Introduction
Proliferative diabetic retinopathy (PDR) continues
to be a major cause of vision loss, and it has been reported that
there were >90 million adults with diabetes in China as of 2010,
and patients with PDR accounted for 2.8% of those with diabetes
(1). Although panretinal
photocoagulation (PRP) has been the treatment of choice for
delaying the diabetic retinopathy process and preventing visual
loss, there remains a high number of patients progressing to the
advanced stages, such as vitreous hemorrhage and tractional retinal
detachment, and subsequently requiring pars plana vitrectomy (PPV)
to clear vitreous haemorrhage (VH) and reattach the retina.
However, PPV for patients with PDR can often be challenging
(2). Vitreous removal, membrane
peeling and membrane delamination can be difficult procedures due
to the tight adhesions formed between the fibrovascular membrane
and the retina, which can lead to intraoperative haemorrhages and
iatrogenic breaks (3).
Furthermore, surgery-related complications, such as recurrent VH
and postoperative reproliferation, represent a major concern for
patients with PDR, resulting in poorer anatomic and functional
visual outcomes (4,5).
Since the introduction of the 25-gauge (25-G)
sutureless transconjunctival system in 2002 by Fujii et al
(6), microincision vitrectomy
surgery (MIVS) has progressively developed towards the use of
smaller gauge instruments. In 2010, Oshima et al (7) first reported 100% anatomic success
and 65% visual improvement of ≥3 lines using a novel 27-gauge
(27-G) PPV system in patients with vitreoretinal diseases.
Small-gauge vitrectomy has become popular due to its notable
advantages, including less trauma, shortened convalescence, reduced
inflammatory response and improved manoeuvrability (8). As a result, the use of 27-G PPV has
expanded from simple macular diseases to complicated cases,
including rhegmatogenous retinal detachment (RRD) (9). With the improvement of instruments,
25-G and 27-G PPV have been widely used in the management of
different vitreoretinal diseases such as VH, retinal detachment,
macular hole and PDR (10).
Smaller gauge vitrectomy cutters may offer a smaller sphere of
influence compared with larger gauge vitrectomy probes (11,12).
Furthermore, the shortened port-tip distance improves access to
surgical tissue planes and facilitates aspiration of preretinal and
subretinal materials (7).
Consequently, 27-G instruments with smaller gauge and shorter
port-tip distance are considered safer compared with 25-G
instruments. However, whether a 27-G system can be used to perform
complex intraocular manipulations, such as fibrovascular membrane
dissection and haemostasis in diabetic vitrectomy, is still a
concern.
Among the various factors involved in the
pathogenesis of PDR, vascular endothelial growth factor (VEGF)
appears to serve an important role (13). Previous reports have indicated high
levels of VEGFs are present in both animal models of diabetes and
patients with diabetes (14,15).
Inhibition of VEGF receptors by anti-VEGF agents has been reported
to induce endothelial cell apoptosis in blood vessels with vascular
regression and to induce normalisation of premature vessels by
increasing pericyte coverage and reducing vessel fenestration in
PDR (16). It has also been
reported that the application of intravitreal anti-VEGF before PPV
in PDR has the effect of reducing operating times, potentially as a
result of facilitating the surgery by reducing the incidence of
intra-operative bleeding (17).
Therefore, anti-VEGF agents have been widely adopted as adjunctive
therapy in patients requiring vitrectomy for PDR with VH and
tractional retinal detachment (18). Conbercept, also known as KH902, a
novel drug that can bind to all isoforms of VEGF-A, placental
growth factor and VEGF-B, has been demonstrated to serve an active
role in treating ocular diseases with choroidal neovascularisation
(19). Previous studies have
reported the efficacy and safety of conbercept for accelerating
postoperative vitreous recovery in 23-gauge (23-G) PPV for PDR
(20,21).
To the best of our knowledge, there has been no
research concerning the difference between 27-G and 25-G PPV in
diabetic retinopathy with preoperative intravitreal injection of
conbercept. Therefore, the purpose of the present study was to
investigate the feasibility, efficiency and safety of 27-G
vitrectomy with preoperative intravitreal conbercept injection for
PDR treatment compared with those of 25-G vitrectomy.
Materials and methods
Ethical approval
The present study was an interventional, comparative
and ambispective longitudinal study. The study was approved by the
Institutional Review Board of Zhongshan Ophthalmic Center at Sun
Yat-sen University (approval no. 2018KYPJ144; Guangzhou, China) and
performed in accordance with the World Medical Association's
Declaration of Helsinki. Written informed consent was obtained from
each subject.
Patient inclusion
A total of 48 consecutive patients (48 eyes) were
included in the present study. The inclusion criteria were as
follows: Patients who were diagnosed with PDR and had been
administered a conbercept intravitreal injection followed by 27-G
or 25-G vitrectomy at the Zhongshan Ophthalmic Center at Sun
Yat-sen University (Guangzhou, China) between March 2016 and
February 2017. The exclusion criteria were as follows: i) A history
of previous PPV; and ii) eyes that had <6 months of follow-up
after PPV. In addition, as the diameter of 27-G is smaller than
25-G, more time is needed to complete silicone oil tamponade with
27-G (22). The operating time of
the two groups (25-G and 27-G) would therefore not be comparable if
silicone oil injection is used. Consequently, patients who needed
silicone oil tamponade were also excluded from the present study.
Preoperative data, including the age, sex, course of the disease
and history of laser photocoagulation of the patient, were
recorded. Preoperative ophthalmologic evaluations included
measurements of the best-corrected visual acuity (BCVA) and
intraocular pressure (IOP), biomicroscopic examination, B-scan,
indirect ophthalmoscopy, and optical coherence tomography.
Patient treatment
The diagnosis, operation and monitoring in both
groups were conducted by one experienced vitreoretinal surgeon. All
patients received an intravitreal injection of conbercept (0.5 mg;
0.05 ml; 10 mg/ml; KH902; Chengdu Kanghong Pharmaceutical Group
Co., Ltd.) in the superior temporal sector 3.5-4.0 mm from the
sclerocorneal limbus 7-14 days before vitrectomy. Patients
underwent a standard three-port PPV using a 27-G or 25-G system
(Constellation Vitrectomy System; Alcon Inc.) under retrobulbar
anaesthesia. After displacement of the conjunctiva, three cannulas
were inserted 4.0 mm posterior to the limbus with the following
method: The trocar-cannula was inserted parallel to the limbus in a
tangential orientation at an angle of 30-40˚ to the sclera. After
insertion of the beveled trocar to the level of the beginning of
the cannula, the trocar-cannula was redirected such that the
cannula entered perpendicular to the sclera. The surgical
parameters were set as follows: i) Cutting rate of 6,000 cuts per
min (cpm) in the 27-G group and 5,000 cpm in the 25-G group; ii)
linear aspiration of 600 mmHg in the 27-G group and 500 mmHg in the
25-G group; iii) duty cycle of 50/50; and iv) shave mode set as the
horizontal mode. Procedures such as fibrovascular membrane
dissection, endodiathermy and PRP were performed as required.
Endolaser photocoagulation was performed for sealing retinal holes
if iatrogenic retinal breaks occurred. For patients with tractional
retinal detachment (TRD), intraoperative perfluorocarbon liquid
injection, gas-fluid exchange and gas tamponade were selectively
performed according to the extent of retinal detachment and
surgeon's experience. After surgery, all sclerotomy sites were
inspected and, if required, a suture was placed to prevent leakage.
Patients who received intraocular tamponade were instructed to
remain face down for 7-10 days. When the follow-up schedule was
adhered to, the patients were followed up at 1 day, 1 week, 1
month, 3 months and 6 months postoperatively.
The records of intraoperative findings focussed on
the operating time, suturing rate and rate of endodiathermy use.
Preoperative BCBA, postoperative BCVA at 1 month and the last date
of follow up, central foveal thickness (CFT), preoperative IOP,
postoperative IOP at 1 day, 1 week, 1 month, 3 months and the last
date of follow up, and complications were also recorded at each
follow-up visit.
Statistical analysis
Snellen visual acuities were recorded and converted
to the logarithm of the minimum angle of resolution for subsequent
analysis. An unpaired t-test was used for the analyses of age,
operating time, CFT and follow-up duration. Pearson χ2
test was used for the analyses of sex, primary indication, suturing
rate, preoperative PRP and tamponade. Two-way mixed ANOVA and
Bonferroni correction were used for the IOP and BCVA analysis
between the two groups at different time points and within the same
group pre- and postoperatively. Fisher's exact test was applied for
the analyses of intraoperative iatrogenic retinal breaks, rate of
endodiathermy use and postoperative VH. The parametric numerical
data are presented as mean ± standard deviation, and the count data
are shown as n (%). Analyses were conducted using the GraphPAD
Prism 8.4.3 software (GraphPad Software; Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
The baseline demographic data of the patients are
summarised in Table I. The
differences in the demographic data were not significant between
the groups. The data of 48 eyes from 48 patients with PDR were
collected for the current study. Of the 48 eyes, 18 (37.5%)
presented with VH, 8 (16.7%) with proliferative membrane, 9 (18.8%)
with VH and proliferative membrane, 9 (18.8%) with proliferative
membrane and TRD, and 4 (8.3%) with TRD. All the eyes were phakic
and none underwent phacoemulsification during PPV. The mean age of
the patients was 52.4±8.4 years (range, 37-69 years) in the 27-G
group and 54.0±7.6 years (range, 39-67 years) in the 25-G group.
Among the patients, 13 were male in each group. The mean follow-up
period was 9.8±3.3 months (range, 6-15 months) in the 27-G group
and 9.1±2.7 months (range, 6-15 months) in the 25-G group. The
clinical findings of the patients in both groups are summarised in
Tables II and III.
 | Table IBaseline characteristics, surgical
procedures and complications. |
Table I
Baseline characteristics, surgical
procedures and complications.
| Characteristic | 27-Gauge
vitrectomy | 25-Gauge
vitrectomy | P-value |
|---|
| Baseline
characteristics | | | |
|
Eyes, n | 23 | 25 | |
|
Male
patients, n (%) | 13(57) | 13(52) | 0.75a |
|
Age,
years | | | |
|
Mean ±
SD | 52.4±8.4 | 54.0±7.6 | 0.53b |
|
Range | 37-69 | 39-67 | |
|
Lens status,
n (%) | | | |
|
Phakic | 23(100) | 25(100) | |
|
Primary
indication, n (%) | | | |
|
Vitreous
hemorrhage | 15(65) | 16(64) | 0.93a |
|
Proliferative
membrane | 13(57) | 17(68) | 0.41a |
|
Traction
retinal detachment | 5(22) | 8(32) | 0.42a |
|
Preoperative
panretinal photocoagulation, n (%) | 11(48) | 13(52) | 0.77a |
|
Follow-up,
months | | | |
|
Mean ±
SD | 9.8±3.3 | 9.1±2.7 | 0.45b |
|
Range | 6-15 | 6-15 | |
| Surgical
procedures | | | |
|
Suturing
rate, n (%) | 3(13) | 10(40) | 0.04a |
|
Operating
time (min) | 40.2±3.0 | 39.2±2.3 | 0.18b |
|
Tamponade, n
(%) | | | |
|
Air | 5(22) | 8(32) | 0.42a |
|
Balanced
salt solution | 18(78) | 17(68) | |
| Complications | | | |
|
Intraoperative
iatrogenic retinal breaks, n (%) | 2(9) | 5(20) | 0.42c |
|
Endodiathermy
rate, n (%) | 2(9) | 3(12) |
>0.99c |
|
Postoperative
VH, n (%) | 1(4) | 2(8) |
>0.99c |
| Mean ± SD CFT,
µm | 258.17±46.44 | 266.88±45.13 | 0.51b |
 | Table IIClinical findings of patients in the
27-gauge pars plana vitrectomy group. |
Table II
Clinical findings of patients in the
27-gauge pars plana vitrectomy group.
| | Best-corrected
visual acuity, logMAR | Intraocular
pressure, mmHg | |
|---|
| Sex | Eye | Primary
indicationa | Preoperative
PRP | Baseline | Final | Baseline | Final | Operating time,
min | Tamponade | Suturing site | Endodiathermy
use | Retinal break | Follow-up,
months | Postoperative
vitreous hemorrhage |
|---|
| M | L | 1 | Y | 1.398 | 1.000 | 15.4 | 14.8 | 38 | BSS | N | N | N | 6 | N |
| M | R | 1 | Y | 1.222 | 0.699 | 19.5 | 20.6 | 37 | BSS | N | N | N | 9 | N |
| F | L | 1+2+3 | Y | 2.600 | 0.523 | 11.6 | 12.5 | 45 | Air | N | N | Y | 6 | N |
| F | L | 2+3 | N | 1.699 | 0.398 | 12.9 | 11.2 | 44 | Air | N | N | N | 12 | N |
| M | L | 1+2 | N | 2.300 | 0.699 | 14.5 | 15.2 | 46 | BSS | N | N | N | 9 | N |
| F | R | 2 | Y | 1.000 | 0.301 | 15.3 | 16.8 | 44 | BSS | N | Y | N | 6 | N |
| M | L | 1 | N | 1.000 | 0.222 | 16.7 | 14.5 | 38 | BSS | IT | N | N | 15 | N |
| M | L | 1 | Y | 1.097 | 0.699 | 17.5 | 18.7 | 39 | BSS | N | N | N | 12 | N |
| F | R | 2 | N | 0.699 | 0.523 | 12.8 | 10.7 | 37 | BSS | N | N | N | 6 | N |
| F | R | 2+3 | Y | 2.300 | 1.097 | 13.6 | 12.5 | 44 | Air | N | N | N | 9 | N |
| M | R | 1+2 | Y | 1.222 | 0.699 | 17.4 | 14.9 | 43 | BSS | N | N | N | 12 | N |
| M | R | 1+2 | N | 2.300 | 1.000 | 18.9 | 15.6 | 42 | BSS | N | N | N | 15 | N |
| F | L | 1 | N | 0.699 | 0.398 | 19.6 | 18.7 | 36 | BSS | IT | N | N | 9 | N |
| F | R | 1+2 | N | 0.523 | 0.222 | 15.4 | 17.5 | 37 | BSS | N | N | N | 6 | N |
| M | L | 1 | Y | 1.097 | 0.699 | 12.7 | 11.8 | 38 | BSS | N | N | N | 9 | N |
| M | L | 2+3 | Y | 1.000 | 0.398 | 13.3 | 12.7 | 43 | Air | N | Y | Y | 15 | N |
| F | R | 1 | N | 0.699 | 0.301 | 14.2 | 14.8 | 38 | BSS | N | N | N | 12 | Y |
| F | L | 1 | Y | 1.000 | 0.523 | 17.5 | 16.9 | 39 | BSS | ST | N | N | 9 | N |
| M | L | 2 | N | 1.000 | 0.222 | 16.9 | 15.5 | 38 | BSS | N | N | N | 9 | N |
| F | R | 1 | N | 0.699 | 0.301 | 18.8 | 17.2 | 39 | BSS | N | N | N | 6 | N |
| M | L | 2 | N | 1.097 | 0.523 | 20.2 | 18.4 | 40 | BSS | N | N | N | 6 | N |
| M | R | 1 | Y | 1.000 | 0.301 | 15.5 | 14.4 | 39 | BSS | N | N | N | 12 | N |
| M | R | 2+3 | N | 2.300 | 0.699 | 13.7 | 12.9 | 41 | Air | N | N | N | 15 | N |
 | Table IIIClinical findings of patients in the
25-gauge pars plana vitrectomy group. |
Table III
Clinical findings of patients in the
25-gauge pars plana vitrectomy group.
| | Best-corrected
visual acuity, logMAR | Intraocular
pressure, mmHg | |
|---|
| Sex | Eye | Primary
indicationa | Preoperative
PRP | Baseline | Final | Baseline | Final | Operating time,
min | Tamponade | Suturing site | Endodiathermy
use | Retinal break | Follow-up,
months | Postoperative
vitreous hemorrhage |
|---|
| M | R | 1 | N | 1.000 | 0.523 | 16.8 | 14.7 | 36 | BSS | N | Y | N | 9 | N |
| F | R | 1+2+3 | Y | 2.600 | 0.398 | 21.7 | 18.9 | 44 | Air | N | N | Y | 6 | N |
| M | L | 1 | Y | 1.097 | 0.699 | 14.5 | 13.5 | 38 | BSS | IT | N | N | 12 | N |
| F | R | 2 | N | 0.699 | 0.301 | 15.3 | 16.8 | 37 | BSS | N | N | N | 9 | N |
| M | L | 1 | Y | 1.097 | 1.000 | 16.7 | 14 | 40 | BSS | N | N | N | 6 | N |
| M | L | 1 | Y | 1.000 | 0.523 | 17.5 | 18.4 | 41 | BSS | ST | N | N | 12 | N |
| F | R | 2+3 | N | 2.300 | 0.699 | 12.8 | 11.7 | 42 | Air | N | N | Y | 15 | N |
| M | L | 1 | Y | 0.699 | 0.398 | 13.6 | 12.5 | 37 | BSS | IT | Y | N | 12 | N |
| F | L | 2 | N | 1.000 | 0.523 | 17.8 | 16.4 | 38 | BSS | N | N | N | 6 | N |
| M | L | 1+2 | N | 1.097 | 0.523 | 18.9 | 15.4 | 39 | BSS | SN | N | N | 6 | Y |
| M | L | 1+2 | Y | 0.699 | 0.398 | 19.6 | 20.7 | 37 | BSS | ST | N | N | 9 | N |
| F | R | 1+2+3 | N | 1.699 | 1.000 | 15.4 | 14.3 | 39 | BSS | N | N | N | 12 | N |
| F | L | 1 | Y | 0.523 | 0.398 | 12.7 | 11.5 | 36 | BSS | ST | N | N | 9 | N |
| M | L | 2+3 | N | 1.699 | 0.699 | 13.3 | 12.8 | 40 | Air | N | N | N | 6 | N |
| F | R | 2+3 | Y | 1.222 | 0.523 | 14.2 | 12.5 | 39 | Air | N | N | N | 12 | N |
| M | R | 2 | Y | 1.000 | 0.398 | 15.4 | 16.2 | 37 | BSS | N | Y | N | 12 | N |
| M | L | 1 | N | 1.097 | 0.699 | 12.7 | 11.5 | 38 | BSS | SN | N | N | 9 | N |
| F | L | 2+3 | Y | 2.300 | 0.523 | 13.3 | 14.7 | 43 | Air | N | N | Y | 9 | Y |
| M | R | 1+2 | Y | 2.300 | 1.000 | 14.2 | 13.3 | 41 | Air | N | N | Y | 6 | N |
| F | L | 1+2 | Y | 1.000 | 0.398 | 17.5 | 15.6 | 39 | BSS | N | N | N | 6 | N |
| M | R | 2 | N | 0.699 | 0.523 | 16.9 | 14.2 | 38 | BSS | IT | N | N | 9 | N |
| F | R | 1+2 | N | 1.097 | 0.699 | 18.8 | 17.6 | 39 | BSS | IT | N | N | 9 | N |
| F | R | 1 | N | 1.000 | 0.398 | 11.5 | 9.5 | 37 | BSS | ST | N | N | 12 | N |
| M | L | 1+2+3 | Y | 1.699 | 0.699 | 15.5 | 16 | 43 | Air | N | N | Y | 9 | N |
| F | L | 2+3 | N | 1.398 | 1.000 | 17.1 | 18.4 | 41 | Air | N | N | N | 6 | N |
BCVA and CFT
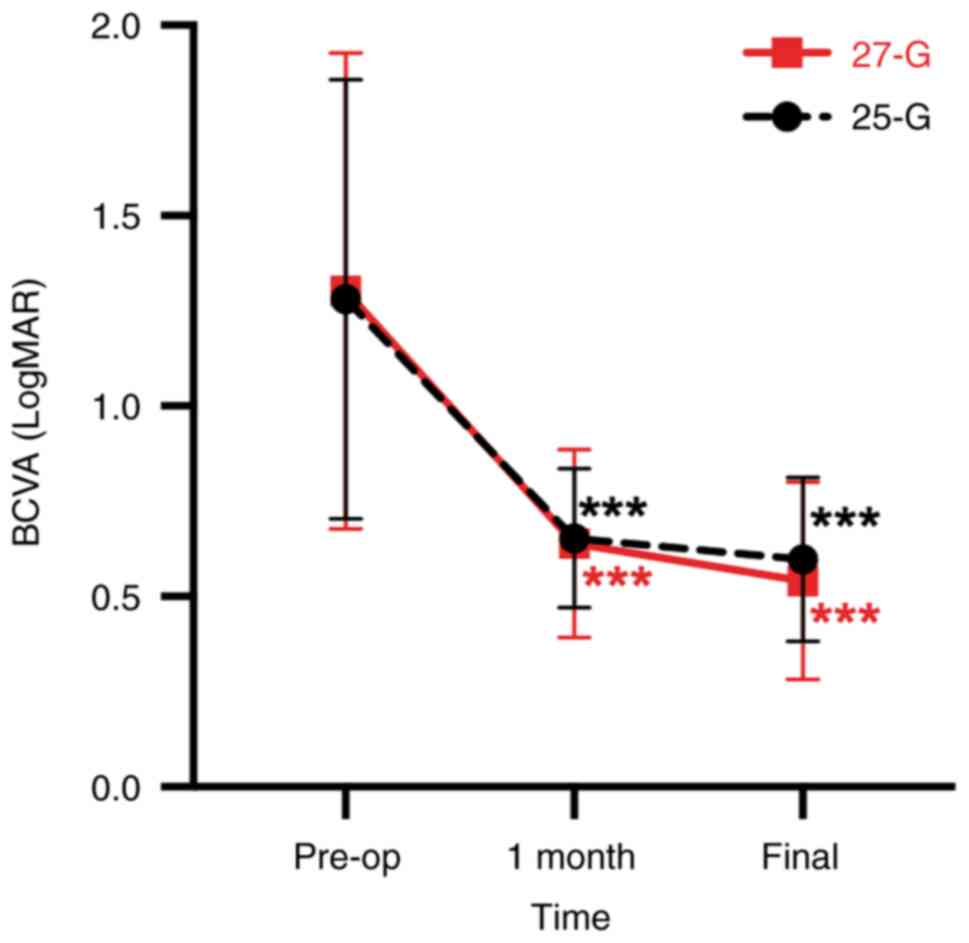
As shown in Fig. 1,
in the 27-G group, the mean BCVA improved from 1.30±0.62
preoperatively to 0.64±0.25 at 1 month after surgery (P<0.001)
and 0.54±0.26 at the final postoperative visit (P<0.001). In the
25-G group, the mean BCVA improved from 1.28±0.58 preoperatively to
0.65±0.18 at 1 month after surgery (P<0.001) and 0.60±0.22 at
the final postoperative visit (P<0.001). The differences in the
mean BCVA changes were not significant between groups at
preoperation (P>0.99), 1 month after surgery (P>0.99) and at
the final postoperative visit (P>0.99). There was no significant
difference in the mean CFT between the 27-G group (258.17±46.44 µm)
and the 25-G group (266.88±45.13 µm) at the final postoperative
visit (P=0.51).
IOP
As shown in Fig. 2,
the mean preoperative IOP was 15.8±2.5 mmHg, while the IOP was
11.1±2.0 mmHg at 1 day after surgery (P<0.001), 14.2±2.2 mmHg at
1 month after surgery (P<0.01), 14.6±2.3 mmHg at 3 months after
surgery (P<0.05) and 15.2±2.7 mmHg at the final follow up
(P=0.36) for the 27-G group. For the 25-G group, the mean
preoperative IOP was 15.8±2.5 mmHg, and the IOP changed to 12.6±2.1
mmHg at 1 day after surgery (P<0.001), 14.2±1.5 mmHg at 1 month
after surgery (P<0.05), 14.7±2.0 mmHg at 3 months after surgery
(P<0.05) and 14.8±2.7 mmHg at the final follow-up (P=0.05). No
case of ocular hypertension (IOP >25 mmHg) or hypotension (IOP
<6 mmHg) was detected in either group after surgery (data not
shown). In both groups, the IOP decreased in the early
postoperative period and recovered at the final follow-up. There
was no significant difference in the IOP between the two groups at
preoperation (P>0.99), and 1 day (P=0.15), 1 month (P>0.99),
3 months (P>0.99) and the final follow up (P>0.99) after
surgery.
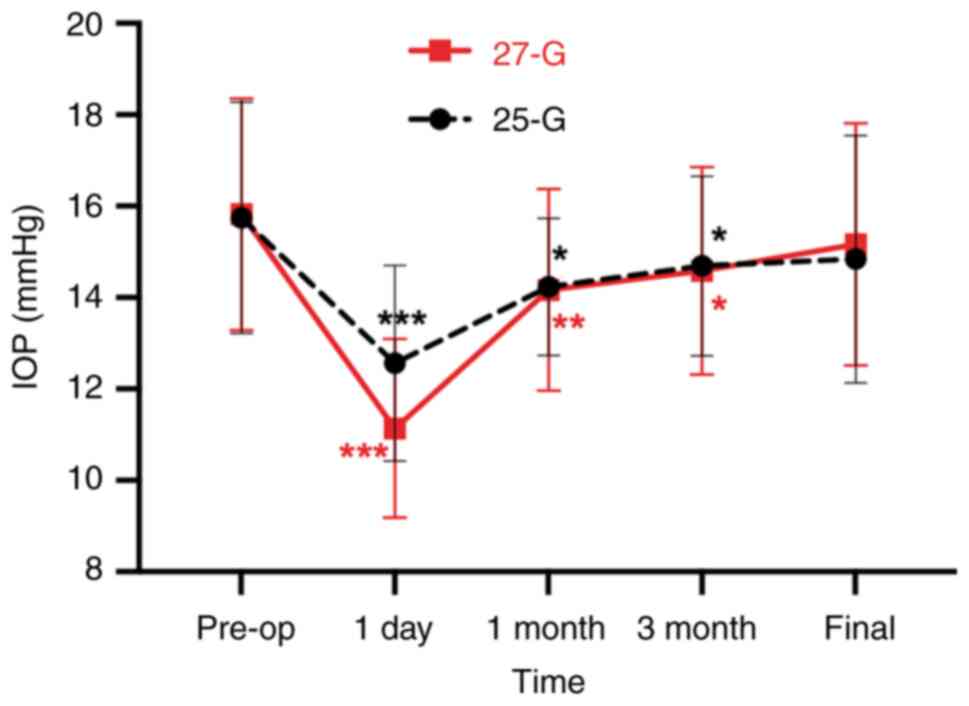 | Figure 2IOP changes during the follow-up
period in the 27-G and 25-G groups. Preoperative intra-group
comparison of IOP in 27-G and 25-G groups, retrospectively, showed
the postoperative IOP was significantly decreased at 1 day, 1 and 3
months, and recovered at the final follow-up. There was no
significant difference in the IOP between the two groups at
preoperation (P>0.99), and 1 day (P=0.15), 1 month (P>0.99),
3 months (P>0.99) and the final follow up (P>0.99) after
surgery. *P<0.05, **P<0.01,
***P<0.001 vs. pre-op. 25-G, 25-gauge; 27-G,
27-gauge; IOP, intraocular pressure; Pre-op, preoperative. |
Operating time and suturing rate
As shown in Table
I, the difference in suturing rate was significant among three
eyes (13%) in 27-G group and 10 eyes (40%) in 25-G group (P=0.04).
There were no statistically significant differences in operating
time between 27-G group (40.2±3.0 min) and 25-G group (39.2±2.3
min) (P=0.18). During the surgery, no case required intravitreal
forceps or scissors to remove the fibrovascular membrane in the
27-G group, and no case required conversion to larger gauge
instrumentation due to severe fibrovascular tissues that were
difficult to remove in both groups.
Surgical complications
As shown in Table
I, intraoperative iatrogenic retinal breaks occurred in two
eyes (9%) in the 27-G group and five eyes (20%) in the 25-G group
(P=0.42). The retinal breaks occurred during membrane removal and
were treated during surgery using endolaser photocoagulation. There
was no significant difference in the rate of endodiathermy use
between the 27-G group (two eyes; 9%) and the 25-G group (three
eyes; 12%) (P>0.99). Postoperative VH (POVH) occurred in one eye
(4%) in the 27-G group and two eyes (8%) in the 25-G group
(P>0.99). No other complications such as postoperative
endophthalmitis, sclerotomy-related retinal tears or choroidal
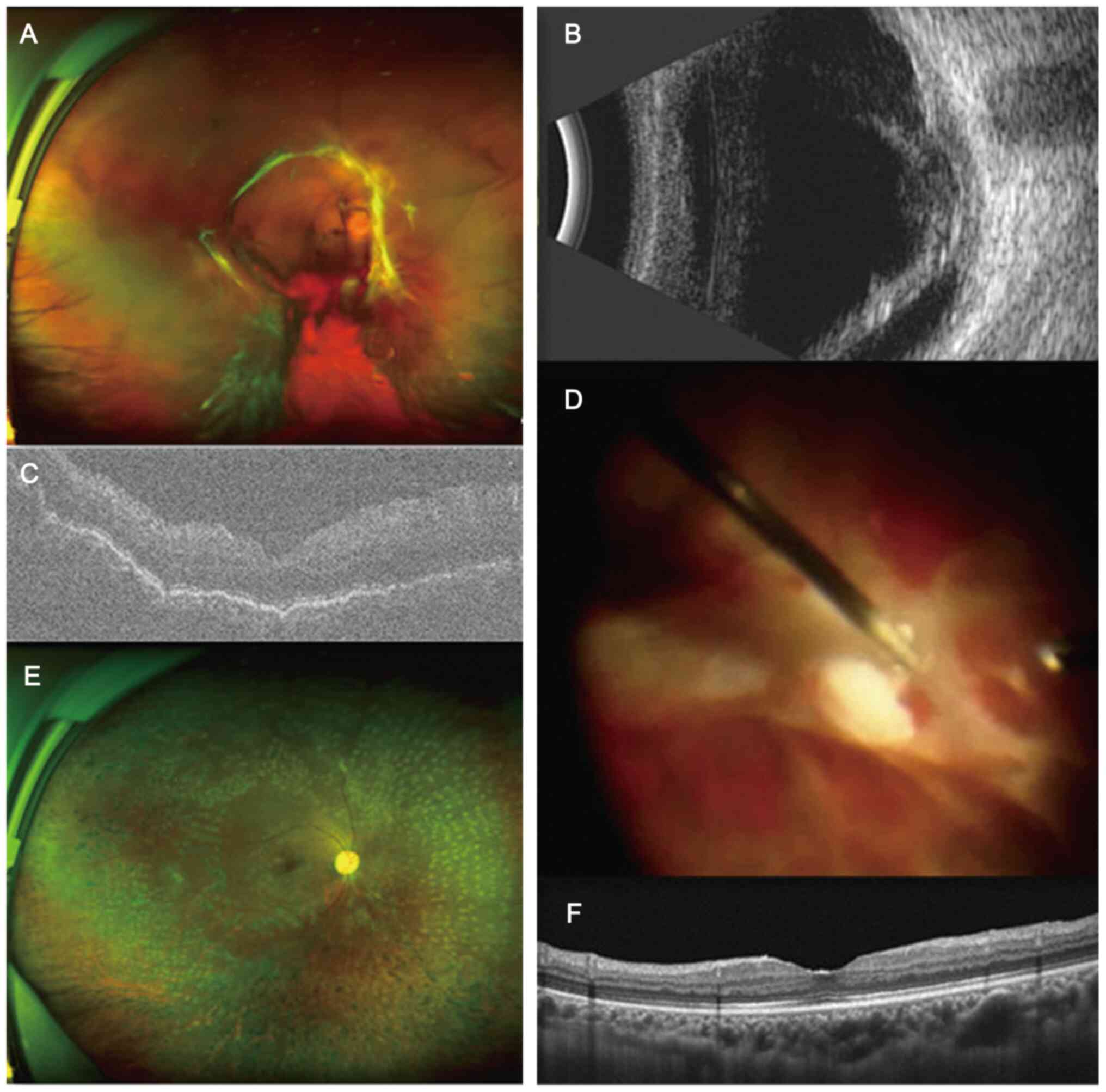
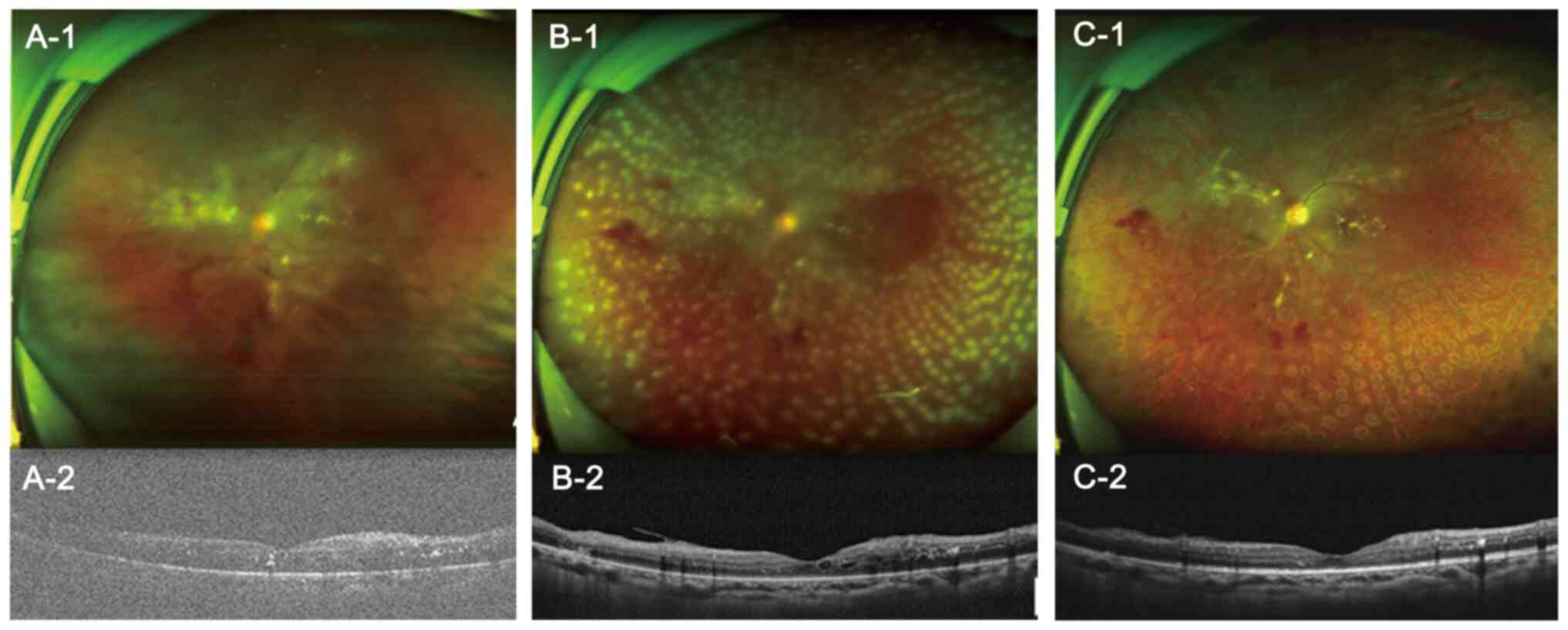
detachments were observed. Typical cases of both groups were shown
in Figs. 3 and 4, which demonstrate 2 patient with PDR
who underwent 27-G and 25-G vitrectomy, respectively, and who
experienced good rehabilitation in terms of vitreoretinal anatomy
after surgery.
Discussion
Since the introduction of MIVS in 2002(23), vitrectomy instruments have been
progressing towards smaller sizes with less trauma caused. Compared
with conventional 20-gauge vitrectomy, smaller gauge PPV has gained
wider adoption with the use of more innovative instruments that
offer less postoperative inflammation, quicker recovery and
improved manoeuvrability (24).
These advantages are crucial for patients with PDR, who are
characterised by a hard-to-remove fibrovascular membrane, new
vessels prone to bleeding during surgery and a higher risk of
nonspecific inflammatory reaction after surgery (25). The use of 27-G instrumentation for
routine macular surgery is well established (26,27).
However, few studies have evaluated its efficacy in PDR (28,29),
and they have not assessed the effect of preoperative intravitreal
anti-VEGF injection. The present study explored the use of 27-G or
25-G PPV combined with preoperative intravitreal injection of
conbercept for the treatment of patients with PDR. At 1 month and
the final follow-up visit, BCVA was significantly improved after
surgery, but no statistical difference in the BCVA and CFT changes
were observed between the two groups. This suggested that the 27-G
technique could be used to obtain equal functional and anatomical
improvements to those achieved with the 25-G system in PDR
treatment.
In the 27-G system, the internal diameter of the
cutter is decreased to 0.275 mm (compared with 0.347 mm in 25-G)
(24), which might raise a concern
on the possible reduction of flow rates during surgery according to
the Hagen-Poiseuille law: The velocity of the steady flow of a
fluid through a narrow tube (such as a blood vessel or a catheter)
varies directly with the pressure and the fourth power of the
radius of the tube, and inversely with the length of the tube and
the coefficient of viscosity (30). Previous studies have reported
longer operating times using the 27-G vitrectomy system for
epiretinal membranes and RRD (31,32).
However, the mean operating time in the 27-G group was similar
compared with that of the 25-G group in the present study. This may
be explained by a reduction in flow rate, which was compensated by
the faster cutting rate in the 27-G system. The
dual-pneumatic-driven ultrahigh-speed 27-G vitrectomy system can
reach a cutting rate as high as 7,500 cpm, while only 5,000 cpm is
observed for the 25-G system (24). Additionally, we consider that the
operating time may be compensated by the improved manoeuvrability
during fibrovascular membrane dissection and lower suturing rate
using the 27-G vitrectomy system. Thus, the present study
demonstrated that, with an appropriate parameter setting, high
efficiency could be achieved even in complex cases such as PDR
using the 27-G vitrectomy system.
In addition to the smaller external diameter of the
cutter in the 27-G system, the opening of the vitrectomy probe is
wider and the distance between the cutting port and the tip is
shortened to 0.221 mm (compared with 0.330 mm in the 25-G
vitrectomy probe) (7). The
improvement in design further enhances the manoeuvrability in
handling the proliferative membrane during PDR surgery. In the
present study, in the region with loose adhesion, the small-gauge
cutter could be more easily inserted into the small space between
the fibrovascular membrane and retina to complete the membrane
dissection and removal. After this step, the whole piece of
membrane was split into smaller pieces. Therefore, the shorter
distance between the cutting port and tip showed a great advantage
in the management of the proliferative membrane, which means the
27-G cutter may be superior to the 25-G cutter. In addition, the
probe could move horizontally on the surface of the retina to shave
the fibrovascular tissue into the cutter port. As mentioned in the
literature (28,33), when using a larger gauge PPV, such
as a 23-G or 25-G PPV, the complex manipulation typically requires
multiple instruments, including a membrane forceps to grasp the
proliferative membrane, scissors to separate the membrane from the
retinal surface and a vitrectomy cutter to remove the membrane.
However, in the present study, there was no need for membrane
forceps or scissors in the 27-G group. The findings of the present
study indicated that 27-G vitrectomy could act as a multifunctional
tool for successful membrane segmentation, dissection and removal,
which reduced the need for instrument change and shortened the
overall operating time.
Intraoperative complications commonly occur during
PPV in patients with PDR. Among these complications, the high
incidence of iatrogenic retinal breaks (3-50%) (34) and intraocular bleeding (>50%)
(35) are two major concerns. In
the present study, the 27-G system had a low incidence of
iatrogenic retinal breaks, endodiathermy use and POVH compared with
the 25-G system. However, the differences between the two systems
were not significant for all complications.
The concept of a ‘sphere of influence’ was first
proposed by Dugel et al (12) in 2012, and this was described as
the affected sphere of the vitreous cutter on adjacent tissue
structures. According to this principle, a smaller gauge vitrectomy
cutter will offer a smaller sphere of influence compared with
larger-gauge vitrectomy probes (11,12).
In addition, with an optimal duty cycle, set at 50/50 in the
present study, the ultrahigh speed vitreous cutter in the 27-G
system makes it easy to cut the vitreous into small pieces
(36), thereby reducing the
vitreal viscosity (37) and
incidence of cutter blockage (38). Consequently, we consider that 27-G
vitrectomy is considered safer due to the reduction of the
vitreoretinal traction from the probe tip. Furthermore, the
shortened port-tip distance further makes it easy to remove
fibrovascular tissue by vitreous cutter. These factors may account
for the lower, although not significantly different, rates of
endodiathermy use, iatrogenic retinal breaks and POVH in the 27-G
PPV group compared with the 25-G PPV group in the present
study.
Wound leakage may increase the risk of hypotony and
endophthalmitis after vitrectomy (39), thus wound self-sealing is another
concern. A previous reports has proposed that the 27-G (0.40-mm)
needle is the optimal size for easy self-sealing of scleral wounds
with a low incidence of complications (24). In the present study, the 27-G
system was demonstrated to be advantageous in self-sealing by
presenting a significantly decreased suturing rate compared with
the 25-G system. By contrast, hypotony due to wound leakage is one
of the major concerns of sutureless 27-G PPV (40). It has been reported that the
incidence of transient postoperative hypotony is 5-9% (26) after PPV using the 27-G system. In
the present study, no case of ocular hypotony was observed in
either the 25-G group or the 27-G group. In addition, there were no
other wound leakage-related complications, such as postoperative
endophthalmitis, sclerotomy-related retinal tears and choroidal
detachments. This may be attributed to the adoption of oblique
incisions and conjunctiva displacement, which can reduce wound
leakage (41).
For patients with PDR, anti-VEGF therapy has been
widely reported to be a promising modality for reducing the
incidence of intraoperative bleeding and postoperative recurrent VH
(42-44).
In most countries, bevacizumab is the most commonly used anti-VEGF
agent that is directed against VEGF-A (45,46).
Compared with bevacizumab, conbercept, a novel recombinant, soluble
fusion protein, has shown its superiority in the treatment of
ocular neovascularisation due to its high affinity for PIGF, VEGF-B
and all isoforms of VEGF-A (47).
According to its molecular structure, conbercept is composed of the
second immunoglobulin (Ig) domain of VEGF receptor 1 (VEGFR1), the
third and the fourth Ig domain of VEGFR2, and the constant region
of human IgG (48). Furthermore,
conbercept also binds to VEGF-B and placental growth factor,
another member of the VEGF superfamily (48,49).
Previous studies have provided sufficient evidence of reducing the
chances of intraoperative bleeding after preoperative intravitreal
injection of conbercept in the management of PDR (20,50).
In the present study, an intravitreal injection of
conbercept was administered 7-14 days before PPV. Due to the low
incidence of intraoperative haemorrhage after intravitreal
conbercept injection, the use of endodiathermy decreased in both
groups compared with that in previous studies without anti-VEGF
treatment (50-52).
This was consistent with the results reported for patients with PDR
administered adjunctive injection of bevacizumab before vitrectomy
(35). The incidence of
postvitrectomy VH in PDR without preoperative anti-VEGF agents has
previously been reported to be 12-32% (35). Li et al (53) demonstrated that the adjunctive use
of preoperative and postoperative intravitreal conbercept injection
decreased early POVH recurrence. The present study demonstrated a
relatively low incidence of postvitrectomy VH in both the 27-G (4%)
and 25-G (8%) groups, which was lower than that reported by Someya
et al (54) (23%). The low
incidence of POVH can be attributed to pretreatment with the
anti-VEGF agent, conbercept (35).
Consequently, we hypothesised that preoperative intravitreal
conbercept injection could achieve comparable effects to those of
bevacizumab in reducing intraoperative and postoperative
intraocular bleeding, subsequently helping to simplify the removal
of the fibrovascular membrane and shortening the operating
time.
The present study had several limitations, such as
its mostly retrospective nature, the small sample size and the
short follow-up period. Further randomised and prospective studies
are required that include patients with PDR with a larger sample
size and longer follow-up period. The main focus of the present
study was to determine whether there were any differences between
27-G and 25-G PPV in PDR after intravitreal conbercept injection.
Whether there are any differences between conbercept and other
anti-VEGFs, such as ranizumab and aflibercept, will be explored in
the future. In addition, the present study focused on the clinical
difference between 27-G and 25-G PPV in PDR after intravitreal
conbercept injection, therefore, only data on surgical-related
indicators were collected. In the future, specimens such as
vitreous body and fibrovascular membrane will be collected and
basic research will be conducted to detect the effect of
inflammatory factors and VEGF in the process of fibrovascular
membrane formation.
In conclusion, the present study reported the
surgical outcomes of 27-G vitrectomy combined with preoperative
intravitreal injection of conbercept for the management of PDR. The
use of 27-G vitrectomy achieved equally favourable anatomical and
functional results, lower suturing rates and good manoeuvrability
without extending the operating time compared with the 25-G system.
With reference to the literature, preoperative intravitreal
conbercept injection is associated with a low incidence of
intraoperative and postoperative complications, and it may be an
effective and safe approach in the management of PDR.
Acknowledgements
This abstract was presented as an electronic poster
at the Asia-Pacific Academy of Ophthalmology Congress on 6-9 Mar
2019, Bangkok, Thailand.
Funding
Funding: The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81570865),
Sun Yat-sen University Vitreoretinal Diseases Foundation (grant no.
83000-3050057), Science and Technology Program of Guangzhou (grant
no. 201803010022) and the Fund for principal investigators in State
Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DF performed the data analyses and wrote the
manuscript. WX critically revised the manuscript. SZ, YW and WX
contributed to the conception of the study and study design, and
helped perform the analysis with constructive discussions. YW and
DF confirm the authenticity of all the raw data. XJ, CX, SH, ZZ and
WX contributed to data interpretation and clinical data collection.
XJ and YW contributed to manuscript preparation. ZZ assisted in
data analyses. SZ took part in the manuscript preparation and
provided useful advice. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was performed with the approval of the
Institutional Review Board of Zhongshan Ophthalmic Center at Sun
Yat-sen University (approval no. 2018KYPJ144; Guangzhou, China).
The requirement for informed consent was waived and all procedures
were in accordance with the principles outlined in the Declaration
of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu L, Wu X, Liu L, Geng J, Yuan Z, Shan Z
and Chen L: Prevalence of diabetic retinopathy in mainland China: A
meta-analysis. PLoS One. 7(e45264)2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kumar K, Baliga G, Babu N, Rajan RP, Kumar
G, Mishra C, Chitra R and Ramasamy K: Clinical features and
surgical outcomes of complications of proliferative diabetic
retinopathy in young adults with type 1 diabetes mellitus versus
type 2 diabetes mellitus-A comparative observational study. Indian
J Ophthalmol. 69:3289–3295. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stewart MW, Browning DJ and Landers MB:
Current management of diabetic tractional retinal detachments.
Indian J Ophthalmol. 66:1751–1762. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hershberger VS, Augsburger JJ, Hutchins
RK, Raymond LA and Krug S: Fibrovascular ingrowth at sclerotomy
sites in vitrectomized diabetic eyes with recurrent vitreous
hemorrhage: Ultrasound biomicroscopy findings. Ophthalmology.
111:1215–1221. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ahn J, Woo SJ, Chung H and Park KH: The
effect of adjunctive intravitreal bevacizumab for preventing
postvitrectomy hemorrhage in proliferative diabetic retinopathy.
Ophthalmology. 118:2218–2226. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fujii GY, De Juan E Jr, Humayun MS, Chang
TS, Pieramici DJ, Barnes A and Kent D: Initial experience using the
transconjunctival sutureless vitrectomy system for vitreoretinal
surgery. Ophthalmology. 109:1814–1820. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Oshima Y, Wakabayashi T, Sato T, Ohji M
and Tano Y: A 27-gauge instrument system for transconjunctival
sutureless microincision vitrectomy surgery. Ophthalmology.
117:93–102.e2. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shahzadi B, Rizwi SF, Qureshi FM, Latif K
and Mahmood SA: Outcomes of transconjunctival sutureless 27-gauge
micro-incision vitrectomy surgery in diabetic vitreous haemorrhage.
Pak J Med Sci. 33:86–89. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Khan MA, Shahlaee A, Toussaint B, Hsu J,
Sivalingam A, Dugel PU, Lakhanpal RR, Riemann CD, Berrocal MH,
Regillo CD and Ho AC: Outcomes of 27 gauge microincision vitrectomy
surgery for posterior segment disease. Am J Ophthalmol.
161:36–43.e1-e2. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ribeiro L, Oliveira J, Kuroiwa D, Kolko M,
Fernandes R, Junior O, Moraes N, Vasconcelos H, Oliveira T and Maia
M: Advances in vitreoretinal surgery. J Clin Med.
11(6428)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dugel PU, Abulon DJ and Dimalanta R:
Comparison of attraction capabilities associated with high-speed,
dual-pneumatic vitrectomy probes. Retina. 35:915–920.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dugel PU, Zhou J, Abulon DJK and Buboltz
DC: Tissue attraction associated with 20-gauge, 23-gauge, and
enhanced 25-gauge dual-pneumatic vitrectomy probes. Retina.
32:1761–1766. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Semeraro F, Cancarini A, dell'Omo R,
Rezzola S, Romano MR and Costagliola C: Diabetic retinopathy:
Vascular and inflammatory disease. J Diabetes Res.
2015(582060)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Qaum T, Xu Q, Joussen AM, Clemens MW, Qin
W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD
and Adamis AP: VEGF-initiated blood-retinal barrier breakdown in
early diabetes. Invest Ophthalmol Vis Sci. 42:2408–2413.
2001.PubMed/NCBI
|
|
15
|
Funatsu H, Yamashita H, Ikeda T, Mimura T,
Eguchi S and Hori S: Vitreous levels of interleukin-6 and vascular
endothelial growth factor are related to diabetic macular edema.
Ophthalmology. 110:1690–1696. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kohno R, Hata Y, Mochizuki Y, Arita R,
Kawahara S, Kita T, Miyazaki M, Hisatomi T, Ikeda Y, Aiello LP and
Ishibashi T: Histopathology of neovascular tissue from eyes with
proliferative diabetic retinopathy after intravitreal bevacizumab
injection. Am J Ophthalmol. 150:223–229.e1. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang DY, Zhao XY, Zhang WF, Meng LH and
Chen YX: Perioperative anti-vascular endothelial growth factor
agents treatment in patients undergoing vitrectomy for complicated
proliferative diabetic retinopathy: A network meta-analysis. Sci
Rep. 10(18880)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Simunovic MP and Maberley DA:
Anti-vascular endothelial growth factor therapy for proliferative
diabetic retinopathy: A systematic review and meta-analysis.
Retina. 35:1931–1942. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang M, Zhang J, Yan M, Luo D, Zhu W,
Kaiser PK and Yu DC: KH902 Phase 1 Study Group. A phase 1 study of
KH902, a vascular endothelial growth factor receptor decoy, for
exudative age-related macular degeneration. Ophthalmology.
118:672–678. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang X, Xu J, Wang R, Mei Y, Lei H, Liu J,
Zhang T and Zhao H: A randomized controlled trial of conbercept
pretreatment before vitrectomy in proliferative diabetic
retinopathy. J Ophthalmol. 2016(2473234)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mao JB, Wu HF, Chen YQ, Zhao SX, Tao JW,
Zhang Y, Zheng B, Wang L and Shen LJ: Effect of intravitreal
conbercept treatment before vitrectomy in proliferative diabetic
retinopathy. Int J Ophthalmol. 11:1217–1221. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kitagawa Y, Shimada H, Yukita M and Naruse
S: Silicone oil injection and removal in 27-gauge vitreous surgery.
Int J Ophthalmol. 16:139–142. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nam Y, Chung H, Lee JY, Kim JG and Yoon
YH: Comparison of 25- and 23-gauge sutureless microincision
vitrectomy surgery in the treatment of various vitreoretinal
diseases. Eye (Lond). 24:869–874. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Osawa S and Oshima Y: 27-Gauge vitrectomy.
Dev Ophthalmol. 54:54–62. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gupta V and Arevalo JF: Surgical
management of diabetic retinopathy. Middle East Afr J Ophthalmol.
20:283–292. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mitsui K, Kogo J, Takeda H, Shiono A,
Sasaki H, Munemasa Y, Kitaoka Y and Takagi H: Comparative study of
27-gauge vs 25-gauge vitrectomy for epiretinal membrane. Eye
(Lond). 30:538–544. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kunikata H, Yasuda M, Aizawa N, Osada U,
Nishiguchi KM, Abe T and Nakazawa T: Retinal sensitivity and vessel
density after macular hole surgery with the superior inverted
internal limiting membrane flap technique. Retina. 41:45–53.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen PL, Chen YT and Chen SN: Comparison
of 27-gauge and 25-gauge vitrectomy in the management of tractional
retinal detachment secondary to proliferative diabetic retinopathy.
PLoS One. 16(e0249139)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Naruse Z, Shimada H and Mori R: Surgical
outcomes of 27-gauge and 25-gauge vitrectomy day surgery for
proliferative diabetic retinopathy. Int Ophthalmol. 39:1973–1980.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rizzo S, Barca F, Caporossi T and Mariotti
C: Twenty-seven-gauge vitrectomy for various vitreoretinal
diseases. Retina. 35:1273–1278. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Romano MR and Cennamo G, Ferrara M,
Cennamo M and Cennamo G: Twenty-seven-gauge versus 25-gauge
vitrectomy for primary rhegmatogenous retinal detachment. Retina.
37:637–642. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rizzo S, Polizzi S, Barca F, Caporossi T
and Virgili G: Comparative study of 27-gauge versus 25-gauge
vitrectomy for the treatment of primary rhegmatogenous retinal
detachment. J Ophthalmol. 2017(6384985)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kasi SK, Hariprasad SM and Hsu J: Making
the jump to 27-gauge vitrectomy: Perspectives. Ophthalmic Surg
Lasers Imaging Retina. 48:450–456. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Issa SA, Connor A, Habib M and Steel DH:
Comparison of retinal breaks observed during 23 gauge
transconjunctival vitrectomy versus conventional 20 gauge surgery
for proliferative diabetic retinopathy. Clin Ophthalmol. 5:109–114.
2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang ZH, Liu HY, Hernandez-Da Mota SE,
Romano MR, Falavarjani KG, Ahmadieh H, Xu X and Liu K: Vitrectomy
with or without preoperative intravitreal bevacizumab for
proliferative diabetic retinopathy: A meta-analysis of randomized
controlled trials. Am J Ophthalmol. 156:106–115.e2. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Abulon DJK and Buboltz DC: Performance
comparison of high-speed dual-pneumatic vitrectomy cutters during
simulated vitrectomy with balanced salt solution. Transl Vis Sci
Technol. 4(6)2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Abulon DJ: Vitreous flow rates through
dual pneumatic cutters: Effects of duty cycle and cut rate. Clin
Ophthalmol. 9:253–261. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Steel DH and Charles S: Vitrectomy
fluidics. Ophthalmologica. 226 (Suppl 1):S27–S35. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dave VP, Pathengay A, Basu S, Gupta N,
Basu S, Raval V, Das T, Sharma S, Mathai A, Narayanan R, et al:
Endophthalmitis after pars plana vitrectomy: Clinical features,
risk factors, and management outcomes. Asia Pac J Ophthalmol
(Phila). 5:192–195. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ibarra MS, Hermel M, Prenner JL and Hassan
TS: Longer-term outcomes of transconjunctival sutureless 25-gauge
vitrectomy. Am J Ophthalmol. 139:831–836. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hsu J, Chen E, Gupta O, Fineman MS, Garg
SJ and Regillo CD: Hypotony after 25-gauge vitrectomy using oblique
versus direct cannula insertions in fluid-filled eyes. Retina.
28:937–940. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhao LQ, Zhu H, Zhao PQ and Hu YQ: A
systematic review and meta-analysis of clinical outcomes of
vitrectomy with or without intravitreal bevacizumab pretreatment
for severe diabetic retinopathy. Br J Ophthalmol. 95:1216–1222.
2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Qu J, Chen X, Liu Q, Wang F, Li M, Zhou Q,
Yao J and Li X: Prophylactic intravitreal injection of aflibercept
for preventing postvitrectomy hemorrhage in proliferative diabetic
retinopathy: A randomized controlled trial. Front Public Health.
10(1067670)2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bahr TA and Bakri SJ: Update on the
management of diabetic retinopathy: Anti-VEGF agents for the
prevention of complications and progression of nonproliferative and
proliferative retinopathy. Life (Basel). 13(1098)2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kaiser SM, Arepalli S and Ehlers JP:
Current and future anti-VEGF agents for neovascular age-related
macular degeneration. J Exp Pharmacol. 13:905–912. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chang E, Josan AS, Purohit R, Patel CK and
Xue K: A network meta-analysis of retreatment rates following
bevacizumab, ranibizumab, aflibercept, and laser for retinopathy of
prematurity. Ophthalmology. 129:1389–1401. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li X, Xu G, Wang Y, Xu X, Liu X, Tang S,
Zhang F, Zhang J, Tang L, Wu Q, et al: Safety and efficacy of
conbercept in neovascular age-related macular degeneration: results
from a 12-month randomized phase 2 study: AURORA study.
Ophthalmology. 121:1740–1747. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang Q, Li T, Wu Z, Wu Q, Ke X, Luo D and
Wang H: Novel VEGF decoy receptor fusion protein conbercept
targeting multiple VEGF isoforms provide remarkable
anti-angiogenesis effect in vivo. PLoS One.
8(e70544)2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ferrara N: Role of vascular endothelial
growth factor in physiologic and pathologic angiogenesis:
Therapeutic implications. Semin Oncol. 29 (6 Suppl 16):S10–S14.
2002.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Su L, Ren X, Wei H, Zhao L, Zhang X, Liu
J, Su C, Tan L and Li X: INTRAVITREAL conbercept (KH902) for
surgical treatment of severe proliferative diabetic retinopathy.
Retina. 36:938–943. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Farahvash MS, Majidi AR, Roohipoor R and
Ghassemi F: Preoperative injection of intravitreal bevacizumab in
dense diabetic vitreous hemorrhage. Retina. 31:1254–1260.
2011.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Modarres M, Nazari H, Falavarjani KG,
Naseripour M, Hashemi M and Parvaresh MM: Intravitreal injection of
bevacizumab before vitrectomy for proliferative diabetic
retinopathy. Eur J Ophthalmol. 19:848–852. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li H, Niu Y, Rong A, Bi Y, Xu W and Cui H:
Effect of adjunctive intravitreal conbercept injection at the end
of 25G vitrectomy on severe proliferative diabetic retinopathy:
6-month outcomes of a randomised controlled trial. Ophthalmol Ther.
12:1173–1180. 2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Someya H, Takayama K, Takeuchi M, Yokoyama
H, Kimura T, Morioka M, Takamura Y, Sameshima S, Ueda T, Ogata N,
et al: Outcomes of 25-gauge vitrectomy for tractional and
nontractional diabetic macular edema with proliferative diabetic
retinopathy. J Ophthalmol. 2019(5304524)2019.PubMed/NCBI View Article : Google Scholar
|