|
1
|
Walbrecq G, Margue C, Behrmann I and Kreis
S: Distinct cargos of small extracellular vesicles derived from
hypoxic cells and their effect on cancer cells. Int J Mol Sci.
21(5071)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zarà M, Guidetti GF, Camera M, Canobbio I,
Amadio P, Torti M, Tremoli E and Barbieri SS: Biology and role of
extracellular vesicles (EVs) in the pathogenesis of thrombosis. Int
J Mol Sci. 20(2840)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Skotland T, Sagini K, Sandvig K and
Llorente A: An emerging focus on lipids in extracellular vesicles.
Adv Drug Deliv Rev. 159:308–321. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang W, Jo H, Park S, Kim H, Kim SI, Han
Y, Lee J, Seol A, Kim J, Lee M, et al: Integrated analysis of
ascites and plasma extracellular vesicles identifies a miRNA-based
diagnostic signature in ovarian cancer. Cancer Lett.
542(215735)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kuhlmann JD, Chebouti I, Kimmig R,
Buderath P, Reuter M, Puppel SH, Wimberger P and Kasimir-Bauer S:
Extracellular vesicle-associated miRNAs in ovarian cancer-design of
an integrated NGS-based workflow for the identification of
blood-based biomarkers for platinum-resistance. Clin Chem Lab Med.
57:1053–1062. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Koshiyama M, Matsumura N and Konishi I:
Subtypes of ovarian cancer and ovarian cancer screening.
Diagnostics (Basel). 7(12)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
US Preventive Services Task Force.
Grossman DC, Curry SJ, Owens DK, Barry MJ, Davidson KW, Doubeni CA,
Epling JW Jr, Kemper AR, Krist AH, et al: Screening for ovarian
cancer: US preventive services task force recommendation statement.
JAMA. 319:588–594. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stewart C, Ralyea C and Lockwood S:
Ovarian cancer: An integrated review. Semin Oncol Nurs. 35:151–156.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Coburn SB, Bray F, Sherman ME and Trabert
B: International patterns and trends in ovarian cancer incidence,
overall and by histologic subtype. Int J Cancer. 140:2451–2460.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kurman RJ: Origin and molecular
pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol. 24
(Suppl 10):x16–x21. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Singer G, Oldt R III, Cohen Y, Wang BG,
Sidransky D, Kurman RJ and Shih IeM: Mutations in BRAF and KRAS
characterize the development of low-grade ovarian serous carcinoma.
J Natl Cancer Inst. 95:484–486. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Karnezis AN, Cho KR, Gilks CB, Pearce CL
and Huntsman DG: The disparate origins of ovarian cancers:
Pathogenesis and prevention strategies. Nat Rev Cancer. 17:65–74.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Prat J: New insights into ovarian cancer
pathology. Ann Oncol. 23 (Suppl 10):x111–x117. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chan JK, Teoh D, Hu JM, Shin JY, Osann K
and Kapp DS: Do clear cell ovarian carcinomas have poorer prognosis
compared to other epithelial cell types? A study of 1411 clear cell
ovarian cancers. Gynecol Oncol. 109:370–376. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yeung TL, Leung CS, Yip KP, Au Yeung CL,
Wong ST and Mok SC: Cellular and molecular processes in ovarian
cancer metastasis. A review in the theme: Cell and molecular
processes in cancer metastasis. Am J Physiol Cell Physiol.
309:C444–C456. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chandra A, Pius C, Nabeel M, Nair M,
Vishwanatha JK, Ahmad S and Basha R: Ovarian cancer: Current status
and strategies for improving therapeutic outcomes. Cancer Med.
8:7018–7031. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kurnit KC, Fleming GF and Lengyel E:
Updates and new options in advanced epithelial ovarian cancer
treatment. Obstet Gynecol. 137:108–121. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Woad KJ, Watkins WJ, Prendergast D and
Shelling AN: The genetic basis of premature ovarian failure. Aust N
Z J Obstet Gynaecol. 46:242–244. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Howell S and Shalet S: Gonadal damage from
chemotherapy and radiotherapy. Endocrinol Metab Clin North Am.
27:927–943. 1998.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ishizuka B: Current understanding of the
etiology, symptomatology, and treatment options in premature
ovarian insufficiency (POI). Front Endocrinol (Lausanne).
12(626924)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang F, Liu Y, Ni F, Jin J, Wu Y, Huang Y,
Ye X, Shen X, Ying Y, Chen J, et al: BNC1 deficiency-triggered
ferroptosis through the NF2-YAP pathway induces primary ovarian
insufficiency. Nat Commun. 13(5871)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Domniz N and Meirow D: Premature ovarian
insufficiency and autoimmune diseases. Best Pract Res Clin Obstet
Gynaecol. 60:42–55. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Goswami D and Conway GS: Premature ovarian
failure. Hum Reprod Update. 11:391–410. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Szeliga A, Calik-Ksepka A,
Maciejewska-Jeske M, Grymowicz M, Smolarczyk K, Kostrzak A,
Smolarczyk R, Rudnicka E and Meczekalski B: Autoimmune diseases in
patients with premature ovarian insufficiency-our current state of
knowledge. Int J Mol Sci. 22(2594)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sullivan SD, Sarrel PM and Nelson LM:
Hormone replacement therapy in young women with primary ovarian
insufficiency and early menopause. Fertil Steril. 106:1588–1599.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang S, Zhu D, Mei X, Li Z, Li J, Xie M,
Xie HJW, Wang S and Cheng K: Advances in biomaterials and
regenerative medicine for primary ovarian insufficiency therapy.
Bioact Mater. 6:1957–1972. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sirmans SM and Pate KA: Epidemiology,
diagnosis, and management of polycystic ovary syndrome. Clin
Epidemiol. 6:1–13. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Meier RK: Polycystic ovary syndrome. Nurs
Clin North Am. 53:407–420. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wolf WM, Wattick RA, Kinkade ON and Olfert
MD: Geographical prevalence of polycystic ovary syndrome as
determined by region and race/ethnicity. Int J Environ Res Public
Health. 15(2589)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Louwers YV and Laven JSE: Characteristics
of polycystic ovary syndrome throughout life. Ther Adv Reprod
Health. 14(2633494120911038)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Patel S: Polycystic ovary syndrome (PCOS),
an inflammatory, systemic, lifestyle endocrinopathy. J Steroid
Biochem Mol Biol. 182:27–36. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pauli JM, Raja-Khan N, Wu X and Legro RS:
Current perspectives of insulin resistance and polycystic ovary
syndrome. Diabet Med. 28:1445–1454. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mitra S, Nayak PK and Agrawal S:
Laparoscopic ovarian drilling: An alternative but not the ultimate
in the management of polycystic ovary syndrome. J Nat Sci Biol Med.
6:40–48. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tian W, Lei N, Zhou J, Chen M, Guo R, Qin
B, Li Y and Chang L: Extracellular vesicles in ovarian cancer
chemoresistance, metastasis, and immune evasion. Cell Death Dis.
13(64)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ingenito F, Roscigno G, Affinito A, Nuzzo
S, Scognamiglio I, Quintavalle C and Condorelli G: The Role of
Exo-miRNAs in cancer: A focus on therapeutic and diagnostic
applications. Int J Mol Sci. 20(4687)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang Y, Lang P, Zhang X, Wu X, Cao S, Zhao
C, Shen R, Ling X, Yang Y and Zhang J: Molecular characterization
of extracellular vesicles derived from follicular fluid of women
with and without PCOS: Integrating analysis of differential miRNAs
and proteins reveals vital molecules involving in PCOS. J Assist
Reprod Genet. 40:537–552. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Park HS, Cetin E, Siblini H, Seok J,
Alkelani H, Alkhrait S, Liakath Ali F, Mousaei Ghasroldasht M,
Beckman A and Al-Hendy A: Therapeutic potential of mesenchymal stem
cell-derived extracellular vesicles to treat PCOS. Int J Mol Sci.
24(11151)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Geng Z, Guo H, Li Y, Liu Y and Zhao Y:
Stem cell-derived extracellular vesicles: A novel and potential
remedy for primary ovarian insufficiency. Front Cell Dev Biol.
11(1090997)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fu YX, Ji J, Shan F, Li J and Hu R: Human
mesenchymal stem cell treatment of premature ovarian failure: New
challenges and opportunities. Stem Cell Res Ther.
12(161)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Dorayappan KDP, Wallbillich JJ, Cohn DE
and Selvendiran K: The biological significance and clinical
applications of exosomes in ovarian cancer. Gynecol Oncol.
142:199–205. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li SP, Lin ZX, Jiang XY and Yu XY:
Exosomal cargo-loading and synthetic exosome-mimics as potential
therapeutic tools. Acta Pharmacol Sin. 39:542–551. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Savina A, Furlán M, Vidal M and Colombo
MI: Exosome release is regulated by a calcium-dependent mechanism
in K562 cells. J Biol Chem. 278:20083–20090. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Villarroya-Beltri C, Baixauli F,
Mittelbrunn M, Fernández-Delgado I, Torralba D, Moreno-Gonzalo O,
Baldanta S, Enrich C, Guerra S and Sánchez-Madrid F: ISGylation
controls exosome secretion by promoting lysosomal degradation of
MVB proteins. Nat Commun. 7(13588)2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yang H, Fu H, Xu W and Zhang X: Exosomal
non-coding RNAs: A promising cancer biomarker. Clin Chem Lab Med.
54:1871–1879. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lin Y, Lu Y and Li X: Biological
characteristics of exosomes and genetically engineered exosomes for
the targeted delivery of therapeutic agents. J Drug Target.
28:129–141. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jalalian SH, Ramezani M, Jalalian SA,
Abnous K and Taghdisi SM: Exosomes, new biomarkers in early cancer
detection. Anal Biochem. 571:1–13. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Makler A and Asghar W: Exosomal biomarkers
for cancer diagnosis and patient monitoring. Expert Rev Mol Diagn.
20:387–400. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sedgwick AE and D'Souza-Schorey C: The
biology of extracellular microvesicles. Traffic. 19:319–327.
2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xu X, Lai Y and Hua ZC: Apoptosis and
apoptotic body: Disease message and therapeutic target potentials.
Bioscience reports. 39(BSR20180992)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhao D, Tao W, Li S, Chen Y, Sun Y, He Z,
Sun B and Sun J: Apoptotic body-mediated intercellular delivery for
enhanced drug penetration and whole tumor destruction. Sci Adv.
7(eabg0880)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chen J, Chen W and Li Y: Conservation of
gene order in human microRNA-neighboring regions. Genome.
55:701–704. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human microRNA targets. PLoS Biol.
2(e363)2004.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Janas T, Janas MM, Sapoń K and Janas T:
Mechanisms of RNA loading into exosomes. FEBS Lett. 589:1391–1398.
2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Rashed MH, Kanlikilicer P,
Rodriguez-Aguayo C, Pichler M, Bayraktar R, Bayraktar E, Ivan C,
Filant J, Silva A, Aslan B, et al: Exosomal miR-940 maintains
SRC-mediated oncogenic activity in cancer cells: A possible role
for exosomal disposal of tumor suppressor miRNAs. Oncotarget.
8:20145–20164. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang H, Wang Q, Zhao Q and Di W: MiR-124
inhibits the migration and invasion of ovarian cancer cells by
targeting SphK1. J Ovarian Res. 6(84)2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang
J and Wu X: Ovarian cancer cell-secreted exosomal miR-205 promotes
metastasis by inducing angiogenesis. Theranostics. 9:8206–8220.
2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Cai J, Gong L, Li G, Guo J, Yi X and Wang
Z: Exosomes in ovarian cancer ascites promote
epithelial-mesenchymal transition of ovarian cancer cells by
delivery of miR-6780b-5p. Cell Death Dis. 12(210)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lian XY, Zhang H, Liu Q, Lu X, Zhou P, He
SQ, Tang RX and Cui J: Ovarian cancer-excreted exosomal miR-199a-5p
suppresses tumor metastasis by targeting hypoxia-inducible
factor-2α in hypoxia microenvironment. Cancer Commun (Lond).
40:380–385. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Cao J, Zhang Y, Mu J, Yang D, Gu X and
Zhang J: Exosomal miR-21-5p contributes to ovarian cancer
progression by regulating CDK6. Human Cell. 34:1185–1196.
2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chen X, Zhou J, Li X and Wang X, Lin Y and
Wang X: Exosomes derived from hypoxic epithelial ovarian cancer
cells deliver microRNAs to macrophages and elicit a tumor-promoted
phenotype. Cancer Lett. 435:80–91. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Li W, Zhang X, Wang J, Li M, Cao C, Tan J,
Ma D and Gao Q: TGFβ1 in fibroblasts-derived exosomes promotes
epithelial-mesenchymal transition of ovarian cancer cells.
Oncotarget. 8:96035–96047. 2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Viaud S, Terme M, Flament C, Taieb J,
André F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz
T, et al: Dendritic cell-derived exosomes promote natural killer
cell activation and proliferation: A role for NKG2D ligands and
IL-15Ralpha. PLoS One. 4(e4942)2009.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Shen X, Wang C, Zhu H, Wang Y, Wang X,
Cheng X, Ge W and Lu W: Exosome-mediated transfer of CD44 from
high-metastatic ovarian cancer cells promotes migration and
invasion of low-metastatic ovarian cancer cells. J Ovarian Res.
14(38)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Alharbi M, Lai A, Guanzon D, Palma C,
Zuñiga F, Perrin L, He Y, Hooper JD and Salomon C: Ovarian
cancer-derived exosomes promote tumour metastasis in vivo: An
effect modulated by the invasiveness capacity of their originating
cells. Clin Sci (Lond). 133:1401–1419. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Guan X, Zong ZH, Liu Y, Chen S, Wang LL
and Zhao Y: circPUM1 promotes tumorigenesis and progression of
ovarian cancer by sponging miR-615-5p and miR-6753-5p. Mol Ther
Nucleic Acids. 18:882–892. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Zong ZH, Du YP, Guan X, Chen S and Zhao Y:
CircWHSC1 promotes ovarian cancer progression by regulating MUC1
and hTERT through sponging miR-145 and miR-1182. J Exp Clin Cancer
Res. 38(437)2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo
JK and Choi C: Biodistribution of exosomes and engineering
strategies for targeted delivery of therapeutic exosomes. Tissue
Eng Regen Med. 18:499–511. 2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Zhang Y, Bi J, Huang J, Tang Y, Du S and
Li P: Exosome: A review of its classification, isolation
techniques, storage, diagnostic and targeted therapy applications.
Int J Nanomedicine. 15:6917–6934. 2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Sharma S, Zuñiga F, Rice GE, Perrin LC,
Hooper JD and Salomon C: Tumor-derived exosomes in ovarian
cancer-liquid biopsies for early detection and real-time monitoring
of cancer progression. Oncotarget. 8:104687–104703. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Rayamajhi S, Nguyen TDT, Marasini R and
Aryal S: Macrophage-derived exosome-mimetic hybrid vesicles for
tumor targeted drug delivery. Acta Biomater. 94:482–494.
2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Xie X, Wu H, Li M, Chen X, Xu X, Ni W, Lu
C, Ni R, Bao B and Xiao M: Progress in the application of exosomes
as therapeutic vectors in tumor-targeted therapy. Cytotherapy.
21:509–524. 2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Hadla M, Palazzolo S, Corona G, Caligiuri
I, Canzonieri V, Toffoli G and Rizzolio F: Exosomes increase the
therapeutic index of doxorubicin in breast and ovarian cancer mouse
models. Nanomedicine (Lond). 11:2431–2441. 2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Liu H, Shen M, Zhao D, Ru D, Duan Y, Ding
C and Li H: The effect of triptolide-loaded exosomes on the
proliferation and apoptosis of human ovarian cancer SKOV3 cells.
Biomed Res Int. 2019(2595801)2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Huang X, Wu W, Jing D, Yang L, Guo H, Wang
L, Zhang W, Pu F and Shao Z: Engineered exosome as targeted lncRNA
MEG3 delivery vehicles for osteosarcoma therapy. J Control Release.
343:107–117. 2022.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si
K, Sun B, Chen B and Xiao Z: Engineered exosomes for targeted
co-delivery of miR-21 inhibitor and chemotherapeutics to reverse
drug resistance in colon cancer. J Nanobiotechnology.
18(10)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Coukos G, Tanyi J and Kandalaft LE:
Opportunities in immunotherapy of ovarian cancer. Ann Oncol. 27
(Suppl 1):i11–i15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Hamanishi J, Mandai M, Ikeda T, Minami M,
Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S,
et al: Safety and antitumor activity of Anti-PD-1 antibody,
nivolumab, in patients with platinum-resistant ovarian cancer. J
Clin Oncol. 33:4015–4022. 2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Tanyi JL, Bobisse S, Ophir E, Tuyaerts S,
Roberti A, Genolet R, Baumgartner P, Stevenson BJ, Iseli C, Dangaj
D, et al: Personalized cancer vaccine effectively mobilizes
antitumor T cell immunity in ovarian cancer. Sci Transl Med.
10(eaao593)2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Sangwan K, Sharma V and Goyal PK:
Pharmacological profile of novel anti-cancer drugs approved by
USFDA in 2022: A review. Curr Mol Med: Jun 22, 2023 (Epub ahead of
print).
|
|
81
|
Zhang L, Conejo-Garcia JR, Katsaros D,
Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H,
Schlienger K, Liebman MN, et al: Intratumoral T cells, recurrence,
and survival in epithelial ovarian cancer. N Engl J Med.
348:203–213. 2003.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Han LY, Fletcher MS, Urbauer DL, Mueller
P, Landen CN, Kamat AA, Lin YG, Merritt WM, Spannuth WA, Deavers
MT, et al: HLA class I antigen processing machinery component
expression and intratumoral T-Cell infiltrate as independent
prognostic markers in ovarian carcinoma. Clin Cancer Res.
14:3372–3379. 2008.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Czystowska-Kuzmicz M, Sosnowska A, Nowis
D, Ramji K, Szajnik M, Chlebowska-Tuz J, Wolinska E, Gaj P, Grazul
M, Pilch Z, et al: Small extracellular vesicles containing
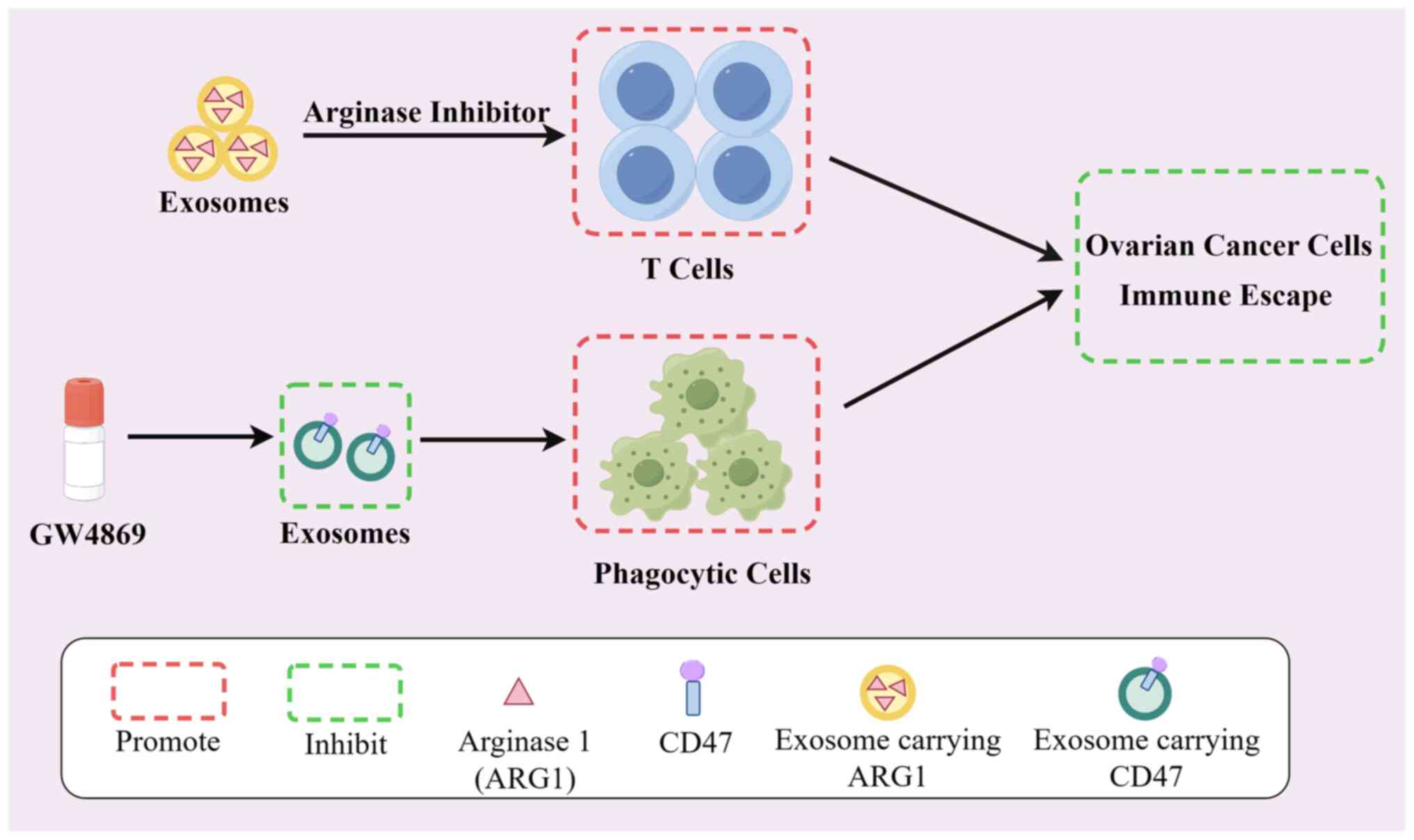
arginase-1 suppress T-cell responses and promote tumor growth in
ovarian carcinoma. Nat Commun. 10(3000)2019.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Labani-Motlagh A, Israelsson P, Ottander
U, Lundin E, Nagaev I, Nagaeva O, Dehlin E, Baranov V and
Mincheva-Nilsson L: Differential expression of ligands for NKG2D
and DNAM-1 receptors by epithelial ovarian cancer-derived exosomes
and its influence on NK cell cytotoxicity. Tumour Biol.
37:5455–5466. 2016.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Koh E, Lee EJ, Nam GH, Hong Y, Cho E, Yang
Y and Kim IS: Exosome-SIRPα, a CD47 blockade increases cancer cell
phagocytosis. Biomaterials. 121:121–129. 2017.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Shimizu A, Sawada K, Kobayashi M, Yamamoto
M, Yagi T, Kinose Y, Kodama M, Hashimoto K and Kimura T: Exosomal
CD47 plays an essential role in immune evasion in ovarian
cancerexosomal CD47 regulates immune evasion in ovarian cancer. Mol
Cancer Res. 19:1583–1595. 2021.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Qiu Y, Yang Y, Yang R, Liu C, Hsu JM,
Jiang Z, Sun L, Wei Y, Li CW, Yu D, et al: Activated T cell-derived
exosomal PD-1 attenuates PD-L1-induced immune dysfunction in
triple-negative breast cancer. Oncogene. 40:4992–5001.
2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Tang MK and Wong AS: Exosomes: Emerging
biomarkers and targets for ovarian cancer. Cancer Lett. 367:26–33.
2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Zhao Z, Ji M, Wang Q, He N and Li Y:
Circular RNA Cdr1as upregulates SCAI to suppress cisplatin
resistance in ovarian cancer via miR-1270 suppression. Mol Ther
Nucleic Acids. 18:24–33. 2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Taylor DD and Gercel-Taylor C: MicroRNA
signatures of tumor-derived exosomes as diagnostic biomarkers of
ovarian cancer. Gynecol Oncol. 110:13–21. 2008.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Cheng L, Wu S, Zhang K, Qing Y and Xu T: A
comprehensive overview of exosomes in ovarian cancer: Emerging
biomarkers and therapeutic strategies. J Ovarian Res.
10(73)2017.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Ryu J and Thomas SN: Quantitative mass
spectrometry-based proteomics for biomarker development in ovarian
cancer. Molecules. 26(2674)2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Cortez AJ, Tudrej P, Kujawa KA and
Lisowska KM: Advances in ovarian cancer therapy. Cancer Chemother
Pharmacol. 81:17–38. 2018.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Lee EH, Han SE, Park MJ, Kim HJ, Kim HG,
Kim CW, Joo BS and Lee KS: Establishment of effective mouse model
of premature ovarian failure considering treatment duration of
anticancer drugs and natural recovery time. J Menopausal Med.
24:196–203. 2018.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Qi Y, Zhu YM and Li B: Comparison of
Animal Models for Premature Ovarian Insufficiency Induced by
Different Doses of Cyclophosphamide: A Network Meta-analysis,
2022.
|
|
96
|
Yang M, Lin L, Sha C, Li T, Zhao D, Wei H,
Chen Q, Liu Y, Chen X, Xu W, et al: Bone marrow mesenchymal stem
cell-derived exosomal miR-144-5p improves rat ovarian function
after chemotherapy-induced ovarian failure by targeting PTEN. Lab
Invest. 100:342–352. 2020.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Sun B, Ma Y, Wang F, Hu L and Sun Y:
miR-644-5p carried by bone mesenchymal stem cell-derived exosomes
targets regulation of p53 to inhibit ovarian granulosa cell
apoptosis. Stem Cell Res Ther. 10(360)2019.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Zhang Q, Sun J, Huang Y, Bu S, Guo Y, Gu
T, Li B, Wang C and Lai D: Human amniotic epithelial cell-derived
exosomes restore ovarian function by transferring microRNAs against
apoptosis. Mol Ther Nucleic Acids. 16:407–418. 2019.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Xiao GY, Cheng CC, Chiang YS, Cheng WT,
Liu IH and Wu SC: Exosomal miR-10a derived from amniotic fluid stem
cells preserves ovarian follicles after chemotherapy. Sci Rep.
6(23120)2016.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Yang W, Zhang J, Xu B, He Y, Liu W and Li
J, Zhang S, Lin X, Su D, Wu T and Li J: HucMSC-Derived exosomes
mitigate the age-related retardation of fertility in female mice.
Mol Ther. 28:1200–1213. 2020.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Cai JH, Sun YT and Bao S: HucMSCs-exosomes
containing miR-21 promoted estrogen production in ovarian granulosa
cells via LATS1-mediated phosphorylation of LOXL2 and YAP. Gen Comp
Endocrinol. 321(114015)2022.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Li H, Huang X, Chang X, Yao J, He Q, Shen
Z, Ji Y and Wang K: S100-A9 protein in exosomes derived from
follicular fluid promotes inflammation via activation of NF-κB
pathway in polycystic ovary syndrome. J Cell Mol Med. 24:114–125.
2020.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Zhao Y, Pan S, Li Y and Wu X: Exosomal
miR-143-3p derived from follicular fluid promotes granulosa cell
apoptosis by targeting BMPR1A in polycystic ovary syndrome. Sci
Rep. 12(4359)2022.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Huang X, Wu B, Chen M, Hong L, Kong P, Wei
Z and Teng X: Depletion of exosomal circLDLR in follicle fluid
derepresses miR-1294 function and inhibits estradiol production via
CYP19A1 in polycystic ovary syndrome. Aging (Albany NY).
12:15414–15435. 2020.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Yuan D, Luo J, Sun Y, Hao L, Zheng J and
Yang Z: PCOS follicular fluid derived exosomal miR-424-5p induces
granulosa cells senescence by targeting CDCA4 expression. Cell
Signal. 85(110030)2021.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Zhao Y, Tao M, Wei M, Du S, Wang H and
Wang X: Mesenchymal stem cells derived exosomal miR-323-3p promotes
proliferation and inhibits apoptosis of cumulus cells in polycystic
ovary syndrome (PCOS). Artif Cells Nanomed Biotechnol.
47:3804–3813. 2019.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Szyposzynska A, Bielawska-Pohl A,
Krawczenko A, Doszyn O, Paprocka M and Klimczak A: Suppression of
ovarian cancer cell growth by AT-MSC microvesicles. Int J Mol Sci.
21(9143)2020.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Guo L, Zhang Y, Wei R, Zhang X, Wang C and
Feng M: Proinflammatory macrophage-derived microvesicles exhibit
tumor tropism dependent on CCL2/CCR2 signaling axis and promote
drug delivery via SNARE-mediated membrane fusion. Theranostics.
10:6581–6598. 2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Mancilla P, Liberona M, Kato S, Barra J,
Gonzalez A and Cuello M: Simvastatin modifies the internalization,
endocytic trafficking, and the content of ovarian cancer
cellderived extracellular microvesicles which are responsible of
inducing migration and invasion in vitro. Int J Gynecol Cancer. 30
(Suppl 3):A192–A193. 2020.
|
|
110
|
Kang F, Zhu J, Wu J, Lv T, Xiang H, Tian
J, Zhang Y and Huang Z: O2-3-Aminopropyl
diazeniumdiolates suppress the progression of highly metastatic
triple-negative breast cancer by inhibition of microvesicle
formation via nitric oxide-based epigenetic regulation. Chem Sci.
9:6893–6898. 2018.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Huang D, Chen J, Yang C and Wang M: TPX2
silencing mediated by joint action of microvesicles and ultrasonic
radiation inhibits the migration and invasion of SKOV3 cells. Mol
Med Rep. 17:7627–7635. 2018.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Yang Z, Du X, Wang C, Zhang J, Liu C, Li Y
and Jiang H: Therapeutic effects of human umbilical cord
mesenchymal stem cell-derived microvesicles on premature ovarian
insufficiency in mice. Stem Cell Res Ther. 10(250)2019.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Faruk EM: El desoky RE, El-Shazly AM and
Taha NM: Does exosomes derived bone marrow mesenchymal stem cells
restore ovarian function by promoting stem cell survival on
experimentally induced polycystic ovary in adult female albino
rats?(Histological and Immunohistochemical Study). J Stem Cell Res
Ther. 8(1000442)2018.
|
|
114
|
Zweemer AJM, French CB, Mesfin J, Gordonov
S, Meyer AS and Lauffenburger DA: Apoptotic bodies Elicit
Gas6-mediated migration of AXL-Expressing tumor cell. Mol Cancer
Res. 15:1656–1666. 2017.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Bergsmedh A, Szeles A, Henriksson M, Bratt
A, Folkman MJ, Spetz AL and Holmgren L: Horizontal transfer of
oncogenes by uptake of apoptotic bodies. Proc Natl Acad Sci USA.
98:6407–6411. 2001.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Muhsin-Sharafaldine MR, Kennedy BR,
Saunderson SC, Buchanan CR, Dunn AC, Faed JM and McLellan AD:
Mechanistic insight into the procoagulant activity of tumor-derived
apoptotic vesicles. Biochim Biophys Acta Gen Subj. 1861:286–295.
2017.PubMed/NCBI View Article : Google Scholar
|