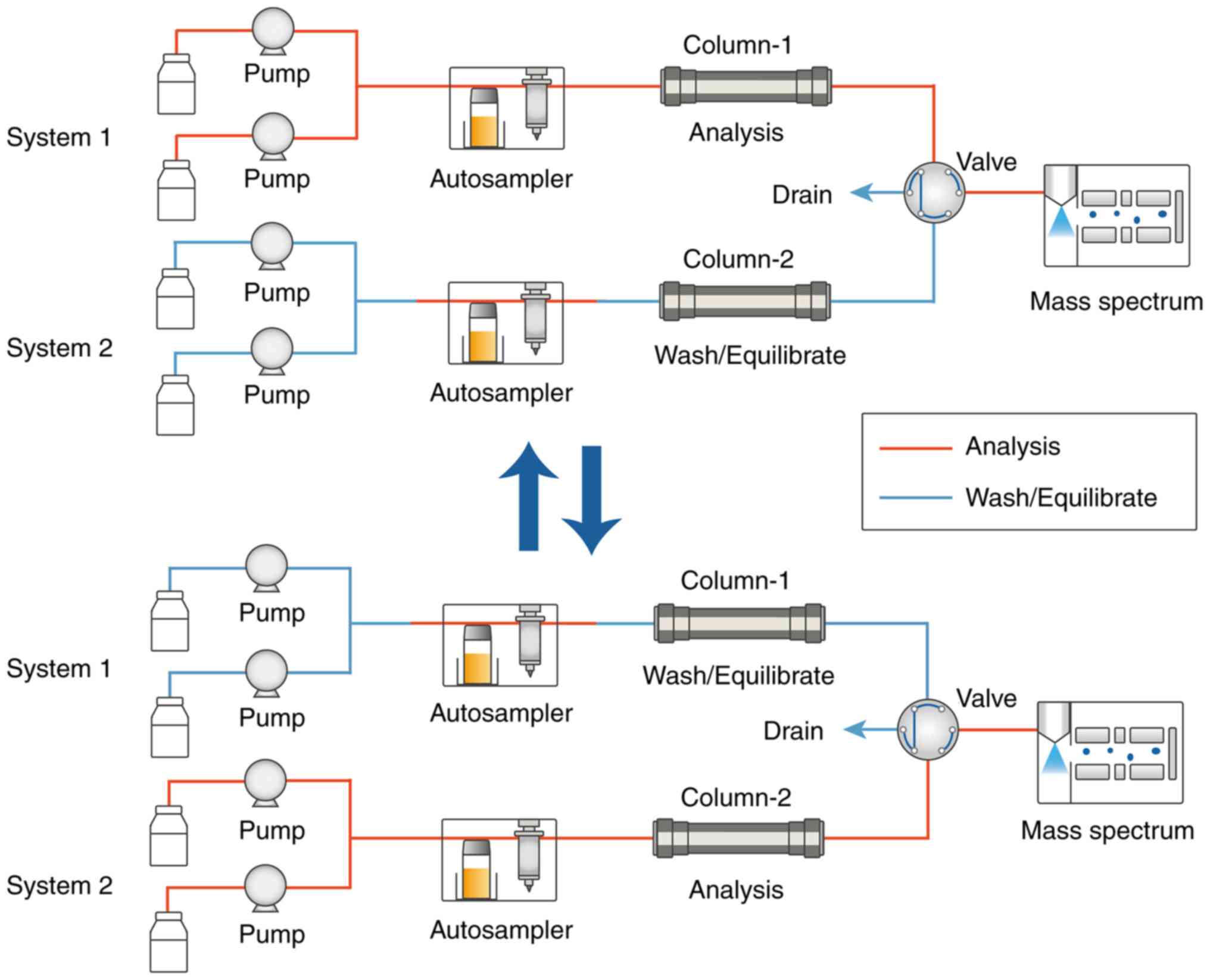
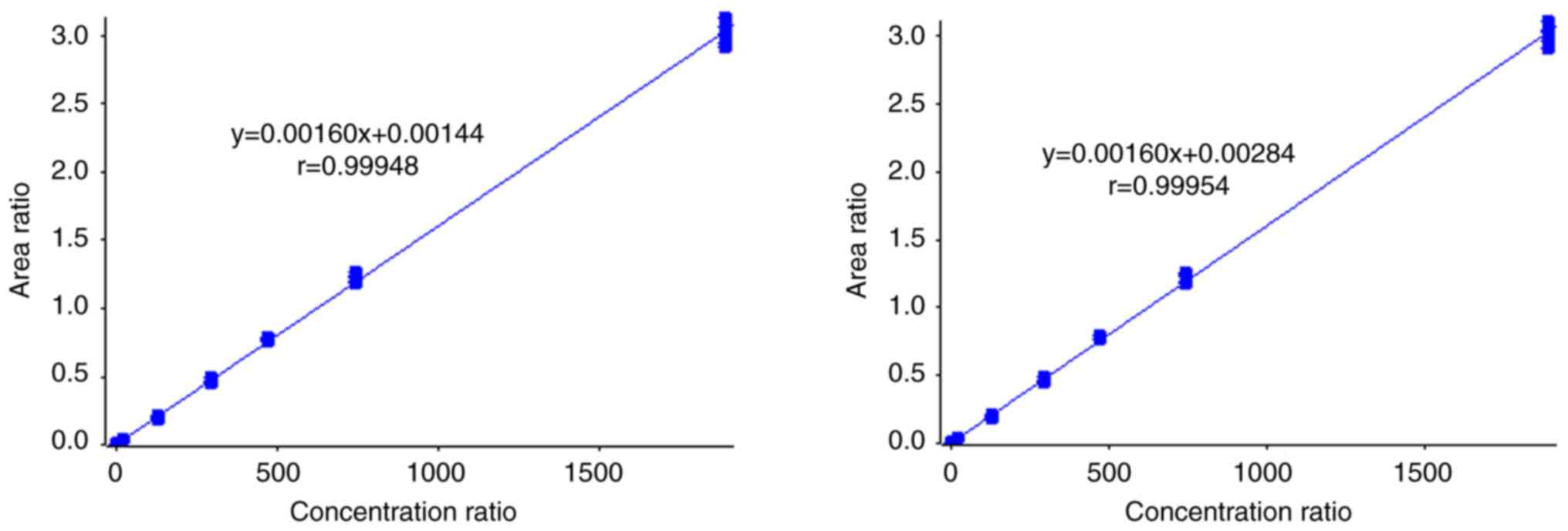
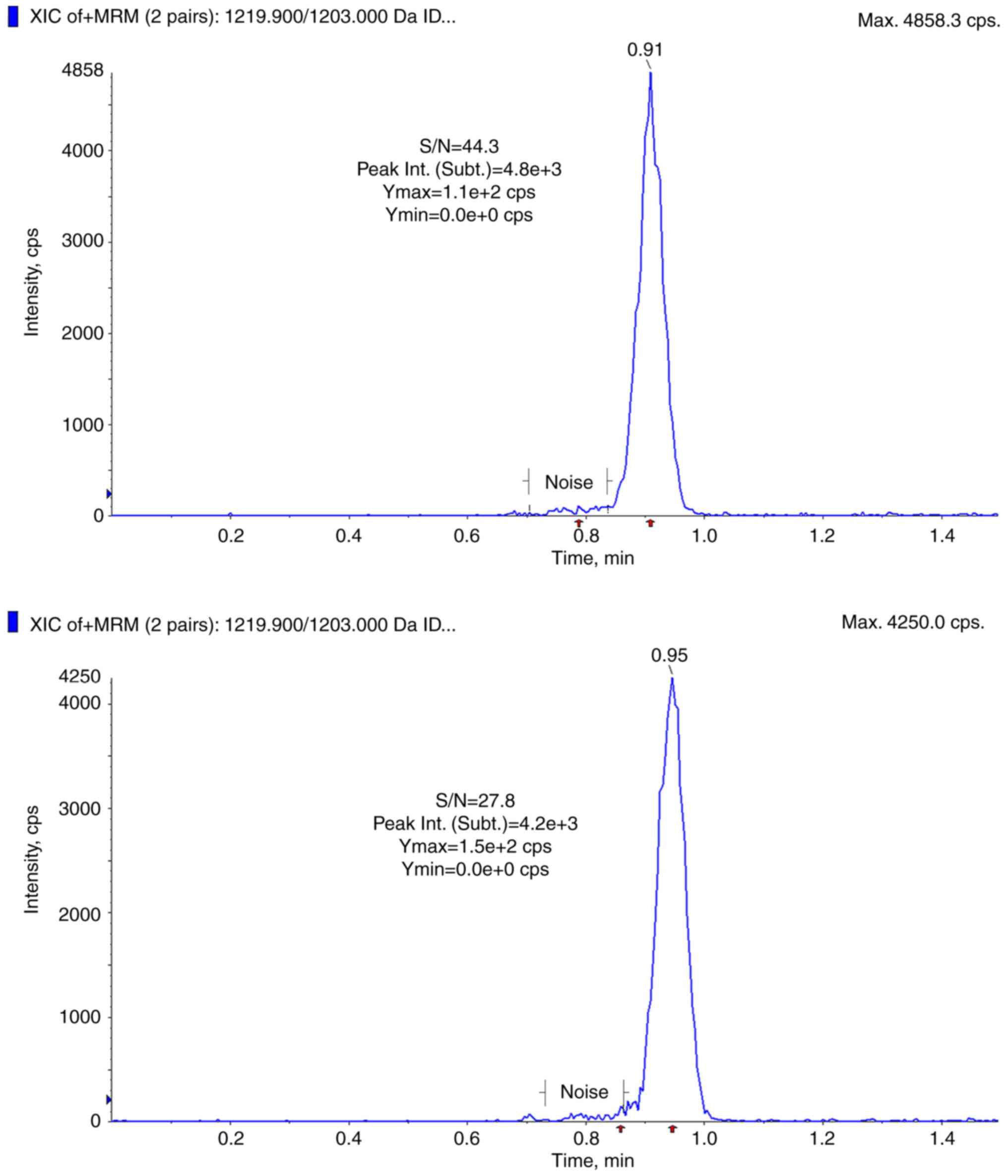
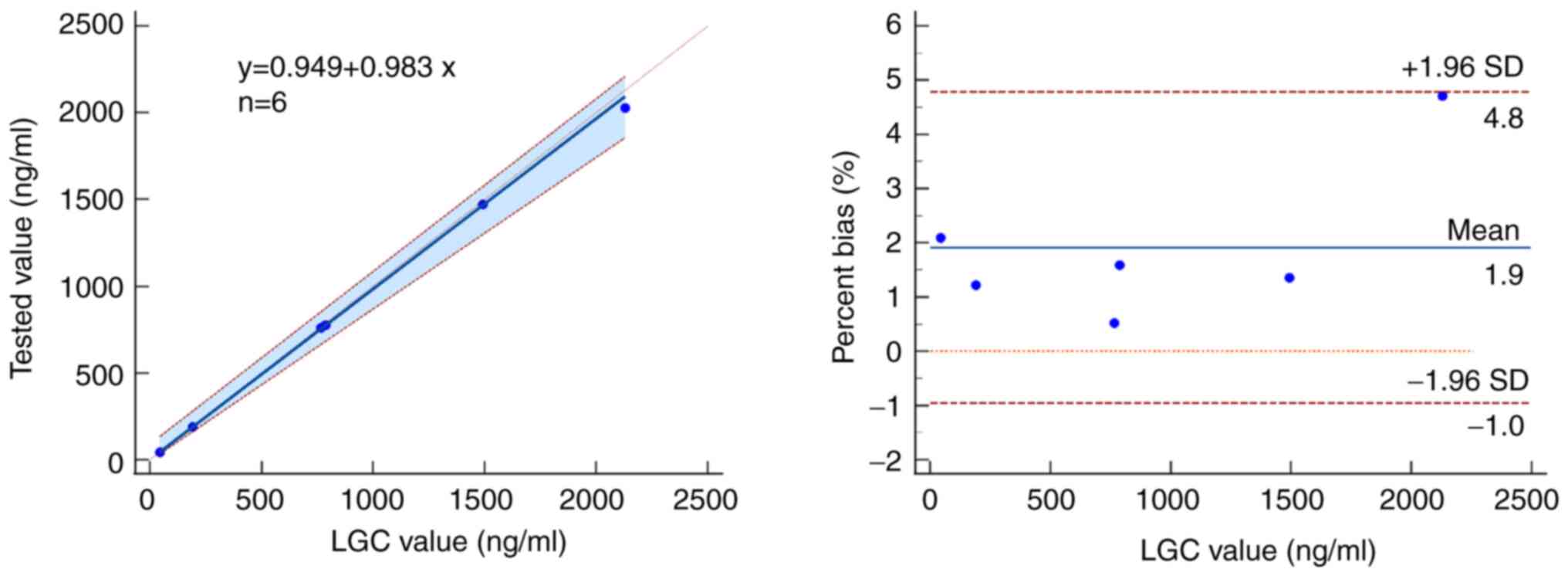
|
1
|
Chighizola CB, Ong VH and Meroni PL: The
use of cyclosporine A in rheumatology: A 2016 comprehensive review.
Clin Rev Allergy Immunol. 52:401–423. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu Q, Wang X, Nepovimova E, Wang Y, Yang H
and Kuca K: Mechanism of cyclosporine A nephrotoxicity: Oxidative
stress, autophagy, and signalings. Food Chem Toxicol. 118:889–907.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Becker A, Backman JT and Itkonen O:
Comparison of LC-MS/MS and chemiluminescent immunoassays for
immunosuppressive drugs reveals organ dependent variation in blood
cyclosporine a concentrations. Clin Chim Acta. 508:22–27.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Drira C, Ben Ayed W, Soussi MA, Razgallah
Khrouf M and Fradi I: Validation of routine analytical method for
injectable cyclosporine preparation control using HPLC-FIA assay.
Ann Pharm Fr. 79:266–274. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu R, Li X, Wei J, Liu S, Chang Y, Zhang
J, Zhang J, Zhang X, Fuhr U, Taubert M and Tian X: A single dose of
baicalin has no clinically significant effect on the
pharmacokinetics of cyclosporine A in healthy chinese volunteers.
Front Pharmacol. 10(518)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Koster RA, Dijkers EC and Uges DR: Robust,
high-throughput LC-MS/MS method for therapeutic drug monitoring of
cyclosporine, tacrolimus, everolimus, and sirolimus in whole blood.
Ther Drug Monit. 31:116–125. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vosough M and Tehrani SM: Development of a
fast HPLC-DAD method for simultaneous quantitation of three
immunosuppressant drugs in whole blood samples using intelligent
chemometrics resolving of coeluting peaks in the presence of blood
interferences. J Chromatogr B Analyt Technol Biomed Life Sci.
1073:69–79. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bjergum MW, Jannetto PJ and Langman LJ:
Simultaneous determination of tacrolimus and cyclosporine A in
whole blood by ultrafast LC-MS/MS. Methods Mol Biol. 1872:111–118.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Salm P, Taylor PJ and Rooney F: A
high-performance liquid chromatography-mass spectrometry method
using a novel atmospheric pressure chemical ionization approach for
the rapid simultaneous measurement of tacrolimus and cyclosporin in
whole blood. Ther Drug Monit. 30:292–300. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gong ZS, Wu ZH, Xu SX, Han WN, Jiang XM,
Liu HP, Yan-Li , Wei-Hu and Yan-Wang : A
high-throughput LC-MS/MS method for the quantification of four
immunosu- ppressants drugs in whole blood. Clin Chim Acta.
498:21–26. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pablo AH, Breaud AR and Clarke W: Analysis
of immunosuppressant drugs in whole blood by liquid
chromatography-tandem mass spectrometry (LC-MS/MS). Curr Protoc
Toxicol. 84(e92)2020.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
CLSI. Liquid Chromatography-Mass
Spectrometry Methods; Approved Guideline. CLSI document C62-A.
Wayne, PA: Clinical and Laboratory Standards Institute; 2014.
|
|
13
|
Seger C, Shipkova M, Christians U, Billaud
EM, Wang P, Holt DW, Brunet M, Kunicki PK, Pawinski T, Langman LJ,
et al: Assuring the proper analytical performance of measurement
procedures for immunosuppressive drug concentrations in clinical
practice: Recommendations of the international association of
therapeutic drug monitoring and clinical toxicology
immunosuppressive drug scientific committee. Ther Drug Monit.
38:170–189. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mei S, Wang J, Chen D, Zhu L, Zhao M, Hu
X, Yang L and Zhao Z: Ultra-high performance liquid chromatography
tandem mass spectrometry for cyclosporine analysis in human whole
blood and comparison with an antibody-conjugated magnetic
immunoassay. Ther Drug Monit. 40:69–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Toole B, Gechtman C, Dreier J, Kuhn J,
Gutierrez MR, Barrett A and Niederau C: Evaluation of the new
cyclosporine and tacrolimus automated electrochemiluminescence
immunoassays under field conditions. Clin Lab. 61:1303–1315.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Butch AW and Fukuchi AM: Analytical
performance of the CEDIA cyclosporine PLUS whole blood immunoassay.
J Anal Toxicol. 28:204–210. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kidney Disease: Improving Global Outcomes
(KDIGO) CKD-MBD Update Work Group: KDIGO 2017 clinical practice
guideline update for the diagnosis, evaluation, prevention, and
treatment of chronic kidney disease-mineral and bone disorder
(CKD-MBD). Kidney Int Suppl. 7:1–59. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tredger JM, Grevel J, Naoumov N, Steward
CM, Niven AA, Whiting B and Williams R: Cyclosporine
pharmacokinetics in liver transplant recipients: Evaluation of
results using both polyclonal radioimmunoassay and liquid
chromatographic analysis. Eur J Clin Pharmacol. 40:513–519.
1991.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shigematsu T, Suetsugu K, Yamamoto N,
Tsuchiya Y and Masuda S: Comparison of 4 commercial immunoassays
used in measuring the concentration of tacrolimus in blood and
their cross-reactivity to its metabolites. Ther Drug Monit.
42:400–406. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang Y and Zhang R: Recent advances in
analytical methods for the therapeutic drug monitoring of
immunosuppressive drugs. Drug Test Anal. 10:81–94. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Watanabe T, Tanaka R, Ono H, Suzuki Y,
Tatsuta R and Itoh H: Sensitive, wide-range and high-throughput
quantification of cyclosporine in whole blood using
ultra-performance liquid chromatography coupled to tandem mass
spectrometry and comparison with an antibody-conjugated magnetic
immunoassay. Biomed Chromatogr. 35(e5128)2021.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Szerkus O, Wolska E, Struck-Lewicka W,
Siluk D, Radwanska A, Wiczling P, Chorazewicz J, Sznitowska M,
Markuszewski MJ and Kaliszan R: Development and validation of UHPLC
method for the determination of cyclosporine A in biological
samples. Biomed Chromatogr. 28:802–809. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Mei S, Wang J, Chen D, Zhu L, Zhao M, Tian
X, Hu X and Zhao Z: Simultaneous determination of cyclosporine and
tacrolimus in human whole blood by ultra-high performance liquid
chromatography tandem mass spectrometry and comparison with a
chemiluminescence microparticle immunoassay. J Chromatogr B Analyt
Technol Biomed Life Sci. 1087-1088:36–42. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang Z and Wang S: Recent development in
application of high performance liquid chromatography-tandem mass
spectrometry in therapeutic drug monitoring of immunosuppressants.
J Immunol Methods. 336:98–103. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Taylor PJ, Brown SR, Cooper DP, Salm P,
Morris MR, Pillans PI and Lynch SV: Evaluation of 3 internal
standards for the measurement of cyclosporin by HPLC-mass
spectrometry. Clin Chem. 51:1890–1893. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Meinitzer A, Gartner G, Pilz S and Stettin
M: Ultra fast liquid chromatography-tandem mass spectrometry
routine method for simultaneous determination of cyclosporine A,
tacrolimus, sirolimus, and everolimus in whole blood using
deuterated internal standards for cyclosporine A and everolimus.
Ther Drug Monit. 32:61–66. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nishino T, Takahashi K, Tomori S, Ono S
and Mimaki M: Cyclosporine A C1.5 monitoring reflects the area
under the curve in children with nephrotic syndrome: A
single-center experience. Clin Exp Nephrol. 26:154–161.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mulder TAM, van Eerden RAG, de With M,
Elens L, Hesselink DA, Matic M, Bins S, Mathijssen RHJ and van
Schaik RHN: CYP3A4*22 genotyping in clinical practice: Ready
for implementation? Front Genet. 12(711943)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ziaei M, Ziaei F and Manzouri B: Systemic
cyclosporine and corneal transplantation. Int Ophthalmol.
36:139–146. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Inoue M, Yumura W, Morishita Y, Ito C,
Hamano Y, Ando Y, Muto S and Kusano E: Patients with adult minimal
change nephrotic syndrome treated with long-term cyclosporine did
not experience a reduction in their eGFR. Clin Nephrol. 79:101–106.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Sommerer C, Giese T, Schmidt J, Meuer S
and Zeier M: Ciclosporin A tapering monitored by NFAT-regulated
gene expression: A new concept of individual immunosuppression.
Transplantation. 85:15–21. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Noreikaitė A, Saint-Marcoux F, Marquet P,
Kaduševičius E and Stankevičius E: Influence of cyclosporine and
everolimus on the main mycophenolate mofetil pharmacokinetic
parameters: Cross-sectional study. Medicine (Baltimore).
96(e6469)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wasilewska A, Zoch-Zwierz W and Pietruczuk
M: Expression of multidrug resistance P-glycoprotein on lymphocytes
from nephrotic children treated with cyclosporine A and
ACE-inhibitor. Eur J Pediatr. 166:447–452. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tuzimski T and Petruczynik A: Review of
chromatographic methods coupled with modern detection techniques
applied in the therapeutic drugs monitoring (TDM). Molecules.
25(4026)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Andre C, Choukroun G, Bennis Y, Kamel S,
Lemaire-Hurtel AS, Masmoudi K, Bodeau S and Liabeuf S: Potential
interactions between uremic toxins and drugs: An application in
kidney transplant recipients treated with calcineurin inhibitors.
Nephrol Dial Transplant. 37:2284–2292. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Trkulja V, Lalić Z, Nađ-Škegro S, Lebo A,
Granić P, Lovrić M, Pasini J and Božina N: Effect of cyclosporine
on steady-state pharmacokinetics of MPA in renal transplant
recipients is not affected by the MPA formulation: Analysis based
on therapeutic drug monitoring data. Ther Drug Monit. 36:456–464.
2014.PubMed/NCBI View Article : Google Scholar
|