1. Introduction
Post-surgical fracture nonunion (PSFN) is defined as
failure to achieve cortical continuity at the fracture site as
determined by radiological examination at 6-9 months after the
orthopedic operation (such as osteotomies and arthrodesis), and had
a prevalence of 4.9-6.8% in patients with fracture during 2011-2019
(1,2). PSFN causes a considerable disease
burden in patients with fractures, including long-term chronic
pain, leading to disability and reducing the quality of life.
Furthermore, it may be associated with an increased risk of death
(3-6).
Currently, the most commonly used treatment modalities for patients
with PSFN include drug therapy (such as skeleton growth factor and
teriparatide) and surgical therapy (autogenous bone grafting and
internal fixation surgery), with surgical therapy being regarded as
the gold standard (7-9).
Although the union rate is as high as 70.4-89.2% (reported in
different countries during 2011-2021) after surgical intervention,
a non-negligible proportion of patients with PSFN still face
post-surgical complications due to its invasiveness (10-12).
Therefore, less invasive methods are needed to improve the
prognosis of patients with PSFN.
Extracorporeal shock wave treatment (ESWT) is a
treatment modality that converts the acoustic pulses to a shock
wave, and it delivers these short and intense acoustic energy
impulses into the targeted bone fracture site through skin and
superficial tissues, which then convert into the kinetic energy and
exert their therapeutic effect (13-15).
Previous studies have reported the efficacy of ESWT in treating
patients with fracture nonunion (14,15).
For instance, a randomized controlled trial enrolled 126 patients
with long-bone nonunions and these patients received ESWT or
surgical treatment (including intramedullary nail fixation, plate
fixation and combined nail and plate fixation), and this study
indicated that ESWT could achieve similar union rates compared with
that achieved with surgical therapy (71 vs. 74%) (14). In another study, ESWT induced a
union rate of 73% in patients with fracture nonunion, which was
similar to that observed in patients receiving surgical treatment
(15). However, to the best of our
knowledge, there are no studies that comprehensively evaluated the
rationale, mechanism and implementation of ESWT in patients with
PSFN, thus the present review aimed to address this issue.
2. Mechanisms of ESWT in treating patients
with PSFN
Restart of the union procedure
The concept of ESWT is as follows: The acoustic
pulses are converted to a shock wave by the lithotripter, and the
shock wave can propagate (the propagation of the shock wave in the
media may be described as the propagation of sound in the media) in
all types of media (including human soft tissue and bone). However,
due to the different acoustic impedance of various media, its
attenuation varies. If the acoustic impedance (which may be
described as the resistance faced by the shock wave during the
transmission of the medium; a higher acoustic impedance is
associated with a higher resistance faced by the shock wave) is
different at the interface of two substances, attenuation will
occur at the interface, which may convert to other energies (such
as kinetic energy). When a shock wave passes through human tissues,
its energy is not easily absorbed by superficial tissues (such as
the fat layer and muscle), but can directly reach the bone tissue.
In the process of transmission to bone tissue, acoustic energy is
lost, and part of the lost acoustic energy is converted to kinetic
energy, which causes bone tissue damage and may further restart the
bone union procedure (16). It has
been reported that ESWT can deliver a high-energy shock wave within
a short life cycle (~10 msec) to the targeted bone fracture site
(17), thus ESWT can affect the
bone tissue without damaging the soft tissues. ESWT can cause tear
and shear forces at transition sites, leading to the formation of
microfractures at the targeted fracture site and dividing the bone
with sclerosis into minor bone fragments (0.1-3.0 mm3).
Finally, the small bone fragments can fill the fracture site,
acting as an autologous bone graft (18). At the same time, local bleeding can
occur at the microfracture site followed by formation of hematoma,
thereby restarting the fracture trauma, aggravating inflammatory
responses, releasing various inflammatory cytokines (such as IL-1β
and IL-6) and recruiting osteoblasts. Consequently, the bone
healing process may be restarted (19,20).
At present, two types of ESWT are commonly applied:
Focused ESWT (fESWT) and radial ESWT (rESWT). The former converts
the acoustic pulses to a focused acoustic pressure shock wave,
creating a high-pressure spot at the targeted fracture nonunion
site, while the latter produces stress waves by striking the metal
applicator, affecting the targeted fracture nonunion (13). There are some differences between
the fESWT and rESWT: i) fESWT can lead to a higher speed of
velocity of the wave in the soft tissue, while the speed of rESWT
is slower (21); ii) the pressure
of f-ESWT would rise in a sharp manner during a very short period,
while the pressure of r-ESWT would increase in a linear manner with
a long rise time duration; and iii) the wave from fESWT is more
focused on the target tissue than that of rESWT, which makes it
easier to reach the lesion at depth. Therefore, fESWT is more
frequently used in treating bone pathology with deep penetration;
in addition, it applies high-energy shock waves and anesthesia is
commonly needed (21,22). By contrast, rESWT involves
mid-low-energy shock waves, which are frequently used in patients
with soft tissue disease (such as carpal tunnel syndrome) (13,23)
and lately for treating fracture nonunion of superficial bones
(such as navicular bone and tibia) (24,25).
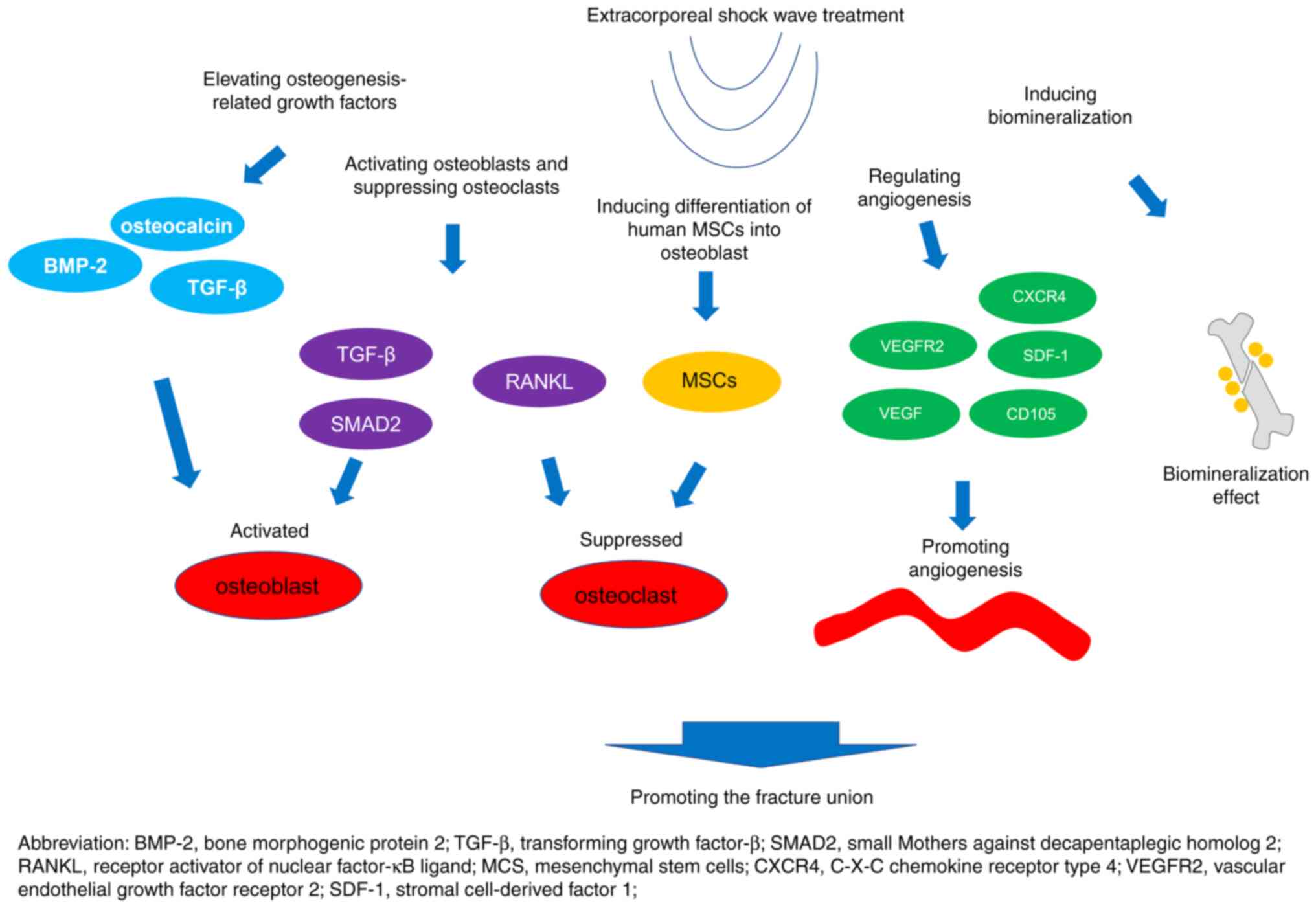
Promotion of osteogenesis-related
growth factors
ESWT can promote fracture union via some
osteogenesis-related growth factors such as bone morphogenic
protein 2 (BMP-2), osteocalcin and TGF-β (26-28).
For instance, an in vivo study demonstrated that ESWT could
achieve improved tibia healing and fracture remodeling, and
increase bone mineral density values and the bone tissue formation
by regulating the VEGF, van Willebrand factor, proliferation cell
nuclear antigen, BMP-2 and osteocalcin released by the
osteoprogenitors (26). Another
study showed that ESWT could improve mineral density, induce bone
formation and increase the expression of type I collagen and
osteocalcin (27). Furthermore,
the ESWT has also been reported to be involved in the regulation of
osteogenesis-related growth factors, such as TGF-β, which further
participates in the fracture union (Fig. 1) (28).
Activation of osteoblasts and
deactivation of osteoclasts
ESWT regulates osteoblast differentiation and
maturation through several pathways, such as the TGF-β/SMAD2
signaling pathway (29), and the
differentiation and maturation of the osteoblasts is reported to be
associated with new bone formation and development (29,30).
Furthermore, it has been reported that ESWT promotes the
differentiation of chondroblasts both in vivo and in
vitro, which further induces endochondral ossification and
implies its potential in facilitating fracture union (31).
In addition, another study established an
osteoporosis rat model and treated the rats with ESWT, finding that
ESWT could suppress the osteoclast activity and further promote
bone healing (32-34)
(Fig. 1). During this process, the
actin-bundling protein L-plastin (LPL) may serve a fundamental
role. It has been reported that LPL may be regulated following the
regulation effect of receptor activator of NF-κB on the
PI3K/AKT/specific protein 1, and the deletion of LPL may inhibit
preosteoclast fusion by regulating the formation of filopodia,
which further participate in the bone union procedure (35).
Differentiation of human mesenchymal
stem cells (MSCs)
Chen et al (36) demonstrated that ESWT promoted the
proliferation, survival and migration of MSCs. In addition, the
same study revealed that ESWT was also involved in osteogenic
differentiation through several mechanisms such as: i) Enhancement
of the activity of alkaline phosphatase; and ii) regulation of the
expression of runt-related transcription factor-2, type I collagen,
osteocalcin and osteopontin. In another study, ESWT with
0.4-mJ/mm2 energy flux density was able to double the
proliferation rate of MSCs (37).
At the same time, ESWT also enhanced the differentiation of MSCs
into osteoblasts, which implied its potential role in promoting
fracture union (37). Chen et
al (38) performed an in
vitro experiment in which bone marrow-derived MSCs were treated
with ESWT, and found that ESWT could stimulate the proliferation
and osteogenic differentiation of bone marrow-derived MSCs
(Fig. 1). Further in vivo
experiments in the same study revealed that seeding ESWT-treated
MSCs on poly-lactic-co-glycolic acid scaffolds could induce faster
bone formation with more mineral apposition inside the defect site
compared with that of MSCs only (38).
Angiogenesis
Angiogenesis serves an essential role in fracture
union (39,40). A recent study revealed that ESWT
could increase the protein expression levels of cell angiogenesis
receptor VEGFR2 and angiogenesis biomarkers (VEGF/C-X-C chemokine
receptor type 4/stromal cell derived factor-1 axis) (41). Another study, which involved an
in vivo mouse model with skin wounds treated with ESWT,
indicated that ESWT could promote wound healing through
regeneration of microcirculation and angiogenesis. In addition, a
higher ESWT pulse was associated with an improved recovery effect
(42). Furthermore, Modena et
al (43) suggested that ESWT
could activate angiogenesis by upregulating the angiogenesis
markers (CD105 and VEGF) (Fig.
1).
Biomineralization
Sternecker et al (44) treated zebra mussel Dreissena
polymorpha with ESWT and analyzed the biological response to
evaluate the molecular mechanism of newly formed mineralized tissue
after ESWT. The study found that ESWT with a 0.4 mJ/mm2
energy density could achieve an increment of bone mineralization
compared with the control. This finding was further supported by a
recent study that proposed a positive association between ESWT
energy and the fluorescence intensity of the mineralized tissue
(45). The two aforementioned
studies suggested that ESWT promoted fracture union by inducing
biomineralization (Fig. 1).
Others
Although there is still no definite conclusion, some
studies have focused on the mechanism of ESWT on the
musculoskeletal and neuromuscular system (46-48).
ESWT could alter the elasticity and extensibility of the muscle,
which would further benefit the bone union (49,50).
However, more studies are needed for further exploration.
3. Efficacy and safety of ESWT in treating
patients with PSFN
ESWT monotherapy
In 2001, Rompe et al (51) performed a study on 42 patients with
PSFN who were previously treated with pseudarthroses after fracture
or corrective osteotomies. All patients received fESWT with 3,000
impulses at 0.6 mJ/mm2 energy flux density for 50-75 min
after local anesthesia with a bone union rate of 72.0% at 9 months
after fESWT. Furthermore, Elster et al (52) re-evaluated the efficacy of fESWT in
172 patients with PSFN who underwent bone fixation (including
external, internal or intramedullary fixation, casting, plaster
cast, bone graft and autograft). The patients with PSFN received
fESWT with a median impulse number of 4,000 at 0.38-0.40
mJ/mm2 energy flux density for 20-60 min after general
or local anesthesia. The results indicated a fracture union rate of
80.2% after a mean follow-up of 4.8±4.0 months (from the first
fESWT to the fracture union).
The wide application of fESWT for the treatment of
patients with PSFN allowed for the efficiency of rESWT in patients
with PSFN to be further recognized and revealed. In 2013, Zhang
et al (53) studied 42
patients with PSFN who previously received external, internal or
intramedullary fixation. The patients were treated with rESWT at
different shock dosages and it was found that the rESWT with 1,000
impulses of shock waves group exhibited a fracture union rate of
only 28.6%, which was lower compared with that in patients with
PSFN receiving rESWT with 2,000 (85.7%) and 3,000 (78.6%) impulses
of shock waves. Based on these findings, the use of an rESWT dosage
of <2,000 impulses of shock waves was excluded from subsequent
clinical trials. In 2017, Kertzman et al (24) reported that the rESWT with 3,000
impulses of shock waves at 0.18 mJ/mm2 energy flux
density per session could achieve a fracture union rate of 72.7% at
6 months after rESWT in patients with PSFN who were previously
treated with internal plates, nails or intramedullary/internal
screw fixations. Furthermore, a recent case report also showed the
efficacy of rESWT with 3,000 impulses of shock waves in a patient
with PSFN (25). Studies reporting
the efficacy of ESWT in patients with PSFN are summarized in
Table I.
 | Table IInformation on the studies reporting
the efficacy of ESWT in patients with post-surgical fracture
nonunion. |
Table I
Information on the studies reporting
the efficacy of ESWT in patients with post-surgical fracture
nonunion.
| A, Efficacy of ESWT
monotherapy |
|---|
| | Treatment
modality | Fracture union
rate | |
|---|
| First author/s,
year | Study type | No. of cases | Previous surgery
type for fracture | Intervention | Control | Intervention | Control | (Refs.) |
|---|
| Rompe et al,
2001 | Cohort | 42 | Pseudarthroses
after fracture or corrective osteotomies | fESWT | - | 72.0% (31/43) | - | (51) |
| Elster et
al, 2009 | Cohort | 172 | Fixation (including
external, internal or intramedullary fixation, casting, plaster
cast, bone graft and autograft) | fESWT | - | 80.2%
(138/172) | - | (52) |
| Zhang et al,
2013 | Cohort | 42 | External, internal
or intramedullary fixation | rESWT with 1,000
(group 1) and 2,000 (group 2) shock dosages | rESWT with 3,000
shock dosages | Group 1, 28.6%
(4/14); and group 2, 85.7% (12/14) | 78.6% (11/14) | (53) |
| Kertzman et
al, 2017 | Cohort | 22 | Internal plates,
nails and intramedullary/ internal screw fixations | rESWT | - | 72.7% (16/22) | - | (24) |
| Yue et al,
2021 | Case report | 1 | Intramedullary
nailing | rESWT | - | 100.0% (1/1) | - | (25) |
| B, ESWT compared
with surgical treatment |
| | Treatment
modality | Fracture union
rate | |
| First author/s,
year | Study type | No. of cases | Previous surgery
type for fracture | Intervention | Control | Intervention | Control | (Refs.) |
| Cacchio et
al, 2009 | RCT | 126 | Orthopedic
operation | Group 1, fESWT with
Dornier lithotripter; and group 2, fESWT with Storz
lithotripter | Surgical
treatment | Group 1, 70.0%
(26/37); and group 2, 71.0% (27/38) | 73.0% (28/38) | (14) |
| Huang et al,
2015 | RCT | 72 | External,
intramedullary fixation | rESWT | Autogenous bone
grafting | 87.2% (31/35) | 93.9% (29/31) | (54) |
| Wu et al,
2021 | RCT | 65 | Intramedullary
nailing | fESWT | Intramedullary
nailing | 97.0% (32/33) | 75.0% (24/32) | (16) |
| C, ESWT combined
with other therapy compared with ESWT monotherapy |
| | Treatment
modality | Fracture union
rate | |
| First author/s,
year | Study type | No. of cases | Previous surgery
type for fracture | Intervention | Control | Intervention | Control | (Refs.) |
| Wang et al,
2006 | Cohort | 42 | External, internal
or intramedullary fixation | fESWT with bone
marrow grafting | fESWT | 84.2% (16/19) | 82.6% (19/23) | (55) |
| Jin et al,
2018 | RCT | 48 | Open reduction and
internal fixation | rESWT with
autologous cell growth factor injection | rESWT | 95.8% (23/24) | 75.0% (18/24) | (56) |
ESWT vs. surgical treatment
The aforementioned surgical treatment (including
external, internal or intramedullary fixation, casting, plaster
cast, bone graft and autograft) is regarded as the gold standard
for the treatment of patients with PSFN (7-9).
Therefore, some studies have also compared the efficacy of ESWT
with that of surgical treatment in patients with PSFN (14,16,54).
In a study by Cacchio et al (14), a total of 126 patients with PSFN
were enrolled and treated with fESWT at 4,000 impulses of shock
waves at 0.40-0.70 mJ/mm2 energy flux density or with
surgical treatment. A fracture union rate of 70-71% was reported in
the fESWT group, which was similar to that in the surgical
treatment group (73%) (14). Huang
et al (54) compared the
efficacy of rESWT with that of autogenous bone grafting in patients
with PSFN and showed that three sessions of rESWT (3,000 impulses
at 80-120 J) with 7-day intervals could achieve a fracture union
rate of 87.2%, which was similar to the fracture union rate
recorded in patients with PSFN receiving autogenous bone grafting
(93.9%).
Furthermore, another study applied a more intensive
ESWT method and compared the efficacy of this intensive method with
that of surgical treatment in patients with PSFN (16). In detail, a total of 65 patients
with PSFN who were previously treated with open reduction and
internal fixation were enrolled. fESWT or intramedullary nailing
were applied in patients with PSFN. In the fESWT group, all
patients received three courses of fESWT. At each course, these
patients received the fESWT for 10 min each, twice a week, for up
to 4 weeks. The patients in the surgical group received the normal
intramedullary nailing surgical treatment instead. The study found
that the fracture union rate could increase to 97.0% in the fESWT
group, which was higher compared with that in the surgical group
(75.0%) (16) (Table I).
ESWT combined with other treatment
modalities
Wang et al (55) compared the efficacy of fESWT
combined with bone marrow grafting with fESWT alone in 42 patients
with PSFN previously treated with external, internal or
intramedullary fixation. fESWT with 2,000 impulses plus the
autologous bone marrow grafting could achieve a fracture union rate
of 84.2%, which was numerically but not statistically significantly
higher compared with that in patients with PSFN receiving fESWT
monotherapy (82.6%). Jin et al (56) determined the efficacy of rESWT with
autologous cell growth factor injection. rESWT with 3,000 impulses
and 0.54 mJ/mm2 energy flux density plus autologous cell
growth factor injection could achieve a fracture union rate of
95.8%, which was higher compared with that in the rESWT group
(75.0%) (Table I).
Safety
The safety profile of ESWT in patients with PSFN is
generally considered acceptable (14,16,24,51,54).
The most common adverse events (AEs) include skin- and
blood-related AEs such as local edema, subcutaneous hematoma and
peripheral blood vessel damage (14,16,24,51,54).
Only a minor proportion of patients report pain (24). In addition to the common AEs,
certain patients with PSFN may suffer from infection, blisters and
skin ulceration (16,54). The safety profile of ESWT in PSFN
is summarized in Table II.
 | Table IISafety profile of ESWT in patients
with post-surgical fracture nonunion. |
Table II
Safety profile of ESWT in patients
with post-surgical fracture nonunion.
| First author/s,
year | ESWT type | Common AEs | (Refs.) |
|---|
| Rompe et al,
2001 | fESWT | Transient local
hematoma | (51) |
| Elster et
al, 2009 | fESWT | Dose-related local
edema, cutaneous petechial hemorrhage and subcutaneous
hematoma | (52) |
| Zhang et al,
2013 | rESWT | N/R | (53) |
| Kertzman et
al, 2017 | rESWT | Pain | (24) |
| Yue et al,
2021 | rESWT | None | (25) |
| Cacchio et
al, 2009 | fESWT | Hematomas | (14) |
| Huang et al,
2015 | rESWT | Local edema,
subcutaneous hematoma and blisters | (54) |
| Wu et al,
2021 | fESWT | Local edema,
infection, skin ulceration, peripheral blood vessel damage and
peripheral nerve damage | (16) |
| Wang et al,
2006 | fESWT | None | (55) |
| Jin et al,
2018 | rESWT | N/R | (56) |
4. Prognostic factors for patients with
PSFN
Prognostic factors of fracture union
in PSFN
Certain studies have explored the predictive factors
for fracture union in patients with PSFN receiving secondary
surgery (57-61).
These prognostic factors mainly focused on demographic
characteristics (such as tobacco usage) and the recovery status of
the fracture nonunion (including the dislocation distance, nonunion
site and the occurrence of callus in the cortex) (57-61).
In a study by Gvozdenovic et al (57), patients with PSFN and minor
dislocation (vs. those PSFN with greater dislocation) at the union
site exhibited a higher fracture union rate after the second
surgical treatment. In a study by Konda et al (59), lower extremity nonunion, tobacco
use, worker's compensation insurance (which is associated with
longer time to return to work and worse functional outcomes
following the surgery), radiographic bone loss and preoperative
short musculoskeletal function assessment function index were
associated with fracture nonunion in patients with PSFN. In
patients with PSFN and femoral neck nonunion, the predictive
factors for revision surgery included a higher preoperative neck
shortening ratio (60). In a study
by Christiano et al (61),
the presentation of the callus and the invisible fracture line in
the cortex could also predict fracture nonunion.
A recent study established a model based on
contrast-enhanced ultrasound that was applied to predict the union
rate for patients with PSFN. The study showed that the peak
enhancement, wash-in area under the curve (defined as the integral
of the signal intensity over time until peak enhancement is
reached) and wash-in perfusion index (defined as the ratio of
wash-in area under the curve to rise time) at the nonunion site
were increased in patients with PSFN and fracture union compared
with those in patients with PSFN and fracture nonunion (60).
Prognostic factors of ESWT in treating
fracture union in PSFN
Although only a small number of studies have
reported the predictive factors for fracture union in patients with
PSFN receiving ESWT, previous evidence has revealed that the shock
wave treatment (vs. no treatment) was associated with an increased
union rate in patients with PSFN (24,52,62).
In addition, a shorter time between fracture and first shock wave
treatment, a shorter interval between the fracture and the surgery,
a good intramedullary stabilization, and an increased number of
extracorporeal shock wave therapy treatments were associated with a
higher fracture union rate (24,52,62).
5. Prospects and limitations
Apart from ESWT, several studies have reported
advances of the treatment in bone regeneration, such as bone
organoid, physical and chemical crosslinked hydrogels,
polyether-ether-ketone (PEEK) and double-network metallopolymer
hydrogels (63-66).
For instance, one study reported that the bone organoid was
constructed in vitro, which could simulate the biological
function of organs in vivo, and a potential strategy for the
construction of bone organoids and their application in bone
reconstruction was described (63). Another study clarified that
biomimetic hydrogels with injectability and compatibility may serve
an essential role in bone defect reconstruction, benefiting from
their numerous advantages, such as extensive selectivity, rapid
gel-forming capacity, tunable mechanical properties and good
biocompatibility (64).
However, several challenges should be considered
before the aforemetioned advances of the treatment's broad
application, such as the poor bonding of PEEK with bone and soft
tissue (65). In terms of the
ESWT, several limitations should also be noted: i) ESWT has a
dose-dependent efficacy but an excessive dose would lead to
excessive damage, while an insufficient dose would not reach the
optimal efficacy, thus finding the optimal dose is a critical issue
that clinicians should consider (53); ii) its effect on other tissues and
organs should be studied more extensively; and iii) the
construction of ESWT equipment deserves further study. Hence, for
the more wide application of these advance methods (including the
ESWT) in the treatment of PSFN, more studies are still needed.
6. Summary
Previous studies have reported that ESWT is able to
promote fracture union in patients with PSFN (14,16,54).
However, to the best of our knowledge, none of these studies
comprehensively evaluated the mechanism, implementation and
prognostic factors of ESWT in patients with PSFN. The present
review aimed to clarify the potential mechanism for ESWT in
promoting the fracture union, which mainly includes: i) Restart of
the bone union process; ii) activation of osteoblasts and
suppression of osteoclasts by elevating osteogenesis-related growth
factors, such as BMP-2 and TGF-β, promoting several pathways, such
as the TGF-β/SMAD2 signaling pathway, and inducing the
differentiation of human MSCs into osteoblasts; iii) promotion of
angiogenesis; and iv) biomineralization induction. The present
review summarizes the efficacy and safety of ESWT in patients with
PSFN, showing that ESWT was effective and tolerable. Furthermore,
the current review considered the potential prognostic factors for
the facture nonunion and efficacy of ESWT in patients with PSFN,
which mainly included demographic characteristics, such as tobacco
usage, recovery status of the fracture nonunion, time interval
between fracture and first shock wave treatment or surgery, and
intramedullary stabilization status. These findings could provide a
theoretical basis for orthopedics specialists to improve
individualized treatments and the application of ESWT in clinical
practice for patients with PSFN. Further high-quality studies are
required to validate these findings.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HW contributed to the study conception and design.
HW and YS drafted and revised the manuscript. Data authentication
is not applicable. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zura R, Watson JT, Einhorn T, Mehta S,
Della Rocca GJ, Xiong Z, Wang Z, Jones J and Steen RG: An inception
cohort analysis to predict nonunion in tibia and 17 other fracture
locations. Injury. 48:1194–1203. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tian R, Zheng F, Zhao W, Zhang Y, Yuan J,
Zhang B and Li L: Prevalence and influencing factors of nonunion in
patients with tibial fracture: Systematic review and meta-analysis.
J Orthop Surg Res. 15(377)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lerner RK, Esterhai JL Jr, Polomano RC,
Cheatle MD and Heppenstall RB: Quality of life assessment of
patients with posttraumatic fracture nonunion, chronic refractory
osteomyelitis, and lower-extremity amputation. Clin Orthop Relat
Res. (295) 28-36:1993.PubMed/NCBI
|
|
4
|
Zura R, Braid-Forbes MJ, Jeray K, Mehta S,
Einhorn TA, Watson JT, Della Rocca GJ, Forbes K and Steen RG: Bone
fracture nonunion rate decreases with increasing age: A prospective
inception cohort study. Bone. 95:26–32. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yin Y, Xu K, Zhang N, Yi Z, Liu B and Chen
S: Clinical and epidemiological features of scaphoid fracture
nonunion: A hospital-based study in Beijing, China. Orthop Surg.
14:2455–2461. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Van Wijck SFM, Van Lieshout EMM, Prins
JTH, Verhofstad MHJ, Van Huijstee PJ, Vermeulen J and Wijffels MME:
Outcome after surgical stabilization of symptomatic rib fracture
nonunion: A multicenter retrospective case series. Eur J Trauma
Emerg Surg. 48:2783–2793. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ho CH, Tzeng SC, Farn CJ and Lee CC:
Teriparatide as an effective nonsurgical treatment for a patient
with basicervical peritrochanteric fracture Nonunion-A case report.
Medicina (Kaunas). 58(983)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kumaran A and Soh HL: Management of
nonunion and malunion after primary mandibular condylar fracture
treatment: A review and recommendations. J Oral Maxillofac Surg.
78:2267–2272. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Neumann MV, Zwingmann J, Jaeger M, Hammer
TO and Sudkamp NP: Non-Union in upper limb fractures-clinical
evaluation and treatment options. Acta Chir Orthop Traumatol Cech.
83:223–230. 2016.PubMed/NCBI
|
|
10
|
Rao BM, Stokey P, Tanios M, Liu J and
Ebraheim NA: A systematic review of the surgical outcomes of
interprosthetic femur fractures. J Orthop. 33:105–111.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang S, Yang Y, Huo Y, Yu J, Sheng L, Sun
X, Liu X, Yin J and Yin Z: Effect of the degree of displacement of
the third fragment on healing of femoral shaft fracture treated by
intramedullary nailing. J Orthop Surg Res. 17(380)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lari A, Kashif S and AlMukaimi A:
Arthroscopic retrograde intramedullary nailing of periprosthetic
fractures after total knee arthroplasty-technique, safety, and
outcomes. Arthroplast Today. 17:47–52. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kwok IHY, Ieong E, Aljalahma MA, Haldar A
and Welck M: Extracorporeal shock wave treatment in foot and ankle
fracture non-unions-A review. Foot (Edinb).
51(101889)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cacchio A, Giordano L, Colafarina O, Rompe
JD, Tavernese E, Ioppolo F, Flamini S, Spacca G and Santilli V:
Extracorporeal shock-wave therapy compared with surgery for
hypertrophic long-bone nonunions. J Bone Joint Surg Am.
91:2589–2597. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Willems A, van der Jagt OP and Meuffels
DE: Extracorporeal shock wave treatment for delayed union and
nonunion fractures: A systematic review. J Orthop Trauma.
33:97–103. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu GB: Effect of extracorporeal shock wave
therapy on fracture nonunion and delayed union. Med Equip.
34:99–100. 2021.(In Chinese).
|
|
17
|
Alkhawashki HM: Shock wave therapy of
fracture nonunion. Injury. 46:2248–2252. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kaulesar Sukul DM, Johannes EJ, Pierik EG,
van Eijck GJ and Kristelijn MJ: The effect of high energy shock
waves focused on cortical bone: An in vitro study. J Surg Res.
54:46–51. 1993.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gadomski BC, McGilvray KC, Easley JT,
Palmer RH, Jiao J, Li X, Qin YX and Puttlitz CM: An investigation
of shock wave therapy and low-intensity pulsed ultrasound on
fracture healing under reduced loading conditions in an ovine
model. J Orthop Res. 36:921–929. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Stojadinovic A, Elster EA, Anam K, Tadaki
D, Amare M, Zins S and Davis TA: Angiogenic response to
extracorporeal shock wave treatment in murine skin isografts.
Angiogenesis. 11:369–380. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ko NY, Chang CN, Cheng CH, Yu HK and Hu
GC: Comparative effectiveness of focused extracorporeal versus
radial extracorporeal shockwave therapy for knee
osteoarthritis-randomized controlled study. Int J Environ Res
Public Health. 19(9001)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sah V, Kaplan S, Ozkan S, Adanas C and
Toprak M: Comparison between radial and focused types of
extracorporeal shock-wave therapy in plantar calcaneal spur: A
randomized sham-controlled trial. Phys Sportsmed. 51:82–87.
2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Saglam G, Cetinkaya Alisar D and Ozen S:
Physical therapy versus radial extracorporeal shock wave therapy in
the treatment of carpal tunnel syndrome: A randomized-controlled
study. Turk J Phys Med Rehabil. 68:126–135. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kertzman P, Csaszar NBM, Furia JP and
Schmitz C: Radial extracorporeal shock wave therapy is efficient
and safe in the treatment of fracture nonunions of superficial
bones: A retrospective case series. J Orthop Surg Res.
12(164)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yue L, Chen H, Feng TH, Wang R and Sun HL:
Low-intensity extracorporeal shock wave therapy for midshaft
clavicular delayed union: A case report and review of literature.
World J Clin Cases. 9:8242–8248. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang CJ, Huang KE, Sun YC, Yang YJ, Ko JY,
Weng LH and Wang FS: VEGF modulates angiogenesis and osteogenesis
in shockwave-promoted fracture healing in rabbits. J Surg Res.
171:114–119. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ginini JG, Emodi O, Sabo E, Maor G, Shilo
D and Rachmiel A: Effects of timing of extracorporeal shock wave
therapy on mandibular distraction osteogenesis: An experimental
study in a rat model. J Oral Maxillofac Surg. 77:629–638.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lu CC, Chou SH, Shen PC, Chou PH, Ho ML
and Tien YC: Extracorporeal shock wave promotes activation of
anterior cruciate ligament remnant cells and their paracrine
regulation of bone marrow stromal cells' proliferation, migration,
collagen synthesis, and differentiation. Bone Joint Res. 9:458–468.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li B, Wang R, Huang X, Ou Y, Jia Z, Lin S,
Zhang Y, Xia H and Chen B: Extracorporeal shock wave therapy
promotes osteogenic differentiation in a rabbit osteoporosis model.
Front Endocrinol (Lausanne). 12(627718)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Song WP, Ma XH, Sun YX, Zhang L, Yao Y,
Hao XY and Zeng JY: Extracorporeal shock wave therapy (ESWT) may be
helpful in the osseointegration of dental implants: A hypothesis.
Med Hypotheses. 145(110294)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kobayashi M, Chijimatsu R, Yoshikawa H and
Yoshida K: Extracorporeal shock wave therapy accelerates
endochondral ossification and fracture healing in a rat femur
delayed-union model. Biochem Biophys Res Commun. 530:632–637.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Inoue S, Hatakeyama J, Aoki H, Kuroki H,
Niikura T, Oe K, Fukui T, Kuroda R, Akisue T and Moriyama H:
Utilization of Mechanical stress to treat osteoporosis: The effects
of electrical stimulation, radial extracorporeal shock wave, and
ultrasound on experimental osteoporosis in ovariectomized rats.
Calcif Tissue Int. 109:215–229. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hu CC, Chang CH, Hsiao YM, Chang Y, Wu YY,
Ueng SWN and Chen MF: Lipoteichoic acid accelerates bone healing by
enhancing osteoblast differentiation and inhibiting osteoclast
activation in a mouse model of femoral defects. Int J Mol Sci.
21(5550)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wallimann A, Magrath W, Pugliese B,
Stocker N, Westermann P, Heider A, Gehweiler D, Zeiter S, Claesson
MJ, Richards RG, et al: Butyrate inhibits osteoclast activity in
vitro and regulates systemic inflammation and bone healing in a
murine osteotomy model compared to antibiotic-treated mice.
Mediators Inflamm. 2021(8817421)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li X, Wang L, Huang B, Gu Y, Luo Y, Zhi X,
Hu Y, Zhang H, Gu Z, Cui J, et al: Targeting actin-bundling protein
L-plastin as an anabolic therapy for bone loss. Sci Adv.
6(eabb7135)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen Q, Xia C, Shi B, Chen C, Yang C, Mao
G and Shi F: Extracorporeal shock wave combined with
teriparatide-loaded hydrogel injection promotes segmental bone
defects healing in osteoporosis. Tissue Eng Regen Med.
18:1021–1033. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Alshihri A, Niu W, Kammerer PW, Al-Askar
M, Yamashita A, Kurisawa M and Spector M: The effects of shock wave
stimulation of mesenchymal stem cells on proliferation, migration,
and differentiation in an injectable gelatin matrix for osteogenic
regeneration. J Tissue Eng Regen Med. 14:1630–1640. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen Y, Xu J, Huang Z, Yu M, Zhang Y, Chen
H, Ma Z, Liao H and Hu J: An innovative approach for enhancing bone
defect healing using PLGA scaffolds seeded with
extracorporeal-shock-wave-treated bone marrow mesenchymal stem
cells (BMSCs). Sci Rep. 7(44130)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang YQ, Tan YY, Wong R, Wenden A, Zhang
LK and Rabie AB: The role of vascular endothelial growth factor in
ossification. Int J Oral Sci. 4:64–68. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Street J, Bao M, deGuzman L, Bunting S,
Peale FV Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL,
Daugherty A, et al: Vascular endothelial growth factor stimulates
bone repair by promoting angiogenesis and bone turnover. Proc Natl
Acad Sci USA. 99:9656–9661. 2002.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sung PH, Yin TC, Chai HT, Chiang JY, Chen
CH, Huang CR and Yip HK: Extracorporeal shock wave therapy salvages
critical limb ischemia in B6 mice through upregulating cell
proliferation signaling and angiogenesis. Biomedicines.
10(117)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sorg H, Zwetzich I, Tilkorn DJ,
Kolbenschlag J, Hauser J, Goertz O, Spindler N, Langer S and Ring
A: Effects of extracorporeal shock waves on microcirculation and
angiogenesis in the in vivo wound model of the diver box. Eur Surg
Res. 62:134–143. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Modena DAO, Soares CD, Candido EC, Chaim
FDM, Cazzo E and Chaim EA: Effect of extracorporeal shock waves on
inflammation and angiogenesis of integumentary tissue in obese
individuals: Stimulating repair and regeneration. Lasers Med Sci.
37:1289–1297. 2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sternecker K, Geist J, Beggel S,
Dietz-Laursonn K, de la Fuente M, Frank HG, Furia JP, Milz S and
Schmitz C: Exposure of zebra mussels to extracorporeal shock waves
demonstrates formation of new mineralized tissue inside and outside
the focus zone. Biol Open. 7(bio033258)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wu W, Maffulli N, Furia JP, Meindlhumer L,
Sternecker K, Milz S and Schmitz C: Exposure of zebra mussels to
radial extracorporeal shock waves: Implications for treatment of
fracture nonunions. J Orthop Surg Res. 16(707)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hsu PC, Chang KV, Chiu YH, Wu WT and
Ozcakar L: Comparative effectiveness of botulinum toxin injections
and extracorporeal shockwave therapy for post-stroke spasticity: A
systematic review and network meta-analysis. EClinicalMedicine.
43(101222)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hsiao MY, Hung CY, Chang KV, Chien KL, Tu
YK and Wang TG: Comparative effectiveness of autologous
blood-derived products, shock-wave therapy and corticosteroids for
treatment of plantar fasciitis: A network meta-analysis.
Rheumatology (Oxford). 54:1735–1743. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chang KV, Chen SY, Chen WS, Tu YK and
Chien KL: Comparative effectiveness of focused shock wave therapy
of different intensity levels and radial shock wave therapy for
treating plantar fasciitis: A systematic review and network
meta-analysis. Arch Phys Med Rehabil. 93:1259–1268. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Gao J, Rubin JM, Chen J and O'Dell M:
Ultrasound elastography to assess botulinum toxin a treatment for
post-stroke spasticity: A feasibility study. Ultrasound Med Biol.
45:1094–1102. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Venkatakrishnan A, Francisco GE and
Contreras-Vidal JL: Applications of brain-machine interface systems
in stroke recovery and rehabilitation. Curr Phys Med Rehabil Rep.
2:93–105. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Rompe JD, Rosendahl T, Schollner C and
Theis C: High-energy extracorporeal shock wave treatment of
nonunions. Clin Orthop Relat Res. (387) 102-111:2001.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Elster EA, Stojadinovic A, Forsberg J,
Shawen S, Andersen RC and Schaden W: Extracorporeal shock wave
therapy for nonunion of the tibia. J Orthop Trauma. 24:133–141.
2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhang LH, Man LB, Huang GL and Xu X:
Effect of different dosage of radial extracorporeal shock waves on
fracture disunite and bone nonunions. Chinese Journal of
Rehabilitation Theory and Practice. 19:978–980. 2013.(In
Chinese).
|
|
54
|
Huang XW, Han W, Liu YJ, Zhang LH, Gong MQ
and Jiang XY: Comparison of the treatment results of hypertrophic
nonunion by using extracorporeal shock wave therapy(ESWT) or
traditional iliac autograft and internal fixation. J Nanjing Med
Univ (Natural Sciences). 35:1432–1436. 2015.(In Chinese).
|
|
55
|
Wang WZ, Xing GY and Zhai L: Clinical
study of extracorporeal shock wave therapy with autograf t of bone
marrow for bone nonunion. Chin J Prim Med Pharm. 13:1057–1059.
2006.(In Chinese).
|
|
56
|
Jin X, Tan YH, Zhang ZY, Ju CJ, Yan W and
Jiang HJ: A clinical study of injection of autologous cell growth
factors combined with extracorporeal shock wave therapy for
treatment of nonunion of lower limb fractures. J Trad Chin Orthop
Trauma. 30:10–13. 2018.(In Chinese).
|
|
57
|
Gvozdenovic R, Presman B, Larsen MB, Radev
DI, Joerring S and Jensen CH: Can CT-scan measurements of humpback
deformity, dislocation, and the size of bony cysts predict union
after surgery for scaphoid nonunion? J Wrist Surg. 10:418–429.
2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Doll J, Waizenegger S, Schmidmaier G,
Weber MA and Fischer C: Contrast-Enhanced Ultrasound: A viable
diagnostic tool in predicting treatment failure after non-union
revision surgery for Upper- and Lower-limb Non-unions. Ultrasound
Med Biol. 47:3147–3158. 2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Konda SR, Carlock KD, Hildebrandt KR and
Egol KA: Predicting functional outcomes following fracture nonunion
repair-development and validation of a risk profiling tool. J
Orthop Trauma. 34:e214–e220. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yin J, Zhu H, Gao Y and Zhang C:
Vascularized fibular grafting in treatment of femoral neck
nonunion: A prognostic study based on long-term outcomes. J Bone
Joint Surg Am. 101:1294–1300. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Christiano AV, Goch AM, Leucht P, Konda SR
and Egol KA: Radiographic union score for tibia fractures predicts
success with operative treatment of tibial nonunion. J Clin Orthop
Trauma. 10:650–654. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Stojadinovic A, Kyle Potter B, Eberhardt
J, Shawen SB, Andersen RC, Forsberg JA, Shwery C, Ester EA and
Schaden W: Development of a prognostic naive bayesian classifier
for successful treatment of nonunions. J Bone Joint Surg Am.
93:187–194. 2011.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chen S, Chen X, Geng Z and Su J: The
horizon of bone organoid: A perspective on construction and
application. Bioact Mater. 18:15–25. 2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Xue X, Hu Y, Wang S, Chen X, Jiang Y and
Su J: Fabrication of physical and chemical crosslinked hydrogels
for bone tissue engineering. Bioact Mater. 12:327–339.
2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Sun C, Kang J, Yang C, Zheng J, Su Y, Dong
E, Liu Y, Yao S, Shi C, Pang H, et al: Additive manufactured
polyether-ether-ketone implants for orthopaedic applications: A
narrative review. Biomater Transl. 3:116–133. 2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Li H, Yang P, Hwang J, Pageni P, Decho AW
and Tang C: Antifouling and antimicrobial cobaltocenium-containing
metallopolymer double-network hydrogels. Biomater Transl.
3:162–171. 2022.PubMed/NCBI View Article : Google Scholar
|