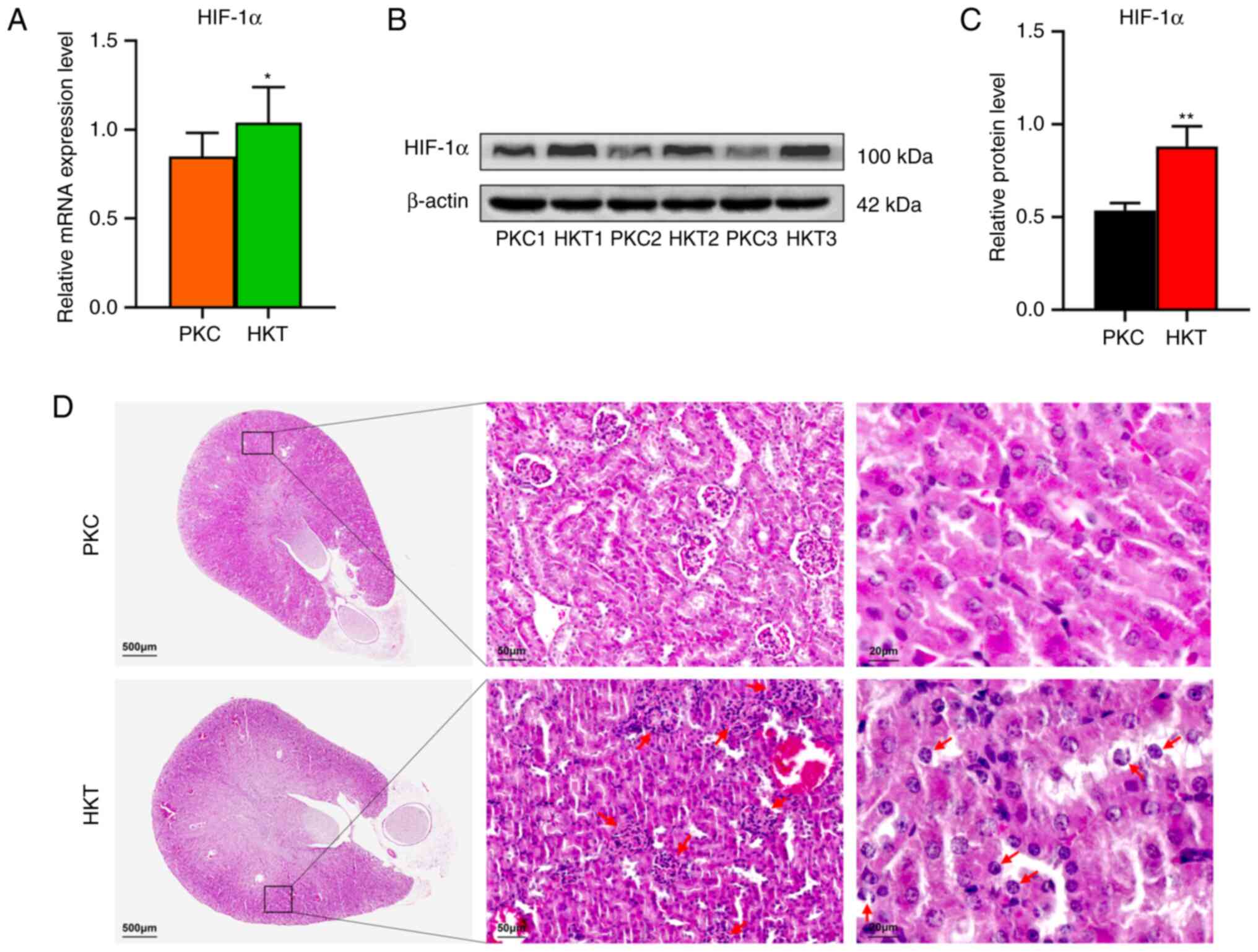
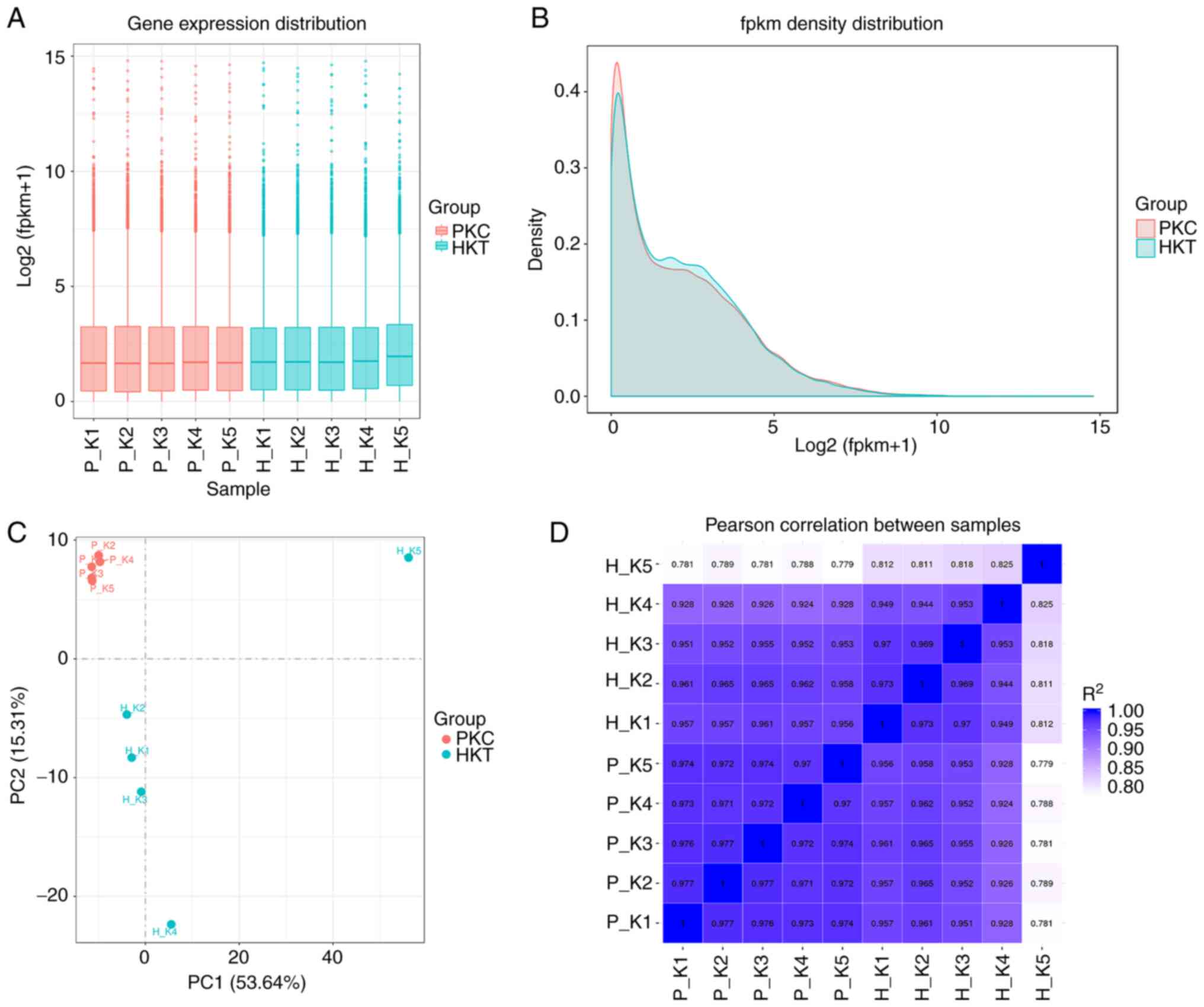
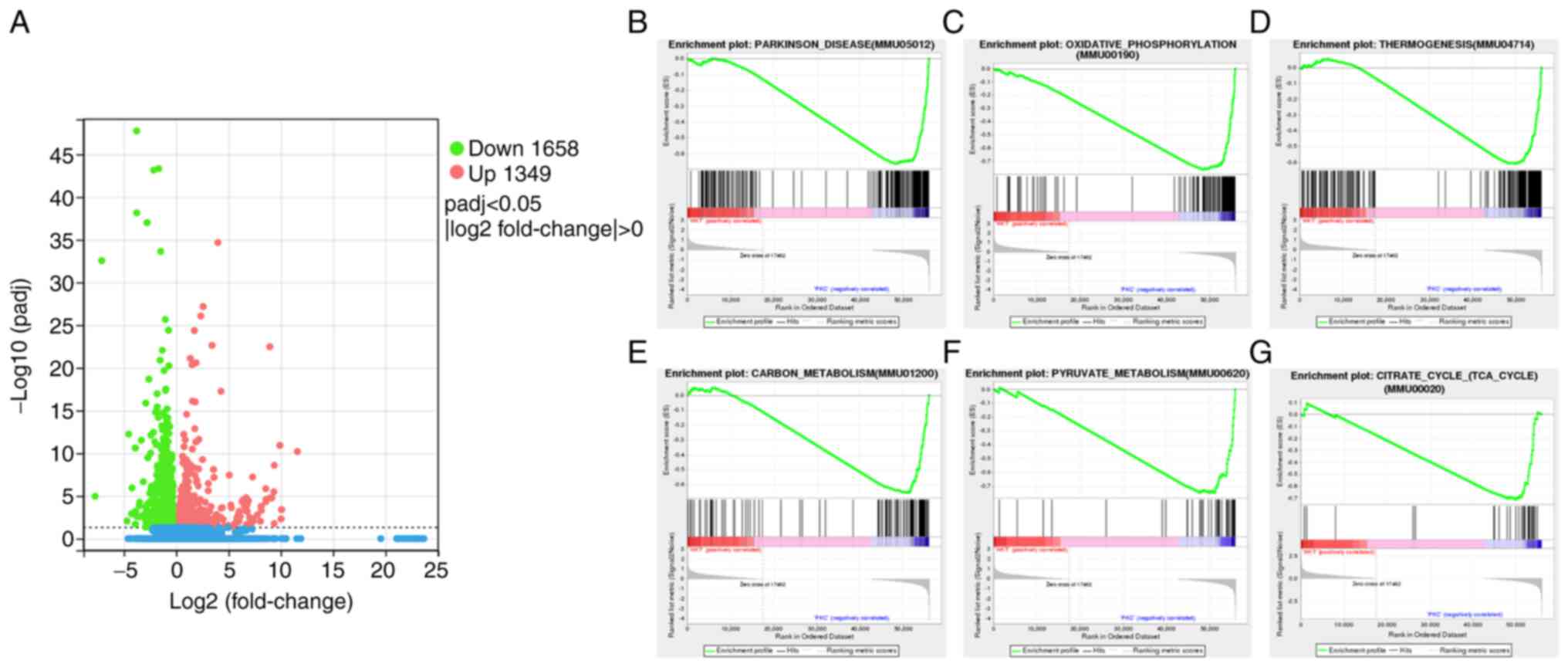
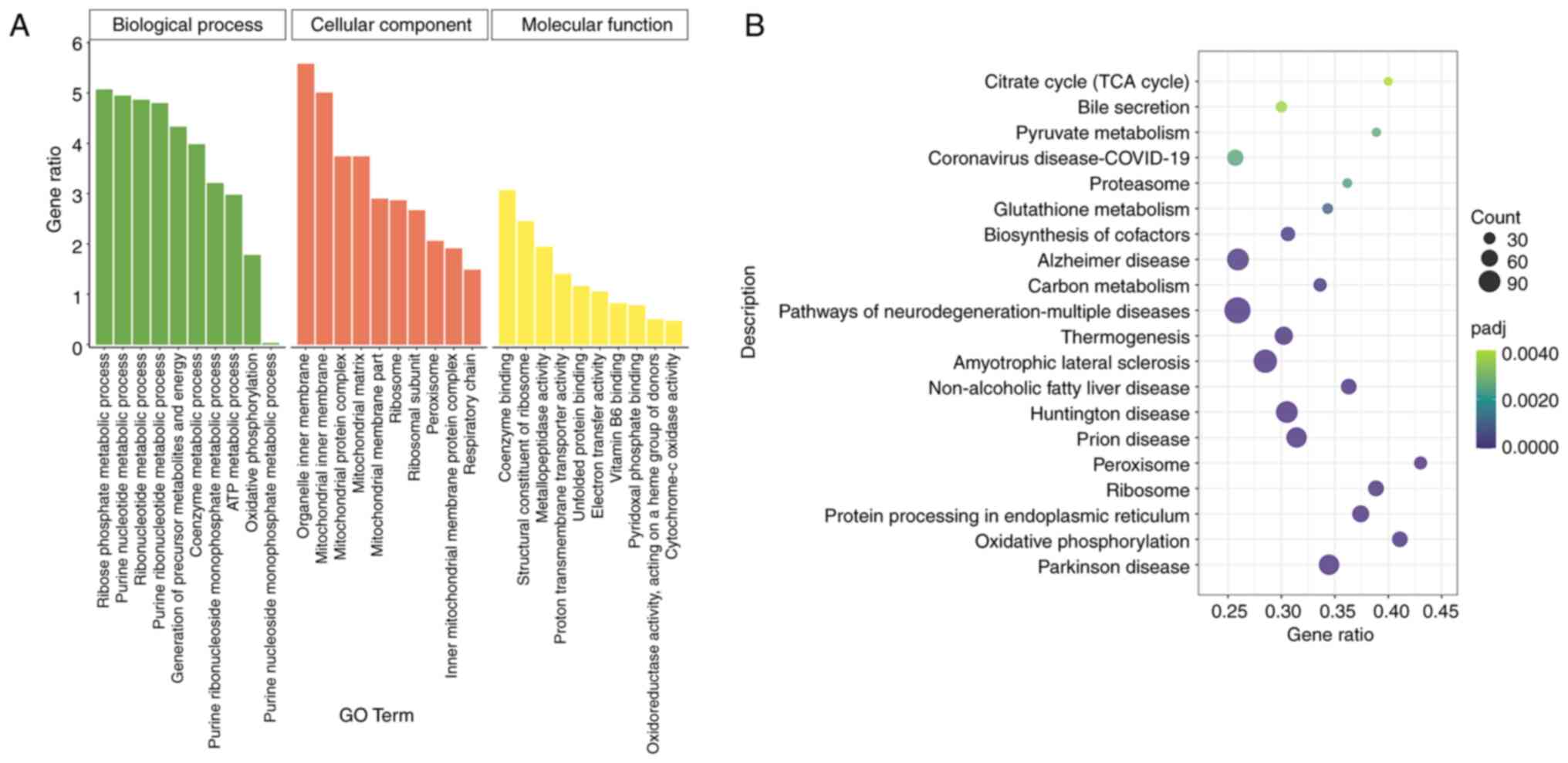
|
1
|
Li Y and Zhang Y and Zhang Y: Research
advances in pathogenesis and prophylactic measures of acute high
altitude illness. Respir Med. 145:145–152. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gaur P, Prasad S, Kumar B, Sharma SK and
Vats P: High-altitude hypoxia induced reactive oxygen species
generation, signaling, and mitigation approaches. Int J
Biometeorol. 65:601–615. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang S and Lian G: ROS and diseases: Role
in metabolism and energy supply. Mol Cell Biochem. 467:1–12.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wilkins MR, Ghofrani HA, Weissmann N,
Aldashev A and Zhao L: Pathophysiology and treatment of
high-altitude pulmonary vascular disease. Circulation. 131:582–590.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Clark AJ and Parikh SM: Mitochondrial
metabolism in acute kidney injury. Semin Nephrol. 40:101–113.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Infantino V, Santarsiero A, Convertini P,
Todisco S and Iacobazzi V: Cancer cell metabolism in hypoxia: Role
of HIF-1 as key regulator and therapeutic target. Int J Mol Sci.
22(5703)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fuhrmann DC and Brüne B: Mitochondrial
composition and function under the control of hypoxia. Redox Biol.
12:208–215. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu X, Du H, Sun Y and Shao L: Role of
abnormal energy metabolism in the progression of chronic kidney
disease and drug intervention. Ren Fail. 44:790–805.
2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cadenas S: Mitochondrial uncoupling, ROS
generation and cardioprotection. Biochim Biophys Acta Bioenerg.
1859:940–950. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Han Y, Xu X, Tang C, Gao P, Chen X, Xiong
X, Yang M, Yang S, Zhu X, Yuan S, et al: Reactive oxygen species
promote tubular injury in diabetic nephropathy: The role of the
mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol.
16:32–46. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Palomba H, Castro I, Yu L and Burdmann EA:
The duration of acute kidney injury after cardiac surgery increases
the risk of long-term chronic kidney disease. J Nephrol.
30:567–572. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wei J, Zhang J, Wang L, Jiang S, Fu L,
Buggs J and Liu R: New mouse model of chronic kidney disease
transitioned from ischemic acute kidney injury. Am J Physiol Renal
Physiol. 317:F286–F295. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang X, Agborbesong E and Li X: The role
of mitochondria in acute kidney injury and chronic kidney disease
and its therapeutic potential. Int J Mol Sci.
22(11253)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang M, Bai M, Lei J, Xie Y, Xu S, Jia Z
and Zhang A: Mitochondrial dysfunction and the AKI-to-CKD
transition. Am J Physiol Renal Physiol. 319:F1105–F1116.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kang W, Suzuki M, Saito T and Miyado K:
Emerging role of TCA cycle-related enzymes in human diseases. Int J
Mol Sci. 22(13057)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jourdain AA, Begg BE, Mick E, Shah H,
Calvo SE, Skinner OS, Sharma R, Blue SM, Yeo GW, Burge CB and
Mootha VK: Loss of LUC7L2 and U1 snRNP subunits shifts energy
metabolism from glycolysis to OXPHOS. Mol Cell. 81:1905–1919.e12.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fuller GG and Kim JK: Compartmentalization
and metabolic regulation of glycolysis. J Cell Sci.
134(jcs258469)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15(550)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huss JM and Kelly DP: Nuclear receptor
signaling and cardiac energetics. Circ Res. 95:568–578.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang Q, Luo P, Chen J, Yang C, Xia F,
Zhang J, Tang H, Liu D, Gu L, Shi Q, et al: Dissection of targeting
molecular mechanisms of aristolochic acid-induced nephrotoxicity
via a combined deconvolution strategy of chemoproteomics and
metabolomics. Int J Biol Sci. 18:2003–2017. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kanehisa M: Toward understanding the
origin and evolution of cellular organisms. Protein Sci.
28:1947–1951. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kanehisa M, Furumichi M, Sato Y, Kawashima
M and Ishiguro-Watanabe M: KEGG for taxonomy-based analysis of
pathways and genomes. Nucleic Acids Res. 51:D587–D592.
2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nissanka N and Moraes CT: Mitochondrial
DNA damage and reactive oxygen species in neurodegenerative
disease. FEBS Lett. 592:728–742. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kan C, Ungelenk L, Lupp A, Dirsch O and
Dahmen U: Ischemia-reperfusion injury in aged livers-the energy
metabolism, inflammatory response, and autophagy. Transplantation.
102:368–377. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vallée A, Guillevin R and Vallée JN:
Vasculogenesis and angiogenesis initiation under normoxic
conditions through Wnt/β-catenin pathway in gliomas. Rev Neurosci.
29:71–91. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
May JL, Kouri FM, Hurley LA, Liu J,
Tommasini-Ghelfi S, Ji Y, Gao P, Calvert AE, Lee A, Chandel NS, et
al: IDH3α regulates one-carbon metabolism in glioblastoma. Sci Adv.
5(eaat0456)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Findlay AS, Carter RN, Starbuck B, McKie
L, Nováková K, Budd PS, Keighren MA, Marsh JA, Cross SH, Simon MM,
et al: Mouse Idh3a mutations cause retinal degeneration and reduced
mitochondrial function. Dis Model Mech.
11(dmm036426)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lambeth DO, Tews KN, Adkins S, Frohlich D
and Milavetz BI: Expression of two succinyl-CoA synthetases with
different nucleotide specificities in mammalian tissues. J Biol
Chem. 279:36621–36624. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Donti TR, Stromberger C, Ge M, Eldin KW,
Craigen WJ and Graham BH: Screen for abnormal mitochondrial
phenotypes in mouse embryonic stem cells identifies a model for
succinyl-CoA ligase deficiency and mtDNA depletion. Dis Model Mech.
7:271–280. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kohno S, Linn P, Nagatani N, Watanabe Y,
Kumar S, Soga T and Takahashi C: Pharmacologically targetable
vulnerability in prostate cancer carrying RB1-SUCLA2 deletion.
Oncogene. 39:5690–5707. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shimozawa Y, Himiyama T, Nakamura T and
Nishiya Y: Structural analysis and reaction mechanism of malate
dehydrogenase from Geobacillus stearothermophilus. J Biochem.
170:97–105. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gray LR, Tompkins SC and Taylor EB:
Regulation of pyruvate metabolism and human disease. Cell Mol Life
Sci. 71:2577–2604. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Laemmle A, Steck AL, Schaller A, Kurth S,
Perret Hoigné E, Felser AD, Slavova N, Salvisberg C, Atencio M,
Mochel F, et al: Triheptanoin-novel therapeutic approach for the
ultra-rare disease mitochondrial malate dehydrogenase deficiency.
Mol Genet Metab Rep. 29(100814)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bergman O and Ben-Shachar D: Mitochondrial
oxidative phosphorylation system (OXPHOS) deficits in
schizophrenia: Possible interactions with cellular processes. Can J
Psychiatry. 61:457–469. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Haapanen O, Reidelbach M and Sharma V:
Coupling of quinone dynamics to proton pumping in respiratory
complex I. Biochim Biophys Acta Bioenerg.
1861(148287)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Grivennikova VG, Gladyshev GV and
Vinogradov AD: Deactivation of mitochondrial NADH:ubiquinone
oxidoreductase (respiratory complex I): Extrinsically affecting
factors. Biochim Biophys Acta Bioenerg. 1861(148207)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhu J, Vinothkumar KR and Hirst J:
Structure of mammalian respiratory complex I. Nature. 536:354–358.
2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rak M and Rustin P: Supernumerary subunits
NDUFA3, NDUFA5 and NDUFA12 are required for the formation of the
extramembrane arm of human mitochondrial complex I. FEBS Lett.
588:1832–1838. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mimaki M, Wang X, McKenzie M, Thorburn DR
and Ryan MT: Understanding mitochondrial complex I assembly in
health and disease. Biochim Biophys Acta. 1817:851–862.
2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen Q, Thompson J, Hu Y, Dean J and
Lesnefsky EJ: Inhibition of the ubiquitous calpains protects
complex I activity and enables improved mitophagy in the heart
following ischemia-reperfusion. Am J Physiol Cell Physiol.
317:C910–C921. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Heidari E, Rasoulinezhad M, Pak N, Reza
Ashrafi M, Heidari M, Banwell B, Garshasbi M and Reza Tavasoli A:
Defective complex III mitochondrial respiratory chain due to a
novel variant in CYC1 gene masquerades acute demyelinating syndrome
or leber hereditary optic neuropathy. Mitochondrion. 60:12–20.
2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sánchez E, Lobo T, Fox JL, Zeviani M,
Winge DR and Fernández-Vizarra E: LYRM7/MZM1L is a UQCRFS1
chaperone involved in the last steps of mitochondrial complex III
assembly in human cells. Biochim Biophys Acta. 1827:285–293.
2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gusic M, Schottmann G, Feichtinger RG, Du
C, Scholz C, Wagner M, Mayr JA, Lee CY, Yépez VA, Lorenz N, et al:
Bi-allelic UQCRFS1 variants are associated with mitochondrial
complex III deficiency, cardiomyopathy, and alopecia totalis. Am J
Hum Genet. 106:102–111. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang Q, Li M, Gan Y, Jiang S, Qiao J,
Zhang W, Fan Y, Shen Y, Song Y, Meng Z, et al: Mitochondrial
protein UQCRC1 is oncogenic and a potential therapeutic target for
pancreatic cancer. Theranostics. 10:2141–2157. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zeng J, Tao J, Xi L, Wang Z and Liu L:
PCSK9 mediates the oxidative low-density lipoprotein-induced
pyroptosis of vascular endothelial cells via the UQCRC1/ROS
pathway. Int J Mol Med. 47(53)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Giannos P, Prokopidis K, Raleigh SM,
Kelaiditi E and Hill M: Altered mitochondrial microenvironment at
the spotlight of musculoskeletal aging and Alzheimer's disease. Sci
Rep. 12(11290)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Han Y, Sun S, Zhao M, Zhang Z, Gong S, Gao
P, Liu J, Zhou J, Ma D, Gao Q and Wu P: CYC1 predicts poor
prognosis in patients with breast cancer. Dis Markers.
2016(3528064)2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Oka SI, Hsu CP and Sadoshima J: Regulation
of cell survival and death by pyridine nucleotides. Circ Res.
111:611–627. 2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yang Y and Sauve AA: NAD(+) metabolism:
Bioenergetics, signaling and manipulation for therapy. Biochim
Biophys Acta. 1864:1787–1800. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ying W: NAD+/NADH and NADP+/NADPH in
cellular functions and cell death: Regulation and biological
consequences. Antioxid Redox Signal. 10:179–206. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Shi F, Zhang Z, Wang J, Wang Y, Deng J,
Zeng Y, Zou P, Ling X, Han F, Liu J, et al: Analysis by
metabolomics and transcriptomics for the energy metabolism disorder
and the Aryl hydrocarbon receptor activation in male reproduction
of mice and GC-2spd cells exposed to PM2.5. Front
Endocrinol (Lausanne). 12(807374)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chouchani ET, Pell VR, James AM, Work LM,
Saeb-Parsy K, Frezza C, Krieg T and Murphy MP: A unifying mechanism
for mitochondrial superoxide production during ischemia-reperfusion
injury. Cell Metab. 23:254–263. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Baldissera MD, Souza CF, Grings M,
Parmeggiani BS, Leipnitz G, Moreira KLS, da Rocha MIUM, da Veiga
ML, Santos RCV, Stefani LM and Baldisserotto B: Inhibition of the
mitochondrial respiratory chain in gills of Rhamdia quelen
experimentally infected by Pseudomonas aeruginosa: Interplay with
reactive oxygen species. Microb Pathog. 107:349–353.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhao M, Wang Y, Li L, Liu S, Wang C, Yuan
Y, Yang G, Chen Y, Cheng J, Lu Y and Liu J: Mitochondrial ROS
promote mitochondrial dysfunction and inflammation in ischemic
acute kidney injury by disrupting TFAM-mediated mtDNA maintenance.
Theranostics. 11:1845–1863. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Hessam S, Gambichler T, Skrygan M, Sand M,
Rüddel I, Scholl L and Bechara FG: Reduced ten-eleven translocation
and isocitrate dehydrogenase expression in inflammatory
hidradenitis suppurativa lesions. Eur J Dermatol. 28:449–456.
2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Alkhater RA, Ahonen S and Minassian BA:
SUCLA2 Arg407Trp mutation can cause a nonprogressive movement
disorder-deafness syndrome. Ann Clin Transl Neurol. 8:252–258.
2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Pei X, Li KY, Shen Y, Li JT, Lei MZ, Fang
CY, Lu HJ, Yang HJ, Wen W, Yin M, et al: Palmitoylation of MDH2 by
ZDHHC18 activates mitochondrial respiration and accelerates ovarian
cancer growth. Sci China Life Sci. 65:2017–2030. 2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Chen J, Jin J, Zhang X, Yu H, Zhu X, Yu L,
Chen Y, Liu P, Dong X, Cao X, et al: Microglial lnc-U90926
facilitates neutrophil infiltration in ischemic stroke via
MDH2/CXCL2 axis. Mol Ther. 29:2873–2885. 2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ma Q, Wang C, Wang M, Li Y, Li P, Wang J,
Cheng L, An Y, Dai H, Duan Y, et al: Investigation of brain damage
mechanism in middle cerebral artery occlusion/reperfusion rats
based on i-TRAQ quantitative proteomics. Exp Brain Res.
239:1247–1260. 2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang B, Li ZL, Zhang YL, Wen Y, Gao YM and
Liu BC: Hypoxia and chronic kidney disease. EBioMedicine.
77(103942)2022.PubMed/NCBI View Article : Google Scholar
|