Introduction
Systemic lupus erythematosus (SLE) is an autoimmune
disease characterized by the production of multiple autoantibodies,
including anti-nuclear antibodies and anti-double-stranded (ds)DNA
antibodies (1,2). The generation of these autoantibodies
was reported to have a detrimental effect on multiple tissues and
organs, such as the skin, joints and kidney (3-5).
Previous studies suggested that SLE is a complex disease involving
the participation of both T and B cells in its progression
(6-8).
Activated B cells differentiate into plasma cells to
secrete antibodies in response to an antigen. CD138 is a marker of
plasma cells (9,10). CD138+ T cells, which
express both CD3 and CD138 on their surface, were previously
reported to be associated with plasmablastic B-cell neoplasms in
clinical cases (11) and were also
identified in SLE murine models (12-14).
CD138+ T cells were reported to significantly accumulate
in Fas-deficient lupus mice (12-14).
Furthermore, previous studies demonstrated that double negative
(DN) T cells serve an important role in the progression of lupus
and significantly contribute to tissue injury in SLE (6,15,16).
Plasma cell accumulation was reported to be a hallmark feature of
SLE (17,18). Our previous study demonstrated that
the majority of CD138+ cells in SLE murine models were
CD138+ T cells (19). A
previous study also reported that CD4+CD138+
T cells could significantly promote autoantibody production both
in vivo and in vitro (12). This suggests that CD138+
T cells may be autoreactive and accumulate in Murphy Roths Large
lymphoproliferative (MRL/lpr) mice to induce an autoimmune
response. Therefore, CD138+ T cells may aid in
elucidating the underlying mechanism of SLE progression. In the
present study, the mechanism by which CD138+ T cells are
involved in the progression of SLE in MRL/lpr mice was
evaluated.
Phorbol 12-myristate 13-acetate (PMA) and ionomycin
(PI) are commonly used to induce in vitro cellular
activation (20,21). A previous study also demonstrated
that T cell receptor (TCR) activation negatively regulates CD138
protein expression in DN T cells (14). PMA and PI are commonly used to
activate T cells and promote cytokine secretion in T cells
(22,23). In the present study, the effect of
PI stimulation on CD138+ T cell accumulation in
splenocytes of MRL/lpr mice was assessed. Furthermore, the function
of CD138 protein expression in CD3+CD138- T
cells and its resulting accumulation in MRL/lpr mice was
evaluated.
Materials and methods
Experimental animals
Female Murphy Roths Large
(MRL/MPJ-Fas+/+; n=8; age,
4-week-old; weight, 20-25 g) and Murphy Roths Large
lymphoproliferative
(MRL/lpr-Fas-/-; n=8; age, 4
weeks; weight, 20-25 g) mice were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. Mice were housed at 22±1˚C with a
relative humidity of 50-60% and a 12-h light/dark cycle. The mice
were allowed free access to water and food. All animal experiments
were compliant with the institutional ethical guidelines of Beijing
Institute of Chinese Medicine (Beijing, China).
Cell culture
At 17-18 weeks of age, mice that did not undergo
intervention or treatment were anesthetized using an
intraperitoneal injection of 1% sodium pentobarbital (80 mg/kg) to
harvest blood for serum samples. Mice were then euthanized by
cervical dislocation and subsequently the spleen was harvested.
Single-cell suspensions of splenocytes were then obtained via
filtration through a 70-µm cell strainer (BD Biosciences).
Splenocytes with 5x10^6 cells in each experiment were then cultured
in RPMI-1640 medium (HyClone; Cytiva) with 10% fetal bovine serum,
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C with 5%
CO2 or in the presence of PMA (50 ng/ml; Thermo Fisher
Scientific, Inc.) and PI (1 µg/ml; Thermo Fisher Scientific, Inc.)
to stimulate the splenocytes (24-26).
Measurement of serum cytokine levels
using the Luminex™ platform
Serum levels of IFN-γ, IFN-α, tumor necrosis factor,
IL-6, IL-10, IL-17, IL-21 and IL-2 were measured using 8-Plex
ProcartaPlex Panel (cat. no. PPX-08; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. Diluted serum
samples with universal assay buffer (from the Luminex kit) were
added onto 96-well plates coated with magnetic beads and incubated
at room temperature for 120 min after vortexing. The beads were
then washed with wash buffer, and the 50X detection antibody
mixture was diluted to 1X with detection antibody diluent, and then
added and incubated for an additional 30 min at room temperature.
After incubation and plate washing with wash buffer, the samples
were analyzed on the Luminex 200 platform.
Flow cytometry
Splenocytes were incubated on ice with CD16/CD32
monoclonal antibodies (cat. no. 14-0161-85;
eBioscience™; Thermo Fisher Scientific, Inc.) for 15
min, and then red blood cells were lysed using lysis buffer (BD
Biosciences). Cells were subsequently incubated with the following
antibodies (100 µl/test as recommended by the manufacturer for flow
cytometry) at room temperature for 30 min for flow cytometric
analysis: Anti-CD3 phycoerythrin (PE)-cyanine7 (cy7) (cat. no.
25-0032-82; eBioscience; Thermo Fisher Scientific, Inc.); anti-CD3
allophycocyanin (APC)-cy7 (cat. no. 47-0032-82; eBioscience; Thermo
Fisher Scientific, Inc.); anti-CD4 fluorescein isothiocyante (FITC)
(cat. no. 53-0041-82; eBioscience; Thermo Fisher Scientific, Inc.);
anti-CD8 peridinin-chlorophyll-protein (PerCP) (cat. no.
45-0081-82; eBioscience; Thermo Fisher Scientific, Inc.); anti-CD8
APC (cat. no. 17-0081-82; eBioscience; Thermo Fisher Scientific,
Inc.); anti-CD19 APC-cy7 (cat. no. 47-0193-82; eBioscience; Thermo
Fisher Scientific, Inc.); anti-CD138 PE (cat. no. 142504;
BioLegend, Inc.); anti-CD69 PE (cat. no. 104508; BioLegend, Inc.);
anti-CD69 APC (cat. no. 17-0691-82; eBioscience; Thermo Fisher
Scientific, Inc.); anti-CD25 APC (cat. no. 17-0251-82; eBioscience;
Thermo Fisher Scientific, Inc.); anti-FasL APC (cat. no.
17-5911-82; eBioscience; Thermo Fisher Scientific, Inc.); anti-B220
PerCP (cat. no. 45-0452-82; eBioscience; Thermo Fisher Scientific,
Inc.); and anti-B220 PE-cy7 (cat. no. 25-0452-82; eBioscience;
Thermo Fisher Scientific, Inc.). Annexin V-FITC and
7-aminoactinomycin D (7-AAD) PerCP (cat. no. 35-6410 KIT; Tonbo
Biosciences, Inc.) were utilized for staining, performed according
to the manufacturer's instructions. Cells were incubated with
annexin V conjugate for 15 min at room temperature and 7-AAD
staining solution was added and incubated at room temperature for 5
min before flow cytometric analysis. Stained cells were analyzed
using BD FACSVerse (BD Biosciences). Data were analyzed using
FlowJo software (version 10.6 for PC; Tree Star, Inc.).
Immunofluorescence
Frozen tissue sections (6 µm thick) were used for
immunofluorescence assays. They were embedded in optimal cutting
temperature compound (cat. no. 4583; Sakura Finetek), fixed with
acetone cooled to 4˚C for 5 min, blocked with 5% donkey serum (cat.
no. S9100; Solarbio Co., Ltd., China) at room temperature for 1 h
and then stained with primary antibodies at room temperature for 1
h, namely anti-CD3 antibody (1:100 dilution; cat. no. ab33429;
Abcam) and anti-CD138 antibody (10 µg/ml; cat. no. AF3190; R&D
Systems), as previously described (27). Sections were visualized using
donkey anti-goat IgG H&L (Alexa Fluor® 488) (1:200
dilution; cat. no. ab150129; Abcam) and donkey anti-rat IgG H&L
(Alexa Fluor® 594) (1:200 dilution; cat. no. ab150156;
Abcam) secondary antibodies at room temperature for 30 min.
Immunofluorescence images were obtained and analyzed using ZEN Blue
lite 2.3 software (Zeiss GmbH). Representative imaging data were
obtained at identical settings with the ZEN Blue lite software and
all assays included negative controls, where the primary antibodies
were omitted (Fig. S1A).
Polymerase chain reaction (PCR)
To detect the genotype of the fas gene in MRL/lpr
mice, total genomic DNA was extracted from the kidney tissue from
all the eight MRL/MPJ and eight MRL/lpr mice using the TIANamp
Genomic DNA Kit (cat. no. DP304; Tiangen Biotech Co., Ltd.)
according to the manufacturer's instructions. The PCR containing
1.1X CataAmp Taq Plus PCR Mix (cat. no. C105; Beijing Catascis
Biotech. Co. Ltd.) was performed according to genotyping protocols
provided by the Jackson Laboratory (Jax Lab). The primer sequences
for PCR were as follows: olMR1678/olMR1680 forward,
5'-GTAAATAATTGTGCTTCGTCAG-3'; olMR1678 reverse,
5'-TAGAAAGGTGCACGGGTGTG-3'; and olMR1680 reverse,
5'-CAAATCTAGGCATTAACAGTG-3'. The PCR protocol was executed as
follows: 94˚C for 3 min for initial denaturation, then 94˚C for 10
sec for denaturation, 65˚C (initially, then decreasing by 0.5˚C per
cycle) for 10 sec for annealing and 68˚C for 30 sec for extension,
repeated for 10 cycles, next 72˚C for 10 sec, 60˚C for 10 sec and
72˚C for 30 sec for 28 cycles. This was followed by 72˚C for 60 sec
for final extension and a hold at 10˚C. The PCR products were
electrophoresed in a 3% agarose gel (cat. no. 1110GR100; BioFroxx)
at a voltage of 80V. The agarose gel was stained with GelRed for 15
min. An image of the agarose gel was then obtained by Amersham
ImageQuant 800 (Amersham Pharmacia Biotech, Inc.). The amplified
products were analyzed and revealed a fragment length of 217 bp for
the mutant and 179 bp for the wild-type (WT).
Statistical analysis
Data from all experiments are representative of 2-3
independent experiments showing reproducibility and are expressed
as mean ± standard deviation. Data were analyzed using SPSS 17.0
(SPSS, Inc.). Comparisons between groups were performed using the
Student's t-test, while comparisons among ≥3 groups were performed
using one-way ANOVA followed by Tukey's post-hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
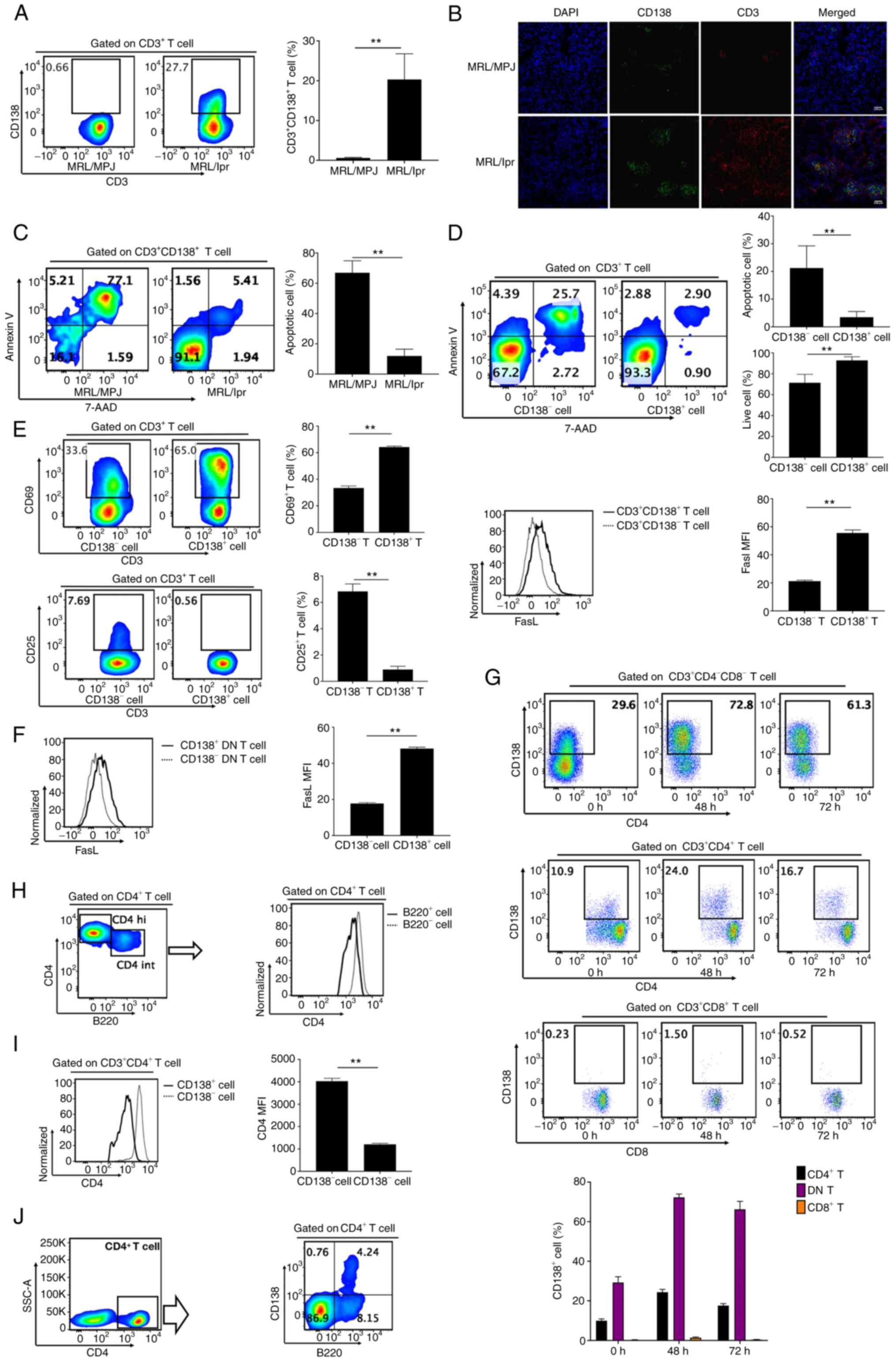
CD138 protein expression in T cells
leads to defective apoptosis of T cells in MRL/lpr mice
Results of PCR showed all MRL/MPJ mice had
homozygous WT fas genes and all MRL/lpr mice had homozygous mutant
fas gene (Fig. S1B). It
demonstrated that the MRL/lpr mice we used were fas-deficient and
SLE murine models were absolutely established in the present study
(Fig. S1B). CD138 protein
expression levels in CD3+ T cells were negligible in
splenocytes of MRL/MPJ mice, compared with the significant
accumulation of abnormal (CD138+) T cells in the
splenocytes of Fas-deficient MRL/lpr mice (Figs. 1A and S1C). In addition, it was demonstrated
that CD138+ T cells infiltrated the kidneys of MRL/lpr
mice, but is negligible in the kidneys of MRL/MPJ mice (Fig. 1B). CD138+ T cells in
MRL/MPJ mice also had significantly higher levels of apoptosis,
compared with MRL/lpr mice, which were found to have low levels
(Fig. 1C). This indicated that
CD138+ T cell apoptosis in mice was Fas-dependent and
Fas deficiency in MRL/lpr mice led to the accumulation of
CD138+ T cells. However, it was demonstrated that
CD138+ T cells in the splenocytes of MRL/lpr mice were
associated with a significant decrease in the number of apoptotic
cells and a significant increase in the number of live cells
compared with CD3+CD138- T cells (Fig. 1D).
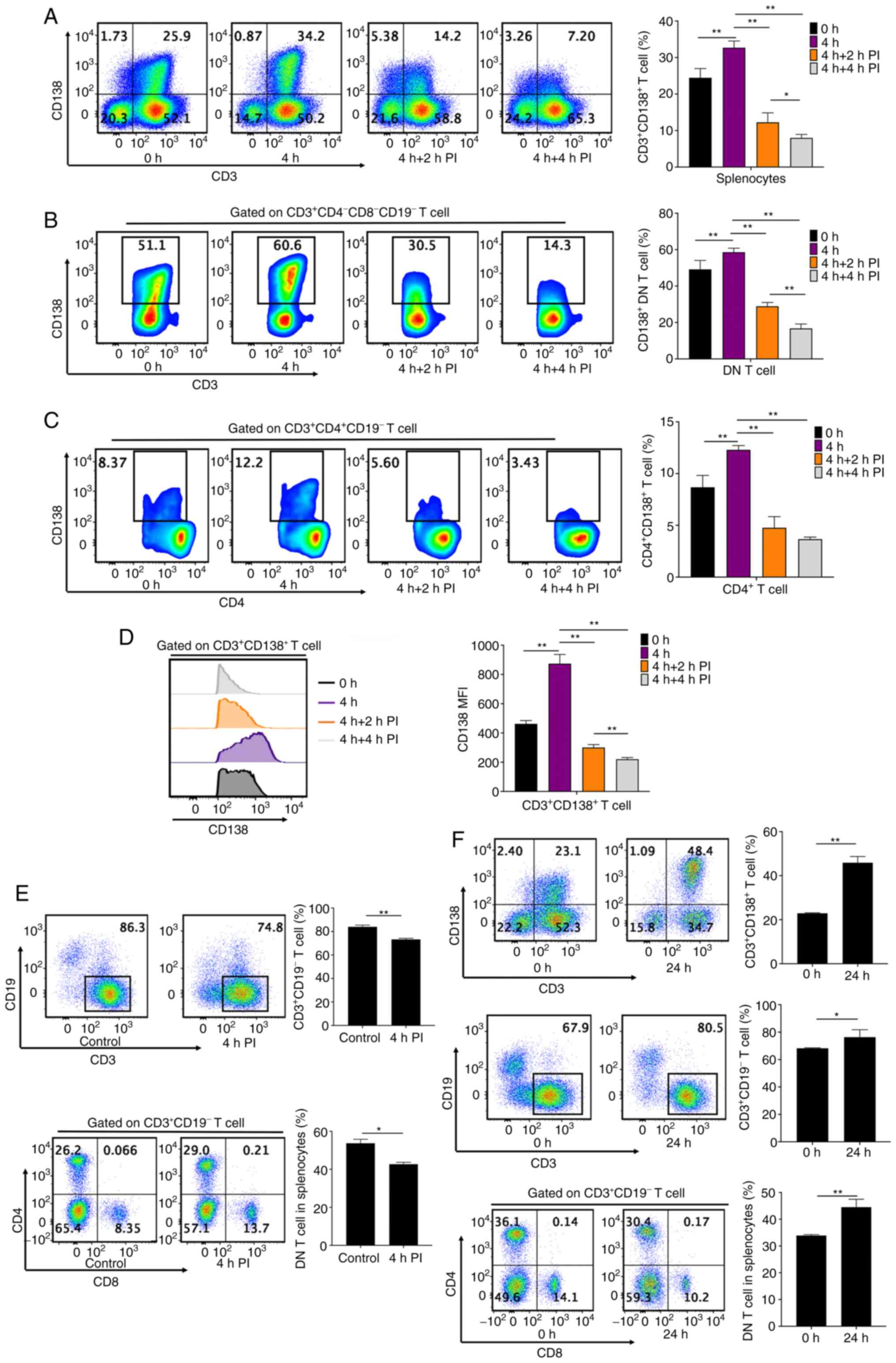
 | Figure 1CD138 expression leads to defective
apoptosis of T cells. (A) Flow cytometry analyses and bar chart
showing the frequencies of CD138+ cells in
CD3+ T cells from fresh splenocytes of MRL/MPJ and
MRL/lpr mice aged 17-18 weeks. (B) Frozen kidney sections stained
with CD3 (red), CD138 (green) and DAPI (blue) demonstrate
CD138+ T cell infiltration into the renal tissues of
MRL/lpr mice (original magnification, x200). (C) Flow cytometry
analyses and bar chart showing the frequency of apoptotic cells in
CD3+CD138+ T cells of fresh splenocytes in
MRL/MPJ and MRL/lpr mice. (D) Flow cytometry analyses and bar
charts demonstrating the frequencies of apoptotic and live cells in
CD3+CD138- and
CD3+CD138+ T cells in fresh splenocytes of
MRL/lpr mice. (E) Flow cytometry analyses and bar charts showing
CD69+ and CD25+ cell frequencies and FasL
expression in CD3+CD138- and
CD3+CD138+ T cells in fresh splenocytes from
MRL/lpr mice. (F) Flow cytometry analyses and bar charts
demonstrating FasL expression in CD138- and
CD138+ DN T cells in fresh splenocytes from MRL/lpr
mice. (G) Flow cytometry analyses and bar chart demonstrating
CD138+ cell frequencies of DN, CD4+ and
CD8+ T cells in fresh splenocytes from MRL/lpr mice, and
in fresh splenocytes from MRL/lpr mice that were cultured in
vitro for 48 and 72 h with no stimulation. (H) Flow cytometric
analyses of CD4 expression in CD4+ T cell subsets.
CD4+ T cells of MRL/lpr mice were demonstrated to have
two cell subsets, namely CD4 hi and CD4 int T cells. CD4 int T
cells had significant downregulation of CD4 expression with
simultaneous expression of B220. (I) Bar chart indicating CD4
expression in CD4+CD138+ and
CD4+CD138- T cells in MRL/lpr mice. (J)
CD4+CD138+ T cells expressed B220 and were in
the B220+CD4 int T cell subsets. n=4-6 per
group/experiment. **P<0.01 by one-way analysis of
variance. MRL/MPJ, Murphy Roths Large; MRL/lpr, Murphy Roths Large
lymphoproliferative; FasL, Fas ligand; DN, double negative; 7-AAD,
7-aminoactinomycin D; MFI, mean or median fluorescence
intensity. |
CD69 and CD25 are markers of early and late active T
cells (12). In the present study,
it was demonstrated that CD138+ T cells in fresh
splenocytes of MRL/lpr mice had a significant increase in
CD69+ cell frequency with a simultaneous significant
decrease in CD25+ cell frequency, compared with
CD138- T cells in fresh splenocytes of MRL/lpr mice
(Fig. 1E). Furthermore, CD138
protein expression significantly increased Fas ligand (FasL)
protein expression in CD3+ T cells of MRL/lpr mice,
compared with CD3- T cells (Fig. 1E). CD138 protein expression also
significantly increased FasL protein expression in
CD138+ DN T cells of MRL/lpr mice, compared with
CD138- DN T cells (Fig.
1F). Therefore, it was concluded that CD138 protein expression
in CD3+ T cells led to defective apoptosis of
CD3+ T cells but prompted activation of CD3+
T cells in Fas-deficiency MRL/lpr mice.
CD4+ T cells and DN T cells
express CD138 in MRL/lpr mice
The majority of CD138+ T cells in MRL/lpr
mice were demonstrated to be CD4 and CD8 double negative T cells,
with a small proportion of CD138+ T cells expressing CD4
and a negligible proportion of cells expressing CD8 (Fig. S1D). A significantly lower
frequency of CD138+ cells was demonstrated in
CD8+ T cells, compared with CD4+ T cells and
DN T cells (Fig. 1G). There was
also a significantly greater frequency of CD138+ cells
in DN T cells than in CD4+ T cells (Fig. 1G). This indicated that the majority
of CD138+ T cells in MRL/lpr mice were derived from
CD138- DN T cells in MRL/lpr mice that had an increased
accumulation of DN T cells. Additionally, it was demonstrated that
CD4+ T cells in MRL/lpr mice were comprised of two
subsets, CD4 hi T cells and CD4 int T cells (Fig. 1H). CD4 int T cells had reduced
expression of CD4 but increased expression of B220, compared with
CD4 hi T cells (Fig. 1H).
CD4+CD138+ T cells simultaneously expressed
B220 with a significant downregulation of CD4 protein expression
compared with CD4+CD138- T cells (Fig. 1I and J).
CD138 protein expression improves
defective activation of CD138- T cells in MRL/lpr
mice
Multiple cytokines were found in the serum of
MRL/lpr mice, of which interferon-γ, tumor necrosis factor (TNF),
interleukin (IL)-6, IL-10, IL-17, and IL-2 were significantly
increased compared with those in MRL/MPJ mice (Fig. 2A). However, there were no
significant changes of IFN-α and IL-21 levels in the serum of
MRL/lpr mice, compared with those in MRL/MPJ mice (Fig. 2A). This suggested that MRL/lpr mice
were undergoing an inflammatory response (Fig. 2A). Unstimulated CD138- T
cells derived from fresh splenocytes of MRL/lpr mice had a
significantly increased frequency of CD69+ cells
compared with that in CD138- T cells from fresh
splenocytes of MRL/MPJ mice (Fig.
2B). However, the frequency of CD69+ cells in
CD138- T cells of MRL/MPJ mice was significantly
increased compared with that in CD138- T cells from
MRL/lpr mice after 5 h of in vitro stimulation of the fresh
splenocytes by PI (Fig. 2C).
Furthermore, both CD69 and FasL protein expression levels in
CD138- T cells were not significantly increased after
in vitro stimulation of splenocytes with 2 or 4 h PI
stimulation compared with the CD138- T cells without PI stimulation
(Fig. 2D). These results
demonstrated a defect in CD138- T cell activation in
MRL/lpr mice.
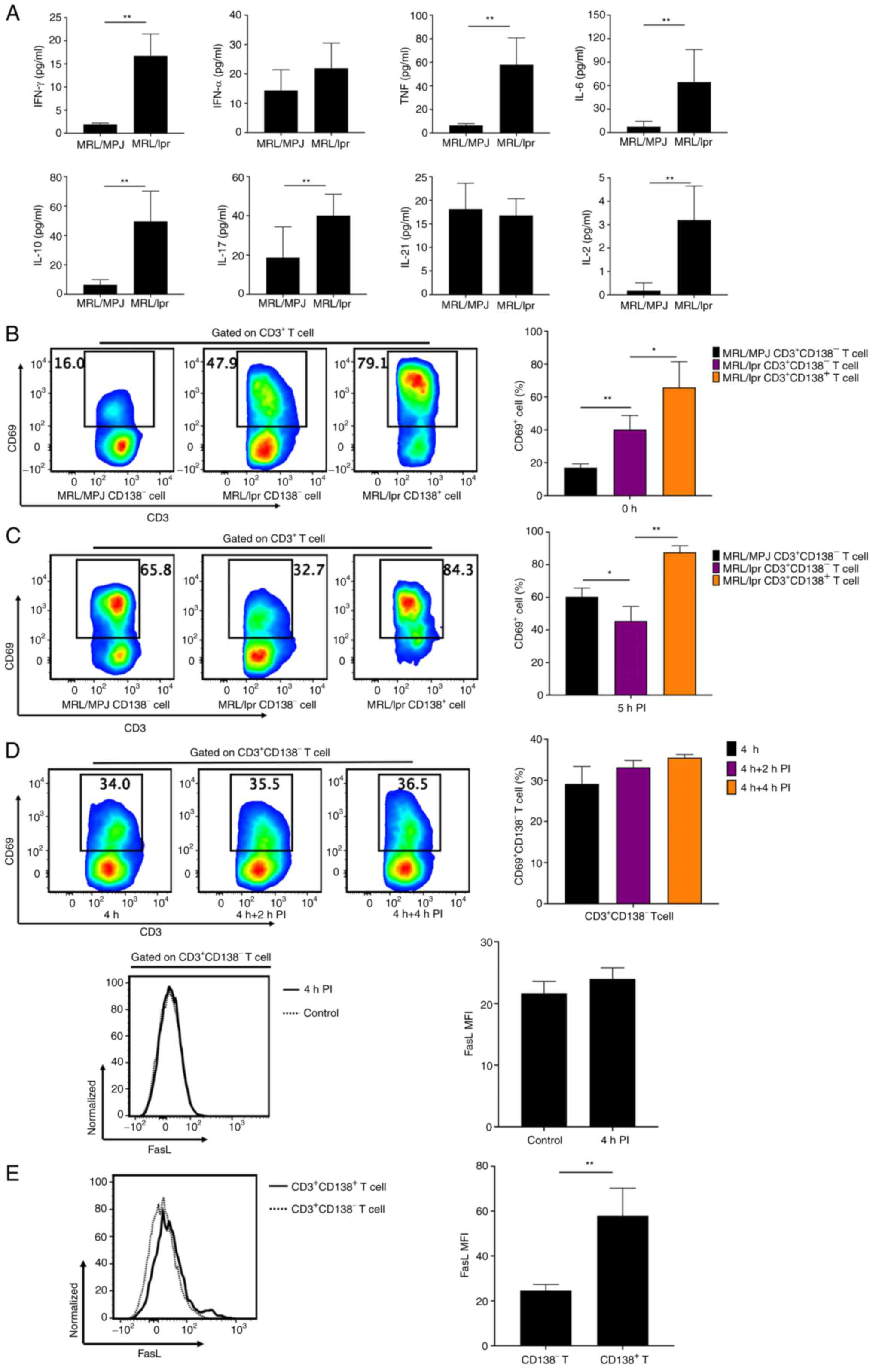 | Figure 2CD138 improves defective activation
of CD138- T cells. (A) Bar charts showing serum
levels of IFN-γ, IFN-α, TNF, IL-6, IL-10, IL-17, IL-21 and IL-2 in
MRL/MPJ and MRL/lpr mice. (B) Flow cytometry analyses and bar chart
demonstrating the frequencies of CD69+ cells in
CD138- T cells in fresh splenocytes from MRL/MPJ mice
aged 17-18 weeks, and in CD138- and CD138+ T
cells in fresh splenocytes from MRL/lpr mice aged 17-18 weeks. (C)
Flow cytometry analyses and bar chart demonstrating the frequency
of CD69+ cells in CD138- T cells in fresh
splenocytes from MRL/MPJ mice aged 17-18 weeks, and in
CD138- and CD138+ T cells in fresh
splenocytes from MRL/lpr mice aged 17-18 weeks after 5 h in
vitro PI stimulation. (D) Flow cytometry analyses and bar
charts demonstrating CD69+ cell frequencies in
CD138- T cells in splenocytes from MRL/lpr mice after 0,
2 and 4 h PI stimulation and FasL expression in CD138- T
cells in splenocytes from MRL/lpr mice with or without 4 h PI
stimulation. (E) Flow cytometry analyses and bar chart
demonstrating FasL expression in CD3+CD138+
and CD3+CD138- T cells from MRL/lpr mouse
splenocytes with 4 h PI stimulation in. n=4-8 per group/experiment.
*P<0.05 and **P<0.01 by one-way
analysis of variance. IFN, interferon; TNF, tumor necrosis factor;
IL, interleukin; MRL/MPJ, Murphy Roths Large; MRL/lpr, Murphy Roths
Large lymphoproliferative; FasL, Fas ligand; PI, ionomycin; MFI,
mean or median fluorescence intensity. |
CD138+ T cells from fresh splenocytes of
MRL/lpr mice had significantly increased CD69+ cell
frequencies (Figs. 1E and 2B) and FasL protein expression (Fig. 1E) compared with CD138- T
cells. In addition, CD138+ T cells showed a
significantly increased CD69+ cell frequency after in
vitro 5 h PI stimulation, as well as significantly increased
FasL protein expression compared with that in CD138- T
cells in splenocytes from MRL/lpr mice (Fig. 2C and E). These results suggested that CD138
protein expression improved the defective activation of
CD138- T cells in MRL/lpr mice.
PI stimulation may significantly
prevent CD138+ T cell accumulation
Splenocytes from MRL/lpr mice were stimulated by PI
in order to demonstrate changes in CD138+ T cell
frequency. In vitro culture of unstimulated splenocytes from
MRL/lpr mice for 4 h resulted in a significant increase in
CD138+ T cell frequency and CD138 protein expression in
CD138+ T cells, compared with that at 0 h (Fig. 3A and D). However, CD138+ T cell
frequencies progressively and significantly reduced after 2 and 4 h
PI stimulation (Fig. 3A). PI
stimulation (both 2 and 4 h) also significantly reduced
CD138+ cell frequencies in DN T cells and
CD4+ T cells compared with the 4 h DN T cells and
CD4+ T cells without PI stimulation (Fig. 3B and C). CD138 protein expression in
CD138+ T cells was also significantly and progressively
downregulated after 2 and 4 h of PI stimulation, compared with the
4 h DN T cells and CD4+ T cells without PI stimulation
(Fig. 3D). These results
demonstrated that PI stimulation prevented CD138+ T cell
accumulation in splenocytes from MRL/lpr mice.
CD138 protein expression in T cells
contributes to DN T cell accumulation in MRL/lpr mice
Four-hour PI stimulation significantly reduced
CD138+ T cell frequency in splenocytes (Fig. 3A), as well as significantly reduced
both CD3+ T cell and DN T cell frequencies in
splenocytes from MRL/lpr mice (Fig.
3E). However, 4-h PI stimulation failed to decrease
CD4+CD138- and CD8+ T cell
frequencies in splenocytes, conversely significantly increasing
CD8+ T cell frequencies (Fig. S1E). Splenocytes from MRL/lpr mice
were then cultured without any stimulation for 24 h.
CD138+ T cell frequency in splenocytes was significantly
increased after 24 h, compared with at 0 h (Fig. 3F). Simultaneously, CD3+
T cell frequency and DN T cell accumulation were significantly
increased at 24 h, compared with at 0 h (Fig. 3F). Conversely, 24-h in vitro
culture without any stimulation significantly decreased the
frequencies of CD4+CD138- and CD8+
T cells in splenocytes (Fig.
S1F). These results demonstrated that CD138 protein expression
significantly contributed to the increase in CD3+ T cell
frequency and DN T cell accumulation in splenocytes from MRL/lpr
mice.
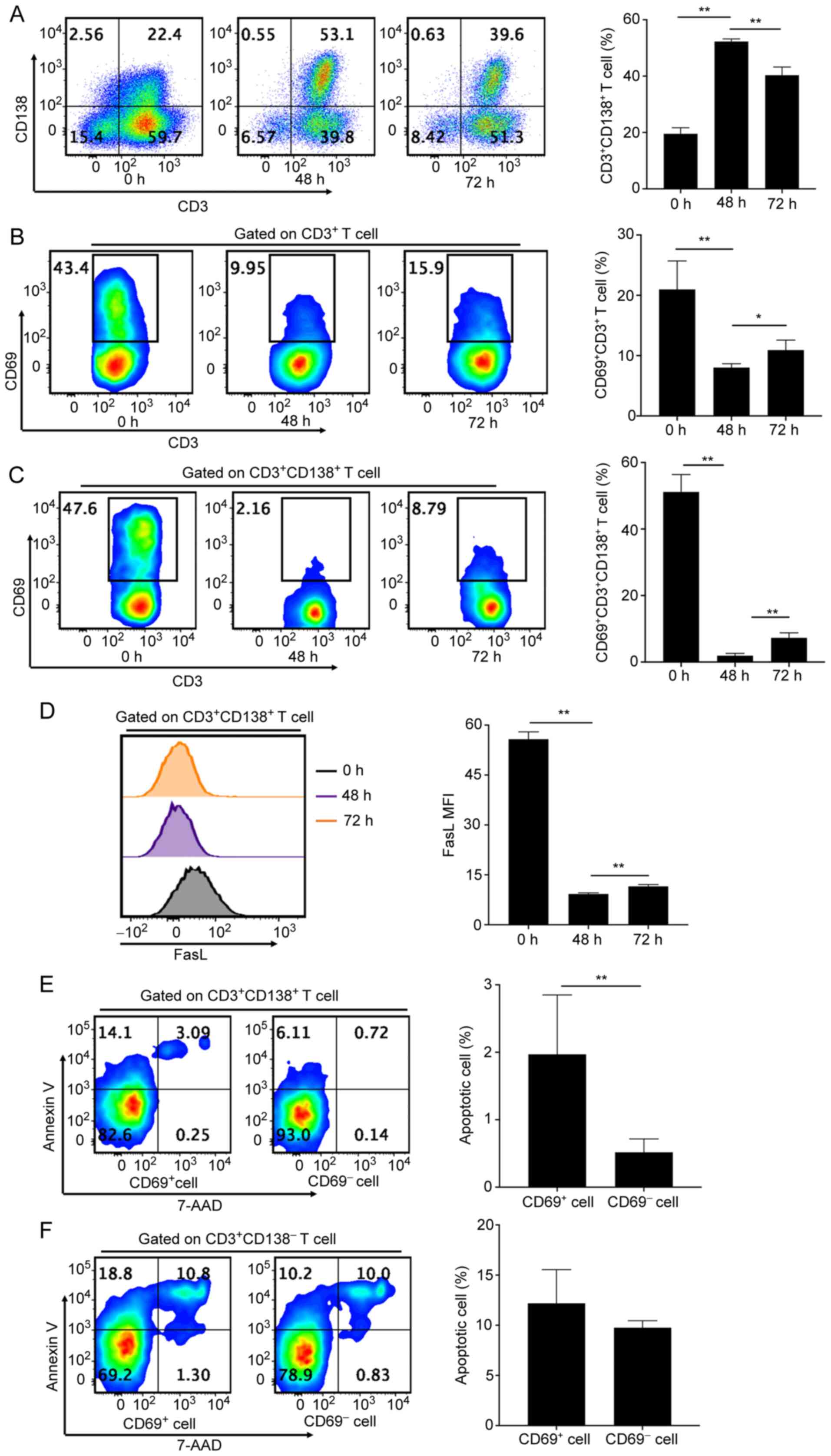
Stimulation with PI induces specific
apoptosis of CD138+ T cells
The mechanism by which PI stimulation prevented the
accumulation of CD138+ T cells in MRL/lpr mice was
assessed. Splenocytes stimulated with 4-h PI demonstrated a
significantly increased number of apoptotic cells and a decreased
frequency of live cells in CD138+ T cells, compared with
controls (Fig. 4A). However, PI
stimulation did not affect the number of apoptotic cells in
CD3+CD138- T cells, compared with controls
(Fig. 4A). Conversely, although
not significantly, PI stimulation even increased the frequency of
live cells, compared with the controls (Fig. 4A). Furthermore, PI stimulation
significantly decreased apoptotic cell numbers and simultaneously
and significantly increased live cell numbers in
CD3-CD138+ plasma cells, compared with the
controls (Fig. 4B). This indicated
that PI stimulation specifically induced cellular apoptosis in
CD3+CD138+ T cells, but not in
CD3+CD138- T cells and
CD3-CD138+ plasma cells. Moreover, the
increased levels of apoptosis in CD138+ T cells induced
by PI stimulation were not caused by the cytotoxic effect of PI
(28,29).
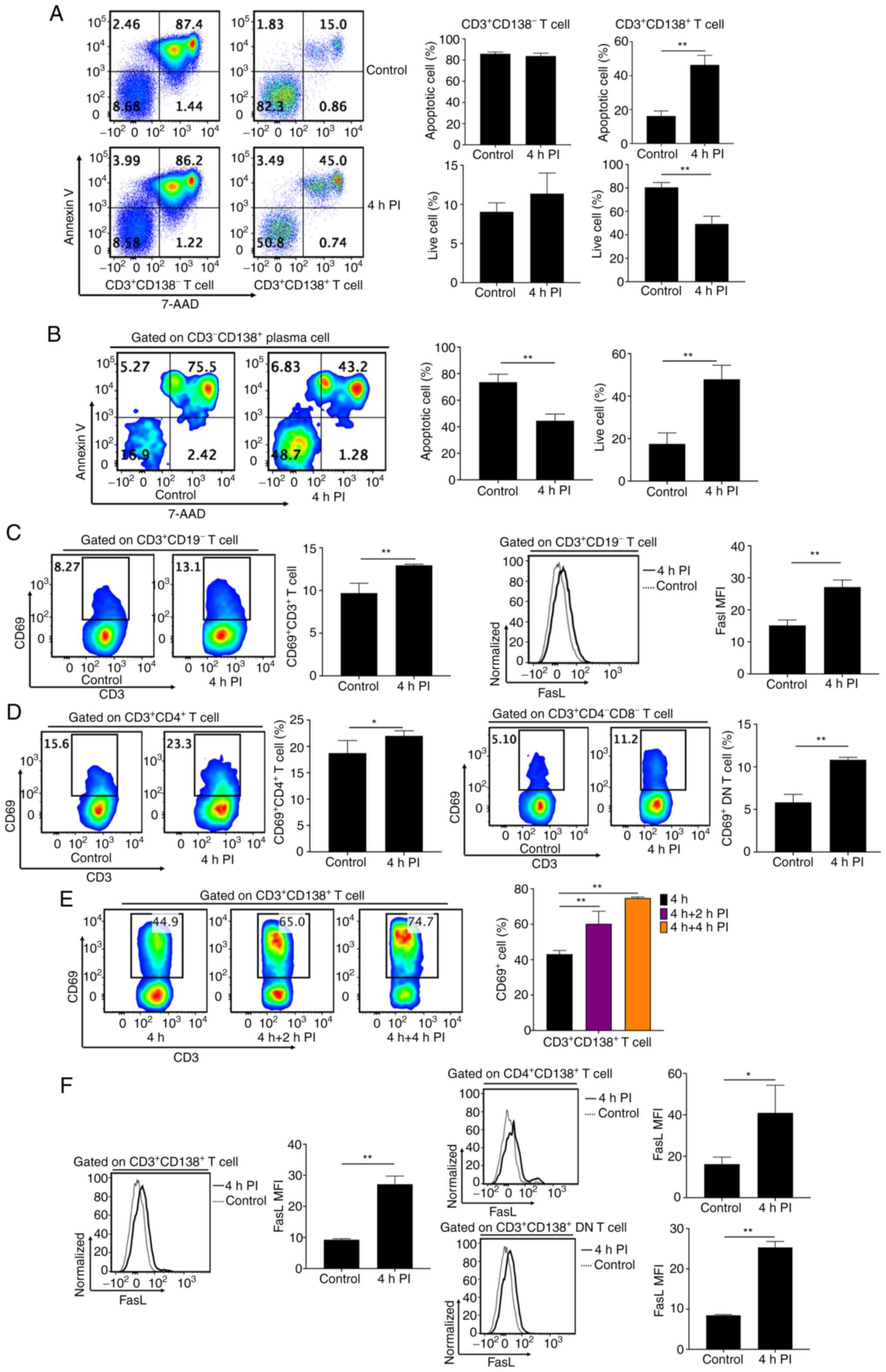 | Figure 4PI stimulation induces specific
apoptosis of CD138+ T cells. (A) Flow cytometry analyses
and bar charts demonstrating frequencies of apoptotic and live
cells in CD3+CD138- and
CD3+CD138+ T cells in splenocytes from
MRL/lpr mice with or without 4 h PI stimulation. (B) Flow cytometry
analyses and bar chart demonstrating the frequencies of apoptotic
and live cells in CD138+CD3- plasma cells in
splenocytes from MRL/lpr mice with or without 4 h PI stimulation.
(C) Flow cytometry analyses and bar charts demonstrating
CD69+ cell frequencies and FasL expression in
CD3+ T cells in splenocytes from MRL/lpr mice with or
without 4 h PI stimulation. (D) Flow cytometry analyses and bar
charts demonstrating CD69+ cell frequencies in
CD4+ T cells and DN T cells of splenocytes from MRL/lpr
mice with or without 4 h PI stimulation. (E) Flow cytometry
analyses and bar charts demonstrating CD69+ cell
frequencies in CD138+ T cells of splenocytes from
MRL/lpr mice after 0, 2, and 4 h PI stimulation. (F) Flow cytometry
analyses and bar charts demonstrating FasL expression in
CD138+, CD4+CD138+, and
CD138+ DN T cells of splenocytes from MRL/lpr mice with
or without 4 h PI stimulation. n=4-5 per group/experiment.
*P<0.05 and **P<0.01 by one-way
analysis of variance. MRL/lpr, Murphy Roths Large
lymphoproliferative; PI, ionomycin; FasL, Fas ligand; DN, double
negative; 7-AAD, 7-aminoactinomycin D; MFI, mean or median
fluorescence intensity. |
Stimulation with PI promotes the
activation of T cells with CD138 expression
PI stimulation was demonstrated to have
significantly increased the activation levels of CD3+ T
cells from MRL/lpr mice, with a significant increase in
CD69+ cell frequency and FasL protein expression,
compared with controls (Fig. 4C).
CD4+ and DN T cells demonstrated significantly increased
frequencies of CD69+ cells after 4-h PI stimulation,
compared with controls (Fig. 4D).
CD69+ cell frequency and FasL protein expression in
CD138+ T cells from MRL/lpr mice were also significantly
increased after 4 h of PI stimulation, compared with the
CD138+ T cells without PI stimulation (Fig. 4E and F). Both CD138+ DN and
CD4+CD138+ T cells demonstrated significantly
increased FasL protein expression after 4-h PI stimulation,
compared with controls (Fig. 4F).
However, PI stimulation failed to significantly promote the
activation of CD138- T cells in MRL/lpr mice (Fig. 2D). This indicated that PI
stimulation resulted in increased apoptosis levels of
CD138+ T cells, whilst simultaneously and significantly
activating CD138+ T cells but not CD138- T
cells in splenocytes from MRL/lpr mice.
The frequency of CD138+ T
cells is inversely associated with the activation levels of
CD138+ T cells
Fresh splenocytes were isolated and cultured from
MRL/lpr mice without any stimulation. CD138+ T cell
frequency significantly increased during the 48-h in vitro
culture (Fig. 5A) and was
accompanied by significantly reduced CD69+ cell
frequency in CD3+ and CD138+ T cells, when
compared with those at 0 h (Fig.
5B-D). Subsequently, the CD138+ T cell frequency
significantly decreased (Fig. 5A)
after 72 h of in vitro culture, accompanied by a significant
increase in activation of CD3+ and CD138+ T
cells, compared with that after 48 h (Fig. 5B-D). This indicated that
CD138+ T cell frequency was inversely associated with
the activation levels of CD3+ and CD138+ T
cells in MRL/lpr mice, suggesting that PI stimulation may have
promoted the activation of CD138+ T cells to induce
specific apoptosis of CD138+ T cells, which prevented
CD138+ T cell accumulation.
CD69 protein expression in
CD138+ T cells results in a significant increase in
apoptosis of CD138+ T cells
The authors hypothesized that the activation of
CD138+ T cells, stimulated by PI, induced their
apoptosis. CD69+ cells in CD138+ T cells of
MRL/lpr mice were demonstrated to have had a significant increase
in apoptosis levels compared with CD69- cells in
CD138+ T cell populations (Fig. 5E). However, CD69 protein expression
failed to significantly increase apoptosis levels of
CD138- T cells in MRL/lpr mice (Fig. 5F). These results suggested that
CD69 protein expression in CD138+ T cells may have
promoted its specific apoptosis. The increase in specific apoptosis
in CD138+ T cells was demonstrated to be due to the
activation of CD138+ T cells via the increase in CD69
protein expression.
Discussion
The present study demonstrated that
CD138+ T cell apoptosis in MRL/MPJ mice operates via a
Fas-dependent pathway. CD138+ T cells were found to
accumulate in MRL/lpr mice and CD138 protein expression in
CD3+ T cells significantly prevented the apoptosis of
CD3+ T cells. This contributed to the accumulation of
CD3+ T and DN T cell subsets whilst simultaneously
promoting CD3+ T cell activation in Fas-deficient
MRL/lpr mice.
CD138 protein expression may also have increased
FasL protein expression in DN T cells to promote their
cytotoxicity. PI stimulation prevented CD138+ T cell
accumulation and decreased CD138+ cell frequencies in DN
and CD4+ T cells by inducing specific apoptosis of
CD138+ T cells. The specific apoptosis was caused by the
activation of CD138+ T cells via an increase in CD69
protein expression.
Fas (CD95) is a member of the TNF receptor family
and interacts with FasL after TCR activation to induce apoptosis
(16). Fas deficiency leads to DN
T cell accumulation in MRL/lpr mice resulting in lymphadenectasis
and splenomegaly (30,31). Results from the present study
demonstrated that apoptosis of CD138+ T cells was via a
Fas-dependent pathway. A previous study reported that Fas
deficiency in MRL/lpr mice led to the accumulation of
CD138+ T cells as they aged and lupus developed
(12). Moreover, the majority of
CD138+ T cells were demonstrated to be CD4-
and CD8- DN T cells in the present study. DN T cells had
been commonly regarded as the abnormal T cells closely related to
SLE. However, most of the DN T cells are also the CD138+ T cells.
It is interesting that these two subjects are related to each other
and suggests CD138 expression in T cells would implicate in
SLE.
CD138 protein expression was demonstrated to have
significantly increased FasL protein expression in CD3+
T cells and their subsets, including DN T cells. However, DN T
cells in MRL/lpr mice were reported to upregulate FasL and be
strongly cytotoxic. This results in autoimmune injuries to multiple
tissues and organs that express small amounts of the Fas receptor
(6,16). In the present study, however, the
results showed CD138 protein expression significantly increased the
cytotoxicity of DN T cells, which in turn promotes lupus
development and tissue injury in MRL/lpr mice.
The increase in CD138+ T cells
significantly decreased the number of apoptotic cells in the
present study, whilst simultaneously increasing the number of live
cells compared with CD138- T cells. This demonstrated
that CD138+ T cells had defective apoptosis in MRL/lpr
mice. Moreover, previous studies reported that CD138+ T
cells had a lower proliferation compared to CD138- T
cell subsets (12,14). Based on these results, it was
concluded that CD138+ T cell accumulation was mainly a
result of their defective apoptosis. CD138 protein expression
significantly contributed to the accumulation of T and DN T cells
in MRL/lpr mice by reducing the number of apoptotic T and DN T
cells.
Autoantibody production has a detrimental effect on
multiple organs and serves a key role in SLE progression (32). Immature T cells undergo both
positive and negative selection to become mature single positive T
cells. During this process, T cells that recognize self-antigens
are eliminated (33,34). Autoreactive T cells are usually
eliminated by Fas-mediated apoptosis during negative selection in
the thymus (35). Fas deficiency
results in autoreactive T cells escaping negative selection
(36,37). CD138+ T cells commonly
express B220, which is also expressed on autoreactive T cells, such
as non-selected CD8+ and DN T cells (35,38).
CD4+CD138+ T cells were reported to be
important for the generation of autoantibodies, such as anti-dsDNA
and anti-smooth muscle antibodies (12). CD138+ T cells were also
reported to promote tissue injury when self-antigens are exposed to
the immune system (7,12). Results from the present study
demonstrated that CD138+ T cells were autoreactive T
cells that escaped Fas-dependent apoptosis during negative
selection (12,14).
The present study demonstrated that CD138 protein
expression in T cells served a key role in the progression of lupus
in MRL/lpr mice. In addition to the significant decrease in
apoptosis levels, CD138+ T cells were demonstrated to be
activated more easily than CD138- T cells. This
indicated that CD138 protein expression could promote the
activation of CD138- T cells in MRL/lpr mice. However,
the mechanism by which CD138 protein is expressed in these abnormal
T cells was not elucidated. The results from the present study
demonstrated that CD138- T cells in MRL/MPJ mice were
more easily activated compared to CD138- T cells in
MRL/lpr mice. PI stimulation failed to significantly activate
CD3+CD138- T cells in MRL/lpr mice. These
results indicated that CD138- T cells in MRL/lpr mice
had defective activation. However, CD138 protein expression
significantly reversed the defective activation of
CD3+CD138- T cells and promoted the
activation of CD3+ T cells in MRL/lpr mice.
PI stimulation could also significantly decrease
CD138 protein expression in CD138+ T cells. PI
stimulation has been used to activate T cells and promote cytokines
secretion in T cells (22,23). However, in the present study, PI
stimulation was able to prevent CD138+ T cell
accumulation by inducing specific apoptosis of CD138+ T
cells. Furthermore, PI stimulation could significantly reduce the
frequency of CD138+ cells in the CD3+ T cell
population and its cell subsets by increasing the activation levels
of CD3+ T and CD138+ T cells. A previous
study demonstrated that TCR activation downregulated CD138 protein
expression in DN T cells (14).
The results from the present study indicated that CD138 protein
expression in CD3+ T cells could be prevented by PI
stimulation to activate CD3+ T cells.
CD69 and CD25 are broadly known as markers for
early and late active T cells (12). Results from the present study
demonstrated that CD138+ T cells had a high level of
CD69 protein expression and low levels of CD25 protein expression.
In addition, it was demonstrated that CD69+ cells in the
CD138+ T cell population had a significant increase in
apoptosis levels compared to CD69- cells in the
CD138+ T cell population. However, both PI stimulation
and TCR activation could significantly activate T cells and
subsequently increase the number of CD69+ cells in the T
cell population (22,39). The present study demonstrated that
the increased apoptosis of CD138+ T cells and subsequent
decreased accumulation of CD138+ T cells were a result
of the activation of CD138+ T cells via the increase of
CD69 protein expression.
A previous study also reported that
CD138+ T cells were autoreactive T cells that promoted
autoantibody production when self-antigens were exposed to the
immune system (12). The results
from the present study demonstrated that CD138+ T cell
frequency was inversely associated with the activation levels of
CD3+ T and CD138+ T cells.
CD3+CD138- T cells in MRL/lpr mice had
defective activation, whilst CD138 protein expression significantly
increased the activation of CD3+CD138- T
cells. PI stimulation activated CD3+ T cells and
simultaneously prevented CD138 protein expression in
CD3+CD138- T cells. These results indicated
that autoreactive CD3+CD138- T cells in
MRL/lpr mice failed to be activated in the absence of self-antigen
exposure, which may have promoted the upregulation of CD138 protein
expression. CD138 protein expression in these T cells therefore
promoted the accumulation of autoreactive T cells and the
autoreactive response in lupus.
The present study provided a novel insight into the
mechanism of autoreactive T cell promotion in the progression of
SLE. However, the findings were based on murine SLE models.
Clinical studies are required to substantiate these findings to
determine the specific marker expressed in human autoreactive T
cells that serves a key role in promoting SLE development. In
addition, the critical proteins that regulate CD138 protein
expression in autoreactive T cells have remained elusive. In the
future, more research needs to be conducted to explore the
underlying mechanisms of the critical proteins regulating CD138
protein expression in autoreactive T cells.
In conclusion, CD138 protein expression in T cells
was implicated in the progression of lupus in MRL/lpr mice. CD138
protein expression in CD3+ T cells prevented apoptosis
of T cells in MRL/lpr mice, simultaneously contributed to
CD3+ and DN T cell accumulation and promoted
CD3+ T cell activation. CD138 protein expression in DN T
cells significantly increased their FasL protein expression,
enhancing the cytotoxicity of the DN T cells. Defective apoptosis,
induced by CD138 protein expression in CD3+ T cells,
resulted in CD138+ T cell accumulation in the spleens of
MRL/lpr mice. Results from the present study indicated that
activation of T cells prevented CD138+ T cell
accumulation by increasing the specific apoptosis of
CD138+ T cells.
Furthermore, the results demonstrated that
CD138- T cells in MRL/lpr mice had defective activation.
Increased activation levels of CD3+ T cells could
prevent CD138 protein expression in CD3+ T cells of
MRL/lpr mice. The results also suggested that CD138 protein
expression in CD3+ T cells of Fas-deficiency MRL/lpr
mice may be caused by the failure of activation in autoreactive T
cells before self-antigen exposure to the immune system.
Supplementary Material
(A) Frozen kidney sections were
stained with or without CD3 (red), or CD138 (green) and DAPI (blue)
for negative control of Fig. 1B,
where primary antibodies were omitted for immunofluorescence
measurement (original magnification, x200). (B) Image of agarose
gels showing the fragment length denoting the genotype of the fas
gene in all of the MRL/MPJ and MRL/lpr mice used in the present
study; mutant: 217 bp, WT: 179 bp. (C) Negative control for flow
cytometry analyses of CD138+ T cells for figures in the
main text. Abnormal T cells express both CD3 and CD138. These
results demonstrate that CD138 protein expression in
CD3+ T cells in flow cytometry was not the result of
fluorescence artifacts or due to PE-cy7 degradation. (D) Flow
cytometry analyses showing double negative, CD4+ and
CD8+ T cell frequencies in CD138+ T cells of
fresh splenocytes from MRL/lpr mice that were cultured in
vitro for 0, 48 and 72 h, respectively. (E) Bar charts
demonstrating CD4+ and CD8+ T cell
frequencies in splenocytes from MRL/lpr mice with or without 4 h
in vitro ionomycin stimulation. (F) Bar charts demonstrating
CD4+ and CD8+ T cell frequencies in
splenocytes from MRL/lpr mice that were cultured in vitro
for 0 and 24 h without stimulation. n=4-5 per group/experiment.
*P<0.05 and **P<0.01 by one-way
analysis of variance. MRL/lpr, Murphy Roths Large
lymphoproliferative; PI, ionomycin; FMO, fluorescence minus
one.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Beijing
Postdoctoral Research Foundation (grant no. ZZ2019-23) and the
MiaoPu Research Foundation of the Beijing Institute of Chinese
Medicine (grant no. MP-2020-45).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
TX conceived the study and wrote the manuscript. TX
and PL designed the experiments. TX and XL performed the laboratory
work. TX and XL performed the data analysis. PL revised and edited
the manuscript. All authors read and approved the final version of
the manuscript. TX and XL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee (IACUC) of the Beijing
Institute of Chinese Medicine (approval no. 2021040202 and were
performed according to institutional ethical guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lisnevskaia L, Murphy G and Isenberg D:
Systemic lupus erythematosus. Lancet. 384:1878–1888.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zharkova O, Celhar T, Cravens PD,
Satterthwaite AB, Fairhurst AM and Davis LS: Pathways leading to an
immunological disease: Systemic lupus erythematosus. Rheumatology
(Oxford). 56 (Suppl 1):i55–i66. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
You M, Dong G, Li F, Ma F, Ren J, Xu Y,
Yue H, Tang R, Ren D and Hou Y: Ligation of CD180 inhibits IFN-α
signaling in a Lyn-PI3K-BTK-dependent manner in B cells. Cell Mol
Immunol. 14:192–202. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dörner T, Giesecke C and Lipsky PE:
Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther.
13(243)2011.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Kotzin BL: Systemic lupus erythematosus.
Cell. 85:303–306. 1996.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Alexander JJ, Jacob A, Chang A, Quigg RJ
and Jarvis JN: Double negative T cells, a potential biomarker for
systemic lupus erythematosus. Precis Clin Med. 3:34–43.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chesnutt MS, Finck BK, Killeen N, Connolly
MK, Goodman H and Wofsy D: Enhanced lymphoproliferation and
diminished autoimmunity in CD4-deficient MRL/lpr mice. Clin Immunol
Immunopathol. 87:23–32. 1998.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nagasu A, Mukai T, Iseki M, Kawahara K,
Tsuji S, Nagasu H, Ueki Y, Ishihara K, Kashihara N and Morita Y:
Sh3bp2 gain-of-function mutation ameliorates lupus phenotypes in
B6.MRL-Faslpr mice. Cells. 8(402)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lu LD, Stump KL, Wallace NH, Dobrzanski P,
Serdikoff C, Gingrich DE, Dugan BJ, Angeles TS, Albom MS, Mason JL,
et al: Depletion of autoreactive plasma cells and treatment of
lupus nephritis in mice using CEP-33779, a novel, orally active,
selective inhibitor of JAK2. J Immunol. 187:3840–3853.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Calame KL: Plasma cells: Finding new light
at the end of B cell development. Nat Immunol. 2:1103–1108.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pan Z, Chen M, Zhang Q, Wang E, Yin L, Xu
Y, Huang Q, Yuan Y, Zhang X, Zheng G and Yuan J: CD3-positive
plasmablastic B-cell neoplasms: A diagnostic pitfall. Mod Pathol.
31:718–731. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu L, Takeda K and Akkoyunlu M: Disease
stage-specific pathogenicity of CD138 (Syndecan 1)-expressing T
cells in systemic lupus erythematosus. Front Immunol.
11(1569)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Seagal J, Leider N, Wildbaum G, Karin N
and Melamed D: Increased plasma cell frequency and accumulation of
abnormal syndecan-1plus T-cells in Igmu-deficient/lpr mice. Int
Immunol. 15:1045–1052. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mohamood AS, Bargatze D, Xiao Z, Jie C,
Yagita H, Ruben D, Watson J, Chakravarti S, Schneck JP and Hamad
AR: Fas-mediated apoptosis regulates the composition of peripheral
alphabeta T cell repertoire by constitutively purging out double
negative T cells. PLoS One. 3(e3465)2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Getachew Y, Cusimano FA, James LP and
Thiele DL: The role of intrahepatic CD3+/CD4-/CD8-double negative T
(DN T) cells in enhanced acetaminophen toxicity. Toxicol Appl
Pharmacol. 280:264–271. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Benihoud K, Bonardelle D, Bobé P and Kiger
N: MRL/lpr CD4- CD8- and CD8+ T cells, respectively, mediate
Fas-dependent and perforin cytotoxic pathways. Eur J Immunol.
27:415–420. 1997.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hidalgo Y, Núñez S, Fuenzalida MJ,
Flores-Santibáñez F, Sáez PJ, Dorner J, Lennon-Dumenil AM, Martínez
V, Zorn E, Rosemblatt M, et al: Thymic B cells promote germinal
center-like structures and the expansion of follicular helper T
cells in lupus-prone mice. Front Immunol. 11(696)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Menon M, Blair PA, Isenberg DA and Mauri
C: A regulatory feedback between plasmacytoid dendritic cells and
regulatory B cells Is aberrant in systemic lupus erythematosus.
Immunity. 44:683–697. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xie T, Liu X, Liu H, Han X, Zhao J, Zhou
D, Wang Y, Zhang H, Wang P and Li P: LangChuangHeJi decoction
ameliorates lupus via preventing accumulation of CD138+ T cells in
MRL/lpr mice. Am J Transl Res. 13:12440–12460. 2021.PubMed/NCBI
|
|
20
|
Chatila T, Silverman L, Miller R and Geha
R: Mechanisms of T cell activation by the calcium ionophore
ionomycin. J Immunol. 143:1283–1289. 1989.PubMed/NCBI
|
|
21
|
Straube F and Herrmann T: Differential
modulation of CD8beta by rat gammadelta and alphabeta T cells after
activation. Immunology. 104:252–258. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Carvalho MUWB, Vendramini P, Kubo CA,
Soreiro-Pereira PV, de Albuquerque RS, Antunes E and Condino-Neto
A: BAY 41-2272 inhibits human T lymphocyte functions. Int
Immunopharmacol. 77(105976)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xie H, Xie S, Wang M, Wei H, Huang H, Xie
A, Li J, Fang C, Shi F, Yang Q, et al: Properties and roles of γδT
Cells in plasmodium yoelii nigeriensis NSM infected C57BL/6 mice.
Front Cell Infect Microbiol. 11(788546)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gao M, Jin W, Qian Y, Ji L, Feng G and Sun
J: Effect of N-methyl-D-aspartate receptor antagonist on T helper
cell differentiation induced by phorbol-myristate-acetate and
ionomycin. Cytokine. 56:458–465. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Han S, Tie X, Meng L, Wang Y and Wu A: PMA
and ionomycin induce glioblastoma cell death: Activation-induced
cell-death-like phenomena occur in glioma cells. PLoS One.
8(e76717)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shan ZG, Zhao YL, Zhang JY, Yan ZB, Wang
TT, Mao FY, Teng YS, Peng LS, Chen WY, Wang P, et al:
FasL+ PD-L2+ identifies a novel
immunosuppressive neutrophil population in human gastric cancer
that promotes disease progression. Adv Sci (Weinh).
9(e2103543)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu Y, He S, Bai B, Zhang L, Xue L, Lin Z,
Yang X, Zhu F, He P, Tang W and Zuo J: Therapeutic effects of the
artemisinin analog SM934 on lupus-prone MRL/lpr mice via inhibition
of TLR-triggered B-cell activation and plasma cell formation. Cell
Mol Immunol. 13:379–390. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Park EK, Jung HS, Yang HI, Yoo MC, Kim C
and Kim KS: Optimized THP-1 differentiation is required for the
detection of responses to weak stimuli. Inflamm Res. 56:45–50.
2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zeng CW, Wang WT, Yu XB, Yang LJ, Chen SH
and Li YQ: Pathways related to PMA-differentiated THP1 human
monocytic leukemia cells revealed by RNA-Seq. Sci China Life Sci.
58:1282–1287. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Martina MN, Noel S, Saxena A, Rabb H and
Hamad ARA: Double negative (DN) αβ T cells: Misperception and
overdue recognition. Immunol Cell Biol. 93:305–310. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Corneth OBJ, Schaper F, Luk F, Asmawidjaja
PS, Mus AMC, Horst G, Heeringa P, Hendriks RW, Westra J and
Lubberts E: Lack of IL-17 receptor a signaling aggravates
lymphoproliferation in C57BL/6 lpr mice. Sci Rep.
9(4032)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tsokos GC, Lo MS, Reis PC and Sullivan KE:
New insights into the immunopathogenesis of systemic lupus
erythematosus. Nat Rev Rheumatol. 12:716–730. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dik WA, Pike-Overzet K, Weerkamp F, de
Ridder D, de Haas EF, Baert MR, van der Spek P, Koster EE, Reinders
MJ, van Dongen JJ, et al: New insights on human T cell development
by quantitative T cell receptor gene rearrangement studies and gene
expression profiling. J Exp Med. 201:1715–1723. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Anderson G and Jenkinson EJ: Lymphostromal
interactions in thymic development and function. Nat Rev Immunol.
1:31–40. 2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Trimble LA, Prince KA, Pestano GA, Daley J
and Cantor H: Fas-dependent elimination of nonselected CD8 cells
and lpr disease. J Immunol. 168:4960–4967. 2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Watanabe-Fukunaga R, Brannan CI, Copeland
NG, Jenkins NA and Nagata S: Lymphoproliferation disorder in mice
explained by defects in Fas antigen that mediates apoptosis.
Nature. 356:314–317. 1992.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Suda T, Takahashi T, Golstein P and Nagata
S: Molecular cloning and expression of the Fas ligand, a novel
member of the tumor necrosis factor family. Cell. 75:1169–1178.
1993.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhou T, Bluethmann H, Eldridge J, Berry K
and Mountz JD: Origin of CD4-CD8-B220+ T cells in MRL-lpr/lpr mice.
Clues from a T cell receptor beta transgenic mouse. J Immunol.
150:3651–3667. 1993.PubMed/NCBI
|
|
39
|
Chun DH, Jung KC, Park WS, Lee IS, Choi
WJ, Kim CJ, Park SH and Bae Y: Costimulatory effect of Fas in mouse
T lymphocytes. Mol Cells. 10:642–646. 2000.PubMed/NCBI View Article : Google Scholar
|