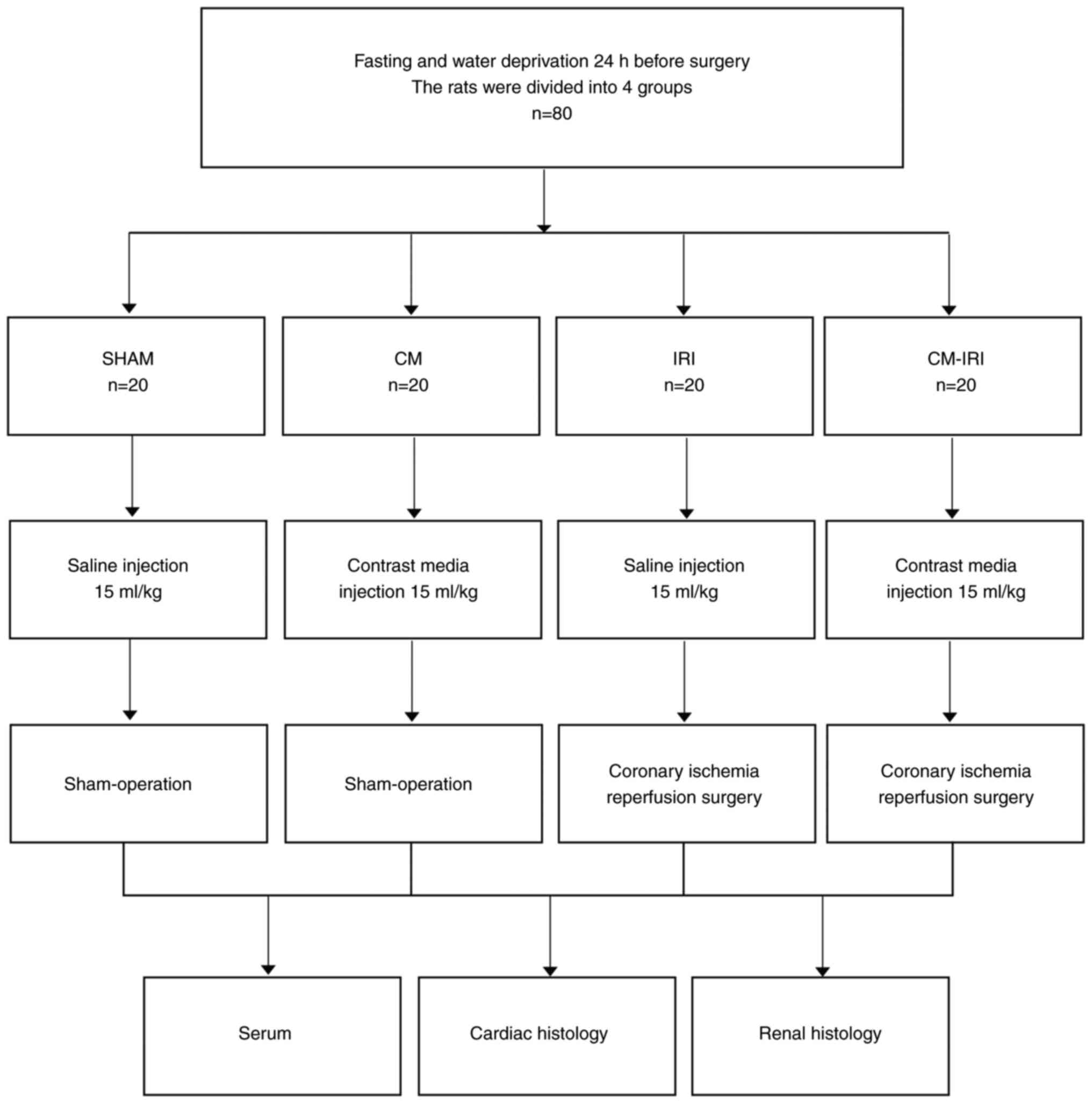
Study design and groups
The rats were divided into four groups, as shown in
Fig. 1. (1) SHAM: sham operation + normal saline
(NS, 15 ml/kg); (2) CM: sham
operation + contrast media (ioprolamine, 15 ml/kg); (3) IRI: IRI surgery + normal saline (NS,
15 ml/kg); (4) CM-IRI: IRI surgery
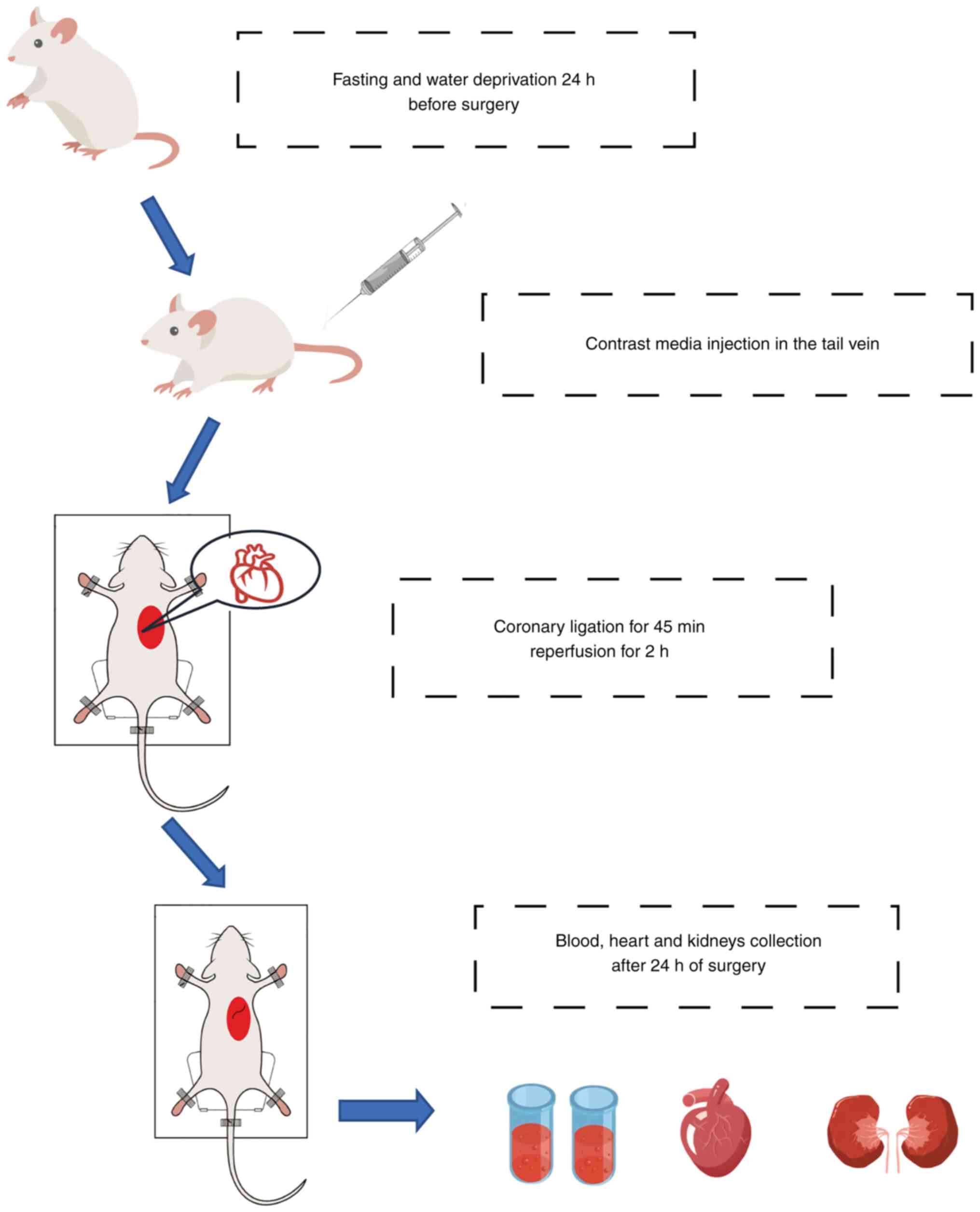
+ contrast media (ioprolamine, 15 ml/kg). After fast and water
restriction for 24 h before surgery, rats were weighed and
anaesthetized using 50 mg/kg pentobarbital sodium and 4 mg/kg
xylazine hydrochloride, intraperitoneally. Then rats received
endotracheal intubation and monitored cardiac rhythm with a typical
lead II electrocardiogram. After that, rats were given a 15 ml/kg
injection of contrast media or normal saline through the tail vein.
Myocardial ischemia-reperfusion surgery was performed as previously
described (9). Briefly, a left
thoracotomy was performed in the fourth intercostal space, and the
pericardium was removed to expose the heart, after which the left
anterior descending (LAD) coronary artery was ligated for 45 min
and repercussed for 2 h. After 24 h of the operation, 1 ml blood
was collected from the tail vein, then all the rats used in the
experiment were euthanized by an overdose of sodium pentobarbital
100 and 8 mg/kg xylazine hydrochloride intraperitoneally and
confirmed by the disappear of pupillary reflex to light,
respiration and heartbeat. Finally, heart and kidneys were removed
for the following detection and histological staining (Fig. 2).
Renal function analysis
Blood samples were collected in a sodium citrate
tube and centrifuged at 2,500 g for 15 min to separate the serum.
Serum creatinine (Scr) and blood urea nitrogen (BUN) levels were
determined using an automatic biochemical analyzer. The level of
eGFR (estimated glomerular filtration rate) was calculated based on
equation (10). The diagnosis of
AKI was defined as an absolute increase in the serum creatinine
≥0.5 mg/dl (44.2 µmol/l) or a relative increase ≥25% from the
baseline within 72 h after contrast administration according to the
European Society of Urogenital Radiology (ESUR) criteria (11). And Kidney Disease Improving Global
Outcomes (KDIGO) defined AKI as an increase in Scr >0.3 mg/dl
within 48 h or a 50% increase from the baseline within 7 days
(12).
Hematoxylin and eosin staining
Kidney was excised and fixed in 4% formaldehyde for
48 h, then embedded in paraffin and each slide was cut into 4 µm
thick sections. Dehydrated sections with gradient alcohol after
rinsing with water for 30 min. Subsequently, continuous slices were
stained with hematoxylin and eosin (HE) and determined under light
microscopy (13).
Measurement of reactive oxygen species
(ROS) level in kidney tissue
After the perfusion of kidney, half of left fresh
kidney tissue was isolated and ground in a sample freeze grinding
machine (LukyM-1). The abrasive solution was centrifuged for 10 min
(4˚C, 5,000 rpm) to collect the supernatant. ROS level was
determined by the ROS assay kit (Bestbio Assay BB-470512). 190
microliters of homogenate supernatant were added to the 96-well
plate, and mixed with O13 reactive oxygen species probe then
incubated the plate at 37˚C for 30 min. Finally, the fluorescein
intensity was detected using excitation at 510 nm and emission at
610 nm wavelengths on a microplate reader.
Western Blotting Analysis
Total protein was extracted from left kidney
tissues. A 12% separating gel and a 5% concentrated gel were
configured separately based on the measured protein molecular
weight. After electrophoresis, proteins were transferred onto the
nitrate cellulose membrane. The primary anti-NGAL antibody (Abcam,
ab216462) and anti-KIM-1 (Abcam, ab233720) antibody were incubated
overnight, then the primary antibodies were washed away and the
secondary antibodies were incubated. After 2 h, the secondary
antibodies were washed away and the developer solution was added
for development. Finally, the relative expression levels of
proteins were obtained by comparing them with the grey levels of
GAPDH internal reference proteins.
Immunohistochemistry Analysis
The tissues were fixed with 4% paraformaldehyde for
3-4 h before dehydration and sectioning. Waiting for the antigen
repair solution to cool naturally to room temperature and probed
with anti-NGAL (Abcam, ab216462) at 4˚C overnight and labeled with
secondary antibodies. The DAB staining solution was configured with
the appropriate concentration according to the instructions. The
stained tissue sections were observed and analyzed under a light
microscope.
Data Analysis
All data are expressed as the mean ± standard
deviation (SD). Statistical analysis was performed using Graph Pad
Prism (GraphPad Software, CA, USA). Data were analyzed for
normality using the Shapiro-Wilk test. One-way ANOVA followed by
post hoc Tukey test was used. The enumeration data were expressed
as rate and analyzed by the Chi-square test. P-value < 0.05 was
considered statistically significant.
Results
Baseline and AKI parameters for each
experimental group
As shown in Table
I, no significant differences in baseline parameters were
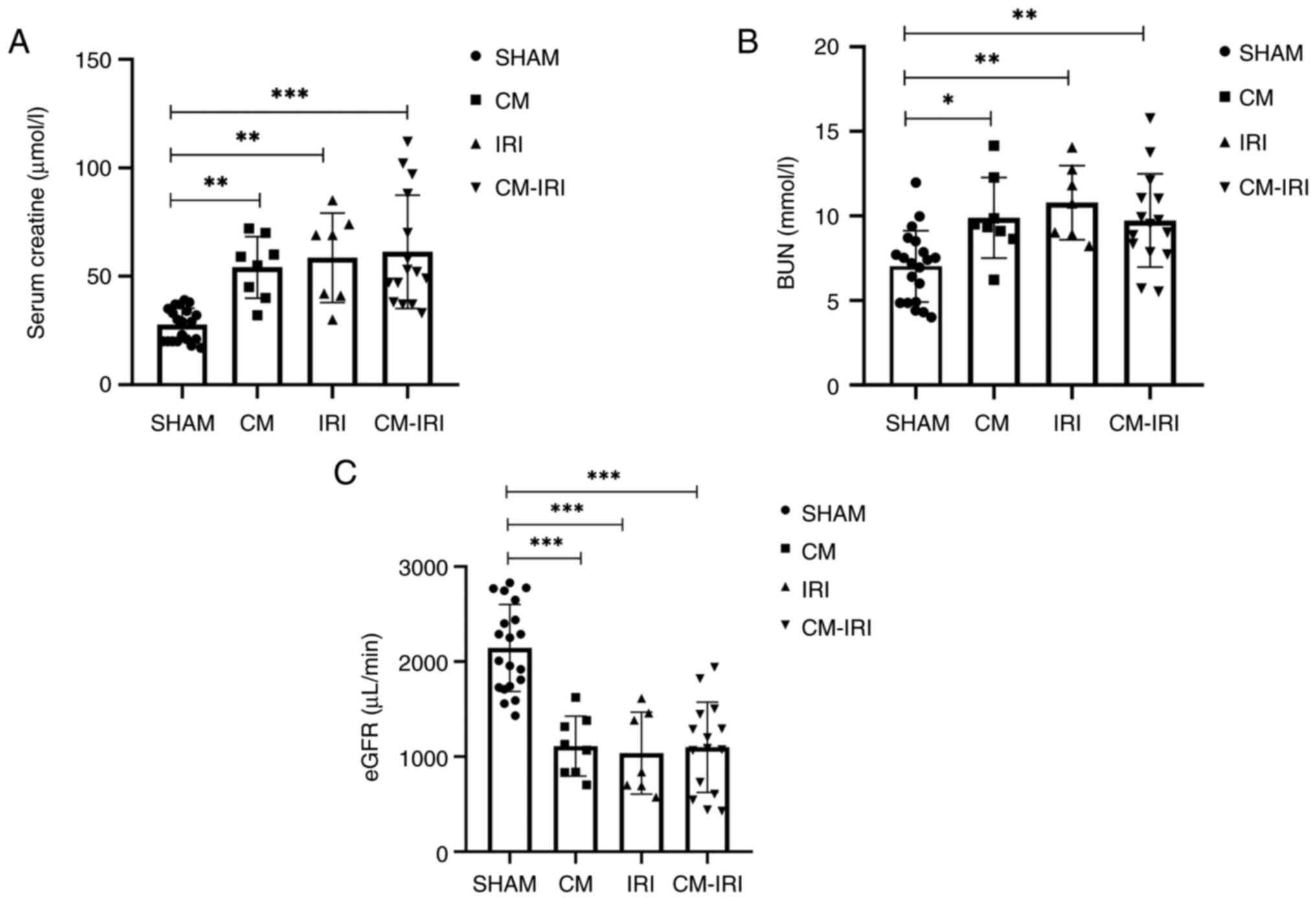
observed among the four groups. Compared with sham group, the level
of BUN and Scr was elevated and eGFR was decreased in CM, IRI and
CM-IRI groups (Fig. 3A-C).
 | Table IAKI baseline parameters for each
group. |
Table I
AKI baseline parameters for each
group.
| Parameter | n | Scr (µmol/l) | BUN (mmol/l) | eGFR (µl/min) |
|---|
| SHAM | 20 | 27.88±5.55 | 4.66±0.98 | 2,631.51±322.68 |
| CM | 20 | 33.63±6.89 | 5.02±1.19 | 2,213.95±394.25 |
| IRI | 20 | 34.00±16.39 | 4.74±1.60 | 2,436.47±885.52 |
| CM-IRI | 20 | 28.60±3.54 | 4.47±1.56 | 2,609.40±566.67 |
The incidence of acute kidney
injury
The sample size is primarily based on the prior
study on the AKI model (14). At
the same time, in the pre-experiment, we assessed the incidence of
AKI in each group in general, so we selected a total of 80 rats to
meet the statistical analysis requirement as well as to better
compared the incidence of AKI in each group. Preoperative and
postoperative levels of creatinine were used to determine the
prevalence of AKI. As shown in Table
II, the incidences of AKI rates were 40, 35 and 75% based on
the ESUR criterion, and were 25, 25 and 55% in each group based on
the KDIGO criterion, CM-IRI group has the highest AKI incidence
rate.
 | Table IIIncidence rate of AKI for each
group. |
Table II
Incidence rate of AKI for each
group.
| Group | Cases of AKI, n
(ESUR) | Incidence of AKI
(ESUR, n %) | P-valuea | Cases of AKI, n
(KDIGO) | Incidence of AKI
(KDIGO, n %) |
P-valuea |
|---|
| SHAM | - | - | | - | - | |
| CM | 8 | 40% (8/20) | 0.055 | 5 | 25% (5/20) | 0.1066 |
| IRI | 7 | 35%
(7/20)b | <0.05 | 5 | 25% (5/20) | 0.1066 |
| CM-IRI | 15 | 75% (15/20) | | 11 | 55% (11/20) | |
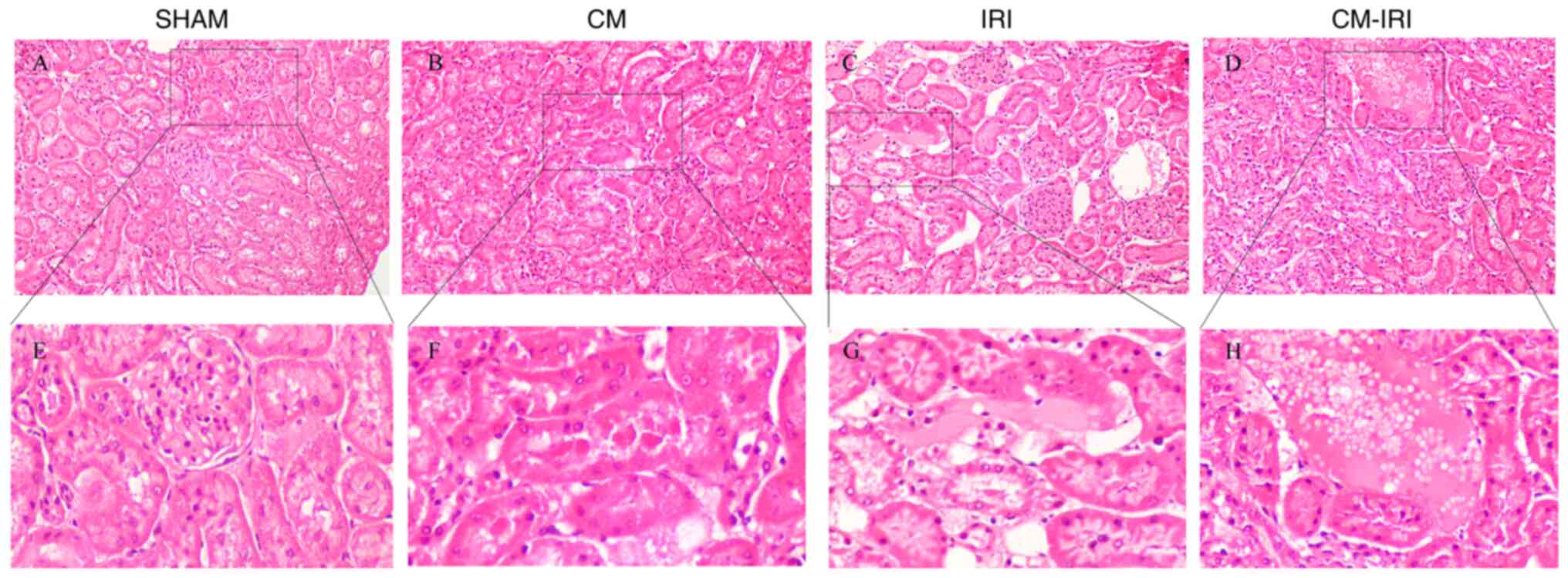
Renal histopathological results
SHAM group showed a normal structure with complete
glomerular structure and no proximal or distal renal tubule damage,
no obvious interstitial edema, cell debris and presence of protein
tubule (Fig. 4A, E). However, CM (Fig. 4B, F), IRI group (Fig. 4C, G) and CM-IRI group (Fig. 4D, H) displayed multiple kinds of renal
tubular injury, including glomerular structure destruction,
interstitial edema, swelling of distal convoluted tubules, protein
degeneration and formation of protein tubes.
The expression of AKI biomarkers and
oxidative stress index
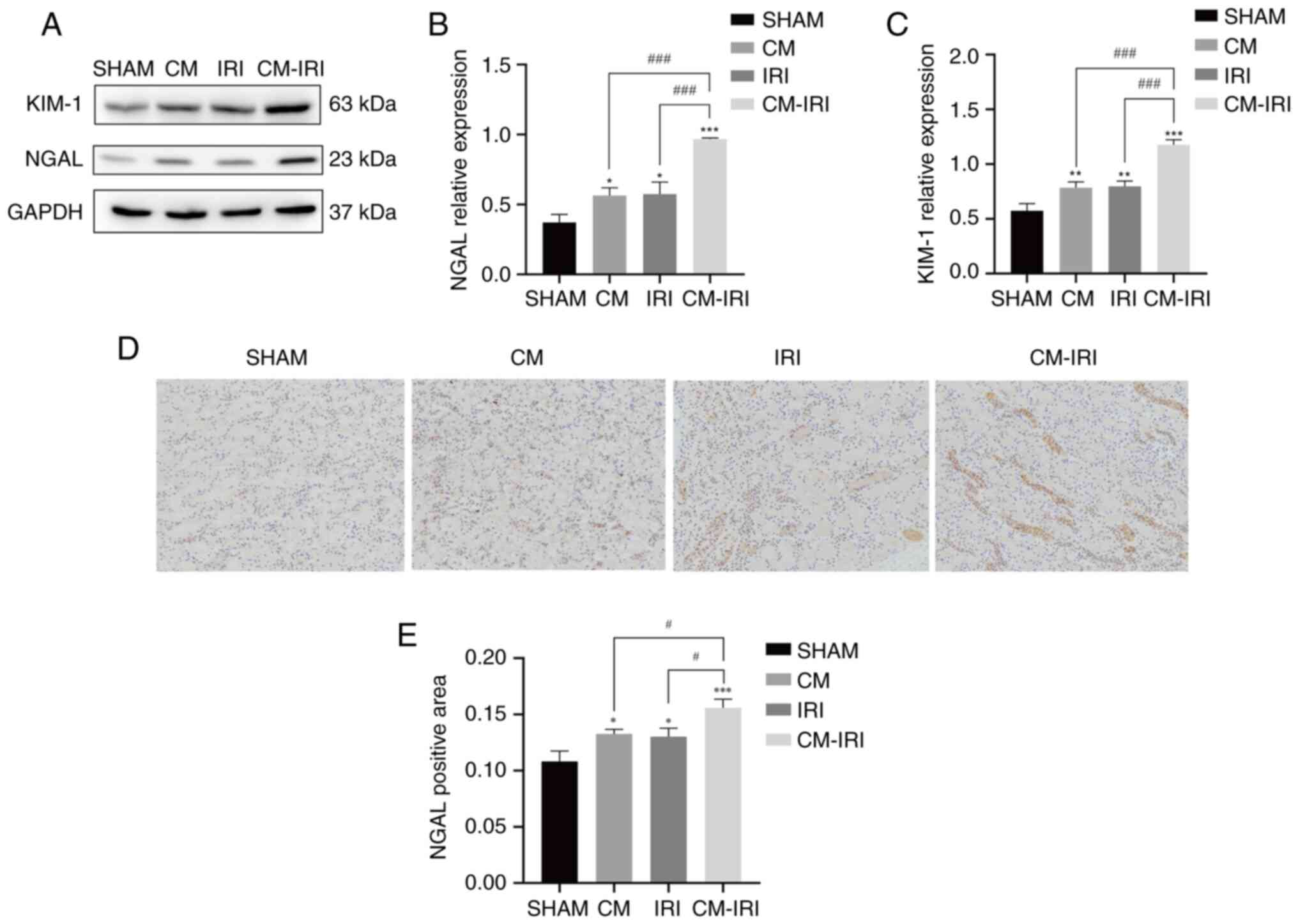
We next examined the expression of AKI biomarkers
and levels of oxidative stress in renal tissues. Western blot
analyses indicated NGAL and KIM-1 levels in the CM-IRI group
increased significantly (Fig.
5A-C), with the CM-IRI group shows a statistically increased
than CM and IRI group. IHC was used to detect the expression and
distribution of NGAL in renal tissue. Compared with the SHAM group,
the expression of NGAL significantly increased in the CM, IRI, and
CM-IRI groups and the positive rate was the highest in the CM-IRI
group (Fig. 5D-E). The level of
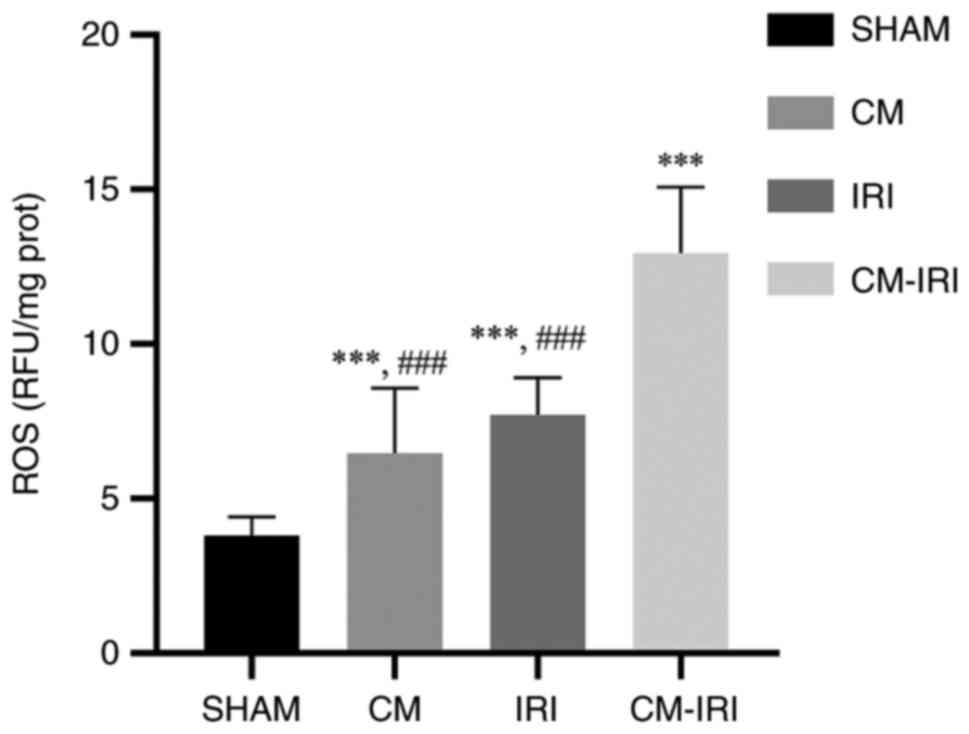
oxidative stress was measured by ROS expression in kidney tissues.
CM, IRI, and CM-IRI groups showed higher ROS levels than sham
group, with the CM-IRI group shows statistically increased than CM
and IRI group (Fig. 6).
Discussion
The present study aimed to establish a post-AMI AKI
model and investigate the stability of model by assessing the
expression of AKI biomarkers, level of renal injury and oxidative
stress. Our study shows that CM combined IRI surgery has a higher
postoperative AKI rate than single factor induced AKI, as well as
more severe renal damage in pathology and a high level of oxidative
stress in pathophysiology, indicating that oxidative stress injury
may play a significant role in post-AMI AKI.
Various preoperative risk factors and mechanisms
have been related to post-AMI AKI, including preoperative declining
kidney function, and excessive use of intraoperative contrast
media, in which contrast media induced renal hypoperfusion and
hypovolemia are pivotal elements in pathophysiology (15). During the PCI surgery, heart
vessels are exposed to a high dose of contrast media, which is
later eliminated by the kidney. Firstly, contrast media in the
circulation will be filtered through the glomeruli and as it
progressively passed through the renal tubules, the concentration
of contrast media in the distal convoluted tubules will gradually
increase (16). Meanwhile, the
viscosity of the fluid in the renal tubules increased due to the
elevated concentration of CM, which prolonged kidney exposure to
contrast media, resulting in congestion of the tubules and reduced
kidney blood flow. This process may be affected by the amount of
contrast media, the character of contrast media, and perioperative
venous hydration. Previous studies show that physicochemical
properties of contrast media can also cause renal vasoconstriction
and hemodynamic change, causing renal hypoperfusion. Under high
osmotic conditions, plasma water flows from vascular lumen to
interstitium, resulting in enrichment of contrast media in vasa
recta, thereby increasing local blood viscosity and local vascular
resistance. Meanwhile, high osmosis can increase urate levels,
resulting in renal tubular obstruction and poor drainage (17).
Furthermore, the state of basic renal function acts
as a major contributor to the development of kidney injury. In the
study of Cheng et.al, the CI-AKI model was constructed with
furosemide combined contrast media demonstrated that dehydration 24
to 48 h before surgery or using diuretics prior to surgery can
reduce basic renal function and more effectively induce AKI
(18). In dehydrated individuals,
most of the water is reabsorbed by the renal tubules during
elimination of contrast media, leaving the high viscosity of
contrast media that may cause damage to renal tubular epithelial
cells. However, the AKI rate in the CM-induced AKI models is
relatively low and solely concentrates on one organ. As a result,
our research focuses on developing an animal model of a contrast
agent associated with IRI surgery that is similar to the incidence
of AKI in clinical patients following PCI or CAG and also better
investigates the connection between the heart and kidney.
Therefore, based on previous research on the AKI model, we used
ioprolamine, a commonly used contrast media in clinical practice to
construct our model. All the rats were dehydrated 24 h before
surgery as a pre-treating procedure. Consistent with previous
research, the model of contrast media after dehydration alone had a
low postoperative AKI rate of around 30% (19). Given extended postoperative nursing
time increases rat mortality, we collected blood, heart, and kidney
24 h following surgery. Compared with other traditional 72-h animal
modeling methods, 24 h was sufficient for rats to meet AKI
standards. In the meantime, the postoperative nursing time for the
rats was reduced, which contributed to shortening the animal
modeling cycle.
However, not all AKI observed after exposure to
contrast media are caused by the contrast media itself,
pathological processes that occur during coronary
ischemia-reperfusion may also lead to kidney injury. During
myocardial infarction, blood vessels of other organs contract to
compensate for the decreased blood volume caused by the infarcted
coronary artery (17). As an organ
with a high blood volume, the kidney may reduce the effective blood
volume to support and maintain the basis cardiac function. Besides,
harmful substances are released into the circulation due to
multiple organ injuries caused by myocardial ischemia-reperfusion,
these harmful substances may cause an additional injury by the
absorption of the kidney (20). As
a result, we combine the preoperative use of a contrast media with
coronary ischemia reperfusion surgery to established the post-AMI
AKI model. This model can better observe the effect of CM and IRI
surgery on kidney injury, which is compatible with the disease
background of post-AMI AKI. Under the double effects of CM and IRI
surgery on kidney blood volume and kidney function, the incidence
rate of AKI in the CM-IRI group is significantly higher than that
of the other two groups.
Clinically, there are two main ways to define AKI
patients: ESUR and KDIGO criteria and using clinical criteria can
better compare the AKI modeling rate between groups. Based on the
ESUR definition, 15 rats in the CM-IRI group met the criteria. And
based on the KDIGO definition, only 11 rats in the CM-IRI group met
the criteria. Because the KDIGO criterion standard is more
stringent than the others, the number of AKI under this threshold
is smaller. The CM-IRI group had the highest incidence of AKI in
both definitions, indicating that contrast media combined with IRI
operation produced more stimulation in rats.
Recent research has demonstrated the importance of
oxidative stress in AKI (21,22).
Excessive production of reactive oxygen species (ROS) causes
oxidative damage to mitochondria and lipids (23). Quintavalle et al. demonstrated that
CM may result in a dose-response increase in reactive oxygen
species production, which activates Jun N-terminal kinases (JNK1/2)
and p38 stress kinases (24). In
our study, markers of oxidative stress such as ROS were
significantly increased in the CM-IRI group. Similar results were
observed in AKI biomarkers, NGAL and KIM-1 are biomarkers of early
renal injury and are highly expressed in kidney tissue of the
CM-IRI group. The results of creatinine and expression of
biomarkers in the kidney were consistent with the increased
expression of ROS suggesting contrast media can exacerbated acute
kidney injury caused by IRI through oxidative stress. And elevated
oxidative stress levels could be the cause of CM-IRI group had the
highest number of postoperative AKI.
This model has some limitations. First, our rats
were dehydrated for 24 h before surgery as a pre-treatment, and
multiple dehydration time points or diuretic can be added to the
model to further reduce renal function. Second, the occurrence of
AMI in our models was determined by an elevated ST segment in an
electrocardiogram. Thus, from the perspective of disease, the
animal model of myocardial infarction in our study is more similar
to STEMI patients in clinical practice. In our study, we used ROS
as the primary indicator of oxidative stress. But some secondary
indicators, like MDA or CAT, can also be used to investigate
oxidative stress, and their importance shouldn't be overlooked.
Therefore, the detection of these secondary indicators can be
considered in subsequent experiments to comprehensively evaluate
oxidative stress. Finally, based on the KDIGO standard, the number
of AKI rats was small. As a consequence, animal research needs to
be improved further, such as expanding sample size as well as
including result analysis at different time points.
In conclusion, we established a reliable and stable
animal model by combined CM with IRI surgery, the model has a high
rate of postoperative AKI and our model can better simulate
clinical features of post-AMI AKI. Meanwhile, we proved the
important role of oxidative stress in our model by detecting ROS
levels. Combined with the detection of kidney injury indicators
such as NGAL and KIM-1, we demonstrated that contrast media can
exacerbate acute kidney injury caused by IRI through oxidative
stress and cause more severe kidney damage in rats. Our study
provides an animal model basis for further exploring the
pathogenesis of the disease. And this work emphasized that reducing
oxidative stress levels may be a potential approach to preventing
or treating the disease of post-AMI AKI.
Acknowledgements
Not applicable.
Funding
Funding: Financial support for the research, authorship, and/or
publication of this article was received from: Guangdong Provincial
Science and Technology Project (grant no. KJ022021049); Guangdong
Provincial Key Laboratory of Coronary Heart Disease Prevention
(grant no. Y0120220151); General Program of Hainan Natural Science
Foundation (grant no. 818MS132); Key Laboratory of Emergency and
Trauma (Hainan Medical University), Ministry of Education (grant
no. KLET-202116) and NSFC Incubation Project of Guangdong
Provincial People's Hospital (grant no. KY0120220041).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY and JC conceived and designed the study. XD, WL
and HL collected data and performed animal surgery. YX wrote the
original draft and performed analysis and interpretation of data.
YZ, KH and JLia analyzed data and the statistical analysis. JX,
JLiu and YL made substantial contributions to the conception and
acquisition of data. YL and JC confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Animal Ethics
Committee of Guangdong Provincial People's Hospital (approval no.
GDRECKY2020-266-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tsai TT, Patel UD, Chang TI, Kennedy KF,
Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Messenger JC,
Rumsfeld JS and Spertus JA: Contemporary incidence, predictors, and
outcomes of acute kidney injury in patients undergoing percutaneous
coronary interventions: Insights from the NCDR Cath-PCI registry.
JACC Cardiovasc Interv. 7:1–9. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tao J, Dai W, Ye C, Yao Q, Zhou M and Li
Y: Preprocedural Lp(a) level and ApoB/ApoA-Ι ratio and the risk for
contrast-induced acute kidney injury in patients undergoing
emergency PCI. Lipids Health Dis. 20(130)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Anzai A, Anzai T, Naito K, Kaneko H, Mano
Y, Jo Y, Nagatomo Y, Maekawa Y, Kawamura A, Yoshikawa T and Ogawa
S: Prognostic significance of acute kidney injury after reperfused
ST-elevation myocardial infarction: Synergistic acceleration of
renal dysfunction and left ventricular remodeling. J Card Fail.
16:381–389. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Khoury S, Margolis G, Ravid D, Rozenbaum
Z, Keren G and Shacham Y: Outcomes of early and reversible renal
impairment in patients with ST segment elevation myocardial
infarction undergoing percutaneous coronary intervention. Eur Heart
J Acute Cardiovasc Care. 9:684–689. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Marenzi G, Cosentino N, Milazzo V, De
Metrio M, Rubino M, Campodonico J, Moltrasio M, Marana I, Grazi M,
Lauri G, et al: Acute kidney injury in diabetic patients with acute
myocardial infarction: Role of acute and chronic glycemia. J Am
Heart Assoc. 7(e008122)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fox CS, Muntner P, Chen AY, Alexander KP,
Roe MT and Wiviott SD: Short-term outcomes of acute myocardial
infarction in patients with acute kidney injury: A report from the
national cardiovascular data registry. Circulation. 125:497–504.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xie XC, Cao Y, Yang X, Xu QH, Wei W and
Wang M: Relaxin attenuates contrast-induced human proximal tubular
epithelial cell apoptosis by activation of the PI3K/Akt signaling
pathway in vitro. Biomed Res Int. 2017(2869405)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhao L, Hu C, Zhang P, Jiang H and Chen J:
Novel preconditioning strategies for enhancing the migratory
ability of mesenchymal stem cells in acute kidney injury. Stem Cell
Res Ther. 9(225)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li Z, Wu J, Wei W, Cai X, Yan J, Song J,
Wang C and Wang J: Association of serum miR-186-5p with the
prognosis of acute coronary syndrome patients after percutaneous
coronary intervention. Front Physiol. 10(686)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Besseling PJ, Pieters TT, Nguyen ITN, de
Bree PM, Willekes N, Dijk AH, Bovée DM, Hoorn EJ, Rookmaaker MB,
Gerritsen KG, et al: A plasma creatinine- and urea-based equation
to estimate glomerular filtration rate in rats. Am J Physiol Renal
Physiol. 320:F518–F524. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Stacul F, van der Molen AJ, Reimer P, Webb
JA, Thomsen HS, Morcos SK, Almén T, Aspelin P, Bellin MF, Clement
O, et al: Contrast induced nephropathy: Updated ESUR contrast media
safety committee guidelines. Eur Radiol. 21:2527–2541.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lameire N and Kellum JA: KDIGO AKI
Guideline Work Group. Contrast-induced acute kidney injury and
renal support for acute kidney injury: A KDIGO summary (part 2).
Crit Care. 17(205)2013.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Cheng H, Fan X, Lawson WE, Paueksakon P
and Harris RC: Telomerase deficiency delays renal recovery in mice
after ischemia-reperfusion injury by impairing autophagy. Kidney
Int. 88:85–94. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu YH, Xue JH, Wu DX, Bei WJ, Wang K, Liu
Y, Chen JY and Tan N: A novel simple experimental model for
low-osmolar contrast-induced acute kidney injury using different
definitions based on the levels of serum creatinine and cystatin C.
BMC Nephrol. 20(243)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou Q, Zhao C, Xie D, Xu D, Bin J, Chen
P, Liang M, Zhang X and Hou F: Acute and acute-on-chronic kidney
injury of patients with decompensated heart failure: Impact on
outcomes. BMC Nephrol. 13(51)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jost G, Lengsfeld P, Lenhard DC, Pietsch
H, Hütter J and Sieber MA: Viscosity of iodinated contrast agents
during renal excretion. Eur J Radiol. 80:373–377. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ye Z, Lu H, Su Q, Guo W, Dai W, Li H, Yang
H and Li L: Clinical effect of trimetazidine on prevention of
contrast-induced nephropathy in patients with renal insufficiency:
An updated systematic review and meta-analysis. Medicine
(Baltimore). 96(e6059)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cheng W, Zhao F, Tang CY, Li XW, Luo M and
Duan SB: Comparison of iohexol and iodixanol induced
nephrotoxicity, mitochondrial damage and mitophagy in a new
contrast-induced acute kidney injury rat model. Arch Toxicol.
92:2245–2257. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sharma A, Kilari S, Cai C, Simeon ML and
Misra S: Increased fibrotic signaling in a murine model for
intra-arterial contrast-induced acute kidney injury. Am J Physiol
Renal Physiol. 318:F1210–F1219. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hašková P, Jansová H, Bureš J, Macháček M,
Jirkovská A, Franz KJ, Kovaříková P and Šimůnek T: Cardioprotective
effects of iron chelator HAPI and ROS-activated boronate
prochelator BHAPI against catecholamine-induced oxidative cellular
injury. Toxicology. 371:17–28. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rubinstein I, Abassi Z, Milman F,
Ovcharenko E, Coleman R, Winaver J and Better OS: Hyperbaric oxygen
treatment improves GFR in rats with ischaemia/reperfusion renal
injury: A possible role for the antioxidant/oxidant balance in the
ischaemic kidney. Nephrol Dial Transplant. 24:428–436.
2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tanaka S, Sugiura Y, Saito H, Sugahara M,
Higashijima Y, Yamaguchi J, Inagi R, Suematsu M, Nangaku M and
Tanaka T: Sodium-glucose cotransporter 2 inhibition normalizes
glucose metabolism and suppresses oxidative stress in the kidneys
of diabetic mice. Kidney Int. 94:912–925. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Boekhoud L, Koeze J, van der Slikke EC,
Bourgonje AR, Moser J, Zijlstra JG, Muller Kobold AC, Bulthuis MLC,
van Meurs M, van Goor H, et al: Acute kidney injury is associated
with lowered plasma-free thiol levels. Antioxidants (Basel).
9(1135)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Quintavalle C, Brenca M, De Micco F, Fiore
D, Romano S, Romano MF, Apone F, Bianco A, Zabatta MA, Troncone G,
et al: In vivo and in vitro assessment of pathways involved in
contrast media-induced renal cells apoptosis. Cell Death Dis.
2(e155)2011.PubMed/NCBI View Article : Google Scholar
|