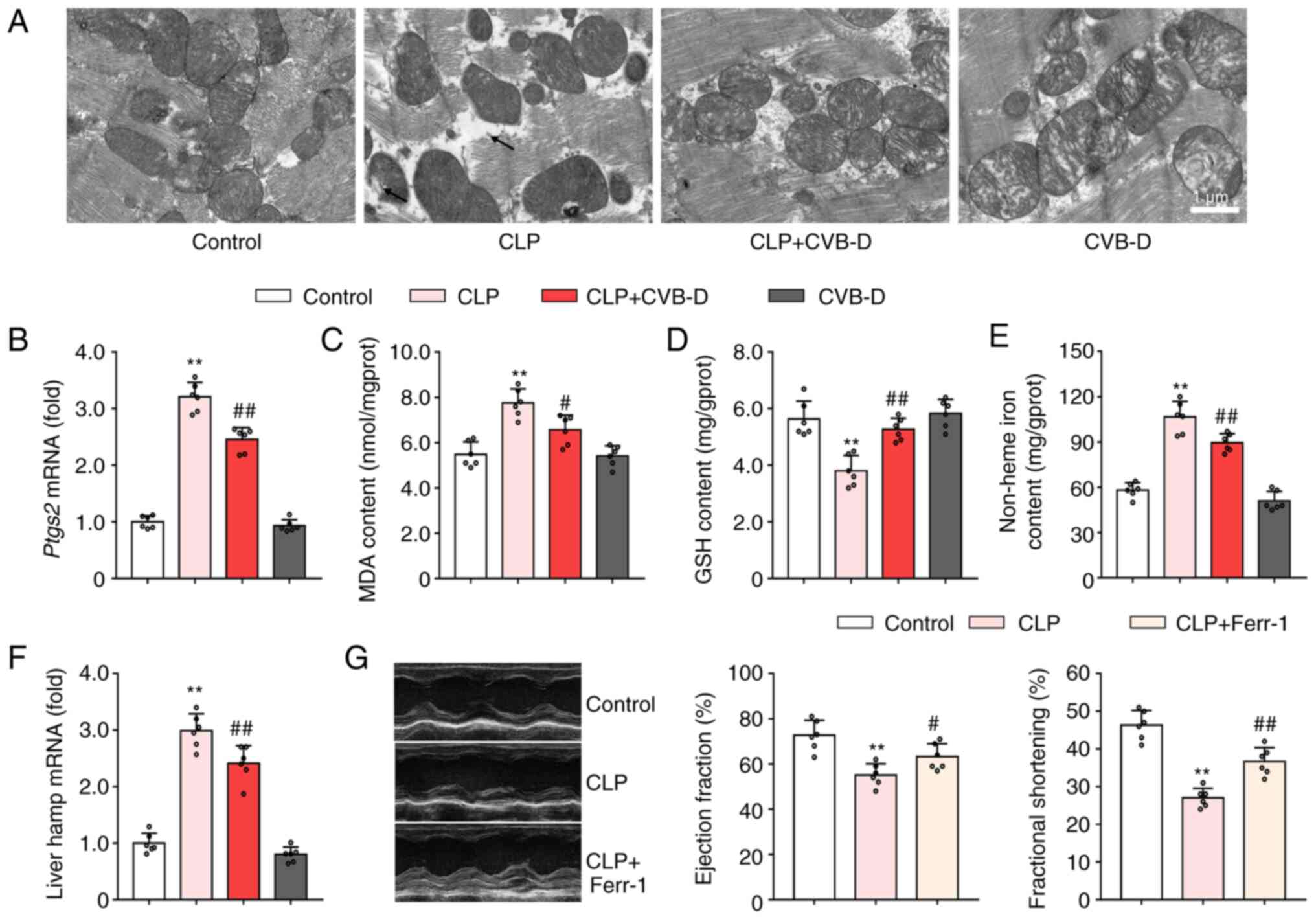
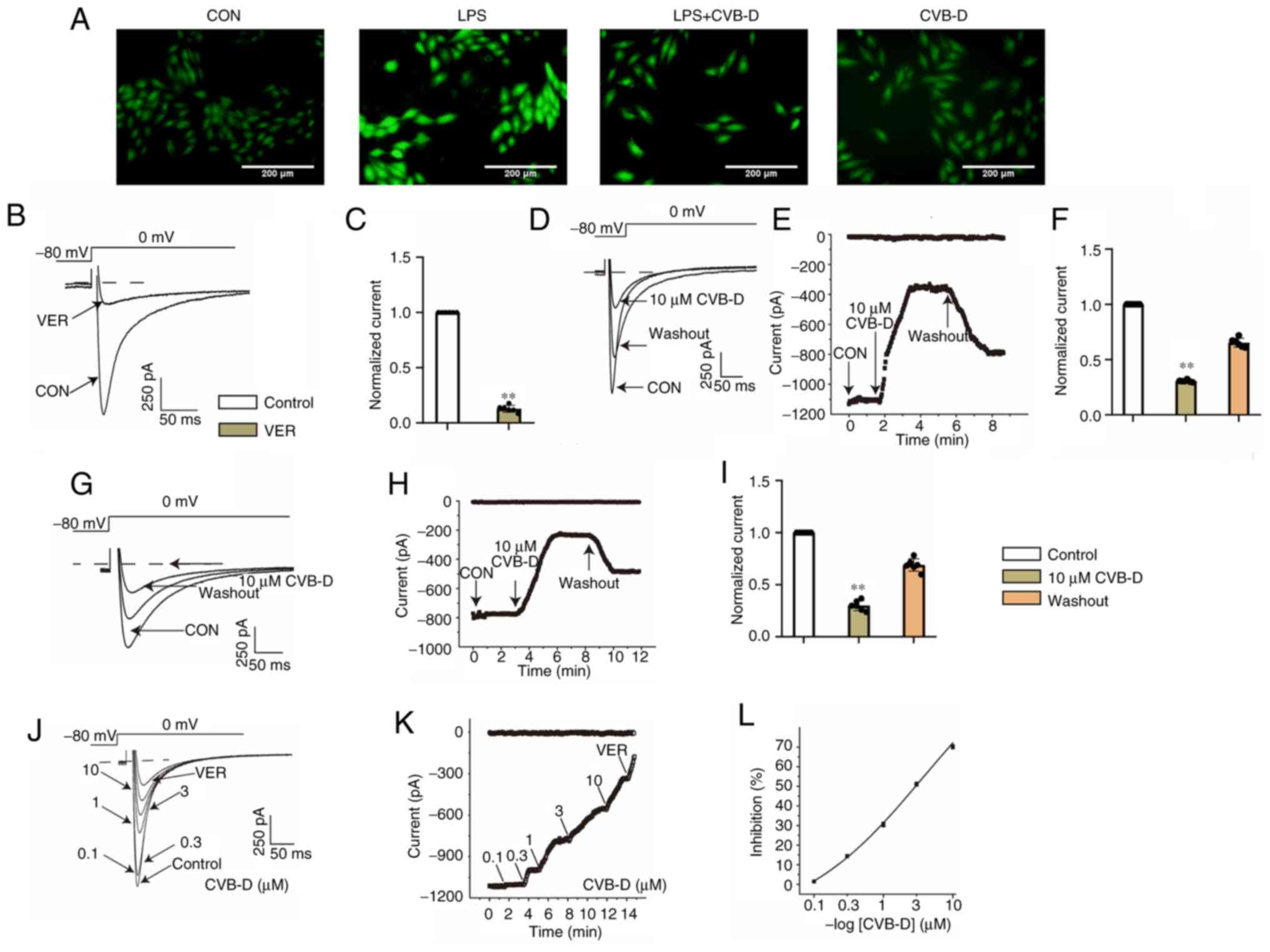
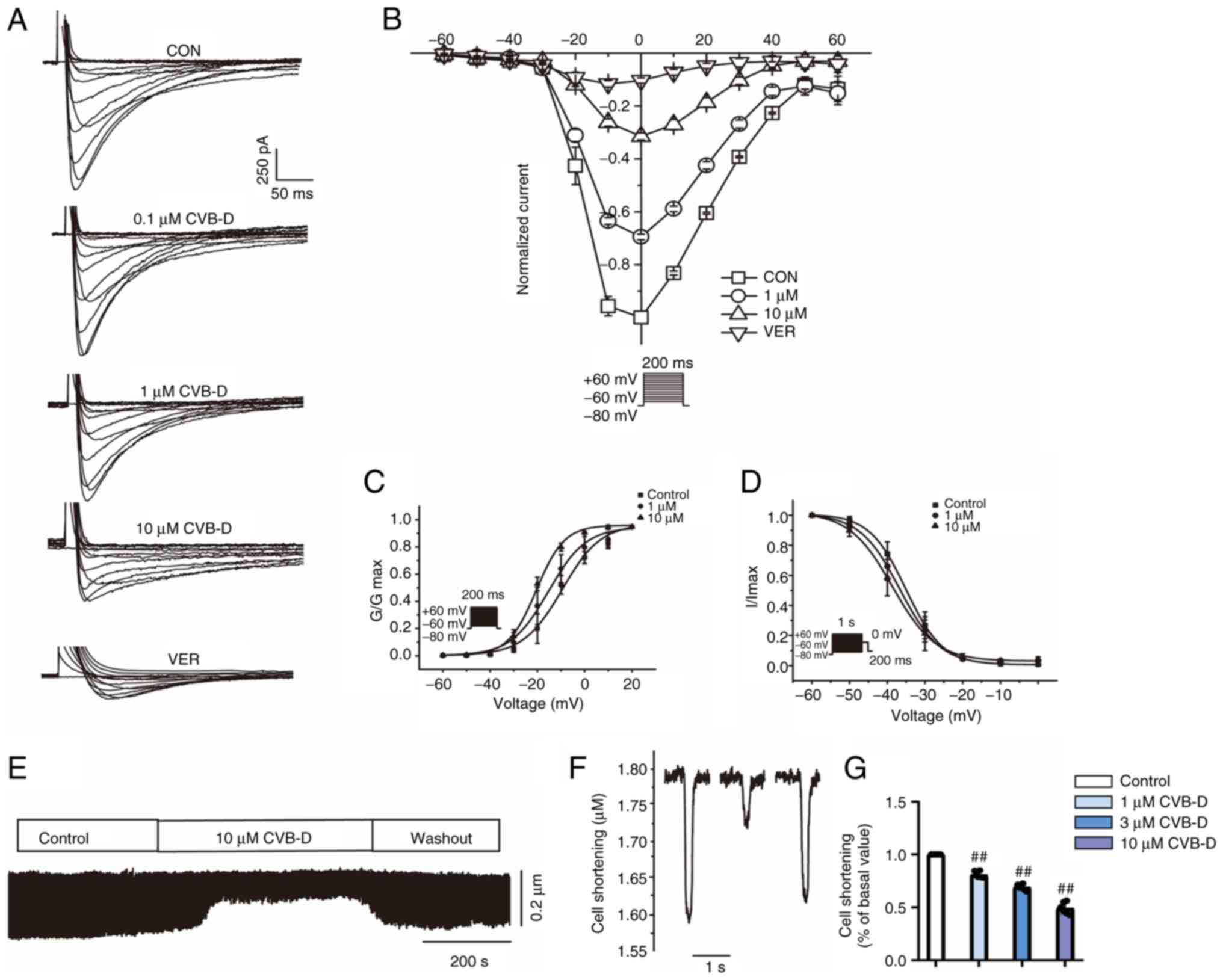
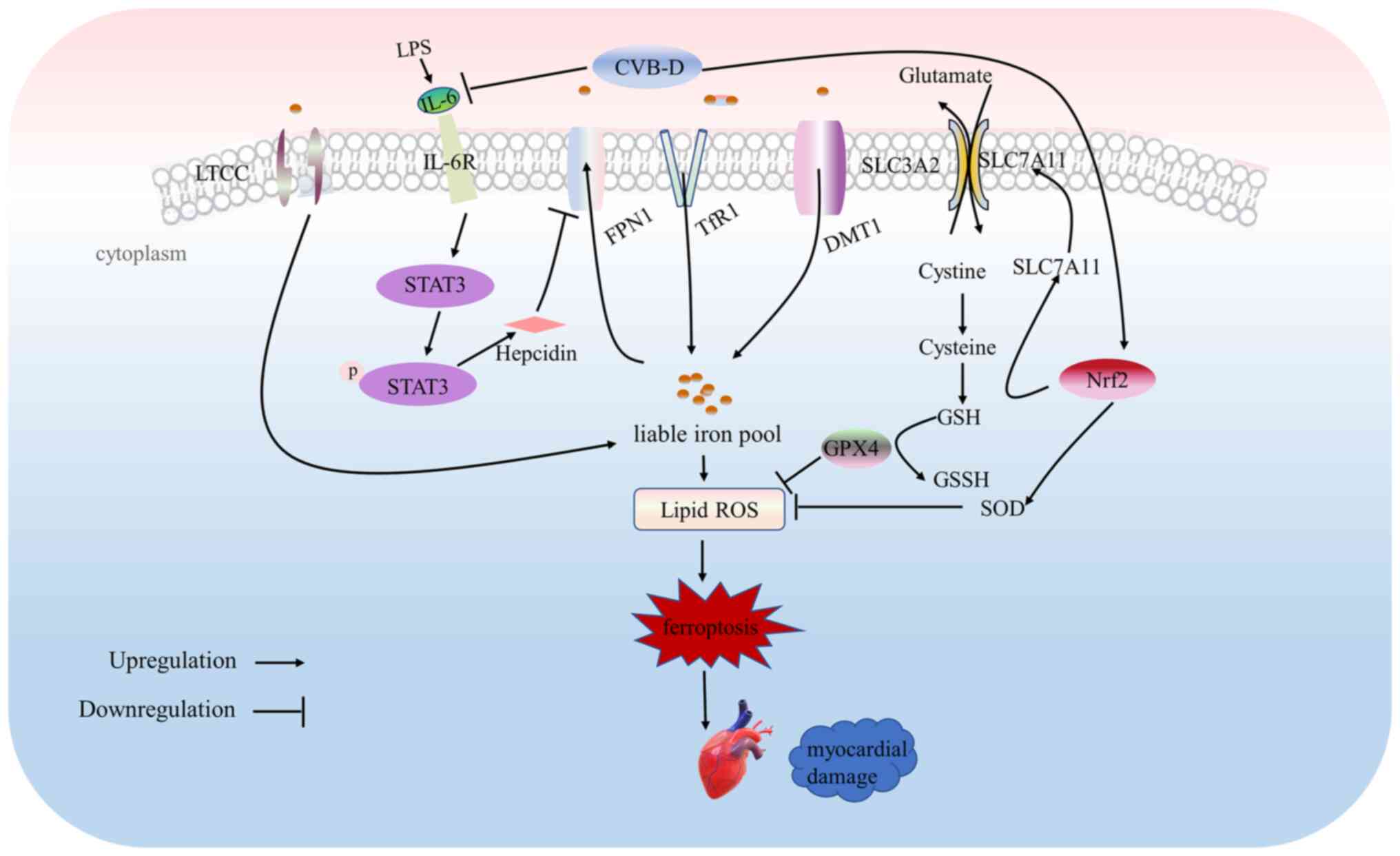
|
1
|
Beesley SJ, Weber G, Sarge T, Nikravan S,
Grissom CK, Lanspa MJ, Shahul S and Brown SM: Septic
cardiomyopathy. Crit Care Med. 46:625–634. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tullo G, Candelli M, Gasparrini I, Micci S
and Franceschi F: Ultrasound in Sepsis and septic shock-from
diagnosis to treatment. J Clin Med. 12(1185)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Flierl MA, Rittirsch D, Huber-Lang MS,
Sarma JV and Ward PA: Molecular events in the cardiomyopathy of
sepsis. Mol Med. 14:327–336. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shang X, Zhang Y, Xu J, Li M, Wang X and
Yu R: SRV2 promotes mitochondrial fission and Mst1-Drp1 signaling
in LPS-induced septic cardiomyopathy. Aging. 12:1417–1432.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zheng Z, Ma H, Zhang X, Tu F, Wang X, Ha
T, Fan M, Liu L, Xu J, Yu K, et al: Enhanced glycolytic metabolism
contributes to cardiac dysfunction in polymicrobial sepsis. J
Infectious Dis. 215:1396–1406. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhu X, Sun M, Guo H, Lu G, Gu J, Zhang L,
Shi L, Gao J, Zhang D, Wang W, et al: Verbascoside protects from
LPS-induced septic cardiomyopathy via alleviating cardiac
inflammation, oxidative stress and regulating mitochondrial
dynamics. Ecotoxicol Environ Saf. 233(113327)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang Y, Lei W, Qian L, Zhang S, Yang W, Lu
C, Song Y, Liang Z, Deng C, Chen Y, et al: Activation of NR1H3
signaling pathways by psoralidin attenuates septic myocardial
injury. Free Radical Biol Med. 204:8–19. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Venkataramani V: Iron homeostasis and
metabolism: Two sides of a coin. Adv Exp Med Biol. 1301:25–40.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fang X, Wang H, Han D, Xie E, Yang X, Wei
J, Gu S, Gao F, Zhu N, Yin X, et al: Ferroptosis as a target for
protection against cardiomyopathy. Proc Natl Acad Sci USA.
116:2672–2680. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rochette L, Dogon G, Rigal E, Zeller M,
Cottin Y and Vergely C: Lipid peroxidation and Iron metabolism: Two
corner stones in the homeostasis control of ferroptosis. Int J Mol
Sci. 24(449)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Islam S, Jarosch S, Zhou J, Parquet Mdel
C, Toguri JT, Colp P, Holbein BE and Lehmann C: Anti-inflammatory
and anti-bacterial effects of iron chelation in experimental
sepsis. J Surg Res. 200:266–273. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li N, Wang W, Zhou H, Wu Q, Duan M, Liu C,
Wu H, Deng W, Shen D and Tang Q: Ferritinophagy-mediated
ferroptosis is involved in sepsis-induced cardiac injury. Free
Radical Biol Med. 160:303–318. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang C, Yuan W, Hu A, Lin J, Xia Z, Yang
CF, Li Y and Zhang Z: Dexmedetomidine alleviated sepsis-induced
myocardial ferroptosis and septic heart injury. Mol Med Rep.
22:175–184. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
National Pharmacopoeia Committee, ed.
Pharmacopoeia of People's Republic of China. Beijing: Chemical
Industry Press, 2010.
|
|
15
|
Guo Q, Guo J, Yang R, Peng H, Zhao J, Li L
and Peng S: Cyclovirobuxine D attenuates doxorubicin-induced
cardiomyopathy by suppression of oxidative damage and mitochondrial
biogenesis impairment. Oxid Med Cell Longev.
2015(151972)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jiang Z, Fu L, Xu Y, Hu X, Yang H, Zhang
Y, Luo H, Gan S, Tao L, Liang G and Shen X: Cyclovirobuxine D
protects against diabetic cardiomyopathy by activating
Nrf2-mediated antioxidant responses. Sci Rep.
10(6427)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xiang ZN, Su JC, Liu YH, Deng B, Zhao N,
Pan J, Hu ZF, Chen FH, Cheng BY, Chen JC and Wan LS: Structurally
diverse alkaloids from Buxus sempervirens with cardioprotective
activity. Bioorg Chem. 109(104753)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fang H, Gong C, Fu J, Liu X, Bi H, Cheng
Y, Liu Y, Tang Y and Wang D: Evaluation of 2 rat models for sepsis
developed by improved cecal ligation/puncture or feces
intraperitoneal-injection. Med Sci Monitor.
26(e919054)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Qin LY, Guan P, Wang JX, Chen Y, Zhao YS,
Yang SC, Guo YJ, Wang N and Ji ES: Therapeutic potential of
Astragaloside IV against adriamycin-induced renal damage in rats
via ferroptosis. Front Pharmacol. 13(812594)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen DD, Wang HW and Cai XJ: Transcription
factor Sp1 ameliorates sepsis-induced myocardial injury via
ZFAS1/Notch signaling in H9C2 cells. Cytokine.
140(155426)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xiong B, Chen L, Huang Y, Lu G, Chen C,
Nong J and Pan H: ZBTB16 eases lipopolysaccharide-elicited
inflammation, apoptosis and degradation of extracellular matrix in
chondrocytes during osteoarthritis by suppressing GRK2
transcription. Exp Ther Med. 25(276)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Varghese J, James J, Vaulont S, McKie A
and Jacob M: Increased intracellular iron in mouse primary
hepatocytes in vitro causes activation of the Akt pathway but
decreases its response to insulin. Biochim Biophys Acta Gen Subj.
1862:1870–1882. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guan P, Sun ZM, Wang N, Zhou J, Luo LF,
Zhao YS and Ji ES: Resveratrol prevents chronic intermittent
hypoxia-induced cardiac hypertrophy by targeting the PI3K/AKT/mTOR
pathway. Life Sci. 233(116748)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu L, Liu F, Sun Z, Peng Z, You T and Yu
Z: LncRNA NEAT1 promotes apoptosis and inflammation in LPS-induced
sepsis models by targeting miR-590-3p. Exp Ther Med. 20:3290–3300.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hu X and Miao H: MiR-539-5p inhibits the
inflammatory injury in septic H9c2 cells by regulating IRAK3. Mol
Biol Rep. 49:121–130. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cheng J, Zhu Y, Xing X, Xiao J, Chen H,
Zhang H, Wang D, Zhang Y, Zhang G, Wu Z and Liu Y:
Manganese-deposited iron oxide promotes tumor-responsive
ferroptosis that synergizes the apoptosis of cisplatin.
Theranostics. 11:5418–5429. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kong Y, Hu L, Lu K, Wang Y, Xie Y, Gao L,
Yang G, Xie B, He W, Chen G, et al: Ferroportin downregulation
promotes cell proliferation by modulating the Nrf2-miR-17-5p axis
in multiple myeloma. Cell Death Dis. 10(624)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kumfu S, Chattipakorn SC, Fucharoen S and
Chattipakorn N: Dual T-type and L-type calcium channel blocker
exerts beneficial effects in attenuating cardiovascular dysfunction
in iron-overloaded thalassaemic mice. Exp Physiol. 101:521–539.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hardy N, Viola HM, Johnstone VP, Clemons
TD, Cserne Szappanos H, Singh R, Smith NM, Iyer KS and Hool LC:
Nanoparticle-mediated dual delivery of an antioxidant and a peptide
against the L-Type Ca2+ channel enables simultaneous reduction of
cardiac. ACS Nano. 9:279–289. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bai T, Wang S, Zhao Y, Zhu R, Wang W and
Sun Y: Haloperidol, a sigma receptor 1 antagonist, promotes
ferroptosis in hepatocellular carcinoma cells. Biochem Biophys Res
Commun. 491:919–925. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xin T and Lu C: SirT3 activates
AMPK-related mitochondrial biogenesis and ameliorates
sepsis-induced myocardial injury. Aging. 12:16224–16237.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu B, Song H, Fan M, You F, Zhang L, Luo
J, Li J, Wang L, Li C and Yuan M: Luteolin attenuates
sepsis-induced myocardial injury by enhancing autophagy in mice.
Int J Mol Med. 45:1477–1487. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xiao Z, Kong B, Fang J, Qin T, Dai C,
Shuai W and Huang H: Ferrostatin-1 alleviates
lipopolysaccharide-induced cardiac dysfunction. Bioengineered.
12:9367–9376. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou B, Zhang J, Chen Y, Liu Y, Tang X,
Xia P, Yu P and Yu S: Puerarin protects against sepsis-induced
myocardial injury through AMPK-mediated ferroptosis signaling.
Aging. 14:3617–3632. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu YC, Yu MM, Shou ST and Chai YF:
Sepsis-induced cardiomyopathy: Mechanisms and treatments. Front
Immunol. 8(1021)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ke Z, Hou X and Jia XB: Design and
optimization of self-nanoemulsifying drug delivery systems for
improved bioavailability of cyclovirobuxine D. Drug Design Dev
Ther. 10:2049–2060. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guo D, Li JR, Wang Y, Lei LS, Yu CL and
Chen NN: Cyclovirobuxinum D suppresses lipopolysaccharide-induced
inflammatory responses in murine macrophages in vitro by blocking
JAK-STAT signaling pathway. Acta Pharmacol Sinica. 35:770–778.
2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kakihana Y, Ito T, Nakahara M, Yamaguchi K
and Yasuda T: Sepsis-induced myocardial dysfunction:
Pathophysiology and management. J Intensive Care.
4(22)2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Baek HS, Min HJ, Hong VS, Kwon TK, Park
JW, Lee J and Kim S: Anti-inflammatory effects of the novel PIM
kinase inhibitor KMU-470 in RAW 264.7 cells through the
TLR4-NF-κB-NLRP3 pathway. Int J Mol Sci. 21(5138)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yu B, Fang TH, Lü GH, Xu HQ and Lu JF:
Beneficial effect of Cyclovirobuxine D on heart failure rats
following myocardial infarction. Fitoterapia. 82:868–877.
2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620.
2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Fang X, Ardehali H, Min J and Wang F: The
molecular and metabolic landscape of iron and ferroptosis in
cardiovascular disease. Na Rev Cardiol. 20:7–23. 2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Broxmeyer HE, Cooper S, Levi S and Arosio
P: Mutated recombinant human heavy-chain ferritins and
myelosuppression in vitro and in vivo: A link between ferritin
ferroxidase activity and biological function. Proc Natl Acad Sci
USA. 88:770–774. 1991.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhang H, Ostrowski R, Jiang D, Zhao Q,
Liang Y, Che X, Zhao J, Xiang X, Qin W and He Z: Hepcidin promoted
ferroptosis through Iron metabolism which is associated with DMT1
signaling activation in early brain injury following subarachnoid
hemorrhage. Oxidative Med Cell Longevity.
2021(9800794)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Pietrangelo A, Dierssen U, Valli L, Garuti
C, Rump A, Corradini E, Ernst M, Klein C and Trautwein C: STAT3 is
required for IL-6-gp130-dependent activation of hepcidin in vivo.
Gastroenterology. 132:294–300. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11(88)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Fotiadis D, Kanai Y and Palacín M: The
SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med.
34:139–158. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lei P, Bai T and Sun Y: Mechanisms of
ferroptosis and relations with regulated cell death: A review.
Front Physiol. 10(139)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23(101107)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ma H, Wang X, Zhang W, Li H, Zhao W, Sun J
and Yang M: Melatonin suppresses ferroptosis induced by high
glucose via activation of the Nrf2/HO-1 signaling pathway in type 2
diabetic osteoporosis. Oxid Med Cell Longev.
2020(9067610)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Dhaouadi N, Vitto VAM, Pinton P, Galluzzi
L and Marchi S: Ca(2+) signaling and cell death. Cell Calcium.
113(102759)2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Neitemeier S, Jelinek A, Laino V, Hoffmann
L, Eisenbach I, Eying R, Ganjam GK, Dolga AM, Oppermann S and
Culmsee C: BID links ferroptosis to mitochondrial cell death
pathways. Redox Biol. 12:558–570. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Li D and Li Y: The interaction between
ferroptosis and lipid metabolism in cancer. Signal Transduct Target
Ther. 5(108)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Xin S, Mueller C, Pfeiffer S, Kraft VAN,
Merl-Pham J, Bao X, Feederle R, Jin X, Hauck SM, Schmitt-Kopplin P
and Schick JA: MS4A15 drives ferroptosis resistance through
calcium-restricted lipid remodeling. Cell Death Differ. 29:670–686.
2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Fu F, Wang W, Wu L, Wang W, Huang Z, Huang
Y, Wu C and Pan X: Inhalable biomineralized liposomes for cyclic
Ca(2+)-Burst-centered endoplasmic reticulum stress enhanced lung
cancer ferroptosis therapy. ACS Nano. 17:5486–5502. 2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Madreiter-Sokolowski CT, Thomas C and
Ristow M: Interrelation between ROS and Ca(2+) in aging and
age-related diseases. Redox Biol. 36(101678)2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Peng TI and Jou MJ: Oxidative stress
caused by mitochondrial calcium overload. Ann N Y Acad Sci.
1201:183–188. 2010.PubMed/NCBI View Article : Google Scholar
|