|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Duan J, Cui L, Zhao X, Bai H, Cai S, Wang
G, Zhao Z, Zhao J, Chen S, Song J, et al: Use of immunotherapy with
programmed cell death 1 vs programmed cell death ligand 1
inhibitors in patients with cancer: A systematic review and
meta-analysis. JAMA Oncol. 6:375–384. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
de Miguel M and Calvo E: Clinical
challenges of immune checkpoint inhibitors. Cancer Cell.
38:326–333. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ma K, Jin Q, Wang M, Li X and Zhang Y:
Research progress and clinical application of predictive biomarker
for immune checkpoint inhibitors Expert Rev Mol. Diagn. 19:517–529.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Powles T, van der Heijden MS, Castellano
D, Galsky MD, Loriot Y, Petrylak DP, Ogawa O, Park SH, Lee JL,
Giorgi UD, et al: Durvalumab alone and durvalumab plus tremelimumab
versus chemotherapy in previously untreated patients with
unresectable, locally advanced or metastatic urothelial carcinoma
(DANUBE): A randomised, open-label, multicentre, phase 3 trial.
Lancet Oncol. 21:1574–1588. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Felip E, Altorki N, Zhou C, Csőszi T,
Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A,
Kenmotsu H, et al: Adjuvant atezolizumab after adjuvant
chemotherapy in resected stage IB-IIIA non-small-cell lung cancer
(IMpower010): A randomised, multicentre, open-label, phase 3 trial.
Lancet. 398:1344–1357. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Weber JS, D'Angelo SP, Minor D, Hodi FS,
Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD,
et al: Nivolumab versus chemotherapy in patients with advanced
melanoma who progressed after anti-CTLA-4 treatment (CheckMate
037): A randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 16:375–384. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brahmer JR, Rodríguez-Abreu D, Robinson
AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A,
Cuffe S, et al: Health-related quality-of-life results for
pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC
(KEYNOTE-024): A multicentre, international, randomised, open-label
phase 3 trial. Lancet Oncol. 18:1600–1609. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hutarew G: PD-L1 testing, fit for routine
evaluation? From a pathologist's point of view. Memo. 9:201–206.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lisberg A and Garon EB: The value of PD-L1
testing in non-small-cell lung cancer. JAMA Oncol. 2:571–572.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jardim DL, Goodman A, de Melo Gagliato D
and Kurzrock R: The challenges of tumor mutational burden as an
immunotherapy biomarker. Cancer Cell. 39:154–173. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
David PC, Reck M, Luis PA, Creelan B, Horn
L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, et
al: First-line nivolumab in stage IV or recurrent non-small-cell
lung cancer. N Engl J Med. 376:2415–2426. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hellmann MD, Nathanson T, Rizvi H, Creelan
BC, Sanchez-Vega F, Ahuja A, Ni A, Novik JB, Mangarin LMB,
Abu-Akeel M, et al: Genomic features of response to combination
immunotherapy in patients with advanced non-small-cell lung cancer.
Cancer Cell. 33:843–852.e4. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alborelli I, Leonards K, Rothschild SI,
Leuenberger LP, Prince SS, Mertz KD, Poechtrager S, Buess M,
Zippelius A, Läubli H, et al: Tumor mutational burden assessed by
targeted NGS predicts clinical benefit from immune checkpoint
inhibitors in non-small cell lung cancer. J Pathol. 250:19–29.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Marcus L, Fashoyin-Aje LA, Donoghue M,
Yuan M, Rodriguez L, Gallagher PS, Philip R, Ghosh S, Theoret MR,
Beaver JA, et al: FDA approval summary: Pembrolizumab for the
treatment of tumor mutational burden-high solid tumors. Clin Cancer
Res. 27:4685–4689. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Goodman A, Kato S, Bazhenova L, Patel SP,
Frampton GM, Miller V, Stephens PJ, Daniels GA and Kurzrock R:
Tumor mutational burden as an independent predictor of response to
immunotherapy in diverse cancers. Mol Cancer Ther. 16:2598–2608.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Palles C, Cazier JB, Howarth KM, Domingo
E, Jones AM, Broderick P, Kemp Z, Spain SL, Guarino E, Salguero I,
et al: Germline mutations in the proof-reading domains of POLE and
POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet.
45:136–144. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Gong J, Wang C, Lee PP, Chu P and Fakih M:
Response to PD-1 blockade in microsatellite stable metastatic
colorectal cancer harboring a POLE mutation. J Natl Compr Canc
Netw. 15:142–147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Postow MA, Manuel M, Wong P, Yuan J, Dong
Z, Liu C, Perez S, Tanneau I, Noel M, Courtier A, et al: Peripheral
T cell receptor diversity is associated with clinical outcomes
following ipilimumab treatment in metastatic melanoma. J Immunother
Cancer. 23:10.1186/s40425-015-0070-4. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Snyder A, Nathanson T, Funt SA, Ahuja A,
Novik JB, Hellmann MD, Chang E, Aksoy BA, Al-Ahmadie H, Yusko E, et
al: Contribution of systemic and somatic factors to clinical
response and resistance to PD-L1 blockade in urothelial cancer: An
exploratory multi-omic analysis. PLoS Med.
14(e1002309)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gao GD, Sun B, Wang XB and Wang SM:
Neutrophil to lymphocyte ratio as prognostic indicator for patients
with esophageal squamous cell cancer. Int J Biol Markers.
32:e409–e414. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Iivanainen S, Ahvonen J, Knuuttila A,
Tiainen S and Koivunen JP: Elevated CRP levels indicate poor
progression-free and overall survival on cancer patients treated
with PD-1 inhibitors. ESMO Open. 4(e000531)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chasseuil E, Saint-Jean M, Chasseuil H,
Peuvrel L, Quéreux G, Nguyen JM, Gaultier A, Varey E, Khammari A
and Dréno B: Blood predictive biomarkers for nivolumab in advanced
melanoma. Acta Derm Venereol. 98:406–410. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
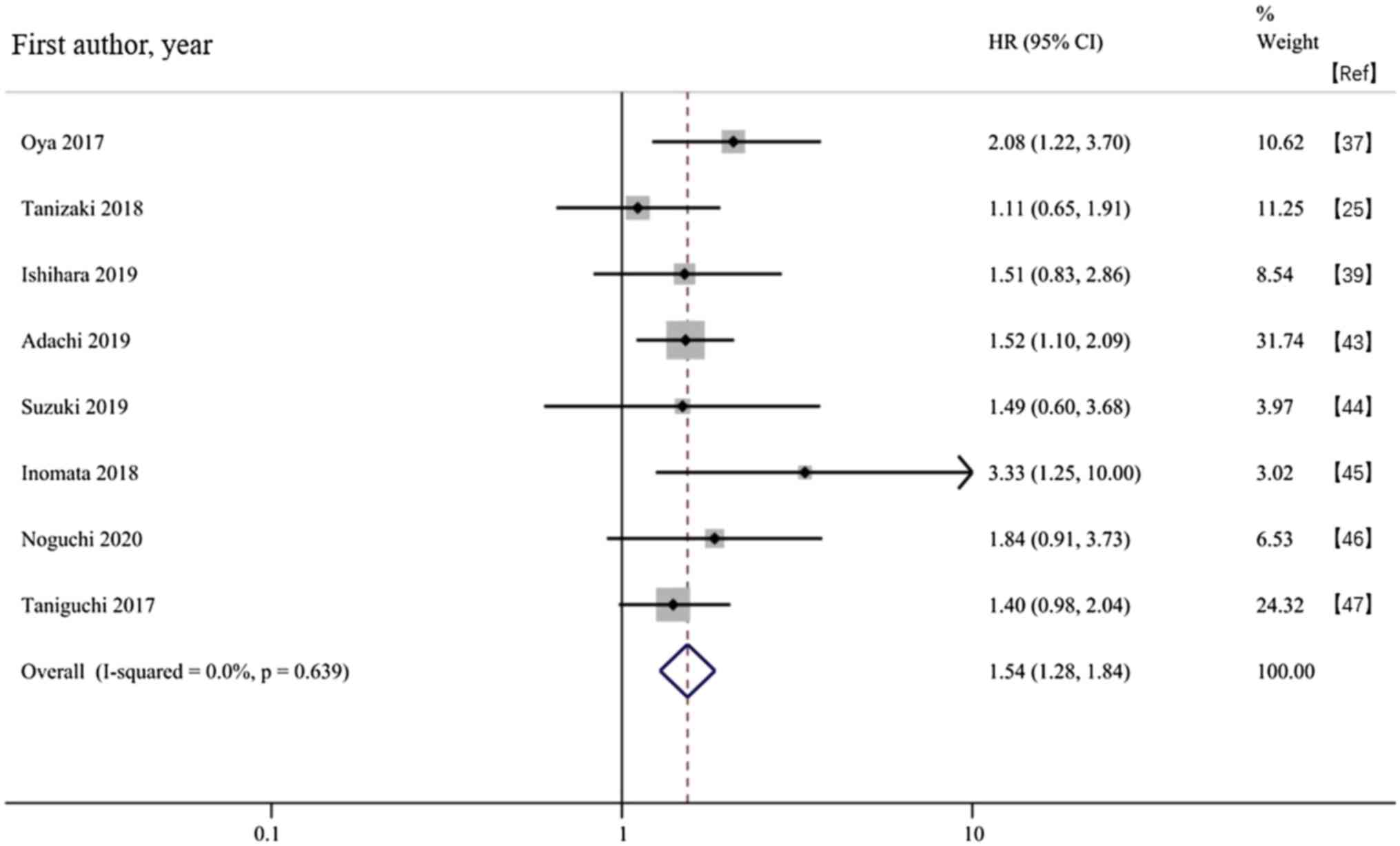
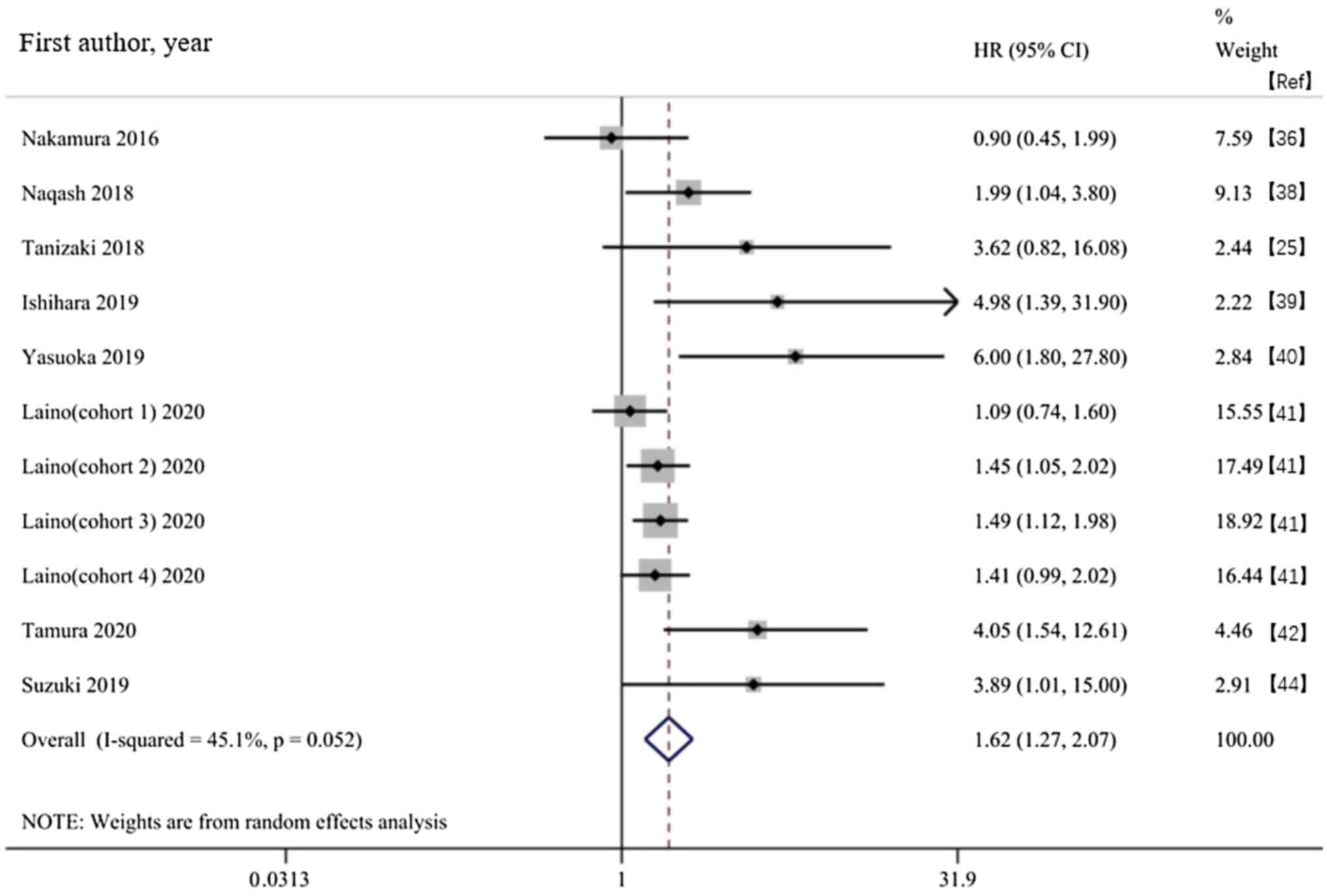
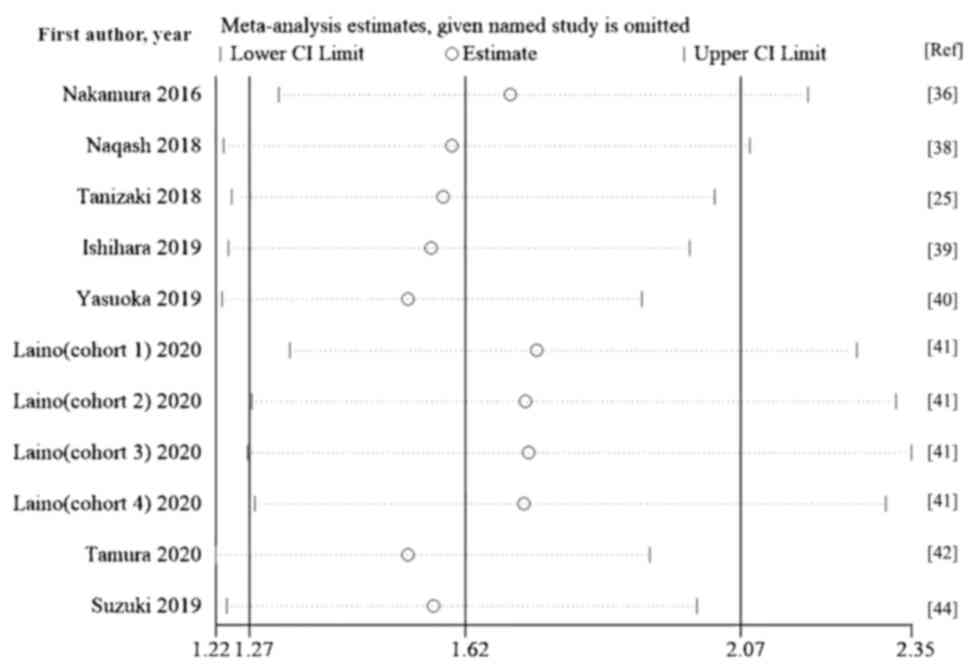
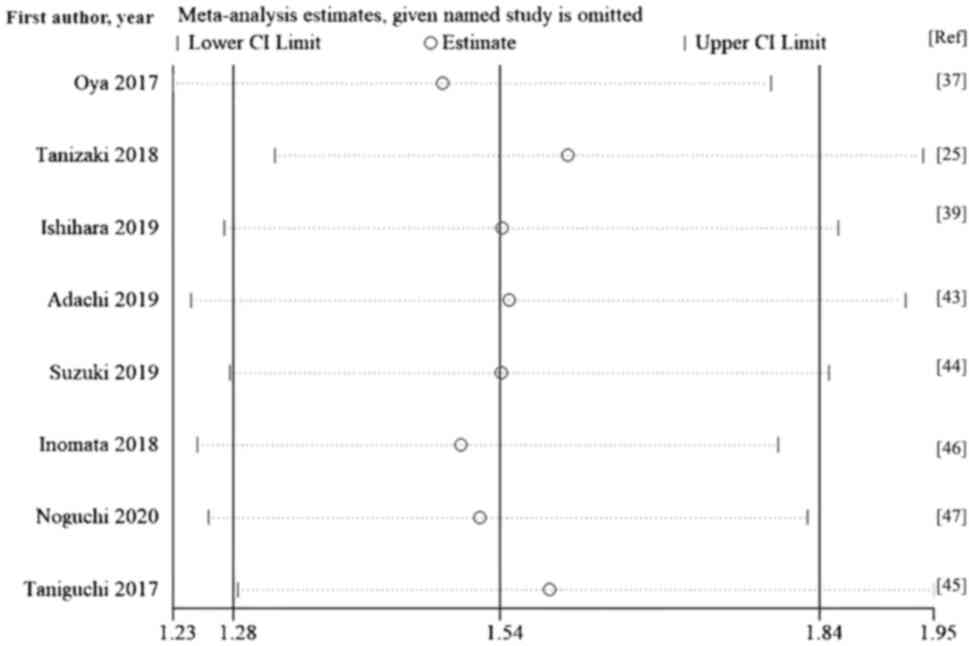
Tanizaki J, Haratani K, Hayashi H, Chiba
Y, Nakamura Y, Yonesaka K, Kudo K, Kaneda H, Hasegawa Y, Tanaka K,
et al: Peripheral blood biomarkers associated with clinical outcome
in non-small cell lung cancer patients treated with nivolumab. J
Thorac Oncol. 13:97–105. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Muto Y, Kitano S, Tsutsumida A, Namikawa
K, Takahashi A, Nakamura Y, Yamanaka T, Yamamoto N and Yamazaki N:
Investigation of clinical factors associated with longer overall
survival in advanced melanoma patients treated with sequential
ipilimumab. J Dermatol. 46:498–506. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shibata Y, Kato T, Shimokawaji T and
Yamada K: P2.01-88 C-reactive protein (CRP) as a predictive marker
for survival in patients with advanced NSCLC treated with first
line Pembrolizumab Monotherapy. J Thorac Oncol. 10(13)2018.
|
|
28
|
Hernandez AV, Marti KM and Roman YM:
Meta-analysis. Chest. 158:S97–S102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Higgins JPT, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.PubMed/NCBI View Article : Google Scholar
|
|
32
|
O'Rourke K, Shea B and Wells GA:
Meta-analysis of clinical trials(M)//Applied Statistics in the
Pharmaceutical Industry. Springer, New York, NY, pp397-424,
2001.
|
|
33
|
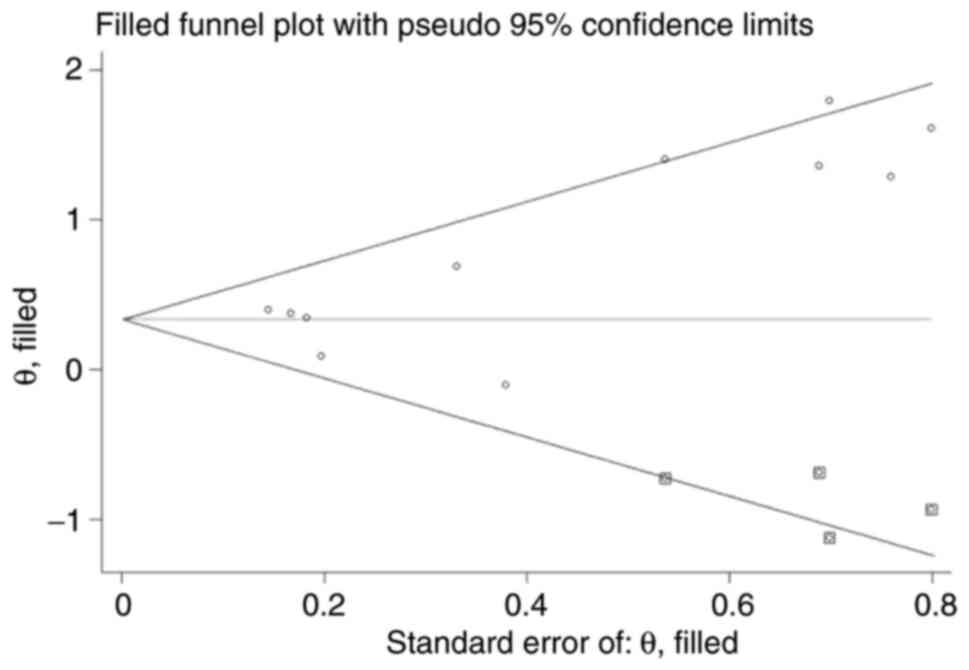
Egger M, Smith GD, Schneider M and Minder
C: Bias in meta-analysis detected by a simple, graphical test. BMJ.
315:629–634. 1997.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994.PubMed/NCBI
|
|
35
|
Duval S and Tweedie R: Trim and fill: A
simple funnel-plot-based method of testing and adjusting for
publication bias in meta-analysis. Biometrics. 56:455–463.
2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nakamura Y, Kitano S, Takahashi A,
Tsutsumida A, Namikawa K, Tanese K, Abe T, Funakoshi T, Yamamoto N,
Amagai M and Yamazaki N: Nivolumab for advanced melanoma:
pretreatment prognostic factors and early outcome markers during
therapy. Oncotarget. 7:77404–77415. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Oya Y, Yoshida T, Kuroda H, Mikubo M,
Kondo C, Shimizu J, Horio Y, Sakao Y, Hida T and Yatabe Y:
Predictive clinical parameters for the response of nivolumab in
pretreated advanced non-small-cell lung cancer. Oncotarget.
8:103117–103128. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Naqash AR, Stroud CRG, Butt MU, Dy GK,
Hegde A, Muzaffar M, Yang LV, Hafiz M, Cherry CR and Walker PR:
Co-relation of overall survival with peripheral blood-based
inflammatory biomarkers in advanced stage non-small cell lung
cancer treated with anti-programmed cell death-1 therapy: Results
from a single institutional database. Acta Oncologica. 57:867–872.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ishihara H, Tachibana H, Takagi T, Kondo
T, Fukuda H, Yoshida K, Iizuka J, Kobayashi H, Okumi M, Ishida H
and Tanabe K: Predictive impact of peripheral blood markers and
C-reactive protein in nivolumab therapy for metastatic renal cell
carcinoma. Target Oncol. 14:453–463. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yasuoka S, Yuasa T, Nishimura N, Ogawa M,
Komai Y, Numao N, Yamamoto S, Kondo Y and Yonese J: Initial
experience of pembrolizumab therapy in Japanese patients with
metastatic urothelial cancer. Anticancer Res. 39:3887–3892.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Laino AS, Woods D, Vassallo M, Qian X,
Tang H, Wind-Rotolo M and Weber J: Serum interleukin-6 and
C-reactive protein are associated with survival in melanoma
patients receiving immune checkpoint inhibition. J Immunother
Cancer. 8(e000842)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tamura D, Jinnouchi N, Abe M, Ikarashi D,
Matsuura T, Kato R, Maekawa S, Kato Y, Kanehira M, Takata R and
Obara W: Prognostic outcomes and safety in patients treated with
pembrolizumab for advanced urothelial carcinoma: Experience in
real-world clinical practice. Int J Clin Oncol. 25:899–905.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Adachi Y, Tamiya A, Taniguchi Y, Enomoto
T, Azuma K, Kouno S, Inagaki Y, Saijo N, Okishio K and Atagi S:
Predictive factors for progression- free survival in non-small cell
lung cancer patients receiving nivolumab based on performance
status. Cancer Med. 9:1383–1391. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Suzuki K, Terakawa T, Furukawa J, Harada
K, Hinata N, Nakano Y and Fujisawa M: C-reactive protein and the
neutrophil-to-lymphocyte ratio are prognostic biomarkers in
metastatic renal cell carcinoma patients treated with nivolumab.
Int J Clin Oncol. 25:135–144. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Inomata M, Hirai T, Seto Z, Tokui K, Taka
C, Okazawa S, Kambara K, Ichikawa T, Imanishi S, Yamada T, et al:
Clinical parameters for predicting the survival in patients with
squamous and non-squamous-cell NSCLC receiving PD-1 inhibitor
therapy. Pathol Oncol Res. 26:327–333. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Noguchi G, Nakaigawa N, Umemoto S,
Kobayashi K, Shibata Y, Tsutsumi S, Yasui M, Ohtake S, Suzuki T,
Osaka K, et al: C-reactive protein at 1 month after treatment of
nivolumab as a predictive marker of efficacy in advanced renal cell
carcinoma. Cancer Chemother Pharmacol. 86:75–85. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Taniguchi Y, Tamiya A, Isa SI, Nakahama K,
Okishio K, Shiroyama T, Suzuki H, Inoue T, Tamiya M, Hirashima T,
et al: Predictive factors for poor progression-free survival in
patients with non-small cell lung cancer treated with nivolumab.
Anticancer Res. 37:5857–5862. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Roxburgh CSD and McMillan DC: Role of
systemic inflammatory response in predicting survival in patients
with primary operable cancer. Future Oncol. 6:149–163.
2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yoshida T, Ichikawa J, Giuroiu I, Laino
AS, Hao Y, Krogsgaard M, Vassallo M, Woods DM, Hodi FS and Weber J:
C reactive protein impairs adaptive immunity in immune cells of
patients with melanoma. J Immunother Cancer.
8(e000234)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Shrotriya S, Walsh D, Bennani-Baiti N,
Thomas S and Lorton C: C-reactive protein is an important biomarker
for prognosis tumor recurrence and treatment response in adult
solid tumors: A systematic review. PLoS One.
10(e0143080)2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Li W, Luo X, Liu Z, Chen Y and Li Z:
Prognostic value of C-reactive protein levels in patients with bone
neoplasms: A meta-analysis. PLoS One. 13(e0195769)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Tong MT: A retrective cohort study to
explore the correlaition between peripheral blood markers and
survival in solid tumor treated with Atezolizumab monotheraphy
(unpublished PhD thesis). Zhejiang University, 2018.
|
|
53
|
Minichsdorfer C, Gleiss A, Aretin MB,
Schmidinger M and Fuereder T: 124P Serum parameters as prognostic
biomarkers for anti PD-1/PD-L1 therapy in patients with solid
tumours: A retrospective data analysis. Ann Oncol. 31:S289–S290.
2020.
|