Introduction
Atypical antipsychotics are currently used in
clinical practice for multiple indications, primarily schizophrenia
spectrum disorders (SSD) and bipolar disorders, but they may be
useful adjuvants in depressive disorders, behavioral and
psychological symptoms associated with neurocognitive disorders,
tic disorders, and autism spectrum disorders, amongst others. These
pharmacological agents are related to various metabolic adverse
effects, ranging from obesity, overweight, or dyslipidemia, to
impaired glucose tolerance and diabetes (1-3).
Negative consequences of the antipsychotic treatment led to a worse
quality of life and unfavorable functional prognosis by adding to
other risk factors in a population already affected by severe
long-term impairments and a high rate of somatic comorbidities
(1-5).
increased risk for cardiovascular diseases and diabetes has been
reported in drug-naïve patients with SSD, likely based on genetic
vulnerability and negative lifestyle factors (4,6).
This biological vulnerability highlights the importance of
investigating the role played by antipsychotics in the onset or
worsening of metabolic dysfunctions in SSD patients.
Pharmacogenomic-focused research could offer insight into the
selection of the antipsychotic agent with the lowest risk of
adverse events in a specific case, or at least could indicate the
need for earlier changes in lifestyle after the initiation of
treatment in vulnerable individuals.
According to a UK national survey of 31,719
participants, a lower life expectancy was reported in association
with major psychiatric illnesses vs. controls, with a reduction of
>14 years for males, and 17 years for females (2). Smoking, diabetes, as well as obesity,
are among the most well-recognized contributors to this difference
in life expectancy (2,7). It is estimated that
antipsychotic-induced weight gain may ultimately reduce life
expectancy by 25 years (8),
although it seems implausible to attribute this kind of impact only
to body weight changes during treatment, as an isolated factor.
Patients diagnosed with SSD have a >2x higher
mortality rate vs. controls, with increased cardiometabolic risk,
which is considered the primary cause of the mortality rate in this
population (9). The participation
of immune reactions and neurotrophic factors in the pathogenesis of
psychiatric disorders, SSD included, and in the modulation of the
atypical antipsychotic effects in these patients has been explored,
and targeting these variables could be of interest for improving
their prognosis (10-13).
Shared pathogenic cascades and biological mechanisms underlying SSD
and type 2 diabetes mellitus (T2DM) were studied, and common gene
functions were related to the onset of both disorders (13). A poor diet, a sedentary lifestyle,
and the adverse effects of antipsychotics are mediators between SSD
and diabetes, making it difficult to distinguish the contribution
of environmental factors from the common biological pathways (such
as molecular basis, inflammation, oxidative stress, and
neuroendocrine dysfunctions) (13). An increased vulnerability to the
metabolic and cardiovascular adverse events of antipsychotics has
been reported in children and adolescents, in particular a trend
towards rapid weight gain during treatment (14).
The pharmacological mechanisms by which atypical
antipsychotics induce metabolic disorders in patients with SSD are
very complex and yet insufficiently clarified. The most extensively
explored pathogenic pathways of the antipsychotics in obesity and
diabetes are the antagonism of serotonin 5HT2C, muscarinic M1, and
histamine H1-receptors, and dysregulations of insulin, cortisol,
glucagon, cholecystokinin, adiponectin, ghrelin, leptin, orexin,
prolactin, and oxytocin (15,16).
Different pharmacological and non-pharmacological
therapies are recommended for the control of metabolic dysfunctions
in schizophrenia-diagnosed patients who are treated with
antipsychotics, especially with atypical agents. Lifestyle changes,
nutritional interventions, and counseling targeting a healthier
lifestyle have been explored in this population in order to
mitigate the trend toward weight gain (17). The switch to a metabolic-neutral
antipsychotic may represent another option, but it may pose the
risk of worsening psychotic symptoms (18). Adding a range of agents with
favorable metabolic profiles to the current antipsychotic regimen
has been suggested; however, the problem of insufficient treatment
adherence and lower tolerability by increasing the number of
medications in a reputedly low-insight population could be critical
(19). Metformin benefits from the
highest level of recommendation for the therapy of
antipsychotic-induced weight gain in patients with SSD, followed by
topiramate (20,21).
Glucagon-like peptide-1 receptor agonists (GLP1-RAs)
enhance insulin secretion as a reaction to high glycemic levels and
decrease the speed of gastric emptying (1). The exact mechanisms of dysfunctions
of glucose metabolism induced by atypical antipsychotic
administration have not been elucidated, but an increase in hepatic
glucose synthesis, a decrease in pancreatic insulin discharge, and
a higher peripheral/brain insulin non-responsivity were suggested
(22-24).
A pharmacological effect of atypical antipsychotics, especially
clozapine, was involved in the acute decrease of GLP-1 signaling,
suggesting this mechanism may explain the onset of obesity and
glucose metabolism dysfunction in patients with SSD (25).
To date, six GLP-1RAs have been explored in clinical
trials, and the recommendations formulated by the American Diabetes
Association (ADA) support the use of these medications as
second-line treatment for T2DM, frequently combined with metformin,
while high doses of liraglutide have been approved for the therapy
of obesity by the Food and Drug Administration (FDA) (8,9). The
specific clinical pharmacology features of each GLP-1RA
(liraglutide, semaglutide, albiglutide, dulaglutide, lixisenatide,
and exenatide) have been described in Table I.
 | Table IClinical pharmacology of
GLP-1Ras. |
Table I
Clinical pharmacology of
GLP-1Ras.
| Pharmacological
agent |
Characteristics | Clinical reports in
the general population | Observations | (Refs.) |
|---|
| Liraglutide | Increases insulin
secretion in peripheral tissues; agonist of the GLP-1R on neurons
in the CNS (brainstem, HPT, and forebrain, NAc included); highly
similar analog of human GLP-1; increases insulin secretion in a
glucose- dependent manner; decreases abnormally high glucagon
secretion. GLP-1R stimulation may reduce the activity of the
rewards pathway | Decrease in body
weight, decrease in body fat mass, regulates the appetite and food
intake; improves glycemic control in patients with T2DM; decreases
cardiovascular mortality and morbidity | Dysfunctions in the
responsiveness of the reward system were found to be a common
causal factor for excessive food intake and for the pathogenesis of
schizophrenia (negative symptoms). Liraglutide is administered once
daily s.c. in the abdomen, thigh, or upper arm | (8,9,26,27) |
| Semaglutide | This is a highly
similar analog of human GLP-1; decreases overall appetite and
decreases the preference for high-fat foods; improves β-cell
function in the pancreas, lowers the fasting and postprandial
glucagon levels, and causes a minor delay of the early postprandial
gastric emptying | Superior to placebo
in overweight or obese adults, according to a meta-analysis (n=4
trials, n=3,447 participants) focused on the change in body weight.
Semaglutide reduced WC and BMI vs. placebo at a significant level
while increasing the quality of life and various cardiometabolic
risk factors. Oral semaglutide administrati on also had favorable
results on body weight in T2DM patients, and, according to another
meta- analysis (n=11 randomized controlled trials, n=9,890
participants), this drug was superior to placebo and several active
comparators (including liraglutide, empagliflozin, and sitagliptin)
in reducing HbA1c levels and body weight (29) | Administered s.c.
once weekly, in the abdomen, thigh, or upper arm | (28,29) |
| Albiglutide | Albiglutide has a
very high sequence similarity to human GLP-1; possibly less
efficient than other GLP-1RAs for decreasing HbA1c | In addition to
albiglutide's positive effects on glycemic management in cases of
insufficiently controlled T2DM, this drug has proven beneficial
effects on body weight, which were preserved during long-term
administrati on for up to 3 years | Administered s.c.
once weekly; has a longer life than the native peptide hormone.
This medication was globally withdrawn due to economic reasons by
its manufacturer in 2018 | (30,31) |
| Dulaglutide | High homology with
human GLP-1 (90%). Increases insulin release in the pancreatic β
cells when the blood glucose levels are high. Decreases glucagon
secretion in T2DM patients. Decreases gastric emptying | Dulaglutide has
proven its capacity to improve HbA1c levels with an efficacy
superior to metformin. Also, it decreased the body weight with a
magnitude of effect similar to or inferior (depending on the dosage
used) to its active comparator (metformin) in T2DM patients at week
52 | Administered s.c.
once weekly in the abdomen, thigh, or upper arm | (32) |
| Lixisenatide | Has low sequence
similarity with GLP-1 (~50%). Increases insulin secretion when
blood glucose levels are increased. Decreases glucagon secretion.
Decreases gastric emptying | BMI significantly
decreased when treated with lixisenatide in obese T2DM patients
(3.2 kg loss after 3.8±1.6 months) in an open- label study with 104
participants. However, in trials with active comparators,
lixisenatide was outperformed by exenatide immediate release and
liraglutide regarding the capacity to induce weight loss | Administered once
daily in the thigh, abdomen, or upper arm | (33-36) |
| Exenatide | Unlike the other
five pharmacological agents mentioned above, exenatide has a low
sequence similarity to human GLP-1 (~53%) | Has been
investigated for metabolic (obesity and diabetes) management, and
also non-metabolic (cognitive functioning) effects in patients with
SSD). The positive influence of GLP-1RAs on cognition observed in
animal studies did not translate significantly in trials with
schizophrenia- diagnosed patients | Administered twice
daily (the immediate-release form) or once weekly (the
extended-release form) s.c. in the abdomen, thigh, or upper
arm | (9,34,37,39) |
| Tirzepatide | This is a dual
GLP-1RA and glucose- dependent insulinotropic polypeptide receptor
agonist. This drug is a synthetic peptide that contains 39 amino
acids and is derived from the original GIP sequence | Pre-clinical
studies and phase I to III trials demonstrated the efficacy of
tirzepatide in lowering blood glucose and body weight, with a
tolerability profile similar to GLP1-RAs, in T2DM patients | Administered s.c.
in the abdomen, thigh, or upper arm, once weekly | (40,41) |
Liraglutide increases insulin secretion in
the peripheral tissues, while at the central level, it exerts its
actions on neurons expressing GLP-1 receptors, which are usually
located in the brainstem, hypothalamus, and forebrain, including
the nucleus accumbens (26,27).
It was shown that GLP-1 receptor stimulation may result in the
reduction of the functioning of the reward pathway (26). This phenomenon was associated with
dysfunctions in the responsivity of the reward system as a common
causal factor for excessive food intake and for the pathogenesis of
schizophrenia (that is, the negative symptoms) (8).
Semaglutide, which is administered once a week, was
superior to placebo in overweight or obese adults, according to a
meta-analysis (n=4 trials, n=3,447 participants) that was focused
on the change in body weight (28). Additionally, semaglutide reduced
waist circumference and body mass index (BMI) vs. placebo,
increased the quality of life, and improved various cardiometabolic
risk factors (28). Oral
semaglutide administration also had favorable results on body
weight in T2DM patients, and, according to another meta-analysis
(n=11 randomized controlled trials, n=9,890 participants), this
drug was superior to placebo and several active comparators
(including liraglutide, empagliflozin, and sitagliptin) in reducing
HbA1c levels and body weight (29).
Albiglutide has a very high sequence similarity to
human GLP-1, is administered subcutaneously once a week, and has a
longer half-life than the native peptide hormone (24). Additionally, the positive effects
of albiglutide on glycemic management in cases of insufficiently
controlled T2DM have proven beneficial effects on body weight,
which were preserved during long-term administration, for up to 3
years (30). Unfortunately, this
medication was globally withdrawn due to economic reasons by its
manufacturer, in 2018(31).
Dulaglutide is administered subcutaneously once a
week and improves HbA1c levels with an efficacy superior to
metformin (26). Additionally, it
decreased the body weight by a similar level to or less than
(depending on the dosage used) its active comparator (metformin) in
T2DM patients at week 52(32).
Lixisenatide has low sequence similarity with GLP-1
(~50%) and is administered once a day (33). BMI was significantly decreased by
lixisenatide in T2DM patients who were obese (3.2 kg after 3.8±1.6
months, in an open-label study with 104 participants (34). However, in trials with active
comparators, lixisenatide was outperformed by exenatide immediate
release and liraglutide regarding the capacity to induce weight
loss (35,36).
Exenatide is a GLP-1RA investigated for metabolic
disease (obesity and diabetes management) but also non-metabolic
diseases (such as cognitive functioning) effects in patients with
SSD (37,38). The positive influence of GLP-1RAs
on cognition observed in animal studies did not translate
significantly in trials with schizophrenia-diagnosed patients
(9,37). Unlike the other five
pharmacological agents mentioned previously (with the exception of
lixisenatide), exenatide has a low sequence similarity to human
GLP-1 (~53%), and it may be administered twice daily (the
immediate-release form) or once weekly (the extended-release form)
(33).
A comparative analysis of the effects of GLP-1RAs in
T2DM patients (34 randomized controlled trials, 24-32 weeks
duration) showed that all representants of this class (except for
semaglutide and albiglutide, for which no data were identified)
reduced the body weight compared to placebo (39). When all GLP-1RAs were compared with
each other, no significant difference between them was observed
regarding weight loss (39). A
higher risk for gastrointestinal adverse events was reported for
all agents compared to the placebo (39).
Tirzepatide was also considered within the
conceptual frame of this review as this agent is a dual GLP-1RA and
glucose-dependent insulinotropic polypeptide (GIP) receptor
agonist. This drug is a synthetic peptide that contains 39 amino
acids and is derived from the original GIP sequence (40). Pre-clinical studies and Phase I-III
trials demonstrated the efficacy of tirzepatide in lowering blood
glucose and body weight, with a tolerability profile similar to
GLP1-RAs, in T2DM patients (40,41).
The primary objective of this review was to
determine the efficacy of GLP-1RAs in the therapeutic management of
antipsychotic treatment-associated metabolic dysfunctions. Another
objective was the exploration of the tolerability of the GLP-1RAs.
The third objective was to formulate clinical recommendations based
on the GRADE criteria (42,43)
for the use of GLP-1RAs in patients with severe mental illnesses
undergoing antipsychotic treatment, who developed metabolic
dysfunctions.
Methods
A systematic review focused on the short- and
long-term effects of GLP-1RAs administration on weight gain in
subjects who received antipsychotics was performed, based on the
PRISMA guidelines (44). For
identifying relevant articles, three electronic databases (PubMed,
https://pubmed.ncbi.nlm.nih.gov/;
Cochrane, https://www.cochrane.org/; and
Clarivate/Web of Science, https://www.webofscience.com/) as well as repositories
for clinical trials (US National Library of Medicine, www.clinicaltrials.gov; EU Clinical Trial Register,
www.clinicaltrialsregister.eu; and
World Health Organization International Clinical Trials Registry
Platform, www.who.int/clinical-trials-registry-platform)
were included. The search criteria used were: ‘glucagon-like
peptide-1 receptor agonists’ OR ‘GLP1-RA’ AND ‘obesity’ OR
‘diabetes mellitus’ OR ‘metabolic syndrome’ OR ‘dyslipidemia’ AND
‘antipsychotics’ OR ‘psychiatric disorders’. All papers published
between January 2000 and November 2022 were included in the primary
search.
As the investigated class of antidiabetics was
launched on the market relatively recently, a more inclusive
methodology was chosen, using the SPIDER algorithm (Table II), which is more appropriate when
quantitative, qualitative, and mixed methods research are expected
to be analyzed than the traditionally recommended PICO algorithm
(45). The main inclusion criteria
referred to the sample (i.e., all patients, regardless of age, were
included, and preclinical studies were also allowed if an
antipsychotic drug was administered), the phenomenon of interest
(i.e., the effects of short and long-term GLP-1RAs administration),
the study design (i.e., all types of clinical and preclinical
studies, case reports, and therapeutic guidelines were explored),
the evaluation (i.e., efficacy, tolerability, and safety of
GLP-1RAs, assessed by structured instruments, laboratory analyses,
behavioral observations, and qualitative research), and the
research type (i.e., qualitative and quantitative methodology). The
main exclusion criteria were: Unspecified demographic parameters of
the study population or insufficient descriptors for the animal
models, lack of data concerning the intervention assessed, unclear
diagnoses or experimental paradigms, the exploration of other
non-GLP-1RA antidiabetics in monotherapy, unspecified design of the
research, and lack of pre-defined measurements for the quantitative
research.
 | Table IISPIDER algorithm for systematic
reviews. |
Table II
SPIDER algorithm for systematic
reviews.
| Dimension | Inclusion
criteria | Exclusion
criteria |
|---|
| Sample | Children,
adolescents, adults, and old age patients were included.
Preclinical studies using any kind of subjects were allowed. All
subjects were currently receiving antipsychotics. All psychiatric
diagnoses or psychiatric disorders paradigms for animal studies
were included | Unspecified
demographic parameters of the study population (clinical research),
or lack of sufficient descriptors for the animal model used
(preclinical studies). Lack of specifics regarding the
antipsychotics administered, or the GLP-1RA treatment regimen.
Unspecified diagnoses for clinical populations or experimental
paradigms for preclinical studies |
| Phenomenon of
interest | The effects of
short- or long-term GLP-1RA administration. Combined, GLP-1RAs and
other antidiabetics were allowed | Other
antidiabetics, if they were used as monotherapy |
| Design | Preclinical
studies, clinical trials, prospective or retrospective, randomized
or not, controlled or not, single/double-blinded or unblinded. Case
reports, systematic reviews, and meta- analyses. Therapeutic
guidelines, observations, document-based research, and experts'
opinions | Unspecified design
of the research. Reviews or meta-analyses with pre-defined
quantifiable objectives that did present different objectives (such
as not including changes in the body weight or BMI) or contained
poorly defined primary outcomes |
| Evaluation | Efficacy,
tolerability, and safety of GLP-1RAs in the pre-defined population.
Structured instruments, laboratory analyses, behavioral
observational methods, and also qualitative research (patients'
self-reports, and questionnaires) | Lack of pre-defined
measurements for the quantitative research outcomes. Qualitative
research, if they did not present clear references to the safety,
efficacy, or tolerability of GLP-1RAs |
| Research type | Qualitative,
quantitative, methods | Imprecise or
undefined method of research |
GRADE recommendations for evaluating the quality of
evidence were applied, using six dimensions: Study design, risk of
bias, inconsistency of results, indirectness of evidence,
imprecision, and publication bias (Table III) (46). The overall quality of evidence was
analyzed for each reviewed source and then rated from ‘very low’ to
‘high’.
 | Table IIIReports included in the review and
their main descriptors. |
Table III
Reports included in the review and
their main descriptors.
| First author/s,
year or trial no. | Sample | Design | Results | Research type | RoB | IoR | IoE | Imp | PB | OQE | (Refs.) |
|---|
| Medak et al,
2020 | Female and male
C57BL/ 6J mice (n=15-18) | Preclinical trial,
OLZ+/- LRGLT or EXND4, 120 min of drug co-administration. Outcomes:
glycemia, insulin, and glucagon plasma levels, serum GLP level | Both GLP1RAs were
associated with complete protection for male mice against OLZ-
related HG. EXND4 did not protect against increases in glucagon
plasma levels | Quantitative | No | No | Yesa | No | No | Mod | (22) |
| Babic et al,
2018 | Female Sprague-
Dawley rats (n=72) | Preclinical trial,
OLZ, CLZ, LRGLT, OLZ+LRGLT, CLZ+LRGLT, 6 weeks. Outcomes: cognitive
performance, body weight, adiposity, and glucose tolerance | LRGLT reduced OLZ-
related WG and adiposity, and improved glucose intolerance and
cognitive functioning | Quantitative | No | No | Yesa | No | No | Mod | (47) |
| Lykkegaard et
al, 2008 | Female Sprague-
Dawley rats (n=40) | Preclinical trial,
OLZ for 28 days, followed by LRGLT or placebo for 14 days.
Outcomes: food intake, body weight, subcutaneous inguinal fat,
mesenteric fat, retroperitoneal fat, and glucose tolerance | LRGLT improved
cumulative food intake, WG, inguinal, mesenteric, and
retroperitoneal fat | Quantitative | No | No | Yesa | No | No | Mod | (48) |
| Ishøy et al,
2013 | Female patient
diagnosed with SCHZ + T2DM + obesity | Case report, CLZ +
LRGLT+ metformin + insulin, 3 months and 2-year follow-up.
Outcomes: HbA1c, BW, insulin doses needed daily | LRGLT led to
favorable outcomes (decreased HbA1c levels, BW, and daily insulin
doses). Positive effects persisted after 2 years | Quantitative and
qualitative | Yesb | No | Yesc | No | No | Low | (49) |
| Siskind et
al, 2016 | Male patient
diagnosed with SCHZ + T2DM | Case report, CLZ +
EXNTD + metformin, 6 months. Outcomes: BPRS score, BW, BMI, waist
circumference, clinical evolution, adverse events | EXNTD decreased BMI
and waist circumference | Quantitative and
qualitative | Yesb | No | Yesc | No | No | Low | (50) |
| Larsen et
al, 2017; | 103 patients
diagnosed | RDBCT, OLZ/CLZ
+ | LRGLT improved
the | Quantitative | No | No | No | No | No | High | (51,52) |
| Svensson et
al, 2019 | with SCHZ spectrum
disorders | LRGLT or placebo,
16 weeks + 1-year follow-up. Outcomes: glucose tolerance, BW, waist
circumference, systolic BP, adverse events, biological parameters
(such as HbA1c and C-peptide levels) | glucose tolerance
vs. placebo significantly at the endpoint. BW and waist
circumference also decreased more vs. placebo. Primarily
gastrointestinal symptoms were reported as adverse events. After 1
year, LRGLT- treated patients in the acute phase of the trial
presented poorer glycemic control but a significant BW loss and BMI
decrease vs. placebo | | | | | | | | |
| Siskind et
al, 2018; Siskind et al, 2020 | 28 outpatients
diagnosed with SCHZ ± T2DM | OLT, randomized,
controlled, 24 weeks + 12- month follow-up, CLZ + EXNTD. Outcomes:
≥5% weight loss (primary outcome), BMI, fasting glucose, HbA1c
(secondary outcomes) | EXNTD improved
primary and secondary outcomes at 6 months vs. the usual care
group. After 12 months, no differences between groups in BMI and BW
were observed | Quantitative | Yesd | No | No | No | No | Low | (38,53) |
| Ishøy et al,
2017 | 45 patients
diagnosed with SCHZ spectrum disorders +/- obesity, without DM | RCT, EXNTD vs.
placebo, 12 weeks. Outcome: BW change | The weight loss did
not differ between groups at week 12 but was significant vs.
baseline | Quantitative | Yese | No | No | No | No | Mod | (54) |
| NCT00845507 | 54 patients
diagnosed with BD, MDD, SCHZ, SCHZ-AF + obesity | RDBCT, OLZ + EXNTD,
28 days. Outcomes: BW change (primary) | EXNTD was superior
to placebo in the primary outcome. SAE and AE rates were higher in
the EXNTD group | Quantitative | Yese | No | No | No | No | Mod | (55) |
| Whicher et
al, 2021 | 50 patients
diagnosed with SCHZ, SCHZ-AF, or FEP | RDBCT, 24 weeks,
LRGLT vs. Placebo. Outcomes: BW (main), BMI, waist circumference,
HbA1c (secondary) | The results
significantly favor LRGLT, and the GLP- 1RA improved all the
secondary outcomes vs. baseline | Quantitative | Yese | No | No | No | No | Mod | (56) |
| Perlis et
al, 2020 | 46 outpatients who
received AP +/- an antidepressant treatment | Retrospective chart
review, 1 year, AP + GLP1Ras or other AD +/- antidepressants,
Outcomes: BW, HbA1c level, waist circumference, and BMI | HbA1c level
decreased in both groups, but BW decreased significantly more with
the GLP1Ras | Quantitative | Yesf | No | No | No | No | Mod | (57) |
| Lee et al,
2021 | 16 patients
diagnosed with SCHZ or BD + obesity | Retrospective chart
review, 16 weeks, LRGLT. Outcomes: BW change (primary), waist
circumference, BMI, plasma glucose levels (secondary); Aes were
assessed | BW change was
significantly superior for patients treated with LRGLT, and the
secondary outcomes also improved vs. Baseline values. Nausea was
the most frequently reported AE | Quantitative | Yesf | No | No | No | No | Mod | (58) |
| Siskind et
al, 2019 | 164 patients
diagnosed with psychotic disorders + obesity | Meta-analysis (n=3
trials), primary outcome- BW change | BW change was
significantly in favor of GLP- 1Ras vs. Controls. Waist
circumference, BMI, glucose, and lipid metabolism- related
variables were significantly lower during GLP1Ras treatment. The
overall tolerability was good | Quantitative | No | No | No | No | No | High | (59) |
| Wang et al,
2021 | 3467 participants
diagnosed with SCHZ | Network
meta-analysis (n=61 trials), primary outcome-BW change | GLP1Ras were
significantly superior to placebo on BW and waist circumference.
The tolerability was good | Quantitative | No | No | No | No | No | High | (60) |
| Cooper et
al, 2016 | Patients undergoing
AP treatment who present WG and metabolic imbalance | Therapeutic
guideline | GLP1Ras (LRGLT,
EXNTD) have a ῾B’ rating, similar to bariatric surgery and
lorcaserin. GLP1Ras may be added to other antidiabetics in the
treatment of T2DM if BMI≥35 kg/m2, or if the BMI is
lower, but there is an impossibility to use insulin therapy or
weight loss is strongly recommended | Qualitative and
quantitative | No | No | No | No | Yesg | Mod | (61) |
| NCT05333003 | 92 patients
(expected enrollment) with SCHZ spectrum disorders + obesity + non-
responsivity to metformin | RDBCT, SMGLT vs.
placebo as an add-on, 32 weeks. Primary outcome: BW change during
the trial | No results yet | Quantitative | Not applicable | | | | | | (62) |
| Sailer et
al, 2019 | 154 patients
(expected enrollment) with SCHZ spectrum disorders | RDBCT, OLZ/ CLZ +
DLGLT vs. Placebo, 24 weeks. Primary outcome: % of BW change at 24
weeks | No results yet | Quantitative | | | | | | | (63) |
| ACTRN12621
001539820 | 120 patients
(expected enrollment) with SCHZ | RDBCT, CLZ + SMGLT
vs. placebo, 24 weeks. The primary outcome: % BW change at the
endpoint | No results yet | Quantitative | | | | | | | (64) |
Results
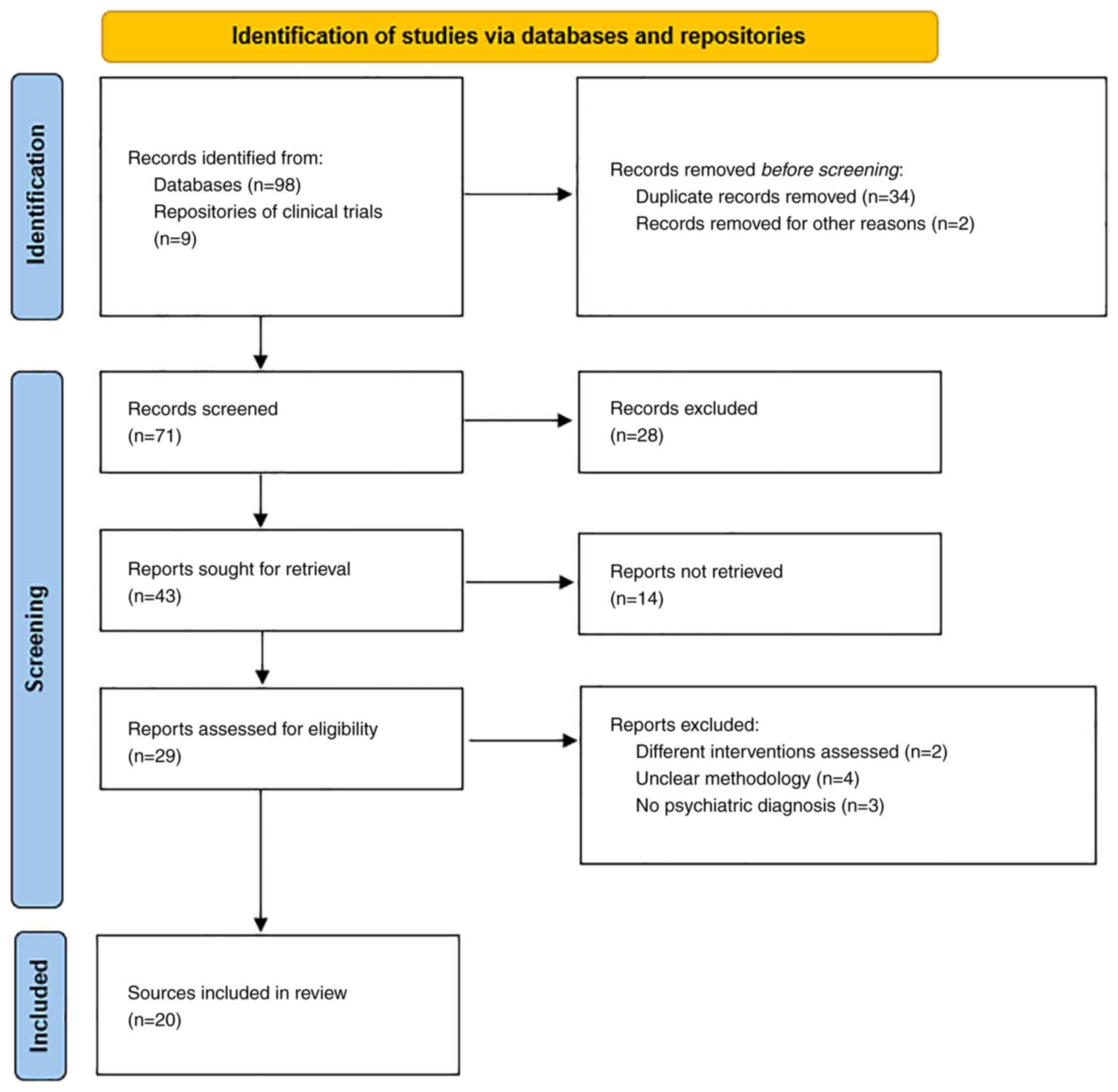
Study inclusion
The primary search identified 107 papers, but only
20 remained after filtering them out according to the inclusion and
exclusion criteria (Fig. 1). A
total of three preclinical studies, two case reports, five
prospective clinical trials (seven references, as two were
follow-up studies), two retrospective studies, two meta-analyses, a
therapeutic guideline, and three ongoing trials were reviewed in
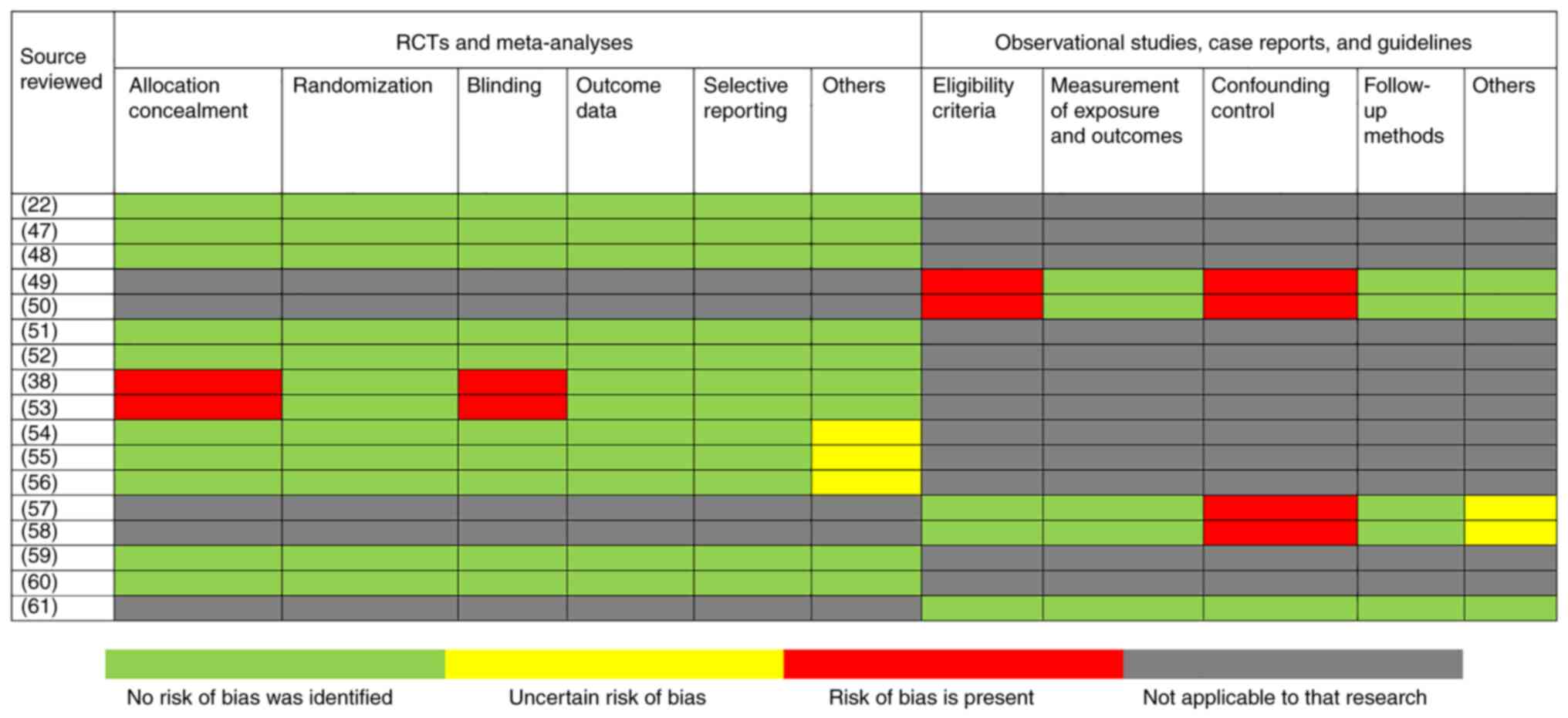
detail (Table III and Fig. 2). The quality of evidence for the
retrieved sources was ‘low’ (n=9), ‘moderate’ (n=4), and ‘high’
(n=4). No quality of evidence rating could be performed for the
ongoing trials, due to the paucity of data available for these
studies.
Preclinical trials
In a preclinical trial, C57BL/6J mice received
olanzapine and/or liraglutide or exendin-4, while the blood glucose
levels were periodically determined (22). The GLP-1RAs offered complete
protection for male mice against increased glycemia due to
olanzapine infusion while increasing circulating insulin (both
agents) and reducing the circulating levels of glucagon (in the
case of liraglutide administration) (22). In the same trial, infusion of
exendin 9-39 (which acts as a GLP-1RA) and olanzapine in female
mice (which did not develop hyperglycemia after acute antipsychotic
administration in the first phase of the experiment) led to
increases in the blood glucose levels, compared with subjects
receiving only olanzapine. Additionally, glucagon levels increased,
and insulin levels decreased when female mice received both the
antipsychotic and the GLP-1RA vs. olanzapine alone. Therefore, it
may be concluded that pharmacological targeting of GLP-1 receptors
may be a useful intervention to mitigate the acute negative
influence of atypical antipsychotics on glucose metabolism
(22).
In rats that received atypical
antipsychotics-olanzapine [2 mg/kg three times a day (t.i.d)] or
clozapine (12 mg/kg t.i.d), a GLP-1RA-liraglutide (0.2 mg/kg twice
a day), combinations of these agents, or inactive comparator for 6
weeks, liraglutide proved beneficial by decreasing the weight gain
and adiposity produced by olanzapine in the first stage of the
study (47). Liraglutide also
improved clozapine-induced glucose intolerance and significantly
reduced the errors made during the Novel Object Recognition Test
discrimination tasks (an outcome related to cognitive functioning)
related to the administration of both antipsychotics (48).
Female Sprague-Dawley rats that received
subcutaneous olanzapine infusion daily or vehicle for four weeks
were given after two weeks liraglutide (0.2 mg/kg) or vehicle twice
daily for another 14 days (48).
Olanzapine administered for 28 days led to higher cumulative food
intake, increased body weight, subcutaneous inguinal fat,
mesenteric fat, retroperitoneal fat, and impaired glucose
tolerance, but these were reduced significantly by liraglutide
administration (48).
Case reports
Liraglutide induced weight loss and lowered the
HbA1c levels in a 60-year-old obese female with dysregulated T2DM
diagnosed with disorganized schizophrenia and a history of drug
abuse, who received treatment with clozapine (49). An initial BMI of 33.5
kg/m2 and baseline HbA1c values of 10% were reported,
before the addition of liraglutide 0.6 mg/day, subcutaneously, to
the already prescribed metformin and insulin. The liraglutide dose
increased gradually to 1.8 mg/day, and after three months the HbA1c
levels decreased to 8.9%, whilst the body weight decreased by 5.1
kg. This trend persisted up to the 2-year visit, with a bodyweight
loss of 7.7 kg and a decrease in her daily need for insulin
(49).
Exenatide was reported to induce weight loss of 41.5
kg after 6 months in a 43-year-old patient with schizophrenia and
newly diagnosed T2DM, who was coincidentally initiated on both
clozapine and the GLP-1 receptor agonist (50). This patient's initial body weight
was 186.9 kg (BMI=46.3 kg/m2) and was already treated
with metformin given his raised glycemic index detected 1 year
before. After the initiation of exenatide, his appetite decreased,
and the only reported adverse event was transient nausea, which
remitted after the dose was lowered. By his last follow-up, his BMI
decreased by 10 kg/m2, while his waist circumference was
reduced by 28 cm (from a baseline value of 160 cm) (50).
Clinical studies
A randomized, double-blind clinical trial enrolled
103 participants diagnosed with SSD who were being treated with
olanzapine or clozapine and received liraglutide (once-daily
subcutaneous injection) or placebo for 4 months (51). The primary outcome was an
improvement in glucose tolerance, and this variable significantly
changed in the active drug group vs. placebo, with 30 vs. 8
patients reaching normal values (64% vs. 16%) at the endpoint. The
body weight decreased with liraglutide vs. placebo (-7 vs. -3.7
kg), and the waist circumference also decreased more with the
GL-1RA treatment (-6 vs. -2.3 cm). Secondary outcomes, like
systolic blood pressure, visceral fat, and low-density lipoprotein
levels were also improved more by liraglutide than by placebo.
Adverse events associated with liraglutide were primarily
gastrointestinal symptoms (51).
However, the liraglutide-treated patients reported better drug
tolerability than patients who received placebo (51).
A total of 1 year after the last visit of this
trial, the body weight increased in patients who received
liraglutide in the initial phase, accompanied by increases in BMI,
waist circumference, and cholesterol levels, while patients in the
placebo group did report only minor changes in HDL levels (52). A comparison of the outcomes in the
two groups showed that patients who initially received liraglutide
were associated with poorer control of glucose metabolism. Other
outcomes, such as fasting glucose, HbA1c levels, C-peptide levels,
and lipids reached the baseline values 12 months after
discontinuing liraglutide. Still, patients who were in the active
treatment group maintained a significant loss of body weight and a
reduction in BMI from baseline to the follow-up vs.
placebo-receiving patients (52).
A 6 month, randomized, open-label trial evaluated
the effects of exenatide on the body weight of obese patients
presenting with SSD ± T2DM undergoing treatment with clozapine
(n=28 outpatients, out of which 5 had T2DM) (38). A total of 6 patients treated with
exenatide reached the primary outcome, which was a ≥5% weight loss
compared to the initial value vs. one patient in the usual care
(control) group. Additionally, exenatide was associated with a
higher body weight decrease and BMI reduction, lower levels of
HbA1c, and lower fasting glucose levels. Therefore, exenatide may
prove as a useful intervention in the reduction of
clozapine-associated cardio-metabolic morbidity and mortality
(38). The 12-month follow-up of
the participants in this study explored the change in weight as the
primary outcome (53). When
compared to the first phase endpoint values, at 12 months, patients
who formerly received exenatide had a significantly greater
increase in BMI, body weight, and percentage of participants
presenting ≥5% weight gain. If the 12-month follow-up values were
matched to the baseline characteristics in the original trial, no
differences were detected between exenatide and treatment as usual
in the evolution of either BMI or body weight (53).
Another trial included 45 patients with SSD and
obesity (without diabetes), undergoing treatment with
antipsychotics, randomized to adjunctive treatment with exenatide
(2 mg administered every week) or placebo for 12 weeks (54). Initial body weight was 118.3±16 kg
and 111.7±18 kg in the active drug and placebo group, respectively,
and at the endpoint, the weight loss was similar in both groups
(~2.24 kg). Patients experienced significant body weight changes
compared with the baseline values, but with no correlation with the
type of intervention they received (54).
Another phase 4, placebo-controlled, randomized
clinical trial enrolled 54 subjects diagnosed with severe
psychiatric disorders and obesity, undergoing treatment with
olanzapine, and explored their evolution during exenatide
administration (5 µg twice daily for 28 days, and increased, if
tolerated, to 10 µg twice daily after this term) (55). According to the unpublished
results, retrieved from the U.S. National Library of Medicine (NLM)
online registry, the primary outcome was the bodyweight change from
baseline to endpoint (week 16) in favor of exenatide (-1.1 vs. +5.9
pounds), while the BMI decreased by 0.2 kg/m2 (exenatide
group) and increased by 1 kg/m2 (placebo). The rate of
serious adverse events was 8.33% in patients receiving exenatide
vs. 3.33% in the placebo group, while the incidence of adverse
events was 58.3% vs. 36.6%, respectively (55).
In a randomized, double-blind, placebo-controlled
pilot study, the effectiveness of liraglutide administration
subcutaneously, once-daily injection (titrated to 3 mg/day), was
compared for 6 months to placebo in 50 patients with acute or
chronic psychotic disorders (56).
Liraglutide had a favorable effect on the weight of the
participants (-5.7±7.9 kg) compared with the placebo (no
significant change). Additionally, the BMI, HbA1c levels, and waist
circumference decreased in the liraglutide-treated patients
(56).
A retrospective chart review included 46 outpatients
who were prescribed antipsychotics and GLP-1RAs (liraglutide,
exenatide, or dulaglutide) or alternative antidiabetic agents
(57). Within 1 year, both groups
presented with a reduction in their HbA1c levels, but the changes
in the patients' weight gain were significantly different between
participants who received GLP-1 analogs (-7.07±2.62 kg compared to
baseline) and controls (+1.93±1.14 kg compared to baseline).
Additionally, it was noted that patients who received an
antipsychotic and an antidepressant had smaller HbA1c reductions
than those who did not have an associated antidepressant in the
absence of a GLP-1RA, whilst patients treated with an antipsychotic
and a GLP-1RA had larger HbA1c reductions vs. controls, independent
of the concomitantly administered antidepressant. However, given
the retrospective nature of this study, it was impossible to
exclude several confounders (such as the addition of GLP-1RAs
selectively in the therapeutic regimen of individuals with a higher
BMI, a lack of assessment of treatment adherence, amongst other
variables) (57).
Another retrospective review included 16 patients
diagnosed with obesity and schizophrenia or bipolar disorder who
received treatment with liraglutide and were monitored for 16 weeks
(58). The endpoint values of body
weight indicated a significant effect of liraglutide (a decrease
from 93.2 to 88.9 kg at the endpoint). Additionally, the waist
circumference, BMI, and plasma glucose levels improved compared to
the baseline. The most commonly reported adverse event was nausea
(37.5% of all subjects). Responders, defined by at least 5% body
weight loss, represented 50% of the 16-week treatment completers
(58).
Meta-analyses
A meta-analysis (n=3 trials) evaluated the effects
of exenatide and liraglutide in 164 patients diagnosed with SSD,
who had a mean body weight at baseline of 105.8±20.8 kg (59). The results showed that after a mean
of 16 weeks of GLP-1ARs administration, the mean body weight loss
reached a value of 3.71 kg (59).
The body weight change was significantly greater for GLP-1Ras vs.
control, with a number-needed-to treat value for a weight loss ≥5%
of 3.8. Additionally, waist circumference, BMI, HbA1c, fasting
glucose, and visceral adiposity were significantly lower during
GLP-1RA treatment. Patients undergoing treatment with olanzapine or
clozapine exhibited greater weight loss than those receiving other
antipsychotics. The tolerability of this type of treatment was
good, with nausea being more common than in the placebo group
(number-needed-to-harm=3.8) (59).
A network meta-analysis compared the impact of
pharmacological strategies on body weight in individuals with
schizophrenia who developed antipsychotic-induced metabolic
abnormalities (n=61 randomized trials, n=3,467 patients) (60). GLP-1RAs were significantly superior
to placebo (weighted mean difference=-3.23), as did topiramate
(-5.4), zonisamide (-3.44), metformin (-3.01), and nizatidine
(-2.14) (60). The superiority of
these agents was also observed in studies evaluating the BMI, and
the tolerability was good, except for topiramate which was inferior
to placebo, GLP-1RAs, and metformin. GLP-1RAs were superior to
placebo in decreasing the waist circumference (60).
Treatment guidelines
The British Association of Psychopharmacology (BAP,
2016) guidelines on the management of antipsychotic
treatment-related adverse events mention the possible use of
GLP-1RAs for obesity, but the lack of a marketing authorization for
this indication precludes its clear recommendation (61). The BAP rating for GLP-1RAs (such as
liraglutide and exenatide) is ‘B’ (derived from the evidence of
efficacy that exists in the general population), which places them
in the same category as bariatric surgery and lorcaserin for these
patients. GLP-1RAs are recommended as a component of the triple
therapy; additionally, metformin and a sulfonylurea agent, for
adults with T2DM who have i) a BMI of at least 35 kg/m2
+ a specific psychological and other medical problems related to
obesity, or ii) a BMI<35 kg/m2 + possible onset of
significant occupational dysfunctions if insulin therapy were to be
initiated, or when weight loss would be beneficial for other
concomitant diseases (61).
Trials in the pipeline
A randomized trial is expected to recruit 92
participants with SSD comorbid with obesity and non-responsive to
metformin who will receive treatment with semaglutide (starting
dose 0.25 mg/week and titrated up to 2 mg/week) or placebo
(62). The primary outcome of this
trial is weight change after 32 weeks, while the secondary outcome
measures are BMI, waist circumference, oral glucose tolerance test,
visceral and hepatic adiposity, fasting lipid profile, and multiple
psychopathological scales, referring to the overall mental status,
depressive symptoms, general functioning, cognitive performance,
quality of life, nicotine dependence, and food cravings (62).
Another published study protocol envisages the
enrollment of 154 participants diagnosed with SSD and newly treated
with olanzapine or clozapine, who will be monitored for 24 weeks
during the treatment with dulaglutide (0.75 mg/week) or placebo
subcutaneously (63). The
described study design is double-blind, multicenter, and
randomized, and it will have as the primary outcome the mean
percentage weight change at the endpoint (63).
Yet another trial protocol envisages enrollment of
120 participants diagnosed with schizophrenia who receive treatment
with clozapine and who will be allocated randomly to semaglutide or
placebo and monitored for 24 weeks (64). The primary variable monitored will
be the change in body weight, and the secondary outcome is the risk
of conversion to type 2 diabetes and/or metabolic syndrome during
the trial (64).
Discussion
The impact of various GLP-1RAs on metabolic
dysfunctions induced by antipsychotics was assessed quantitatively
and/or qualitatively in 20 sources identified during this
systematic review. To the best of our knowledge, there is little
data in favor of other GLP-1RAs used in this specific population,
except for liraglutide and exenatide, although isolated references
to semaglutide and dulaglutide exist. According to the retrieved
data, the variables related to body weight (including BMI, waist
circumference, and body weight change) may be improved during
treatment with GLP-1Ras (38,47,49-52,55-61).
Follow-up studies (up to 1 year) are not unanimously supportive of
the superiority of GLP-1RAs over placebo (52,53),
while a single case report with a 2-year follow-up was more
positive in this direction (48).
The favorable effect of GLP-1RAs on glucose
metabolism (fasting glycemia, HbA1c, insulin, and glucagon blood
levels) in subjects who received antipsychotics is supported by a
large volume of clinical and preclinical data (22,38,47-50,51,56-59,61).
GLP-1RAs also have been associated with positive
effects on lipid metabolism (i.e., lipids blood levels, visceral
adiposity) in the reviewed papers (47,51,59,61).
Regarding the tolerability of GLP-1RAs in patients
with psychiatric disorders and metabolic dysfunctions, the results
are consistent with reviews that evaluated these parameters in
other groups, supporting an overall good safety profile and low
incidence of adverse events, primarily gastrointestinal symptoms
(including transient nausea, diarrhea, constipation, and dyspepsia)
(50,51,58).
For comparison, a systematic review of case reports including
patients treated with GLP-1RAs (n=140 participants, treated with
exenatide, liraglutide, dulaglutide, semaglutide, albiglutide, or
lixisenatide) showed the most frequently reported adverse events
were gastrointestinal manifestations, followed by renal,
dermatologic, hepatic, immunologic, metabolic, hematologic,
angioedema, neurologic, cardiovascular, and very rare psychiatric,
reproductive, or generalized edema symptoms (65).
The GRADE recommendations formulated were A (high),
B (moderate), C (low), or D (very low) (42,43),
according to the level of confidence that the clinical use of
GLP-1RAs will improve the outcome of patients with severe
psychiatric disorders presenting metabolic dysfunctions related to
the antipsychotic treatment (Table
IV).
 | Table IVGRADE recommendations based on the
reviewed papers. |
Table IV
GRADE recommendations based on the
reviewed papers.
| Pharmacological
agents | GRADE
recommendations | Observations | (Refs.) |
|---|
| GLP-1RAs (as a
pharmacological class) | C: These agents may
be used as add-ons to metformin in patients diagnosed with severe
mental disorders undergoing AP treatment, especially OLZ/CLZ, for
the purpose of controlling BW changes | Based on the
favorable results of a therapeutic guideline, two meta- analyses,
and one retrospective review | (57,59-61) |
| | C: For improvement
of glucose metabolism dysfunctions (as add-ons) | | |
| | C: For the
treatment of lipid metabolism dysfunctions (as add-ons) | | |
| Liraglutide | B: As an
augmentative agent to metformin for individuals diagnosed with
severe mental disorders undergoing AP treatment, especially
OLZ/CLZ, for the management of BW | Based on the
favorable conclusions of a case report, one RCT, two meta-analyses,
two retrospective chart reviews, one therapeutic guideline, and
three preclinical trials | (22,47,48,56-61) |
| | B: For the
improvement of glucose metabolism dysfunctions (as an add-on). | | |
| | C: For the
improvement of lipid metabolism dysfunctions (as an add-on) | | |
| Exenatide | B: If added to
metformin for patients diagnosed with severe mental disorders
undergoing AP treatment, especially OLZ/CLZ, for the control of BW
changes | Based on the
favorable results of three RCTs, one retrospective chart review,
two meta-analyses, one therapeutic guideline, and a case
report | (50,53-55,57,59-61) |
| | B: For the
improvement of glucose metabolism dysfunctions (as an add-on) | | |
| | C: For the
improvement of lipid metabolism dysfunctions (as an add-on) | | |
| Semaglutide | D: Not enough data
to formulate a clinical recommendation | One RCT is
ongoing | (64) |
| Albiglutide
Dulaglutide Lixisenatide Tirzepatide | D: Not enough data
to support a clinical recommendation | | |
As a limitation of the review, it must be mentioned
that it includes three references to trials that are ongoing,
therefore, their results are not yet available, but once known, it
is possible they will modify the recommendations. Additionally, the
strength of the GRADE recommendations is dependent on the quality
of the reviewed body of research, and the majority of the presented
sources (n=9) have been considered of moderate quality.
As strengths of the current systematic review, it
included both primary and secondary reports, referring to both
preclinical and clinical research. Additionally, this is the first
systematic review focused on the efficacy and tolerability of
GLP-1RAs in the treatment of a psychiatric population which also
included a set of clinical recommendations.
Future directions of research regarding SSD patients
may target finding the pharmacogenetic variables in individuals
receiving antipsychotics that correlate with a higher risk for
metabolic disease. In the same line of personalized medicine,
exploration of the interaction between diet, physical exercise,
antipsychotic treatment, and individual biological vulnerability
could help in the improvement of these patients' metabolic status
in the medium and long term. Head-to-head comparison trials
investigating GLP-1RAs and other new-generation antidiabetics, such
as inhibitors of dipeptidyl peptidase 4, or sodium-glucose
transport protein 2 inhibitors may provide novel and important
information regarding the specific effect of each class of agents
in SSD patients. Finally, the pharmacogenetics of GLP-1RAs and
other antidiabetics is certainly worth further exploration for the
potential identification of sub-groups of responders and
non-responders in this vulnerable population.
In conclusion, there are favorable results on a
large set of metabolic variables for liraglutide and exenatide in
patients with severe mental disorders, but there is enough ground
to further explore the effects of semaglutide and dulaglutide in
the same population. Due to the significant negative impact of
metabolic dysfunctions on the quality of life, evolution of
comorbid diseases, and life expectancy, second-generation
antidiabetics may represent an important therapeutic resource for
the management of SSD patients. The good tolerability of GLP-1RAs
is essential in this population, where the high rate of treatment
discontinuation due to adverse events, and the existence of poor
insight and care for own health are very common.
Although the number of retrieved sources in the
literature was not very high, this may be considered a consequence
of the relative novelty of GLP-1RAs. It is expected that larger
trials, with a longer duration of monitoring, will explore the
efficacy and tolerability of GLP-1RAs both in the general
population and in individuals with mental disorders.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Author's contributions
OV conceived and designed the study, collected and
analyzed the data, and wrote and revised the manuscript. OV
confirmed the authenticity of the raw data, and read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
Cernea S, Dima L, Correll CU and Manu P:
Pharmacological management of glucose dysregulation in patients
treated with second-generation antipsychotics. Drugs. 80:1763–1781.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chang CK, Hayes RD, Perera G, Broadbent
MT, Fernandes AC, Lee WE, Hotopf M and Stewart R: Life expectancy
at birth for people with serious mental illness and other major
disorders from a secondary mental health care case register in
London. PLoS One. 6(e19590)2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kessing LV, Vradi E, McIntyre RS and
Andersen PK: Causes of decreased life expectancy over the life span
in bipolar disorder. J Affect Disord. 180:142–147. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dieset I, Andreassen OA and Haukvik UK:
Somatic comorbidity in schizophrenia: Some possible biological
mechanisms across the life span. Schizophr Bull. 42:1316–1319.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vasiliu O, Vasile D, Făinărea AF, Pătrașcu
MC, Morariu EA, Manolache R, Alexandru I and Androne FT: Analysis
of risk factors for antipsychotic-resistant schizophrenia in young
patients-a retrospective analysis. Rom J Mil Med. (CXXI): 25–29.
2018.
|
|
6
|
Rajkumar AP, Horsdal HT, Wimberley T,
Cohen D, Mors O, Børglum AD and Gasse C: Endogenous and
antipsychotic-related risks for diabetes mellitus in young people
with schizophrenia: A Danish population-based cohort study. Am J
Psychiatry. 174:686–694. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vasiliu O: Therapeutic management of
schizophrenia and substance use disorders dual diagnosis-clinical
vignettes. Rom J Mil Med. 121:26–34. 2018.
|
|
8
|
Ebdrup BH, Knop FK, Ishøy PL, Rostrup E,
Fagerlund B, Lublin H and Glenthøj B: Glucagon-like peptide-1
analogs against antipsychotic-induced weight gain: Potential
physiological benefits. BMC Med. 10(92)2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kouidrat Y and Amad A: GLP-1 agonists for
metabolic disorders in schizophrenia. Schizophr Res. 204:448–449.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vasiliu O: Esketamine for
treatment-resistant depression: A review of clinical evidence
(Review). Exper Ther Med. 25(111)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hatziagelaki E, Tsiavou A, Gerasimou C,
Vavougios GD, Spathis A, Laskos E, Papageorgiou C and Douzenis A:
Effects of olanzapine on cytokine profile and brain-derived
neurotrophic factor in drug-naïve subjects with first-episode
psychosis. Exp Ther Med. 17:3071–3076. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ermakov EA, Melamund MM, Buneva VN and
Ivanova SA: Immune system abnormalities in schizophrenia: An
integrative view and translational perspectives. Front Psychiatry.
13(880568)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mizuki Y, Sakamoto S, Okahisa Y, Yada Y,
Hashimoto N, Takaki M and Yamada N: Mechanisms underlying the
comorbidities of schizophrenia and type 2 diabetes mellitus. Int J
Neuropsychopharmacol. 24:367–382. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Libowitz MR and Nurmi EL: The burden of
antipsychotic-induced weight gain and metabolic syndrome in
children. Front Psychiatry. 12(623681)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen J, Huang XF, Shao R, Chen C and Deng
C: Molecular mechanisms of antipsychotic drug-induced diabetes.
Front Neurosci. 11(643)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Goh KK, Chen CYA, Wu TH, Chen CH and Lu
ML: Crosstalk between schizophrenia and metabolic syndrome: The
role of oxytocinergic dysfunction. Int J Mol Sci.
23(7092)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Carey ME, Barnett J, Doherty Y, Barnard K,
Daly H, French P, Gossage-Worrall R, Hadjiconstantinou M, Hind D,
Mitchell J, et al: Reducing weight gain in people with
schizophrenia, schizoaffective disorder, and first episode
psychosis: Describing the process of developing the Structured
lifestyle Education for People with SchizophrEnia (STEPWISE)
intervention. Pilot Feasibility Stud. 4(186)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Budisteanu M, Andrei E, Lica F, Hulea DS,
Velicu AC, Mihailescu I, Riga S, Arghir A, Papuc SM, Sirbu CA, et
al: Predictive factors in early onset schizophrenia. Exp Ther Med.
20(210)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vasiliu O: Impact of SGLT2 inhibitors on
metabolic status in patients with psychiatric disorders undergoing
treatment with second-generation antipsychotics (Review). Exp Ther
Med. 25(125)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fitzgerald I, O'Connell J, Keating D,
Hynes C, McWilliams S and Crowley EK: Metformin in the management
of antipsychotic-induced weight gain in adults with psychosis:
Development of the first evidence-based guideline using GRADE
methodology. Evid Based Ment Health. 25:15–22. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hiluy JC, Nazar BP, Gonçalves WS, Coutinho
W and Appolinario JC: Effectiveness of pharmacologic interventions
in the management of weight gain in patients with severe mental
illness: A systematic review and meta-analysis. Prim Care Companion
CNS Disord. 21(19r02483)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Medak KD, Shamshoum H, Peppler WT and
Wright D: GLP1 receptor agonism protects against acute
olanzapine-induced hyperglycemia. Am J Physiol Endocrinol Metab.
319:E1101–E1111. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Boyda HN, Tse L, Procyshyn RM, Wong D, Wu
TH, Pang CC and Barr AM: A parametric study of the acute effects of
antipsychotic drugs on glucose sensitivity in an animal model. Prog
Neuropsychopharmacol Biol Psychiatry. 34:945–954. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chintoh AF, Mann SW, Lam L, Lam C, Cohn
TA, Fletcher PJ, Nobrega JN, Giacca A and Remington G: Insulin
resistance and decreased glucose-stimulated insulin secretion after
acute olanzapine administration. J Clin Psychopharmacol.
28:494–499. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mayfield K, Siskind D, Winckel K, Russell
AW, Kisely S, Smith G and Hollingworth S: Glucagon-like peptide-1
agonists combating clozapine-associated obesity and diabetes. J
Psychopharmacol. 30:227–236. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Deutch AY: Liraglutide for the treatment
of antipsychotic drug-induced weight gain. JAMA Psychiatry.
74:1172–1173. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Heppner KM, Kirigiti M, Secher A, Paulsen
SJ, Buckingham R, Pyke C, Knudsen LB, Vrang N and Grove KL:
Expression and distribution of glucagon-like peptide-1 receptor
mRNA, protein and binding in the male nonhuman primate (Macaca
mulatta) brain. Endocrinolog. 156:255–267. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhong P, Zeng H, Huang M, Fu W and Chen Z:
Efficacy and safety of once-weekly semaglutide in adults with
overweight or obesity: A meta-analysis. Endocrine. 75:718–724.
2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Avgerinos I, Michailidis T, Liakos A,
Karagiannis T, Matthews DR, Tsapas A and Bekiari E: Oral
semaglutide for type 2 diabetes: A systematic review and
meta-analysis. Diabetes Obes Metab. 22:335–345. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Blair HA and Keating GM: Albiglutide: A
review of its use in patients with type 2 diabetes mellitus. Drugs.
75:651–663. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chudleigh RA, Platts J and Bain SC:
Comparative effectiveness of long-acting GLP-1 receptor agonists in
type-2 diabetes: A short review on the emerging data. Diabetes
Metab Syndr Obes. 13:433–438. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Umpierrez G, Tofé Povedano S, Pérez Manghi
F, Shurzinske L and Pechtner V: Efficacy and safety of dulaglutide
monotherapy versus metformin in type 2 diabetes in a randomized
controlled trial (AWARD-3). Diabetes Care. 37:2168–2176.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Madsbad S: Review of head-to-head
comparison of glucagon-like peptide-1 receptor agonists. Diabetes
Obes Metab. 18:317–332. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Roca-Rodriguez MM, Muros de Fuentes MT,
Piédrola-Maroto G, Quesada-Charneco M, Maraver-Selfa S, Tinahones
FJ and Mancha-Doblas I: Lixisenatide in patients with type 2
diabetes and obesity: Beyond glycamic control. Aten Primaria.
49:294–299. 2017.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
35
|
Rosenstock J, Raccah D, Korányi L, Maffei
L, Boka G, Miossec P and Gerich JE: Efficacy and safety of
lixisenatide once daily versus exenatide twice daily in type 2
diabetes inadequately controlled on metformin: A 24-week,
randomized, open-label, active-controlled study (GetGoal-X).
Diabetes Care. 36:2945–2951. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kapitza C, Forst T, Coester HV, Poitiers
F, Ruus P and Hincelin-Méry A: Pharmacodynamic characteristics of
lixisenatide once daily versus liraglutide once daily in patients
with type 2 diabetes insufficiently controlled on metformin.
Diabetes Obes Metab. 15:642–649. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ishøy PL, Fagerlund B, Broberg BV, Bak N,
Knop FJ, Glenthøj BY and Ebdrup BH: No cognitive-enhancing effect
of GLP-1 receptor agonism in antipsychotic-treated, obese patients
with schizophrenia. Acta Psychiatr Scand. 136:52–62.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Siskind DJ, Russell AW, Gamble C, Winckel
K, Mayfield K, Hollingworth S, Hickman I, Siskind V and Kisely S:
Treatment of clozapine-associated obesity and diabetes with
exenatide in adults with schizophrenia: A randomized controlled
trial (CODEX). Diabetes Obes Metab. 20:1050–1055. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Htike ZZ, Zaccardi F, Papamargaritis D,
Webb DR, Khunti K and Davies MJ: Efficacy and safety of
glucagon-like peptide-1 receptor agonists in type 2 diabetes: A
systematic review and mixed-treatment comparison analysis. Diabetes
Obes Metab. 19:524–536. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Min T and Bain SC: The role of
tirzepatide, dual GIP and GLP-1 receptor agonist, in the management
of type 2 diabetes: The SURPASS clinical trials. Diabetes Ther.
12:143–157. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ludvik B, Giorgino F, Jódar E, Frias JP,
Fernández Landó L, Brown K, Bray R and Rodríguez Á: Once-weekly
tirzepatide versus once-daily insulin degludec as add-on to
metformin with or without SGLT2 inhibitors in patients with type 2
diabetes (SURPASS-3): A randomized, open-label, parallel-group,
phase 3 trial. Lancet. 398:583–598. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Brozek JL, Akl EA, Compalati E, Kreis J,
Terraciano L, Fiocchi A, Ueffing E, Andrews J, Alonso-Coello P,
Meerpohl JJ, et al: Grading quality of evidence and strength of
recommendations in clinical practice guidelines part 3 of 3. The
GRADE approach to developing recommendations. Allergy. 66:588–595.
2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Neumann I, Santesso N, Akl EA, Rind DM,
Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE,
Schünemann H and Guyatt GH: A guide for health professionals to
interpret and use recommendations in guidelines developed with the
GRADE approach. J Clin Epidemiol. 72:45–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cooke A, Smith D and Booth A: Beyond PICO:
The SPIDER tool for qualitative evidence synthesis. Qual Health
Res. 22:1435–1443. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Schünemann H, Brożek J, Guyatt G and Oxman
A (eds): GRADE handbook for grading quality of evidence and
strength of recommendations. Updated October 2013. The GRADE
Working Group, 2013. guidelinedevelopment.org/handbook. Accessed January
7, 2023.
|
|
47
|
Babic I, Gorak A, Engel M, Sellers D, Else
P, Osborne AL, Pai N, Huang XF, Nealon J and Weston-Green K:
Liraglutide prevents metabolic side-effects and improves
recognition and working memory during antipsychotic treatment in
rats. J Psychopharmacol. 32:578–590. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lykkegaard K, Larsen PJ, Vrang N, Bock C,
Bock T and Knudsen LB: The once-daily human GLP-1 analog,
liraglutide, reduces olanzapine-induced weight gain and glucose
intolerance. Schizophr Res. 103:94–103. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ishøy PL, Knop FK, Vilsbøll T, Glenthøj BY
and Ebdrup BH: Sustained weight loss after treatment with a
glucagon-like peptide-1 receptor agonist in an obese patient with
schizophrenia and type 2 diabetes. Am J Psychiatry. 170:681–682.
2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Siskind D, Wysoczanski D, Russell A and
Ashford M: Weight loss associated with exenatide in an obese man
with diabetes commenced on clozapine. Aust N Z J Psychiatry.
50:702–703. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Larsen JR, Vedtofte L, Jakobsen MSL,
Jespersen HR, Jakobsen MI, Svensson CK, Koyuncu K, Schjerning O,
Oturai PS, Kjaer A, et al: Effect of liraglutide treatment on
prediabetes and overweight or obesity in clozapine- or
olanzapine-treated patients with schizophrenia spectrum disorder: A
randomized clinical trial. JAMA Psychiatry. 74:719–728.
2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Svensson CK, Larsen JR, Vedtofte L,
Jakobsen MSL, Jespersen HR, Jakobsen MI, Koyuncu K, Schjerning O,
Nielsen J, Ekstrøm CT, et al: One-year follow-up on liraglutide
treatment for prediabetes and overweight/obesity in clozapine- or
olanzapine-treated patients. Acta Psychiatr Scand. 139:26–36.
2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Siskind D, Russell A and Kisely S: T44.
12-Month follow-up of metabolic measures following a randomised
controlled trial of treatment of clozapine associated obesity and
diabetes with exenatide (CODEX). Schizophr Bull. 46
(Suppl.1)(S248)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ishøy PL, Knop FK, Broberg BV, Bak N,
Andersen UB, Jørgensen NR, Holst JJ, Glenthøj BY and Ebdrup BH:
Effect of GLP-1 receptor agonist treatment on body weight in obese
antipsychotic-treated patients with schizophrenia: A randomized,
placebo-controlled trial. Diabetes Obes Metab. 19:162–171.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
U.S. National Library of Medicine:
Exenatide for the treatment of weight gain associated with
olanzapine in obese adults. NCT00845507. https://clinicaltrials.gov/ct2/show/results/NCT00845507.
Accessed June 10, 2022.
|
|
56
|
Whicher CA, Price HC, Phiri P, Rathod S,
Barnard-Kelly K, Ngianga K, Thorne K, Asher C, Peveler RC, McCarthy
J and Holt RIG: The use of liraglutide 3.0 mg daily in the
management of overweight and obesity in people with schizophrenia,
schizoaffective disorder and first episode psychosis: Results of a
pilot randomized, double-blind, placebo-controlled trial. Diabetes
Obes Metab. 23:1262–1271. 2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Perlis LT, Lamberti JS and Miedlich SU:
Glucagon-like peptide analogs are superior for diabetes and weight
control in patients on antipsychotic medications: A retrospective
cohort study. Prim Care Companion CNS Disord.
22(19m02504)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lee SE, Lee NY, Kim SH, Kim KA and Kim YS:
Effect of liraglutide 3.0 mg treatment on weight reduction in obese
antipsychotic-treated patients. Psychiatry Res.
299(113830)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Siskind D, Hahn M, Correll CU, Fink-Jensen
A, Russell AW, Bak N, Broberg BV, Larsen J, Ishøy PL, Vilsbøll T,
et al: Glucagon-like peptide-1 receptor agonists for
antipsychotic-associated cardio-metabolic risk factors: A
systematic review and individual participant data meta-analysis.
Diabetes Obes Metab. 21:293–302. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang Y, Wang D, Cheng J, Fang X, Chen Y,
Yu L, Ren J, Tian Y and Zhang C: Efficacy and tolerability of
pharmacological interventions on metabolic disturbances induced by
atypical antipsychotics in adults: A systematic review and network
meta-analysis. J Psychopharmacol. 35:1111–1119. 2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Cooper SJ and Reynolds GP: With expert
co-authors (in alphabetical order). Barnes T, England E, Haddad PM,
Heald A, Holt R, Lingford-Hughes A, Osborn D, et al: BAP guidelines
on the management of weight gain, metabolic disturbances and
cardiovascular risk associated with psychosis and antipsychotic
drug treatment. J Psychopharmacol. 30:717–748. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
U.S. National Library of Medicine.
Semaglutide in comorbid schizophrenia spectrum disorder and obesity
(Sema). NCT05333003. Retrieved online at https://clinicaltrials.gov/ct2/show/NCT05333003
(accessed June 10, 2022).
|
|
63
|
Sailer CO, Sulieman I, Macedo CT, Parodi
MR, Machuca M, Sanchez CJS, Murrieta CGI, Delgoshaie N, Nanbu DY,
Sarmento R, et al: The glucagon like peptide-1 analogue dulaglutide
to prevent antipsychotic induced weight gain-a study protocol
proposal. Prin Pract Clin Res. 5:19–26. 2019.
|
|
64
|
Queensland Centre for Mental Health
Research: Cadence-CoaST clinical trial. https://qcmhr.org/research/studies-recruiting-participants/2759-2/.
Accessed June 10, 2022.
|
|
65
|
Shetty R, Basheer FT, poojari PG, Thunga
G, Chandran VP and Acharya LD: Adverse drug reactions of GLP-1
agonists: A systematic review of case reports. Diabetes Metab
Syndr. 16(102427)2022.PubMed/NCBI View Article : Google Scholar
|