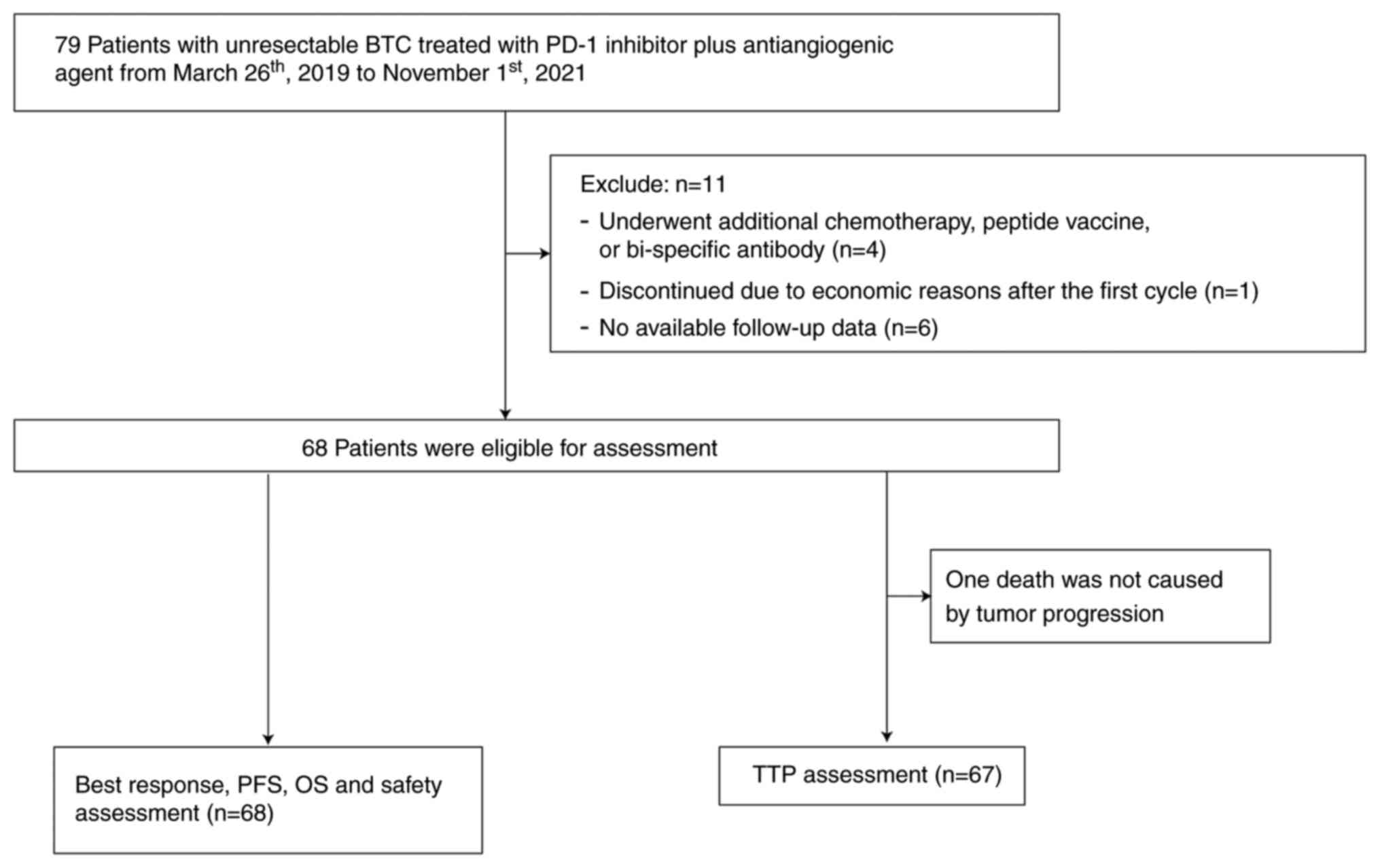
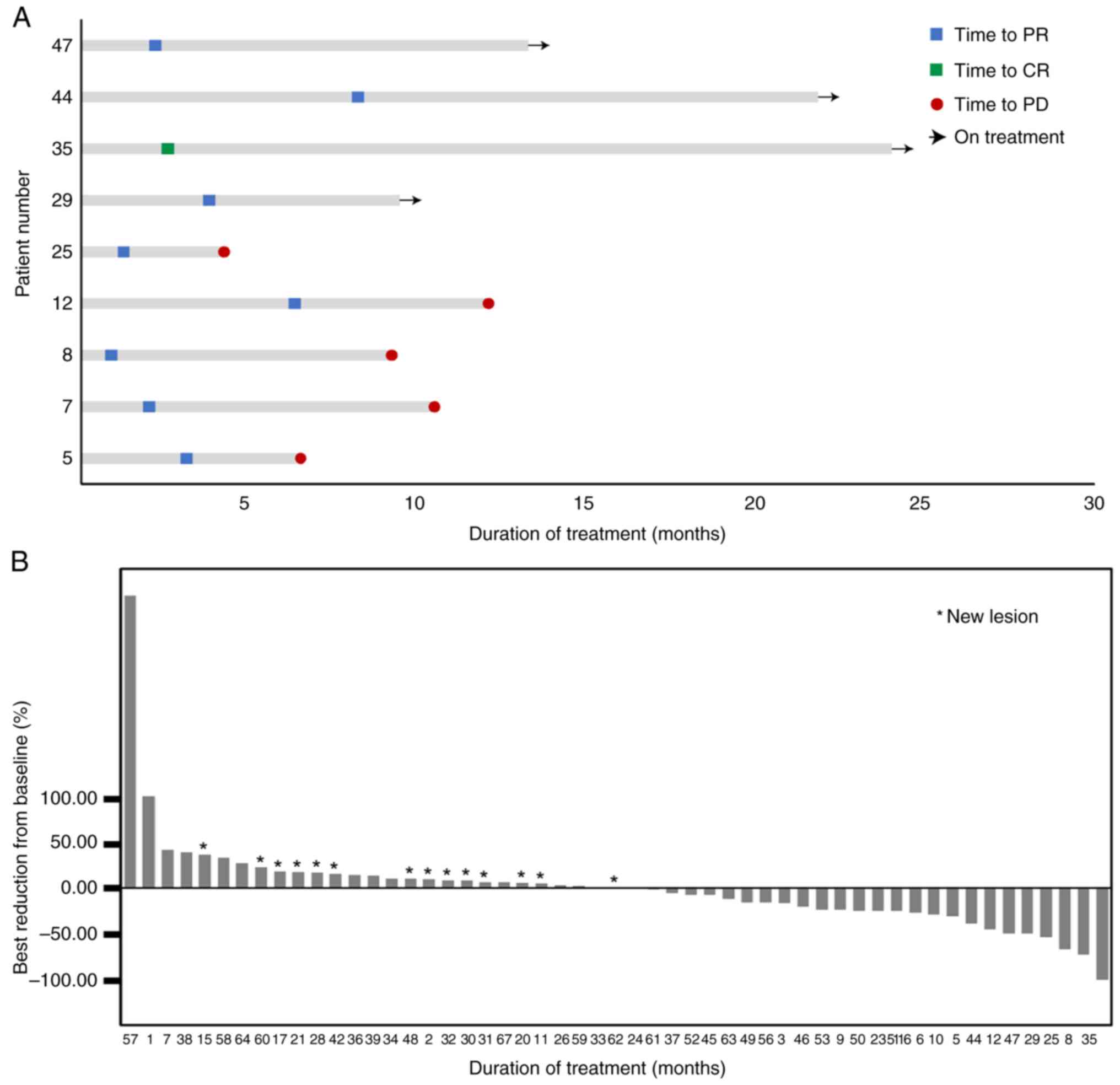
|
1
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Eckel F and Schmid RM: Chemotherapy in
advanced biliary tract carcinoma: A pooled analysis of clinical
trials. Br J Cancer. 96:896–902. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chu T, Zhong R, Zhong H, Zhang B, Zhang W,
Shi C, Qian J, Zhang Y, Chang Q, Zhang X, et al: Phase 1b study of
sintilimab plus anlotinib as first-line therapy in patients with
advanced NSCLC. J Thorac Oncol. 16:643–652. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yau T, Park JW, Finn RS, Cheng AL,
Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, et
al: Nivolumab versus sorafenib in advanced hepatocellular carcinoma
(CheckMate 459): A randomised, multicentre, open-label, phase 3
trial. Lancet Oncol. 23:77–90. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mao J, Yang X, Lin J, Yang X, Wang D,
Zhang L, Bai Y, Bian J, Long J, Xie F, et al: Apatinib as
non-first-line treatment in patients with Intrahepatic
Cholangiocarcinoma. J Cancer. 12:1555–1562. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ueno M, Ikeda M, Sasaki T, Nagashima F,
Mizuno N, Shimizu S, Ikezawa H, Hayata N, Nakajima R and Morizane
C: Phase 2 study of lenvatinib monotherapy as second-line treatment
in unresectable biliary tract cancer: Primary analysis results. BMC
Cancer. 20(1105)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim RD, Sanoff HK, Poklepovic AS, Soares
H, Kim J, Lyu J, Liu Y, Nixon AB and Kim DW: A multi-institutional
phase 2 trial of regorafenib in refractory advanced biliary tract
cancer. Cancer. 126:3464–3470. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun W, Patel A, Normolle D, Patel K, Ohr
J, Lee JJ, Bahary N, Chu E, Streeter N and Drummond S: A phase 2
trial of regorafenib as a single agent in patients with
chemotherapy-refractory, advanced, and metastatic biliary tract
adenocarcinoma. Cancer. 125:902–909. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Piha-Paul SA, Oh D, Ueno M, Malka D, Chung
HC, Nagrial A, Kelley RK, Ros W, Italiano A, Nakagawa K, et al:
Efficacy and safety of pembrolizumab for the treatment of advanced
biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028
studies. Int J Cancer. 147:2190–2198. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim RD, Chung V, Alese OB, El-Rayes BF, Li
D, Al-Toubah TE, Schell MJ, Zhou JM, Mahipal A, Kim BH and Kim DW:
A Phase 2 multi-institutional study of nivolumab for patients with
advanced refractory biliary tract cancer. JAMA Oncol. 6:888–894.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hao Z and Wang P: Lenvatinib in management
of solid tumors. Oncologist. 25:e302–e310. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lwin Z, Gomez-Roca C, Saada-Bouzid E, Im
SA, Castanon E, Senellart H, Graham D, Voss M, Doherty M, Lopez J,
et al: LBA41 LEAP-005: Phase II study of apa (len) plus
pembrolizumab (pembro) in patients (pts) with previously treated
advanced solid tumours. Ann Oncol. 31(S1170)2020.
|
|
13
|
Cousin S, Cantarel C, Guegan JP, Mazard T,
Gomez-Roca C, Metges JP, Bellera C, Adenis A, Korakis I, Poureau
PG, et al: Regorafenib-avelumab combination in patients with
biliary tract cancer (REGOMUNE): A single-arm, open-label, phase II
trial. Eur J Cancer. 162:161–169. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Arkenau H, Martin-Liberal J, Calvo E,
Penel N, Krebs MG, Herbst RS, Walgren RA, Widau RC, Mi G, Jin J, et
al: Ramucirumab plus pembrolizumab in patients with previously
treated advanced or metastatic biliary tract cancer: Nonrandomized,
open-label, phase I trial (JVDF). Oncologist. 23:1407–e136.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ueno M, Ikeda M, Morizane C, Kobayashi S,
Ohno I, Kondo S, Okano N, Kimura K, Asada S, Namba Y, et al:
Nivolumab alone or in combination with cisplatin plus gemcitabine
in Japanese patients with unresectable or recurrent biliary tract
cancer: A non-randomised, multicentre, open-label, phase 1 study.
Lancet Gastroenterol Hepatol. 4:611–621. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Oh DY, He AR, Qin S, Chen LT, Okusaka T,
Vogel A, Kim JW, Suksombooncharoen T, Lee MA, Kitano M, et al: A
phase 3 randomized, double-blind, placebo-controlled study of
durvalumab in combination with gemcitabine plus cisplatin (GemCis)
in patients (pts) with advanced biliary tract cancer (BTC):
TOPAZ-1. J Clin Oncol. 40(378)2022.
|
|
17
|
Salas-Benito D, Pérez-Gracia JL,
Ponz-Sarvisé M, Rodriguez-Ruiz ME, Martínez-Forero I, Castañón E,
López-Picazo JM, Sanmamed MF and Melero I: Paradigms on
immunotherapy combinations with chemotherapy. Cancer Discov.
11:1353–1367. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Monge C, Pehrsson EC, Xie C, Duffy AG,
Mabry D, Wood BJ, Kleiner DE, Steinberg SM, Figg WD, Redd B, et al:
A phase II study of pembrolizumab in combination with capecitabine
and oxaliplatin with molecular profiling in patients with advanced
biliary tract carcinoma. Oncologist. 27:e273–e285. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Feng K, Liu Y, Zhao Y, Yang Q, Dong L, Liu
J, Li X, Zhao Z, Mei Q and Han W: Efficacy and biomarker analysis
of nivolumab plus gemcitabine and cisplatin in patients with
unresectable or metastatic biliary tract cancers: Results from a
phase II study. J Immunother Cancer. 8(e000367)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Moehler M, Maderer A, Schimanski C,
Kanzler S, Denzer U, Kolligs FT, Ebert MP, Distelrath A, Geissler
M, Trojan J, et al: Gemcitabine plus sorafenib versus gemcitabine
alone in advanced biliary tract cancer: A double-blind
placebo-controlled multicentre phase II AIO study with biomarker
and serum programme. Eur J Cancer. 50:3125–3135. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Valle JW, Vogel A, Denlinger CS, He AR,
Bai LY, Orlova R, Van Cutsem E, Adeva J, Chen LT, Obermannova R, et
al: Addition of ramucirumab or merestinib to standard first-line
chemotherapy for locally advanced or metastatic biliary tract
cancer: A randomised, double-blind, multicentre, phase 2 study.
Lancet Oncol. 22:1468–1482. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen X, Qin S, Gu S, Ren Z, Chen Z, Xiong
J, Liu Y, Meng Z, Zhang X, Wang L, et al: Camrelizumab plus
oxaliplatin-based chemotherapy as first-line therapy for advanced
biliary tract cancer: A multicenter, phase 2 trial. Int J Cancer.
149:1944–1954. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Champiat S, Dercle L, Ammari S, Massard C,
Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A,
Soria JC and Ferté C: Hyperprogressive disease is a new pattern of
progression in cancer patients treated by Anti-PD-1/PD-L1. Clin
Cancer Res. 23:1920–1928. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Martin-Romano P, Castanon E, Ammari S,
Champiat S, Hollebecque A, Postel-Vinay S, Baldini C, Varga A,
Michot JM, Vuagnat P, et al: Evidence of pseudoprogression in
patients treated with PD1/PDL1 antibodies across tumor types.
Cancer Med. 9:2643–2652. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kanjanapan Y, Day D, Wang L, Al-Sawaihey
H, Abbas E, Namini A, Siu LL, Hansen A, Razak AA, Spreafico A, et
al: Hyperprogressive disease in early-phase immunotherapy trials:
Clinical predictors and association with immune-related toxicities.
Cancer. 125:1341–1349. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Saâda-Bouzid E, Defaucheux C, Karabajakian
A, Coloma VP, Servois V, Paoletti X, Even C, Fayette J, Guigay J,
Loirat D, et al: Hyperprogression during anti-PD-1/PD-L1 therapy in
patients with recurrent and/or metastatic head and neck squamous
cell carcinoma. Ann Oncol. 28:1605–1611. 2017.PubMed/NCBI View Article : Google Scholar
|