Introduction
Osteomyelitis is an inflammatory reaction in which
bacteria or inflammatory mediators invade periosteum, sclerotin and
bone marrow in various ways, which can result in progressive bone
deterioration, bone neoformation and severe inflammatory responses,
leading to substantial mortality and morbidity in patients
(1). Staphylococcus aureus
(S. aureus) is the commonest bacterial infection (80-90%),
followed by Streptococcus and Escherichia coli
(2). Due to the anatomical and
physiological characteristics of bone, antimicrobial therapy for
bone and joint infections has not achieved a high success rate in
most infectious diseases, so osteomyelitis is still considered one
of the most difficult to treat infectious diseases (3). Osteomyelitis is featured by
progressive devastating of the bone and the forming of sequestra,
which acted as the major directors of net bone formation or
resorption during normal physiological turnover of bone and
following infection. Promoting osteoblast formation is the key to
inhibit progressive bone destruction and bone isolation caused by
osteomyelitis (4).
Wnt is a secreted L-cysteine rich glycoprotein with
an important role in regulating the differentiation of mesenchymal
stem cells (MSCs), containing three main receptors including LDL
receptor-related protein (LRP-5/6), frizzled receptors (FZDs) and
β-catenin protein (5). β-catenin
serves an important role in the regulation of bone formation and
the osteoblasts differentiation (6). In addition, Runx2 can directly
stimulate the Wnt/β-catenin signaling pathway, thus regulate genes
transcription such as osteocalcin (OCN), type I collagen (COL1A1),
osteopontin (OPN) and collagenase 3 during osteoblast proliferation
and differentiation of bone marrow mesenchymal stem cells (BMSCs)
(7). The Wnt/β-catenin signal
transduction pathway is also involved in regulating the expression
of downstream matrix metalloproteinases (MMPs) family, which
include the main enzymes destroying the extracellular matrix (ECM)
of articular cartilage, such as MMP-2 and MMP-3, which are closely
associated with the pathogenesis of osteomyelitis (8,9).
Frizzled related protein (FRZB), as a competitive
inhibitor of the Wnt signal pathway, can competitively bind to the
transmembrane frizzle receptor and LRP-5/6 complex receptor, thus
inhibiting the Wnt signal pathway (10). Secreted frizzled related protein 3
(sFRP3) is an important member of the frizzled related protein
family, which is encoded by the FRZB gene (10). FRZB has been reported to be
involved in the process regulation of osteoarthritis,
cardiovascular disease and types of cancer (11-13).
The high expression of FRZB may be closely associated with bone
metastasis in patients with liver cancer (14). The knockout of FRZB in gastric
cancer cells increases cell growth and migration/invasion, which is
also accompanied by activation of Wnt/β-catenin and the downstream
targets (11). In a mouse model,
deletion of the FRZB not only increases articular cartilage loss
during arthritis arising from enzyme damage or inflammation, but
also leads to cortical bone thickening, increased stiffness after
loading and cortical apical bone formation (15). A study on patients with early RA
reports that the basic serum level of FRZB was high, but was
reduced following treatment with anti-rheumatic drugs (16). As for the genetic study of this
protein, a study conducted in patients with arthritis reported that
FRZB was negatively correlated with this disease (12). Therefore, the mechanism of FRZB in
bone related diseases needs further exploration. It is important to
highlight that the intrinsic mechanism of the FRZB gene associated
with the osteomyelitis remains to be elucidated.
In the present study, the expression profiles of the
FRZB gene were primarily confirmed in human bone marrow derived
stem cells (hBMSCs) and patients with osteomyelitis, then the
influences of FRZB on cell activity, apoptosis and differentiation
of hBMSCs treated with or without Staphylococcus aureus (S.
aureus) were detected in different expression states. Further,
it was verified that whether silencing of FRZB restrained S.
aureus-induced osteomyelitis by regulating Wnt/β-catenin
signal pathway.
Materials and methods
Cell culture
hBMSCs (cat. no. SCSP-405) were purchased from Cell
Bank of the Chinese Academy of Sciences (Shanghai, China). Basic
medium ingredients were low-glucose DMEM (Gibco; Thermo Fisher
Scientific, Inc.) with 10% FBS, 100 U/ml streptomycin and 100 U/ml
penicillin. Culture conditions were 37˚C, 5% CO2, 95%
air humidity with half changed every 48 h. The cells of the 3rd to
5th generation were used for the experiments (17). A previous study has shown that
activation of Wnt/β-catenin pathway contributes to the BMSC
osteogenic differentiation and osteogenesis (18). The present study used inhibitor of
β-catenin responsive transcription [ICRT-3;
2-[[[2-(4-ethylphenyl)-5-methyl-4-oxazolyl]methyl]thio]-N-(2-phenylethyl)acetamide]
as an inhibitor of Wnt signaling pathway that could inhibit the
occurrence of osteogenic differentiation and osteogenesis in
control and infected BMSCs. Hence, ICRT3 was used to investigate
the function of Wnt/β-catenin signaling pathway in the
FRZB-mediated osteomyelitis inhibition. hBMSCs were seeded in 96
well plates at a density of 2x105 cells per ml. At 24 h
after transfection, cells were pre-treated with 10 µM ICRT-3
(MilliporeSigma) or vehicle for 50 min at 37˚C and then infected
with S. aureus or vehicle.
Bacterial culture and infection
The S. aureus (ATCC; cat. no. 53657)
strain was cultured overnight in brain heart infusion broth medium
(OXOID Ltd.) at 37˚C under 160 rpm rotation on a shaker (Shanghai
Fuma Laboratory Instrument Co., Ltd.). Then the cultures were
centrifuged (10,000 x g, 4˚C, 10 min) and washed before
re-suspended in PBS to a final concentration of 0.5x106
colony forming units (CFU) per µl. For bacterial infection process,
hBMSCs were infected with a 100 MOI of S. aureus and
incubated for 72 h under 37˚C. Extracellular S.
aureus was removed with 20 mg/ml lysostaphin. Fresh medium
was added to cells every 2-3 days to remove the bacteria in the
supernatant.
FRZB overexpression and knockdown
vectors
FRZB overexpression construct was obtained by
sub-cloning PCR. FRZB was amplified from the cDNA ORF clone (Sino
Biological) into the pcDNA 3.1 vector (Invitrogen; Thermo Fisher
Scientific, Inc.). Empty pcDNA 3.1 vector (vector) was used as the
overexpression vector negative control. To complete short hairpin
(sh)RNA knockdown, synthesized shRNA oligonucleotide sequences of
FRZB (Shanghai Shenggong Biology Engineering Technology Service,
Ltd.) were subcloned into the lentiviral pSilencer 4.1 vector
backbone (Thermo Fisher Scientific, Inc.). The shRNA sequences for
FRZB were as follows:
3'-GGAGATTCTAAAGTCCTCTTTCAAGAGAAGAGGACTTTAGAATCTCC-5'. A scramble
shRNA was used as negative control: forward,
5'-AGGCGATTAAGTTGGGTA-3'; reverse, 5'-CGGTAGGCGTGTACGGTG-3'.
Plasmids were transfected into hBMSCs for 48 h at 37˚C by using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocols.
At 48 h post-transfection, the subsequent experiments were
performed.
The 3rd generation lentiviral system was used for
lentivirus packaging of sh-FRZB, including pSilencer
4.1-FRZB-shRNA, pCMV Delta R8.2 plasmid (Addgene, Inc.) and pCMV
VSVG plasmid (Addgene, Inc.). Transfection mix was prepared as
follows: Opti-MEM reduced serum medium (Thermo Fisher Scientific,
Inc.) 1 ml; pCMV delta R8.2, 2 µg; pCMV-VSV-G, 0.5 µg; pSilencer
4.1-FRZB-shRNA, 1.5 µg; Xtreme gene 9 transfection reagent
(MilliporeSigma), 12 µl. 2x106 HEK293T cells (ATCC) were
seeded in a 10 cm2 plate and cultured for 24 h at 37˚C
and 5% CO2. The medium was replaced with fresh DMEM.
After incubation for 20 min at 37˚C, the transfection mix was added
to the HEK293T cells and the cells were incubated for 48 h at 37˚C
and 5% CO2. Subsequently, the viral supernatant was
collected and filtered with a 0.45 µm PVDF filter (MilliporeSigma)
and then centrifuged for 15 min at 4,000 x g and 4˚C with Plus-20
centrifugal ultrafiltration (MilliporeSigma) to obtain a high-titer
lentivirus stock. The lentivirus without the transgene was used as
the negative control and was produced in the same manner. hBMSCs
were seeded at 1.0x105 cells per well in 24-well plates
in DMEM containing 10% FBS. After 24 h incubation at 37˚C, the
hBMSCs were transduced with or without lentivirus (MOI=20) and then
incubated for an additional 48 h at 37˚C. Following that, puromycin
(2 µg/ml) was added to hBMSCs and fresh media with puromycin was
added every two days until all hBMSCs not treated with any
lentivirus were dead. Successfully screened hBMSCs were used for
subsequent experiments. Successful transduction was demonstrated
through reverse transcription-quantitative (RT-q) PCR and western
blotting.
RT-qPCR
Expression of FRZB in 1x104 hBMSCs was
examined by RT-qPCR. Total RNA was extracted by the
TRIzol® reagent (Thermo Fisher Scientific, Inc.) from
the harvested hBMSCs. cDNA synthesis was accomplished using a
PrimeScript™ RT Master Mix (Takara Bio, Inc.) according to the
manufacturer's protocols. qPCR was performed using SYBR®
Premix EX Taq™ (Takara Bio, Inc.) according to the manufacturer's
protocols. The qPCR conditions were as follows: Initial
denaturation at 95˚C for 10 min, followed by 40 cycles of 95˚C for
30 sec, 60˚C for 30 sec and 72˚C for 30 sec. The set of primers for
FRZB were as follows: Forward, 5'-GAGGAGCTGCCAGTGTACGAC-3' and
Reverse, 5'-GAAAATCAGCTCCGTCCGC-3'; GAPDH: Forward,
5'-GGACCTGACCTGCCGTCTAG-3' and Reverse, 5'-GTAGCCCAGGATGCCCTTGA-3'
respectively. The 2-ΔΔCq (19) method was used to calculate the
relative mRNA expression level and GAPDH was used as an internal
parameter. The experiments were replicated three times.
Western blotting
Total protein was extracted using RIPA reagent and
then measured by a BCA Protein Assay kit (Beyotime Institute of
Biotechnology). The proteins (40 µg/lane) were separated by a 10%
SDS-PAGE gel before being blotted onto a PVDF membrane. Then, the
membranes were blocked with 5% skimmed milk powder in
phosphate-buffered saline solution with 0.05% Tween (PBST) for 1 h
at room temperature and incubated with the specific primary
antibody at 4˚C overnight. The primary antibodies used were
anti-FRZB (1:1,000; Abcam; ab273582), anti-β catenin (1:1,000;
Abcam; ab32572), anti-runt-related transcription factor 2 (RUNX2;
1:1,000; Abcam; ab236639), anti-alkaline phosphatase (ALP; 1:1,000;
Abcam; ab224335), anti-COL1A1 (1:1,000; Abcam; ab138492),
anti-osterix (Osx; 1:2500, Abcam; ab209484), anti-osteocalcin (OCN;
1:1,000; Abcam; ab133612) and anti-GAPDH (1:1,000; Abcam; ab8245).
The membranes were washed with PBST and incubated with
corresponding horseradish peroxidase-conjugated secondary
antibodies [Goat Anti-Rabbit IgG H&L (1:5,000; ab96899; Abcam)
or Goat Anti-Mouse IgG H&L (1:5,000; ab96879, Abcam)] for 30
min at 37˚C. Protein signals were visualized using enhanced
chemiluminescence reagent (Beyotime Institute of Biotechnology).
Densitometric analysis was performed using ImageJ software V1.52a
(National Institutes of Health) and values were normalized to
GAPDH.
Caspase-3 activity detection
As in a previous study (20), caspase-3 activity was assessed by
Caspase-3 Assay kit (MilliporeSigma) according to the
manufacturer's instructions. Briefly, cells in each group were
lysed on ice for 10 min with lysate (2x104 cells/µl),
then the supernatant was collected at 4˚C at 12,000 x g for 1 min.
Protein quantification was carried out through a BCA detection kit
(Beyotime Institute of Biotechnology). Supernatant (45 µl) was
mixed with 50 µl of 2X reaction buffer (provided in the kit) and 5
µl reaction substrate (Ac-DEVD-pNA; provided in the kit) before
incubating at 37˚C for 2 h. The free pNA was assessed at 450 nm
with microplate reader (Bio-Rad Laboratories, Inc.) and the
concentration of pNA was calculated through a standard curve
obtained from the detection of a series of known concentrations of
pNA. The number of nanomoles of pNA per 1 mg of total protein per
minute was used to represent caspase-3 activity. The experiment was
repeated three times in each group.
Alizarin red sulfate (ARS)
staining
ARS staining was conducted to analyze the
differentiation of hBMSCs. Briefly, after washing twice in PBS, the
cells were fixed with 95% ethanol for 10 min at room temperature.
The cells were then incubated with ARS staining buffer solution at
37˚C for 30 min. The mineralization nodules were imaged using an
optical light microscope (magnification, x200) in five randomly
selected fields of view. The darker the intensity of the red dots,
the higher the number of calcium nodules and therefore the higher
degree of differentiation. For semi-quantitative assessment of the
formation of mineralization nodules, 10% cetylpyridinium chloride
in 10 mM Na2HPO4 was added to the wells and
incubated for 10 min at room temperature. Then absorbance at 562 nm
was measured with a microplate reader.
Alkaline phosphatase (ALP) staining
and ALP activity
ALP, as a marker of osteoblast differentiation
(21), was assessed by an ALP
staining kit (Beijing Solarbio Science & Technology Co., Ltd.).
The cells were fixed for 10 min with 4% paraformaldehyde at room
temperature and then washed with distilled water. ALP staining
solution was added with and incubated for 20 min at 37˚C. After
washing with distilled water, the results were examined using an
optical light microscope (magnification, x200) in five randomly
selected fields of view. The darker intensity of the red dots was
associated with higher ALP activity.
ALP activity was evaluated using a commercial ALP
activity colorimetric kit (BioVision, Inc.). The cell cultures were
rinsed with pre-cooled PBS and treated with 1% Triton X-100
(MilliporeSigma), then transferred to distilled water. The
absorbance at 405 nm was determined with microplate reader (Bio-Rad
Laboratories, Inc.). Total protein concentration was detected by
BCA protein Assay kit (Beyotime Institute of Biotechnology). ALP
activity level was quantified by dividing the absorbance to the
protein concentration.
Cells activity
Activity of the hBMSCs was detected by Cell Counting
Kit-8 (MedChemExpress). hBMSCs were centrifuged at 1,000 x g for 5
min at 4˚C before being suspended in DMEM low-glucose medium
containing 10% FBS and counted. The cells were inoculated at
5x104 cells/well in 24-well plates; the volume of each
well was 1,000 µl. A total of five multiple wells were made for
each group and medium only wells were used as blank controls. PBS
was added to the surrounding wells to slow liquid evaporation
before adding 10 µl CCK-8 solution to each well 5 days post cell
inoculation. Cells were cultured at 37˚C, 5% CO2 for 2
h, followed by measuring the absorbance at 450 nm with a microplate
reader.
Statistical analysis
The data was analyzed using SPSS 18.0 software
(SPSS, Inc.) and the results were presented as mean ± standard
deviation. Unpaired t-test was used to compare two groups.
One-way ANOVA (followed by Tukey post hoc test) was used for
multiple comparisons. SPSS 18.0 software was used for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
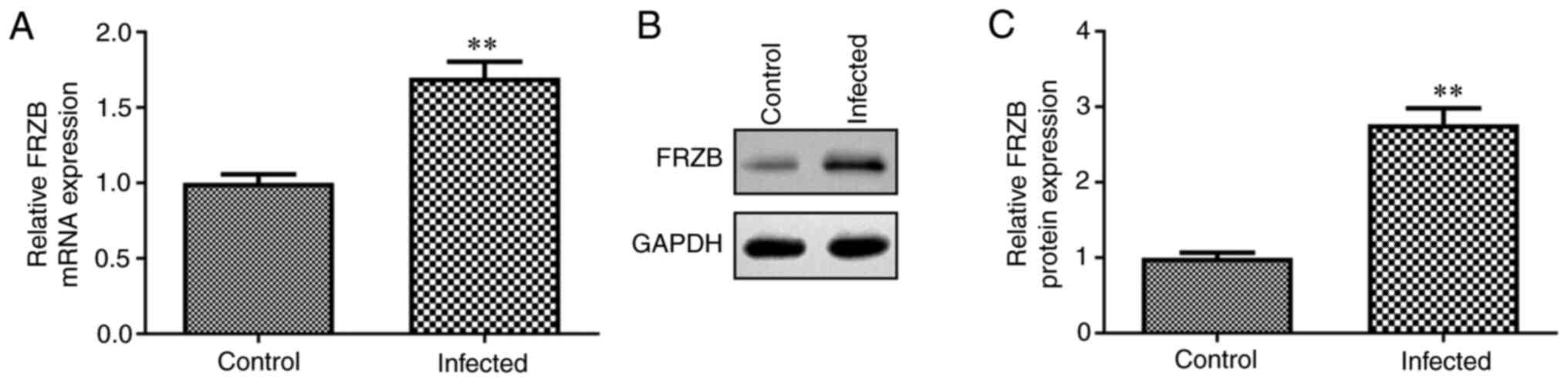
FRZB is highly expressed in S. aureus
infected hBMSCs
As FRZB is an essential factor contributing to the
osteoarthritis pathogenesis (22),
the role of FRZB in osteomyelitis was explored in the present
study. S. aureus infected hBMSCs were used as a model
of S. aureus-induced osteomyelitis in vitro
and the mRNA and protein expression of FRZB in S.
aureus infected hBMSCs was detected by RT-qPCR and western
blotting, respectively. It was found that transcription level and
protein expression level of FRZB in infected hBMSCs were all
significantly higher compared with those in control group (Fig. 1A-C). The results suggested that
S. aureus induced the upregulation of FRZB mRNA and
protein in hBMSCs.
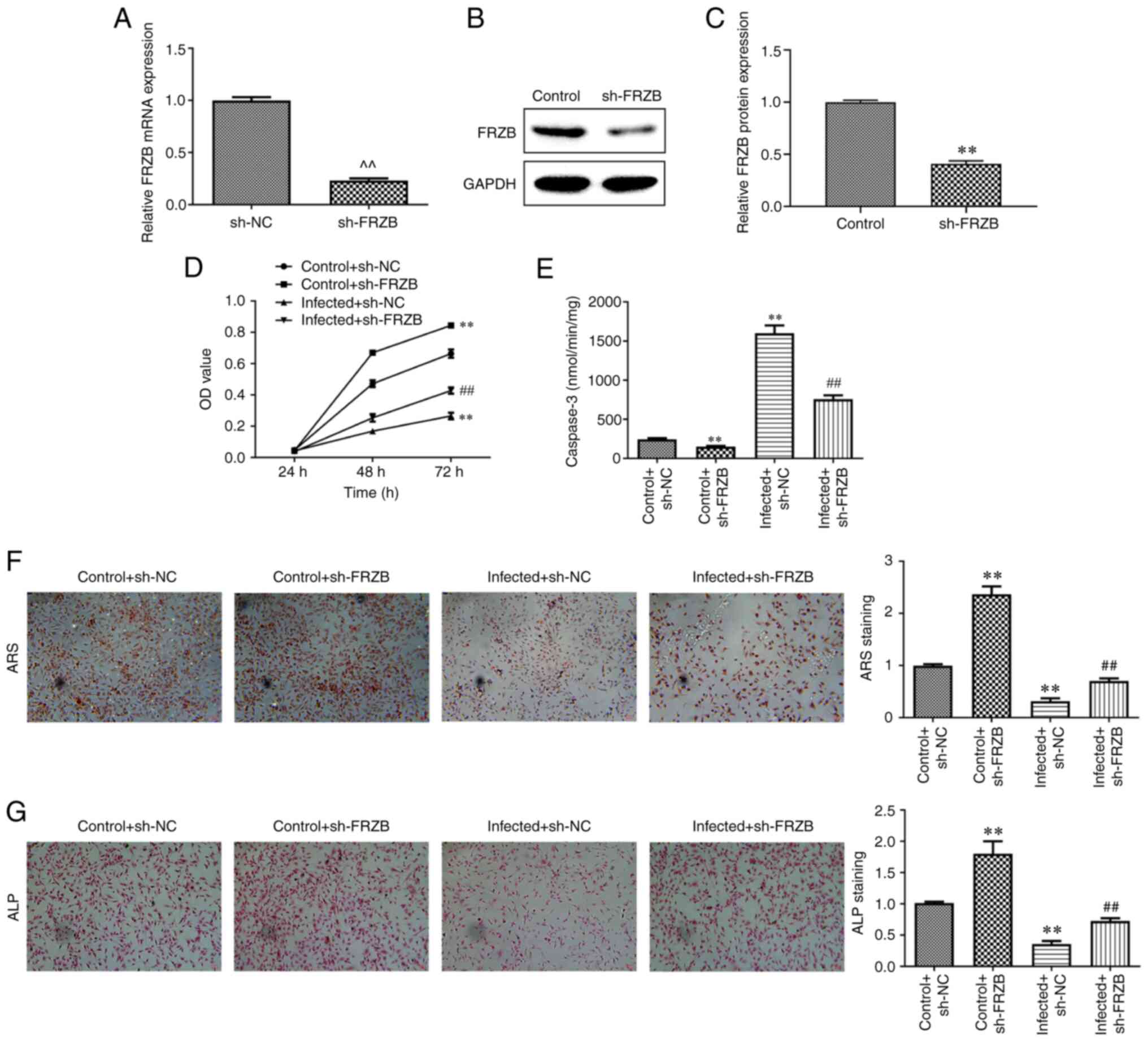
FRZB downregulation represses
osteomyelitis by reducing apoptosis and promoting differentiation
of hBMSCs
To study the FRZB-mediated effects in osteomyelitis,
shFRZB or sh-NC was transfected into hBMSCs. The results showed
that FRZB mRNA and protein expression was repressed in hBMSCs
transfected with shFRZB (Fig.
2A-C). Meanwhile, apart from the significant inhibition effects
caused by S. aureus, cell viability of either the
sh-FRZB group or the S. aureus + sh-FRZB group showed
an increase due to downregulation of FRZB (Fig. 2D). The results revealed a
significant decrease of apoptosis in sh-FRZB group compared with
control group and in S. aureus + sh-FRZB group
compared with S. aureus group (Fig. 2E). ARS and ALP staining results
showed that the sh-FRZB group and infected + sh-FRZB group
exhibited different promoting effects compared with the sh-NC group
and S. aureus infected sh-NC group, respectively
(Fig. 2F-G). Further investigation
into the pathways regulating cell differentiation using western
blotting indicated that β-catenin, RUNX2, ALP, COL1A1, Osx and OCN
allied to the Wnt/β-catenin signaling pathway all exhibited a
significant elevating in sh-FRZB group and S. aureus
+ sh-FRZB group compared with their control groups, severally
(Fig. 3A-H). These results
indicated that silencing of FRZB promoted cell viability and
osteogenic differentiation, while inhibiting apoptosis in S.
aureus infected hBMSCs.
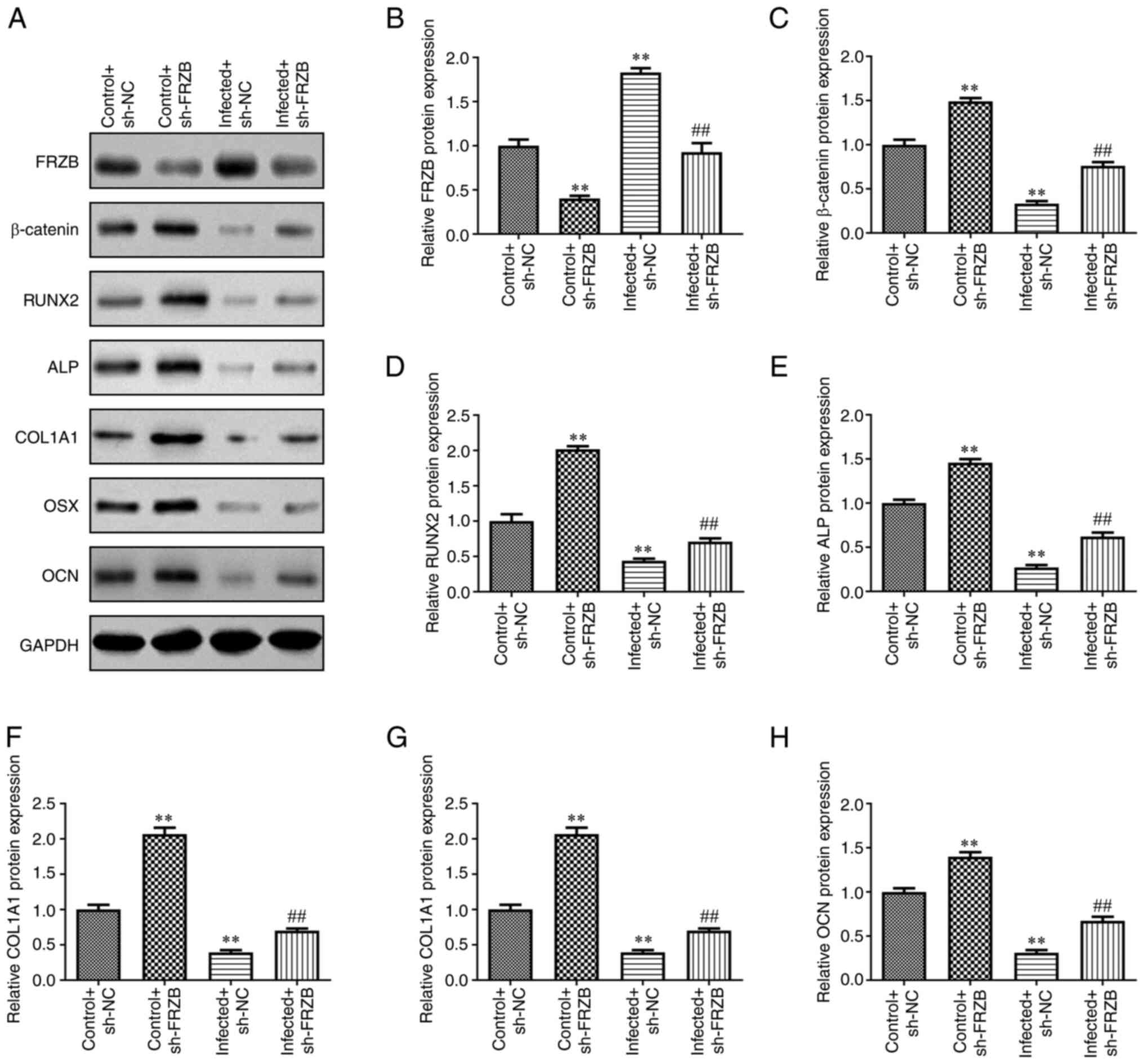 | Figure 3Downregulation of FRZB amplifies the
protein expression of osteoblast-specific markers. (A) The protein
expression of (B) FRZB, (C) β-catenin, (D) RUNX2, (E) ALP, (F)
COL1A1, (G) Osx and (H) OCN were measured by western blotting.
**P<0.01 vs. control + sh-NC group,
##P<0.01 vs. infected + sh-NC. FRZB, frizzled related
protein; RUNX2, runt-related transcription factor 2; ALP, alkaline
phosphatase; COL1A1, type I collagen; Osx, osterix; OCN,
osteocalcin; sh, short hairpin; NC, negative control. |
FRZB upregulation promotes
osteomyelitis by increasing hBMSCs apoptosis and reducing
differentiation
To identify the mechanism responsible for FRZB
mediation in osteomyelitis, the overexpression of FRZB in hBMSCs
was conducted through transfection of pcDNA-FRZB. The expression of
FRZB was upregulated at the mRNA and protein level as determined by
RT-qPCR (Fig. 4A-C). The effect of
transient overexpression of FRZB on cell viability was measured
using the CCK8 assay and the results showed that hBMSC viability of
both the OE-FRZB group and the S. aureus + OE-FRZB
group were reduced compared with their controls separately
(Fig. 4D). From cell apoptosis
assay, a significant enhancement of caspase-3 activity could be
observed corresponding to the FRZB upregulation (Fig. 4E). To further confirm the mode of
action of FRZB on cell differentiation, ARS and ALP staining were
conducted. Staining intensities of OE-FRZB group and the S.
aureus + OE-FRZB group were weaker than their separate
controls (Fig. 4F and G). The phenotypical quantification was
also supported by a reduction in osteogenic markers such as
β-catenin, RUNX2, ALP, COL1A1, Osx and OCN following FRZB
overexpression (Fig. 5A-H). These
results demonstrated that upregulation of FRZB repressed cell
viability and osteogenic differentiation while enhancing apoptosis
in S. aureus infected hBMSCs.
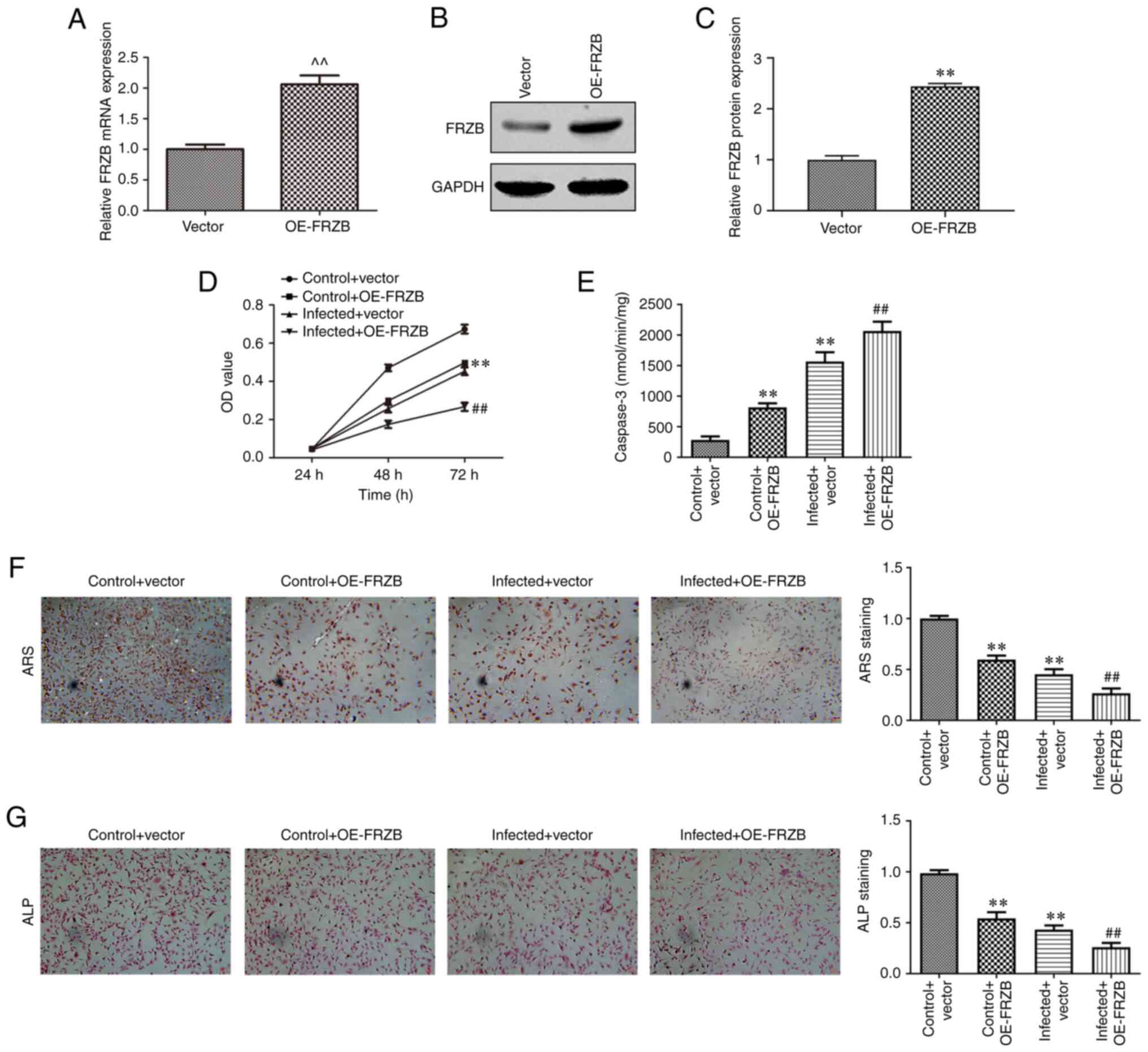 | Figure 4Upregulation of FRZB limits cell
viability and osteogenic differentiation and enhances apoptosis in
hBMSCs. (A) After transfection with pcDNA-FRZB or vector, the mRNA
expression of FRZB was measured by reverse
transcription-quantitative PCR. (B and C) the protein expression of
FRZB was measured by western blotting. (D) Cell viability was
assessed by CCK8 assay. (E) The apoptosis was detected by caspase-3
activity assay. Osteogenic differentiation was estimated by (F) ARS
(magnification, x200) and (G) ALP staining (magnification, x200).
^^P<0.01 vs. vector group, **P<0.01 vs.
control + vector group, ##P<0.01 vs. infected +
vector. FRZB, frizzled related protein; hBMSCs, human bone marrow
derived stem cells; OE, overexpression; ARS, Alizarin red sulfate;
ALP, alkaline phosphatase. |
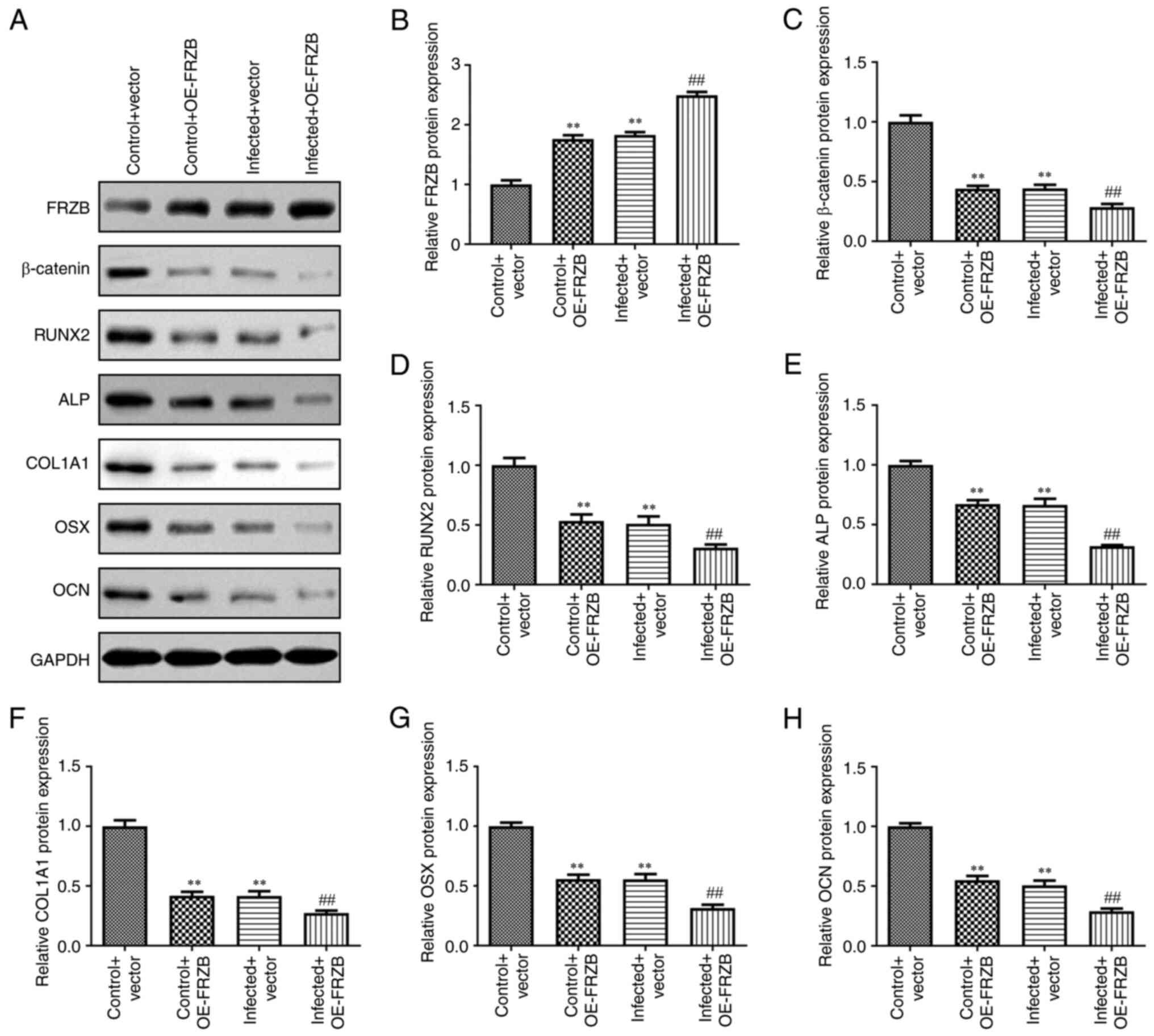 | Figure 5Upregulation of FRZB suppresses the
protein expression of osteoblast-specific markers. (A) The protein
expression of (B) FRZB, (C) β-catenin, (D) RUNX2, (E) ALP, (F)
COL1A1, (G) Osx and (H) OCN were measured by western blotting.
**P<0.01 vs. vector group, **P<0.01 vs.
control + vector group, ##P<0.01 vs. infected +
vector. FRZB, frizzled related protein; RUNX2, runt-related
transcription factor 2; ALP, alkaline phosphatase; COL1A1, type I
collagen; Osx, osterix; OCN, osteocalcin; OE, overexpression. |
FRZB downregulation inhibits
osteomyelitis by activating the Wnt/β-catenin signaling
pathway
To further investigate the function of Wnt/β-catenin
signaling pathway in the FRZB-mediated osteomyelitis inhibition,
hBMSCs were treated with ICRT3(23), an inhibitor of both Wnt and
β-catenin responsive transcription. CCK8 assay was conducted to
evaluate the effect of FRZB and the inhibitor to the activity of
hBMSCs and the observable promotion due to FRZB downregulation was
counteracted by the Wnt inhibitor in the normal hBMSCs group and
the S. aureus infected group (Fig. 6A). In the cell apoptosis detection
assay, activities of the caspase-3 for the sh-FRZB group and the
S. aureus + sh-FRZB were both markedly increased
while all raised to their comparative levels subsequently after
joining of the Wnt inhibitor (Fig.
6B). As for hBMSCs differentiation, staining intensities of ARS
and ALP staining all depicted a significant decrease attributed to
the effect of the Wnt inhibitor when compared with their FRZB
downregulation controls, respectively (Fig. 6C-E). These results showed that
activation of Wnt/β-catenin signaling pathway participated in the
effect of FRZB on hBMSCs induced by S. aureus.
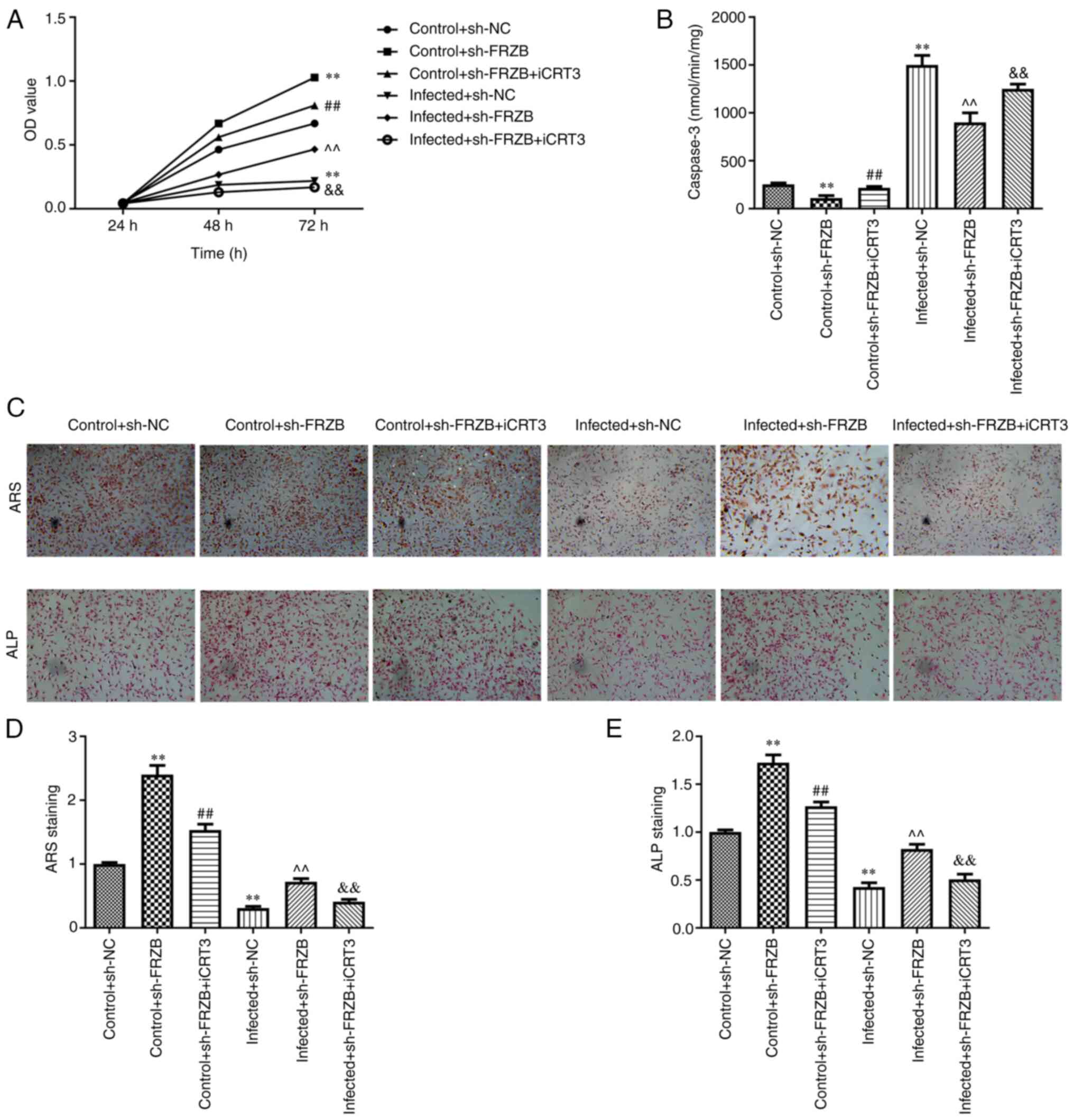 | Figure 6Silencing of FRZB represses
Staphylococcus aureus-induced osteomyelitis in hBMSCs by
regulating the Wnt/β-catenin pathway. (A) The cell viability was
assessed by CCK8 assay. (B) The apoptosis was detected by caspase-3
activity assay. (C-E) The osteogenic differentiation was estimated
by ARS staining (magnification, x200) and ALP staining
(magnification, x200). **P<0.01 vs. control + sh-NC
group, ##P<0.01 vs. control + sh-FRZB group,
^^P<0.01 vs. infected + sh-NC,
&&P<0.01 vs. infected + sh-FRZB group. FRZB,
frizzled related protein; hBMSCs, human bone marrow derived stem
cells; ARS, Alizarin red sulfate; ALP, alkaline phosphatase; sh,
short hairpin; NC, negative control; ICRT-3,
2-[[[2-(4-ethylphenyl)-5-methyl-4-oxazolyl]methyl]thio]-N-(2-phenylethyl)acetamide. |
Discussion
Bone marrow mesenchymal stem cells (BMSCs) have
osteogenic differentiation ability and serve an important role in
bone formation (18,24). Osteomyelitis usually occurs during
bone healing and is known to inhibit osteogenesis and bone
formation (25,26). However, the regulatory mechanism of
osteomyelitis on osteogenic differentiation of BMSCs remains to be
elucidated. In vivo, the inflammatory environment of
osteomyelitis is complex, so it is difficult to replicate the
inflammatory environment of osteomyelitis. S. aureus is the
most common bacterial species causing bone infection, causing 80%
of osteomyelitis secondary to bone infection (27). Previous studies have shown that
S. aureus can induce inflammation in vitro (28,29).
In the present study, S. aureus was selected to trigger the
inflammatory environment in hBMSCs to build a cell model that could
partially replicate the inflammatory environment of osteomyelitis
in vitro. The present study then investigated the role of
FRZB in hBMSCs infected by S. aureus and confirmed that FRZB
participated in the regulation of bone differentiation of hBMSCs
infected by S. aureus by affecting Wnt/β-catenin signaling
pathway.
In previous studies, FRZB has been implicated in a
range of developmental processes and diseases (30-32).
FRZB serves as a secreted Wnt antagonist that can decrease growth
and invasiveness of fibrosarcoma cells (33). In addition, FRZB expression level
also shows a deep relationship with the Wnt/β-catenin pathway in
gastric cancer (11,34). However, the correlation between
FRZB and osteomyelitis, along with the possible relevant regulatory
mechanisms therein, remain to be elucidated. The present study
found that FRZB was upregulated in hBMSCs with S. aureus
infection. To investigate the function of FRZB, FRZB expression was
firstly downregulated using shRNA. The role of FRZB on osteogenic
differentiation of hBMSCs treated with S. aureus was then
assessed. Based on these results, FRZB silencing ameliorated S.
aureus-inhibited proliferation and osteogenic differentiation
in hBMSCs. It has been reported that FRZB antagonizes Wnt signaling
by binding to the Wnt ligands and induces signal transduction
(10). This initiates the
transcription of osteogenic canonical transducers, such as Runx2
and β-catenin, which further regulate cell proliferation and
differentiation (35,36). As the results of the present study
showed, downregulation of FRZB in S. aureus-infected BMSCs
could amplify the expression of β-catenin, RUNX2, ALP, COL1A1, OSC
and OCN, considered as markers of osteogenesis and bone formation
(37). Hence, a role of FRZB in
either direct or indirect regulation of the osteomyelitis through
inhibiting apoptosis and facilitating differentiation can be
presumed in hBMSCs.
As well as the potential salutary effects of the
FRZB downregulation, FRZB was overexpressed in hBMSCs to further
affirm its functional role. The results indicated that FRZB
expression effectively activated caspase-3 and inhibited osteogenic
differentiation in S. aureus-infected BMSCs. FRZB also acted
as a major suppressor to the various transducers in Wnt signaling
pathway. Thus, FRZB was confirmed to be a key factor affecting
osteomyelitis and it can be hypothesized that the regulation center
was located in Wnt signaling pathway. To verify this hypothesis, an
inhibitor (ICRT-3) targeting the Wnt/β-catenin signaling pathway
was used to block the crosstalk between Wnt and other transducers.
FRZB cannot validly bind to the receptors and cause the inhibition
of β-catenin nuclear transcription (38,39).
With Wnt inhibition, the elevation in cell activity, drive to
differentiation and apoptosis resistance caused by downregulation
of FRZB were all eliminated almost completely. Previous studies
show that aberrant changes in FRZB expression are associated with
pathophysiological states including osteoarthritis and cancer
(attenuated expression) (40,41)
and limb-girdle muscular dystrophy (increased expression) (42). Combined with the findings of the
present study, it could be concluded that silencing of FRZB may
inhibit osteomyelitis under the mediation of Wnt/β-catenin
signaling pathway.
However, S. aureus-induced
osteomyelitis is associated with multiple signaling pathways
besides the Wnt/β-catenin, such as the NF-κB (43), SMAD (44), MyD88 and IL-1R signaling pathways
(45). Whether FRZB participates
in the regulation of osteomyelitis by mediating other signaling
pathways needs further research. Meanwhile, lack of clinical
samples and in vivo experimental data are two limitations to
the present study. In addition, the effects of ICRT-3 alone on
untransfected control and infected cells were not assessed in the
present study, which was a limitation in experimental grouping.
Those limitations will be studied in future work.
The present study thereby proposed a novel
controlling gene involving FRZB in the pathogenesis of
osteomyelitis, whereby during S. aureus infection, FRZB
inhibited the Wnt/β-catenin signaling pathway, which in turn
reduced osteogenic differentiation of hBMSCs, contributing to
osteomyelitis. FRZB may also serve as therapeutic targets for
treatment against osteomyelitis.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Guizhou Provincial
Science and Technology Projects (grant no. [2021]008), the Guizhou
Provincial Science and Technology Projects (grant no. [2019]1210),
and the Guizhou Health Commission Science and Technology Projects
(grant no. GZWKJ2021-253).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and XL conceived and designed the present study
and wrote the main manuscript text. XL and WP performed the
experiments and data acquisition. HF and HW analyzed and
interpreted the data and performed literature searches. HF and HW
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cui Y, Lu S, Tan H, Li J, Zhu M and Xu Y:
Silencing of long non-coding RNA NONHSAT009968 ameliorates the
staphylococcal protein A-Inhibited osteogenic differentiation in
human bone mesenchymal stem cells. Cell Physiol Biochem.
39:1347–1359. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Josse J, Velard F and Gangloff SC:
Staphylococcus aureus vs. Osteoblast: Relationship and consequences
in osteomyelitis. Front Cell Infect Microbiol. 5(85)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fantoni M, Taccari F and Giovannenze F:
Systemic antibiotic treatment of chronic osteomyelitis in adults.
Eur Rev Med Pharmacol Sci. 23 (2 Suppl):S258–S270. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sanchez CJ Jr, Ward CL, Romano DR, Hurtgen
BJ, Hardy SK, Woodbury RL, Trevino AV, Rathbone CR and Wenke JC:
Staphylococcus aureus biofilms decrease osteoblast viability,
inhibits osteogenic differentiation, and increases bone resorption
in vitro. BMC Musculoskelet Disord. 14(187)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu W, Konermann A, Guo T, Jäger A, Zhang
L and Jin Y: Canonical Wnt signaling differently modulates
osteogenic differentiation of mesenchymal stem cells derived from
bone marrow and from periodontal ligament under inflammatory
conditions. Biochim Biophys Acta. 1840:1125–1134. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang Y, Li YP, Paulson C, Shao JZ, Zhang
X, Wu M and Chen W: Wnt and the Wnt signaling pathway in bone
development and disease. Front Biosci (Landmark Ed). 19:379–407.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Qin X, Jiang Q, Komori H, Sakane C,
Fukuyama R, Matsuo Y, Ito K, Miyazaki T and Komori T: Runt-related
transcription factor-2 (Runx2) is required for bone matrix protein
gene expression in committed osteoblasts in mice. J Bone Miner Res.
36:2081–2095. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kawaguchi H: Regulation of osteoarthritis
development by Wnt-beta-catenin signaling through the endochondral
ossification process. J Bone Miner Res. 24:8–11. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang H, Sun W, Ma J, Pan Y, Wang L and
Zhang WB: Biglycan mediates suture expansion osteogenesis via
potentiation of Wnt/β-catenin signaling. J Biomech. 48:432–440.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kwan T, Kazamel M, Thoenes K, Si Y, Jiang
N and King PH: Wnt antagonist FRZB is a muscle biomarker of
denervation atrophy in amyotrophic lateral sclerosis. Sci Rep.
10(16679)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qin S, Zhang Z, Li J and Zang L: FRZB
knockdown upregulates β-catenin activity and enhances cell
aggressiveness in gastric cancer. Oncol Rep. 31:2351–2357.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fernández-Torres J, Zamudio-Cuevas Y,
López-Reyes A, Garrido-Rodríguez D, Martínez-Flores K, Lozada CA,
Muñóz-Valle JF, Oregon-Romero E and Martínez-Nava GA: Gene-gene
interactions of the Wnt/β-catenin signaling pathway in knee
osteoarthritis. Mol Biol Rep. 45:1089–1098. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ma Z, Wang X, Lv Q, Gong Y, Xia M, Zhuang
L, Lu X, Yang Y, Zhang W, Fu G, et al: Identification of underlying
hub genes associated with hypertrophic cardiomyopathy by integrated
bioinformatics analysis. Pharmgenomics Pers Med. 14:823–837.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang J, Hu W, Lin X, Wang X and Jin K:
FRZB up-regulated in hepatocellular carcinoma bone metastasis. Int
J Clin Exp Pathol. 8:13353–13359, eCollection 2015. 2015.PubMed/NCBI
|
|
15
|
Killock D: Osteoarthritis: Frzb knockout
reveals the complexity of Wnt signaling in joint homeostasis. Nat
Rev Rheumatol. 8(123)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Corallini F, Secchiero P, Castellino G,
Montecucco M, Trotta F and Zauli G: Circulating levels of
frizzled-related protein (FRZB) are increased in patients with
early rheumatoid arthritis and decrease in response to
disease-modifying antirheumatic drugs. Ann Rheum Dis. 69:1733–1734.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li H, Zhang S, Nie B, Long T, Qu X and Yue
B: KR-12-a5 reverses adverse effects of lipopolysaccharides on
HBMSC osteogenic differentiation by influencing BMP/Smad and P38
MAPK signaling pathways. Front Pharmacol. 10(639)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Y, Zhang X, Shao J, Liu H, Liu X and
Luo E: Adiponectin regulates BMSC osteogenic differentiation and
osteogenesis through the Wnt/β-catenin pathway. Sci Rep.
7(3652)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Guo C, Wang SL, Xu ST, Wang JG and Song
GH: SP600125 reduces lipopolysaccharide-induced apoptosis and
restores the early-stage differentiation of osteoblasts inhibited
by LPS through the MAPK pathway in MC3T3-E1 cells. Int J Mol Med.
35:1427–1434. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jin Q, Yuan K, Lin W, Niu C, Ma R and
Huang Z: Comparative characterization of mesenchymal stem cells
from human dental pulp and adipose tissue for bone regeneration
potential. Artif Cells Nanomed Biotechnol. 47:1577–1584.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lories RJ, Peeters J, Bakker A,
Tylzanowski P, Derese I, Schrooten J, Thomas JT and Luyten FP:
Articular cartilage and biomechanical properties of the long bones
in Frzb-knockout mice. Arthritis Rheum. 56:4095–4103.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xiao H, Zeng Y, Wang Q, Wei S and Zhu X: A
novel positive feedback loop between NTSR1 and Wnt/β-Catenin
contributes to tumor growth of glioblastoma. Cell Physiol Biochem.
43:2133–2142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li F, Wang X and Niyibizi C: Bone marrow
stromal cells contribute to bone formation following infusion into
femoral cavities of a mouse model of osteogenesis imperfecta. Bone.
47:546–555. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jin T, Zhu YL, Li J, Shi J, He XQ, Ding J
and Xu YQ: Staphylococcal protein A, Panton-Valentine leukocidin
and coagulase aggravate the bone loss and bone destruction in
osteomyelitis. Cell Physiol Biochem. 32:322–333. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Beck-Broichsitter BE, Smeets R and Heiland
M: Current concepts in pathogenesis of acute and chronic
osteomyelitis. Curr Opin Infect Dis. 28:240–245. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nasser A, Azimi T and Ostadmohammadi S and
Ostadmohammadi S: A comprehensive review of bacterial osteomyelitis
with emphasis on Staphylococcus aureus. Microb Pathog.
148(104431)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dubus M, Varin J, Papa S, Chevrier J,
Quilès F, Francius G, Audonnet S, Mauprivez C, Gangloff SC, Siboni
R, et al: Bone marrow mesenchymal stem cells offer an
immune-privileged niche to Cutibacterium acnes in case of
implant-associated osteomyelitis. Acta Biomater. 137:305–315.
2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ding R, Wei S and Huang M: Long non-coding
RNA KCNQ1OT1 overexpression promotes osteogenic differentiation of
staphylococcus aureus-infected human bone mesenchymal stem cells by
sponging microRNA miR-29b-3p. Bioengineered. 13:5855–5867.
2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Oh CK, Ko Y, Park JJ, Heo HJ, Kang J, Kwon
EJ, Kang JW, Lee Y, Myung K, Kang JM, et al: FRZB as a key molecule
in abdominal aortic aneurysm progression affecting vascular
integrity. Biosci Rep. 41(BSR20203204)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Thysen S, Luyten FP and Lories RJ: Loss of
Frzb and Sfrp1 differentially affects joint homeostasis in
instability-induced osteoarthritis. Osteoarthritis Cartilage.
23:275–279. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Person AD, Garriock RJ, Krieg PA, Runyan
RB and Klewer SE: Frzb modulates Wnt-9a-mediated beta-catenin
signaling during avian atrioventricular cardiac cushion
development. Dev Biol. 278:35–48. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Guo Y, Xie J, Rubin E, Tang YX, Lin F, Zi
X and Hoang BH: Frzb, a secreted Wnt antagonist, decreases growth
and invasiveness of fibrosarcoma cells associated with inhibition
of Met signaling. Cancer Res. 68:3350–3360. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shi Q, Zhou C, Xie R, Li M, Shen P, Lu Y
and Ma S: CircCNIH4 inhibits gastric cancer progression via
regulating DKK2 and FRZB expression and Wnt/β-catenin pathway. J
Biol Res (Thessalon). 28(19)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim HY, Choi S, Yoon JH, Lim HJ, Lee H,
Choi J, Ro EJ, Heo JN, Lee W, No KT and Choi KY: Small molecule
inhibitors of the Dishevelled-CXXC5 interaction are new drug
candidates for bone anabolic osteoporosis therapy. EMBO Mol Med.
8:375–387. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zuo GL, Zhang LF, Qi J, Kang H, Jia P,
Chen H, Shen X, Guo L, Zhou HB, Wang JS, et al: Activation of HIFa
pathway in mature osteoblasts disrupts the integrity of the
osteocyte/canalicular network. PLoS One.
10(e0121266)2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tang CY, Chen W, Luo Y, Wu J, Zhang Y,
McVicar A, McConnell M, Liu Y, Zhou HD and Li YP: Runx1
up-regulates chondrocyte to osteoblast lineage commitment and
promotes bone formation by enhancing both chondrogenesis and
osteogenesis. Biochem J. 477:2421–2438. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Goldring SR: The osteocyte: Key player in
regulating bone turnover. RMD Open. 1(Suppl
1)(e000049)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Matsumoto Y, La Rose J, Lim M, Adissu HA,
Law N, Mao X, Cong F, Mera P, Karsenty G, Goltzman D, et al:
Ubiquitin ligase RNF146 coordinates bone dynamics and energy
metabolism. J Clin Invest. 127:2612–2625. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bovolenta P, Esteve P, Ruiz JM, Cisneros E
and Lopez-Rios J: Beyond Wnt inhibition: New functions of secreted
Frizzled-related proteins in development and disease. J Cell Sci.
121(Pt 6):737–746. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lories RJ, Corr M and Lane NE: To Wnt or
not to Wnt: The bone and joint health dilemma. Nat Rev Rheumatol.
9:328–339. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sáenz A, Azpitarte M, Armañanzas R,
Leturcq F, Alzualde A, Inza I, García-Bragado F, De la Herran G,
Corcuera J, Cabello A, et al: Gene expression profiling in
limb-girdle muscular dystrophy 2A. PLoS One.
3(e3750)2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ren LR, Wang H, He XQ, Song MG, Chen XQ
and Xu YQ: Staphylococcus aureus Protein A induces
osteoclastogenesis via the NF-κB signaling pathway. Mol Med Rep.
16:6020–6028. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Song B, Estrada KD and Lyons KM: Smad
signaling in skeletal development and regeneration. Cytokine Growth
Factor Rev. 20:379–388. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Putnam NE, Fulbright LE, Curry JM, Ford
CA, Petronglo JR, Hendrix AS and Cassat JE: MyD88 and IL-1R
signaling drive antibacterial immunity and osteoclast-driven bone
loss during Staphylococcus aureus osteomyelitis. PLoS Pathog.
15(e1007744)2019.PubMed/NCBI View Article : Google Scholar
|