Introduction
Colon cancer is a malignant tumor of the digestive
tract that occurs in the colon mucosa (1,2).
Diet, environment and genetic factors affect the pathogenesis of
colon cancer (3,4). Currently, colon cancer is primarily
treated using surgery; however, most patients are already in the
advanced stage when they are diagnosed because the disease has no
clear symptoms in the early stage (5,6).
Chemotherapy and radiation therapy are key strategies for antitumor
therapy when the tumor progresses to mid- and late-stage (7-10).
However, the ionizing radiation associated with radiation therapy
causes severe side effects such as loss of appetite, fatigue,
headache, dizziness, nausea, vomiting and bone marrow suppression
(11-14).
The existing chemotherapeutic drugs, such as sorafenib,
doxorubicin, 5-fluorouracil and cisplatin, have definite antitumor
mechanisms and effects, but shortcomings such as low cytotoxic
selectivity, poor water solubility, serious side effects, low
bioavailability and drug resistance limit their application
(15-18).
Therefore, it is necessary to explore novel drugs with efficient
anticancer effects and low acute and long-term toxicity to improve
the disease-free survival time and decrease the postoperative
recurrence rate.
Several plant-based compounds have been shown to be
potent anticancer drugs or chemosensitizers or to reverse drug
resistance in different tumors (19-22).
Compared with small molecule cytotoxic drugs, plant-based compounds
show advantages such as multi-targeting, low toxicity, precise
function and easy synthesis. Procyanidins (PCs) are a class of
natural polyphenolic compounds found in a variety of plants, such
as grape seeds, apples, peanut skin and cranberries (23-25).
Owing to the strong antioxidant capacity and free radical
scavenging ability, PC exhibits a wide range of applications in
protecting cardiovascular circulation, anti-inflammatory and
immunity enhancement (26-28).
In addition, acute and long-term toxicity evaluation showed
excellent biocompatibility, which is the basis for its biomedical
applications (23). Currently, the
application of PC in biomedicine has been extended to tumor therapy
but studies on the antitumor effects of PC are limited to their
total extracts (29-31).
To the best of our knowledge, no anticancer activity evaluation of
PC with specific polymerization degree and structural
characteristics has been reported so far.
Materials and methods
Cell culture
Human colon carcinoma cell line HCT-116, colorectal
adenocarcinoma epithelial cell line DLD-1 and colon cancer cell
line SW620 were purchased from the Cell Bank of Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China) and
cultured in a 5% CO2 incubator at 37˚C. HCT-116 cells
were cultured in McCoy's 5A supplemented with 10% fetal bovine
serum (FBS), 1% streptomycin and penicillin (all Gibco; Thermo
Fisher Scientific, Inc.). DLD-1 cells were cultured in RPMI-1640
(Gibco) supplemented with 10% FBS and 1% streptomycin and
penicillin. SW620 cells were cultured in Leibovitz's L-15 (Gibco)
supplemented with 10% FBS and 1% streptomycin and penicillin. For
the CCK-8, apoptosis and cell cycle assay, HCT-116, DLD-1 and SW620
cells were treated with PCB1 (purity >95%, Shanghai Yuanye
Biotechnology Co. LTD) (0, 6, 12, 25, 50, and 100 µg/ml) or DOX
(100 nM) (MCE) in a serum-free culture medium at 37˚C for 24, 48,
or 72 h.
CCK-8 assay
CCK-8 assay was used to assess viability of HCT-116,
DLD-1 and SW620 cells incubated with PCB1 or DOX. HCT-116, DLD-1 or
SW620 cells were seeded into a 96-well plate at a density of
5x103 cells/well and incubated overnight at 37˚C.
Subsequently, culture medium was replaced with that containing PCB1
or DOX. After incubation at 37˚C for 24, 48 and 72 h, 10 µl of
CCK-8 reagent was added to each well and incubated with cells for 2
h. Then the absorbance of cells at 450 nm was measured with a
microplate reader.
Apoptosis assay
Apoptosis assay was performed to measure early or
late apoptotic HCT-116 cells following treatment with PCB1 or DOX.
Briefly, HCT-116 cells were first seeded into 6-well plates at the
density of 2x105 cells per well and incubated overnight
at 37˚C. Following treatment with PCB1 or DOX for 48 h at 37˚C,
HCT-116 cells were washed twice with PBS and re-suspended in
binding buffer at a density of 1x106 cells/ml. HCT-116
cells (100 µl), Annexin V-FITC (5 µl) and PI (5 µl) were mixed and
transferred to a tube for incubation at 37˚C for 15 min. The
binding buffer (400 µl) was added to the stained HCT-116 cells,
which were analyzed using a flow cytometer (BD FACS Calibur; BD
Biosciences) and FlowJo 10.8.1 (BD Biosciences).
Cell cycle analysis
Cell Cycle Assay Kit, DOJINDO) was used to analyze
the effect of PCB1 on the cell cycle. Briefly, HCT-116 cells first
seeded into a 6-well plate at the density of 2x105 cells
per well and incubated overnight at 37˚C. After treatment with PCB1
or DOX for 48 h at 37˚C, HCT-116 cells were added to PBS and fixed
in cold ethanol (70%, 4˚C) for 4 h. The mixed solution was
centrifugated at 37˚C (500 x g, 5 min) for removing ethanol and
then added to DNA staining solution (500 µl). Following incubation
at 37˚C for 30 min, HCT-116 cells were analyzed using a flow
cytometer (BD FACS Calibur; BD Biosciences). The data were analyzed
by FlowJo 10.8.1 (BD Biosciences).
Reverse transcription-quotative
(RT-q)PCR
Following treatment with PCB1 or DOX at 37˚C for 48
h, total RNA was extracted from HCT-116 cells using TRIzol™ (Thermo
Fisher Scientific, Inc.; Invitrogen). Total RNA was
reverse-transcribed into cDNA (1 µg) using the PrimeScript™ RT
Reagent kit (Takara Bio, Inc.), according to the manufacturer's
protocol. qPCR was performed using SYBR®-Green Premix Ex
Taq™ (Takara Bio, Inc.) on a QuantStudio™ 5 Real-Time PCR Detection
System (Applied Biosystems; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The following primer
pairs were used for qPCR: Caspase-3 forward,
5'-GGTGCTATTGTGAGGCGGTT-3' and reverse, 5'-TGAGAATGGGGGAAGAGGCA-3';
Ki67 forward, 5'-ATGGAGAGGTGGCCAAGAAC-3' and reverse,
5'-TGTGTGGTCTGTGTGAGCTG-3'; Bcl-2 forward,
5'-CTTTGAGTTCGGTGGGGTCA-3' and reverse, 5'-GGGCCGTACAGTTCCACAAA-3';
Bax forward, 5'-CCCGAGAGGTCTTTTTCCGAG-3' and reverse,
5'-CCAGCCCATGATGGTTCTGAT-3' and β-actin forward
5'-GGACTTCGAGCAAGAGATGG-3' and reverse, 5'-AGCACTGTGTTGGCGTACAG-3'.
The mRNA levels were quantified using the 2-ΔΔCq method
(32) and normalized to the
internal reference gene β-actin.
Western blotting
HCT-116 cells were seeded into a 6-well plate at a
density of 2x105 cells per well and incubated overnight
at 37˚C. After treatment with PCB1 at 37˚C for 24 h, HCT-116 cells
were lysed in cold RIPA lysis and extraction buffer (cat. no.
89900; Thermo Fisher Scientific, Inc.) at 4˚C for 30 min. The
protein extract was centrifugated at 12,000 x g for 5 min at room
temperature to collect the cell lysate. Total protein was
quantified using the BCA Protein Detection kit (cat. no. 23227;
Thermo Fisher Scientific, Inc.) and 20 µg protein/lane was
separated by SDS-PAGE on 10 and 12% gels. The separated proteins
were transferred onto a PVDF membrane using a western blot system
(Bio-Rad Laboratories, Inc.). PVDF membranes were blocked with 5%
skimmed milk powder for 1 h at 4˚C and then incubated with primary
antibodies at 4˚C overnight. The following antibodies were used at
a dilution of 1:1,000: Anti-cleaved-Caspase-3 (cat. no. 9661T; Cell
Signaling Technology, Inc.), anti-Ki67 (cat. no. ab16667; Abcam),
anti-Bax (cat. no. 14796S; Cell Signaling Technology, Inc.) and
anti-Bcl2 (cat. no. ab59348; Abcam). The PVDF membranes were washed
three times with TBST (8.8 g NaCl + 20 ml of Tris-HCL (1 M) + 0.5
ml of Tween 20) and then incubated with secondary antibodies for 1
h at room temperature as follows: Anti-rabbit IgG (1:5,000; cat.
no. 7074; Cell Signaling Technology, Inc.) or anti-mouse IgG,
horseradish peroxidase-linked antibody (1:5,000; cat. no. 7076;
Cell Signaling Technology, Inc.). After incubation, the membranes
were washed, added with ECL reagent (SuperSignal West Femto, Thermo
Scientific) and observed by enhanced chemiluminescent detection
systems (chemiscope6100, Shanghai Qinxiang Scientific Instrument
Co., LTD) and analyzed by ChemiScope Analysis software (Shanghai
Qinxiang Scientific Instrument Co., LTD).
Solid tumor treatment
A total of 25 female BALB/c nude mice (age, 5 weeks,
weight, ~20 g) were purchased from Slack Experimental Animal Center
(Shanghai, China). All animal experiments were approved by the
Biology Ethics Committee of Shihezi University (approval no.
A2022-046). All mice were housed in an animal facility under
constant environmental conditions (room temperature, 22±1˚C,
relative humidity, 40-70% and a 12 h light-dark cycle) and allowed
free access to autoclaved water and irradiated food HCT-116 tumor
models were established by subcutaneous injection of
6x106 cells into the right shoulder of nude mice. The
mice were randomly divided into five groups (n=5/group) after the
tumor volume reached ~50 mm3 as follows: Control (PBS),
PCB1 (20 mg/kg), PCB1 (40 mg/kg), PCB1 (60 mg/kg) and DOX (5 mg/kg)
groups. The mice received intragastric PCB1 or DOX every other day
via gavage. The tumor size was measured every day and calculated
using as follows: (Length x width2)/2. Animals were
euthanized when the individual tumor volume reached 1000
mm3. No animals were sacrificed according to the
endpoints before the end of the experiment. After 18 days of
administration, the experiment was terminated. The mice were
euthanized after being photographed. Euthanasia was performed using
5% isoflurane inhalation followed by cervical dislocation. The
tumors were collected, photographed and weighed to evaluate the
anticancer activity of PCB1.
Statistical analysis
Data are expressed as the mean ± standard deviation
and analyzed by Graphpad Prism 8.0 (GraphPad Software). To ensure
the accuracy of the experiments, at least three replicates were
performed. When variances were homogeneous, the statistical
analysis was performed through one-way ANOVA. If not, the data were
analyzed using the Kruskal-Wallis non-parametric test. If there was
significant difference, the data were analyzed using Dunnett's post
hoc test.
Results
Viability of HCT-116 cells incubated
with PCB1 using CCK-8 assay
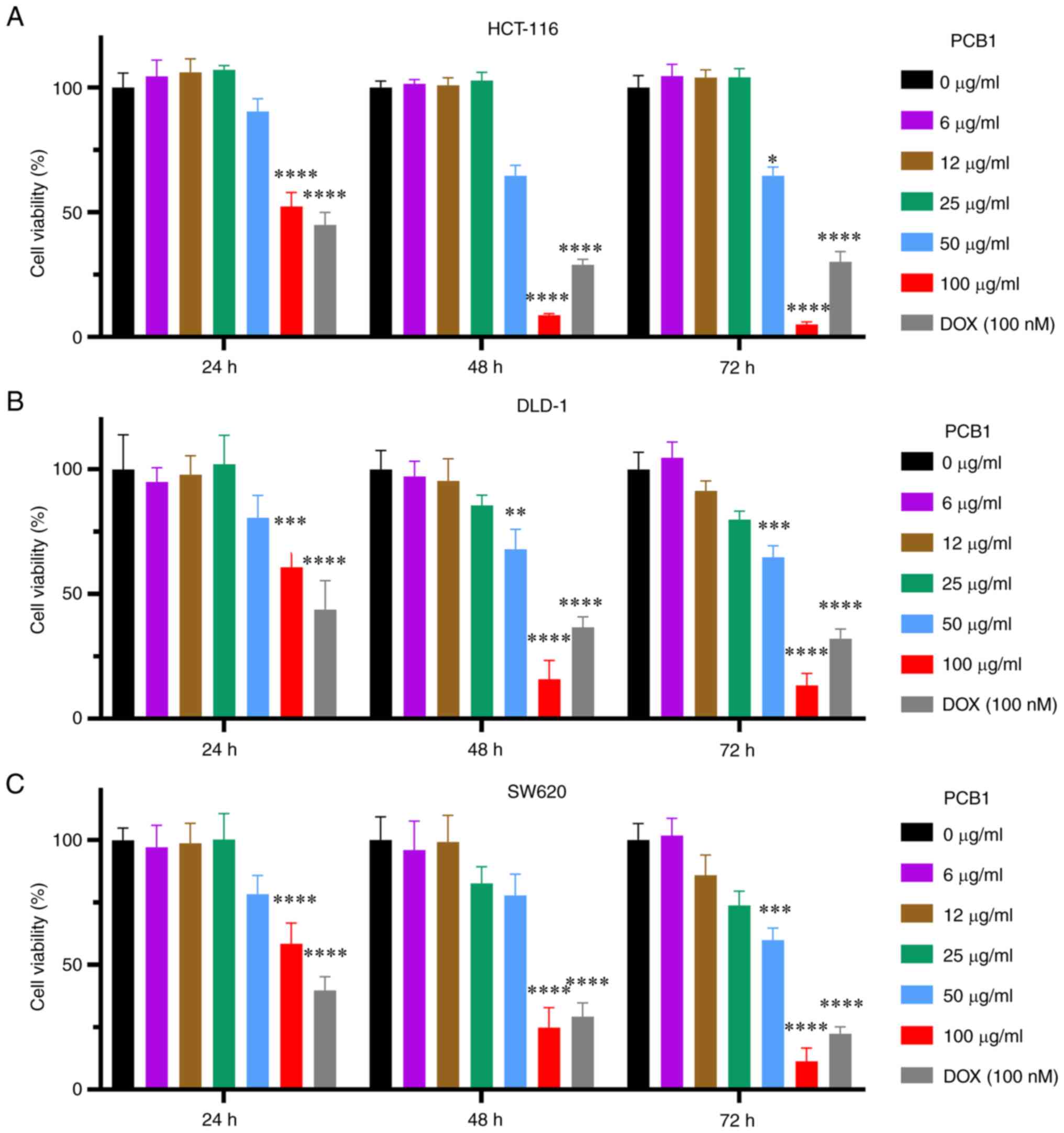
The concentration-dependent cytotoxicity of PCB1 is
reported in Fig. 1A. PCB1
decreased the number of live HCT-116 cells; 100 µg/ml PCB1 induced
a 52.3±5.0% decrease in live cells after treating for 24 h. By
contrast, viability of HCT-116 cells incubated with PCB1 for 48 or
72 h was decreased to 8.7±0.5 and 5.0±0.9%, respectively, which was
lower than that of DOX at the same incubation length (28.9±2.2 and
30.1±4.1% for 48 and 72 h, respectively). Moreover, a significant
decrease in cell viability was observed for DLD-1 and SW620 cells
following incubation with PCB1, with 100 µg/ml PCB1 showing the
greatest effect on cancer cells (Fig.
1B and C). DOX was chosen as a
positive control because it is a widely used and studied anticancer
drug (33-35).
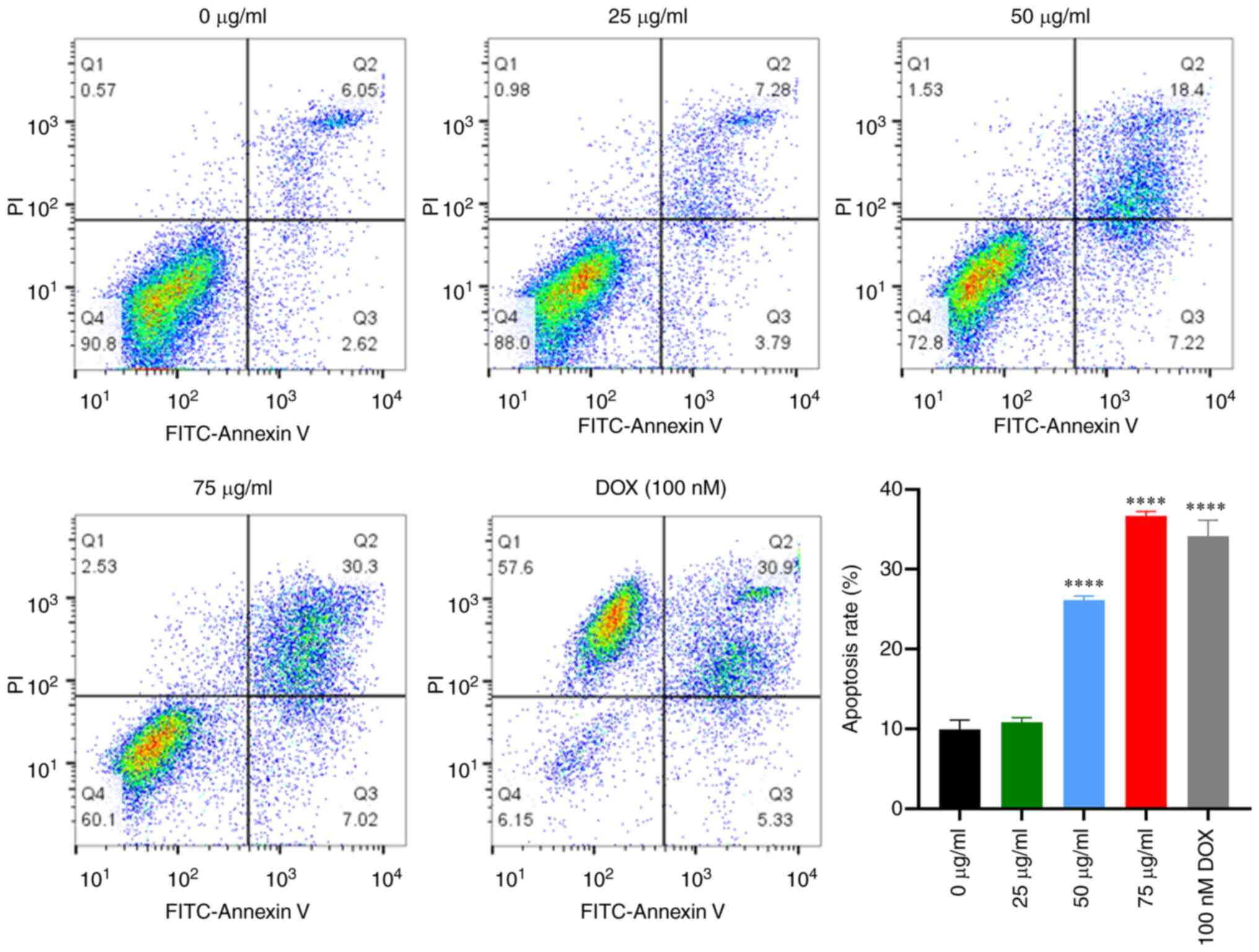
Apoptosis analysis
The mechanism of cell death induced by PCB1 was
investigated using flow cytometry. A significant increase in
apoptotic HCT-116 cells was found following treatment with PCB1 or
DOX for 48 h. Moreover, apoptosis of HCT-116 cells induced by PCB1
was concentration-dependent (Fig.
2). Specifically, the apoptosis rate of HCT-116 cells at 48 h
increased from 11.07 to 37.32% as the concentration of PCB1
increased. Furthermore, levels of apoptotic HCT-116 cells following
incubation with PCB1 for 48 h were higher than in the DOX group
(37.32 vs. 36.23%), indicating that PCB1 efficiently induced cell
apoptosis.
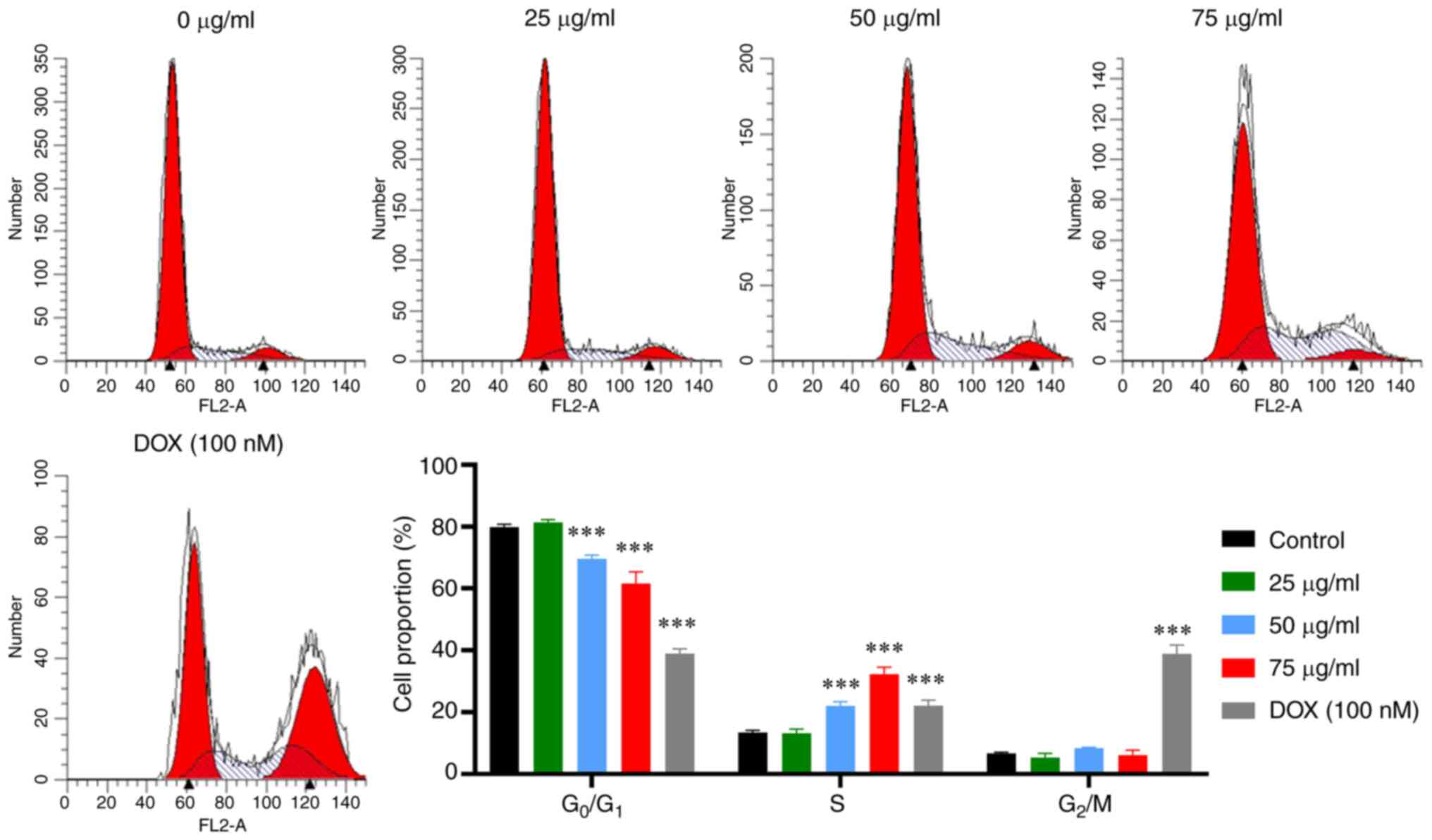
Cell cycle analysis
Given the cell apoptosis induced by PCB1, cell cycle
analysis was performed to verify if PCB1 caused cell cycle arrest.
The proportion of HCT-116 cells in each phase was measured
following treatment for 48 h with either PCB1 or DOX using a flow
cytometer. The accumulation of HCT-116 cells treated with DOX in S
phase and G2/M phase was accompanied by reduction in
G0/G1, demonstrating the DOX-induced S phase
and G2/M phase arrest (Fig.
3). By contrast, the proportion of HCT-116 cells in S phase
following treatment with PCB1 was increased compared with that in
the control group, while that in G0/G1 phase
was significantly decreased. Furthermore, the proportion of cells
in the S phase increased in a concentration-dependent behavior,
suggesting HCT-116 cell cycle progression was arrested in S phase
by PCB1, thereby inducing cell apoptosis.
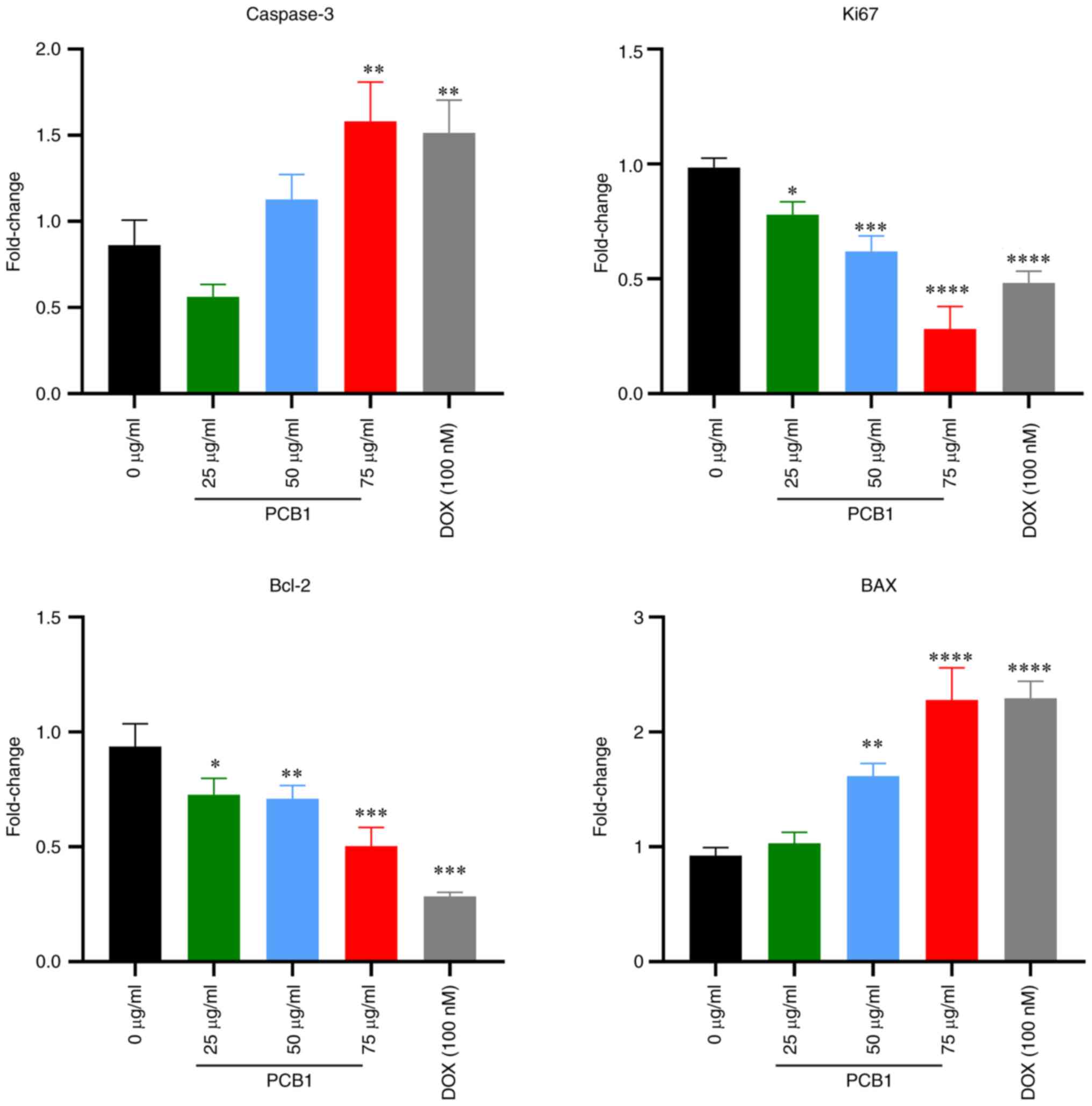
RT-qPCR analysis and western
blotting
Considering that cell apoptosis and cell cycle
arrest were induced by PCB1, RT-qPCR and western blotting were
performed to investigate the possible apoptotic pathways. Following
treatment with PCB1, the PCB1 group (75 µg/ml) showed an increase
in mRNA expression levels of pro-apoptosis proteins caspase-3 and
BAX compared with those in the control group. By contrast, the mRNA
expression of anti-apoptosis protein Bcl-2 in HCT-116 cells treated
with PCB1 significantly decreased as the concentration of PCB1
increased (Fig. 4).
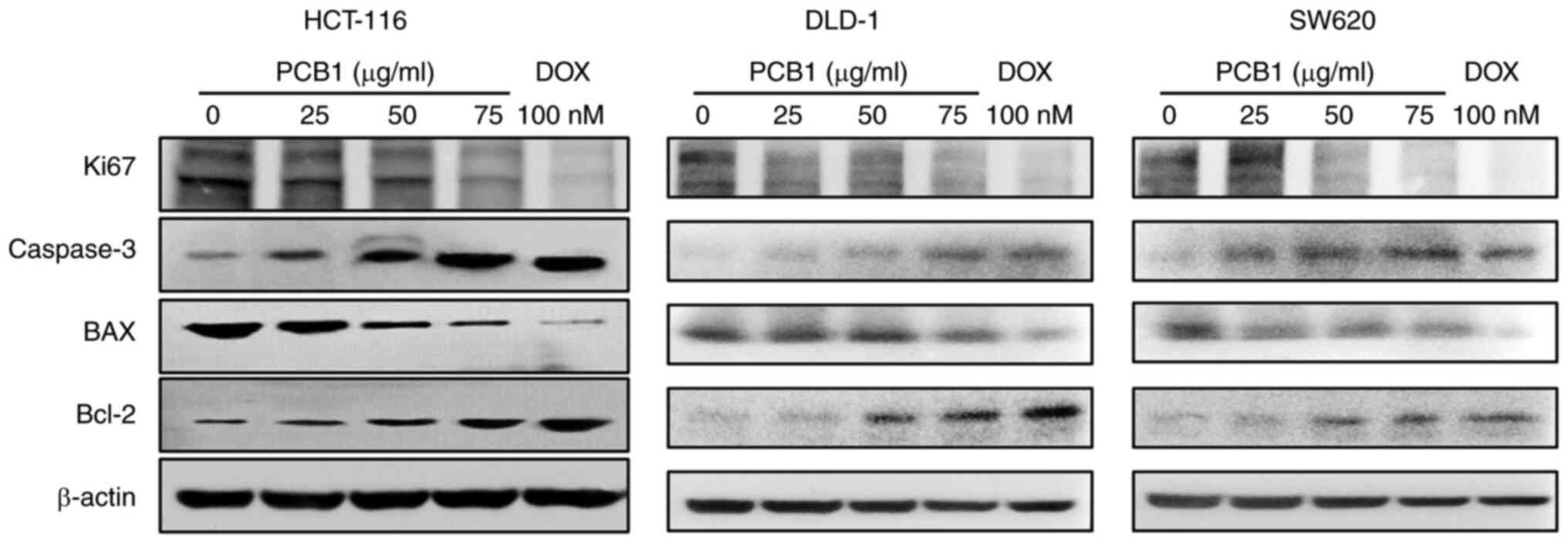
In addition, the protein expression of caspase-3 and
BAX in HCT-116, DLD-1 and SW620 cells increased compared with the
control group, while that of Bcl-2 was notably decreased (Fig. 5). These results demonstrated that
the PCB1 treatment induced caspase-associated apoptosis pathway.
Moreover, mRNA and protein expression levels of Ki-67 were also
decreased in the PCB1 group compared with those in the untreated
group, confirming that PCB1 inhibited cell proliferation.
In vivo therapeutic efficacy of
PCB1
The efficient anticancer and apoptotic activity of
PCB1 at the cellular level suggested that PCB1 may be potentially
applied in clinical practice. Therefore, the in vivo
therapeutic efficacy of PCB1 on an HCT-116 xenograft tumor model
induced via intragastric administration was investigated.
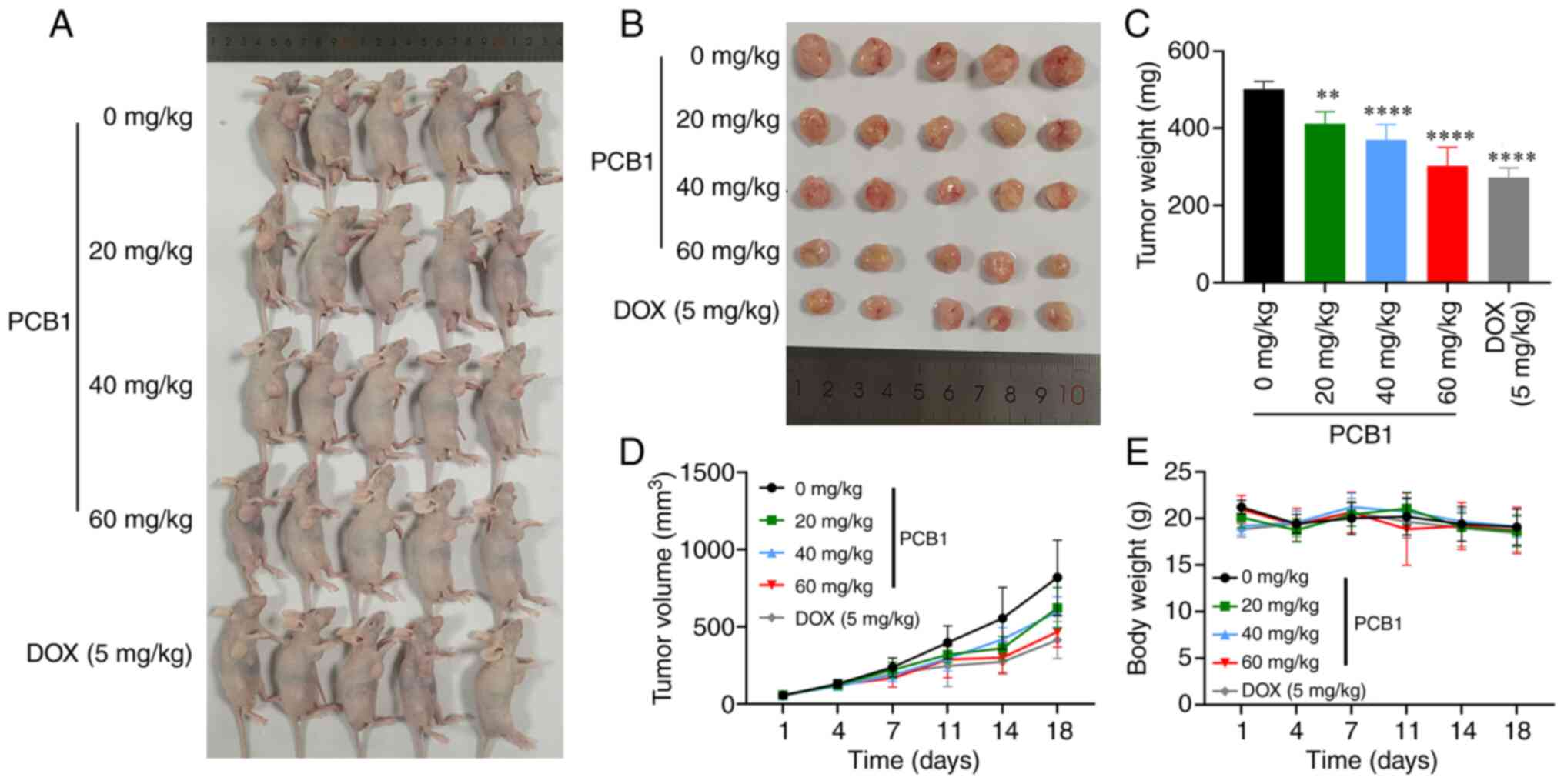
Representative photographs, tumor volume and weight and body weight
of mice treated with PBS, PCB1 (25 µg/ml, 20 mg/kg), PCB1 (50
µg/ml, 40 mg/kg), PCB1 (75 µg/ml, 60 mg/kg) and DOX (100 nM, 5
mg/kg) are presented in Fig. 6. No
inhibitory effect on the tumor growth was observed in the control
group (Fig. 6D). By contrast, the
tumor volume in the low-dose (20 mg/kg) and mid-dose (40 mg/kg)
PCB1 groups were significantly lower than that in the control
group, suggesting that PCB1 had an efficient antitumor activity in
this model. Moreover, the tumor growth inhibition in the high-dose
(60 mg/kg) PCB1 group was comparable with the DOX (100 nM) group
(Fig. 6A and B) In addition, the tumor weight in the
high-dose PCB1 (60 mg/kg) and DOX groups were significantly lower
than that in the control group (Fig.
6C), further confirming the antitumor ability of PCB1. No
significant change in body weight in each group was observed
(Fig. 6E), suggesting good
biocompatibility and undetectable off-target toxicity of PCB1.
Discussion
Existing chemotherapeutic drugs, such as
5-fluorouracil, oxaliplatin and capecitabine, for the treatment of
colon cancer, have definite antitumor mechanisms and effects but
also shortcomings, such as low cytotoxicity selectivity, poor water
solubility, serious side effects, low bioavailability and drug
resistance, that limit their clinical application (36-38).
By contrast, the present study showed that PCB1 may be a potential
novel anticancer agent that originates from natural polyphenolic
compounds and possesses good biocompatibility. To the best of our
knowledge, although the application of PC in biomedicine has been
extended to tumor therapy, studies on the antitumor effects of PC
are limited to their full extracts (29-31);
moreover, no anticancer activity of PC with specific polymerization
degree and structural characteristics has been reported.
CCK-8 assay revealed that PCB1 effectively decreased
the number of viable HCT-116 cells compared with the small-molecule
cytotoxic drug doxorubicin (DOX). Moreover, the expression levels
of several important genes are assessed to establish the anticancer
mechanism of PCB1 as a potential chemotherapy drug. Further
analysis demonstrated that PCB1 blocked the cell cycle of HCT-116
cells in the S phase by decreasing the expression of Ki67 and Bcl-2
and increasing the expression of Caspase-3 and BAX. In vivo
experiments on a xenograft mouse model indicated that PCB1
significantly inhibited tumor growth, comparably to the effect of
DOX.
In the present study, the increased proportion of
cells in S phase suggested that PCB1 arrested HCT-116 cell cycle
progression, thereby inducing cell apoptosis. Mammalian cells
mainly possess two major apoptotic pathways including the
death-receptor and the mitochondrial pathways. Considering that the
two pathways converge at the caspase-3 protein, we thus detected
the expression of caspase-3 in the mRNA and protein levels to
investigate the apoptotic pathways. RT-qPCR and western blot
analysis further indicated that PCB1 increased the protein and mRNA
levels of caspase-3 and BAX and decreased those of Bcl-2. Although
RT-qPCR and western blot experiments have suggested that PCB1
induces cell apoptotic, the potential anticancer mechanism of PCB1
has not been investigated. More experiments will be performed in
future to investigate the anticancer mechanism of PCB1, such as the
comet assay, topoisomerase inhibition assay and lactate
dehydrogenase assay. In addition, the potential anticancer activity
of PCB1 against non-transformed cell lines would be carefully
evaluated in the future. Unlike DOX (34), the plant-based compound PCB1 does
not induce notable damage to normal tissues and important organs.
However, the in vivo therapeutic effect of PCB1 was limited.
Therefore, further studies should investigate the combined
therapeutic effect of PCB1 and commonly used first-line
chemotherapy drugs. Although the present results suggested PCB1 as
a potential chemotherapy drug to inhibit tumor growth, the current
study was not comprehensive and the anticancer mechanism of PCB1
was not investigated at the cellular and molecular levels.
Investigation of the anticancer mechanism of PCB1 compared with
other anticancer drugs should be performed in the future. In
addition, the in vivo long-term toxicity of PCB1 should be
evaluated in the future.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Independent
Funded Project of Science and Technology Program from Tibet
Autonomous Region (grant no. XZ202101ZY0007N).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC designed the study. YL, XD and ZZ collected and
analyzed data. YL wrote the manuscript. All authors have read and
approved the final manuscript. YL and JC confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
All animal experiments were approved by the Biology
Ethics Committee of Shihezi University (approval no.
A2022-046).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu F, Wang XD and Du SY: Production of
gold/silver doped carbon nanocomposites for effective photothermal
therapy of colon cancer. Sci Rep. 10(7618)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang X, Zhong X, Lei H, Geng Y, Zhao Q,
Gong F, Yang Z, Dong Z, Liu Z and Cheng L: Hollow Cu2Se nanozymes
for tumor photothermal-catalytic therapy. Chem Mater. 31:6174–6186.
2019.
|
|
3
|
Roper J, Tammela T, Cetinbas NM, Akkad A,
Roghanian A, Rickelt S, Almeqdadi M, Wu K, Oberli MA,
Sanchez-Rivera FJ, et al: In vivo genome editing and organoid
transplantation models of colorectal cancer and metastasis. Nat
Biotechnol. 35:569–576. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Vasaikar S, Huang C, Wang X, Petyuk VA,
Savage SR, Wen B, Dou Y, Zhang Y, Shi Z, Arshad OA, et al: Clinical
proteomic tumor analysis, proteogenomic analysis of human colon
cancer reveals new therapeutic opportunities. Cell. 177:1035–1049.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Araghi M, Soerjomataram I, Jenkins M,
Brierley J, Morris E, Bray F and Arnold M: Global trends in
colorectal cancer mortality: Projections to the year 2035. Int J
Cancer. 144:2992–3000. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Feletto E, Yu XQ, Lew JB, St John DJB,
Jenkins MA, Macrae FA, Mahady SE and Canfell K: Trends in colon and
rectal cancer incidence in australia from 1982 to 2014: Analysis of
data on over 375,000 cases. Cancer Epidemiol Biomarkers Prev.
28:83–90. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
MacKay JA, Chen M, McDaniel JR, Liu W,
Simnick AJ and Chilkoti A: Self-assembling chimeric
polypeptide-doxorubicin conjugate nanoparticles that abolish
tumours after a single injection. Nat Mater. 8:993–999.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Xu L, Gordon R, Farmer R, Pattanayak A,
Binkowski A, Huang X, Avram M, Krishna S, Voll E, Pavese J, et al:
Precision therapeutic targeting of human cancer cell motility. Nat
Commun. 9(2454)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Keklikoglou I, Cianciaruso C, Guc E,
Squadrito ML, Spring LM, Tazzyman S, Lambein L, Poissonnier A,
Ferraro GB, Baer C, et al: Chemotherapy elicits pro-metastatic
extracellular vesicles in breast cancer models. Nat Cell Biol.
21:190–202. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Park NH, Cheng W, Lai F, Yang C, Florez de
Sessions P, Periaswamy B, Wenhan Chu C, Bianco S, Liu S,
Venkataraman S, et al: Addressing drug resistance in cancer with
macromolecular chemotherapeutic agents. J Am Chem Soc.
140:4244–4252. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Baskar R, Lee KA, Yeo R and Yeoh KW:
Cancer and radiation therapy: Current advances and future
directions. Int J Med Sci. 9:193–199. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang Z, Wang Y, Yao D, Wu J, Hu Y and
Yuan A: Nanoscale coordination polymers induce immunogenic cell
death by amplifying radiation therapy mediated oxidative stress.
Nat Commun. 12(145)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moding EJ, Kastan MB and Kirsch DG:
Strategies for optimizing the response of cancer and normal tissues
to radiation. Nat Rev Drug Discov. 12:526–542. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Song G, Cheng L, Chao Y, Yang K and Liu Z:
Emerging nanotechnology and advanced materials for cancer radiation
therapy. Adv Mater: Jun 23, 2017 (Epub ahead of print).
|
|
15
|
Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu
YL, Liu LF and Yeh ET: Identification of the molecular basis of
doxorubicin-induced cardiotoxicity. Nat Med. 18:1639–1642.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Zhu AX, Rosmorduc O, Evans TR, Ross PJ,
Santoro A, Carrilho FJ, Bruix J, Qin S, Thuluvath PJ, Llovet JM, et
al: SEARCH: A phase III, randomized, double-blind,
placebo-controlled trial of sorafenib plus erlotinib in patients
with advanced hepatocellular carcinoma. J Clin Oncol. 33:559–566.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Breglio AM, Rusheen AE, Shide ED,
Fernandez KA, Spielbauer KK, McLachlin KM, Hall MD, Amable L and
Cunningham LL: Cisplatin is retained in the cochlea indefinitely
following chemotherapy. Nat Commun. 8(1654)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sara JD, Kaur J, Khodadadi R, Rehman M,
Lobo R, Chakrabarti S, Herrmann J, Lerman A and Grothey A:
5-fluorouracil and cardiotoxicity: A review. Ther Adv Med Oncol.
10(1758835918780140)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Banik K, Ranaware AM, Harsha C, Nitesh T,
Girisa S, Deshpande V, Fan L, Nalawade SP, Sethi G and Kunnumakkara
AB: Piceatannol: A natural stilbene for the prevention and
treatment of cancer. Pharmacol Res. 153(104635)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ko HS, Lee HJ, Kim SH and Lee EO:
Piceatannol suppresses breast cancer cell invasion through the
inhibition of MMP-9: Involvement of PI3K/AKT and NF-κB pathways. J
Agric Food Chem. 60:4083–4089. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee YM, Lim DY, Cho HJ, Seon MR, Kim JK,
Lee BY and Park JH: Piceatannol, a natural stilbene from grapes,
induces G1 cell cycle arrest in androgen-insensitive DU145 human
prostate cancer cells via the inhibition of CDK activity. Cancer
Lett. 285:166–173. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu Q, Fu Q, Li Z, Liu H, Wang Y, Lin X, He
R, Zhang X, Ju Z, Campisi J, et al: The flavonoid procyanidin C1
has senotherapeutic activity and increases lifespan in mice. Nat
Metab. 3:1706–1726. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang L, Wang S, Liu Z, Zhang L, Wang S
and Wang B: Procyanidin, a kind of biological flavonoid, induces
protective anti-tumor immunity and protects mice from lethal B16F10
challenge. Int Immunopharmacol. 47:251–258. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shang XJ, Yao G, Ge JP, Sun Y, Teng WH and
Huang YF: Procyanidin induces apoptosis and necrosis of prostate
cancer cell line PC-3 in a mitochondrion-dependent manner. J
Androl. 30:122–126. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lei Y, Ren X, Chen J, Liu D and Ruan J:
Protective effects of grape seed-derived procyanidin extract
against carrageenan-induced abacterial prostatitis in rats. J Funct
Foods. 7:416–424. 2014.
|
|
26
|
Li S, Kodama EN, Inoue Y, Tani H, Matsuura
Y, Zhang J, Tanaka T and Hattori T: Procyanidin B1 purified from
Cinnamomi cortex suppresses hepatitis C virus replication. Antivir
Chem Chemother. 20:239–248. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Na W, Ma B, Shi S, Chen Y, Zhang H, Zhan Y
and An H: Procyanidin B1, a novel and specific inhibitor of Kv10.1
channel, suppresses the evolution of hepatoma. Biochem Pharmacol.
178(114089)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Koteswari LL, Kumari S, Kumar AB and Malla
RR: A comparative anticancer study on procyanidin C1 against
receptor positive and receptor negative breast cancer. Nat Prod
Res. 34:3267–3274. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee Y: Cancer chemopreventive potential of
procyanidin. Toxicol Res. 33:273–282. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Okamoto S, Ishihara S, Okamoto T, Doi S,
Harui K, Higashino Y, Kawasaki T, Nakajima N and Saito A:
Inhibitory activity of synthesized acetylated Procyanidin B1
analogs against HeLa S3 cells proliferation. Molecules.
19:1775–1785. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mao JT, Xue B, Smoake J, Lu QY, Park H,
Henning SM, Burns W, Bernabei A, Elashoff D, Serio KJ and Massie L:
MicroRNA-19a/b mediates grape seed procyanidin extract-induced
anti-neoplastic effects against lung cancer. J Nutr Biochem.
34:118–125. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cagel M, Grotz E, Bernabeu E, Moretton MA
and Chiappetta DA: Doxorubicin: Nanotechnological overviews from
bench to bedside. Drug Discov Today. 22:270–281. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tacar O, Sriamornsak P and Dass CR:
Doxorubicin: An update on anticancer molecular action, toxicity and
novel drug delivery systems. J Pharm Pharmacol. 65:157–170.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ghosh P, Tiwari H, Lakkakula J, Roy A,
Emran TB, Rashid S, Alghamdi S, Rajab BS, Almehmadi M, Allahyani M,
et al: A decade's worth of impact: Dox loaded liposomes in
anticancer activity. Mater Today Adv. 16(100313)2022.
|
|
36
|
van Pelt-Sprangers MJ, Geijteman EC, Alsma
J, Boere IA, Mathijssen RH and Schuit SC: Oromandibular dystonia: A
serious side effect of capecitabine. BMC Cancer.
15(115)2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
James E, Podoltsev N, Salehi E, Curtis BR
and Saif MW: Oxaliplatin-induced immune thrombocytopenia: Another
cumulative dose-dependent side effect? Clin Colorectal Cancer.
8:220–224. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhao J, Zhang X, Cui X, Wang D, Zhang B
and Ban L: Loss of fingerprints as a side effect of capecitabine
therapy: Case report and literature review. Oncol Res. 28:103–106.
2020.PubMed/NCBI View Article : Google Scholar
|