Introduction
Chronic thromboembolic pulmonary hypertension
(CTEPH), which may be diagnosed by the identification of
precapillary pulmonary hypertension (PH) using right heart
catheterization (RHC) and imaging results consistent with chronic
thromboembolism, is a long-term complication of pulmonary embolism
(1) with a poor prognosis
(2). Due to the persistence of
thrombotic material, the pulmonary arteries become obstructed,
which triggers secondary right heart dysfunction and the impairment
of pulmonary hemodynamics, accompanied by systemic malperfusion
(3). Pulmonary endarterectomy
(PEA) is currently used as an established treatment method with the
potential to be curative (2).
However, the use of PEA is not applicable in approximately
one-third of patients, mainly due to the presence of peripheral
lesions (4). In such cases,
balloon pulmonary angioplasty (BPA) is emerging as an alternative
interventional treatment (5).
Renal insufficiency is one of the most common
comorbidities in patients who experience dysfunction of the right
heart attributed to pulmonary arterial hypertension (6,7). It
has been suggested that renal failure in patients with pulmonary
arterial hypertension is caused by an interaction between factors
associated with renal and cardiac functions (7). It has been demonstrated that in
patients with CTEPH, the elevation of N-terminal pro-brain
natriuretic peptide (NT-proBNP) levels is reversed in most patients
when BPA is performed, although not in cases with chronic failure
of the right heart (8). The
accumulation of NT-proBNP can be attributed to renal function
impairment characterized by a reduced glomerular filtration rate
(GFR), which complicates its prognostic utility in CTEPH (9,10). A
study has shown that chronic kidney disease (CKD) is an independent
predictor of outcome in patients with pulmonary arterial
hypertension and is a marker of disease severity (11). BPA facilitates right ventricular
recovery and the improvement of pulmonary hemodynamics, which
promotes systemic circulation and ameliorates venous congestion,
thereby increasing perfusion of the systemic organs (7). It is possible that renal function may
reflect the effects of BPA on the systemic circulation and serve as
an indicator for these effects.
It is not yet known whether GFR levels have any
impact on the clinical prognosis of patients with CTEPH during
follow-up. Therefore, the objective of the present study was to
investigate the prognostic implications of GFR in patients with
CTEPH who have undergone BPA.
Materials and methods
Study population
The data in the present study were obtained from two
prospective studies conducted at The Second Affiliated Hospital of
Harbin Medical University (Harbin, China), with ethical approval
numbers SYDWGZR-2010-152 and SYDWGZR-2013-088. Each patient
included in this retrospective study provided written informed
consent during follow-up. The study population comprised 47
patients (25 male patients and 22 female patients; mean age,
65.34±8.84) with confirmed CTEPH as verified by a pulmonary
ventilation/perfusion scan, pulmonary angiography and chest
computed tomography (CT) scan, at least two of which revealed areas
of deficient pulmonary blood flow (12,13).
The diagnostic criteria for CTEPH included: i) Mean pulmonary
artery pressure ≥25 mmHg at rest measured by RHC; ii) mismatched
perfusion defects stronger than ventilation defects observed by
lung scanning; iii) evidence of chronic and organized thrombi or
emboli in proximal pulmonary arteries revealed by CT/magnetic
resonance imaging or pulmonary angiography; iv) exclusion of left
heart disease, respiratory disorders or other causes of PH
(12,13). The exclusion criteria included
cardiogenic shock, active bleeding or high risk of bleeding,
interstitial lung disease on high-resolution CT, severe hepatic
impairment (Child-Pugh class C liver disease) and left ventricular
heart failure with an ejection fraction of <30%, severe renal
impairment, severe liver disease, inability to comply with study
procedures and follow-up visits and a life expectancy <6
months.
Patients who were treated with BPA and received
intensive care in the period from December 2012 to September 2020
were included in the present study. The patients were grouped into
two categories based on the renal function levels assessed by
estimated GFR upon admission. The GFR levels were as defined as low
level (GFR ≤53 ml/min/1.73 m2) and high level (GFR
>53 ml/min/1.73 m2).
The pre- and post-operative management of patients
with CTEPH was performed as described in a previous study (14). In summary, all patients underwent a
series of examinations including clinical examinations,
echocardiography, 12-lead electrocardiography, laboratory tests,
6-min walk tests, CT scans, RHC and pulmonary angiography. The
final diagnosis of CTEPH was established based on the 2015 European
Society of Cardiology and European Respiratory Society guidelines
(15). An interdisciplinary CTEPH
conference was held for all patients, with the aim of
conceptualizing the therapeutic approach. BPA was performed as a
staged procedure based on standard clinical practice, with
interventional radiologists, cardiologists and thoracic surgeons
planning the procedure together. BPA sessions were held every 4-8
weeks. Before the BPA sessions, examinations adapted to the
requirements of each patient were followed up, with assessment of
laboratory test results and clinical status. During the study, all
patients received BPA therapy four times, and after the final BPA
procedure, a 6-month follow-up with a comprehensive assessment
including vital signs, laboratory tests, RHC and major adverse
events (MAEs) was performed (Fig.
1). MAEs were defined as all-cause mortality, death from right
heart failure (RHF) and rehospitalization associated with RHF.
Echocardiography
All echocardiograms were performed by expert
sonographers with a 2.5-MHz transducer using Vivid 5
ultrasonography equipment (GE Healthcare). All examinations were
subjected to offline analysis by another experienced investigator
at the center. Offline assessment was conducted using commercially
available software (EchoPAC, version 8; GE Healthcare). A
qualitative wall motion score assessed the right ventricle (RV)
hypokinesis, which was determined at the following four locations
along the free wall of the right ventricle in the apical 4-chamber
view: Apex, midapical free wall, midbasal free wall and base
(16). Corresponding
echocardiographic parameters for evaluating right heart function
such as pulmonary artery systolic pressure (PASP), pulmonary artery
diastolic pressure (PADP), mean pulmonary artery pressure,
pulmonary vascular resistance (PVR), RV diameter and RV hypokinesis
were listed. An M-mode cursor was utilized to measure tricuspid
annular plane systolic excursion (TAPSE) in the lateral tricuspid
annulus from the apical 4-chamber view (17).
RHC
The diagnostic work-up included RHC as a key
component (15), which was
routinely carried out via the internal jugular vein on the right
side of the body using a standard Swan-Ganz catheter and 6F sheath.
Prior to or during RHC, no medication modifications were made to
the regimens of the patients. In particular, no vasoactive agents
were administered. The RHC assessment indicated that treatment for
PH was acceptable for all patients, with drugs including riociguat,
phosphodiesterase-5 inhibitors or endothelin receptor antagonists
being administered. Right heart catheterization hemodynamic
characteristics including PASP, PADP, right ventricular mean
pressure, pulmonary arterial oxygen saturation percentage (PAO%),
right atrial mean pressure, cardiac output, cardiac index (CI) and
PVR from baseline to follow-up between the two groups according to
GFR were also evaluated.
BPA
All patients underwent a comprehensive clinical
evaluation before the first BPA (baseline), before each BPA session
and 4-8 months after the last BPA. Assessment at baseline and the
last evaluation included New York Heart Association functional
class, 6-min walk distance, blood gases on room air, serum levels
of NT-proBNP and complete RHC. Femoral or jugular access was used
to perform BPA, which was carried out under moderate sedation in
stages as previously described (Fig.
2) (18). All patients
accepted rivaroxaban as an anticoagulant, which was paused on the
day of intervention; in addition, no bridging therapy with
low-molecular-weight heparin was administered. However, during the
evaluation, to maintain an activated clotting time of ≥250 sec,
~100 IU/kg heparin was administered intravenously to each patient.
A 6F sheath (Terumo Interventional Systems) was placed in the
pulmonary artery and intubation of the partially obstructed
segmental arteries was accomplished via the insertion of a 6F
guiding catheter (JR4; Covidien; Medtronic) into the pulmonary
artery. As the guidewire (Runthrough® NS-PTCA; Terumo
Interventional Systems) was passed through the obstructing
endoluminal material, it was directed into the subsegmental
arterial branches. Multiple inflations of semi-compliant balloons
(Emerge; Boston Scientific) were then used to dilate the
subsegmental branches. The post-procedure morphologic result was
documented by a final fluoroscopic exam.
Blood sampling and laboratory
assessment
Each patient participating in the study underwent
blood sampling from an elbow vein. NT-proBNP and troponin I were
assessed on admission. Elecsys® NT-proBNP automated
electrochemiluminescent sandwich immunoassays (Roche Diagnostics
GmbH) were used to measure NT-proBNP in plasma. Commercially
available immunonephelometric kinetic assays (BN ProSpec System;
Siemens Healthineers) with CardioPhase CRP reagents (Siemens
Healthineers) were used to measure C-reactive protein (CRP) levels.
Biochemistry measurements were also made using a Hitachi 7600
autoanalyzer (Hitachi, Ltd.) using the Jaffe kinetic method.
During the baseline evaluation, before and after
each BPA procedure, and at the end of the study, plain tubes were
used to collect venous blood for the determination of creatinine
and urea levels in serum. A modified diet that was designed to
reduce proteinuria and hyperlipidemia while ensuring adequate
nutrition by optimizing the intake of calories, high quality
proteins, sodium, potassium, phosphorus, fluids and lipids was
administered, and the GFR was calculated in order to evaluate
chronic renal function (19). The
estimated GFR was determined using the modification of diet in
renal disease formula as follows: GFR=186.3 (serum
creatinine)-1.154x(age)-0.203 (x 0.742 if
female) (20). Based on the Acute
Kidney Injury (AKI) Network recommendations, acute renal failure
was considered as an increase in serum creatinine of >0.3 mg/dl
(26.4 mmol/l) or ≥150% from baseline (21). In most cases, if contrast-induced
renal failure were to develop it would occur within 72 h of
exposure (22).
Treatment
Several prevention strategies were applied, which
involved the use of low-osmolar contrast media in small doses
(Visipaque; GE Healthcare), nephroprotective drugs and hydration
during hospitalization. A composition comprising 500 ml saline, 20
mg furosemide and 1 g potassium chloride was administered to all
patients who underwent BPA intervention once a day before BPA
treatment, once as soon as possible after BPA treatment and once
during the BPA treatment. Adaptations were made based on serum
electrolyte levels if necessary.
Statistical analysis
Quantitative data are presented as the mean ±
standard deviation, and qualitative data are presented as frequency
(%). Comparisons between groups were made using an independent
two-sample t-test. Comparisons of categorical variables were
carried out using Chi-square and Fisher's exact tests. Correlations
between the residual GFR and NT-proBNP were assessed using
Pearson's correlation analysis. Univariate and multivariate
logistic regression analysis were performed in order to identify
independent predictors of MAEs at final follow-up. For the
comparison of RHC hemodynamic characteristics between two groups,
the data are presented as box plots with a median bar, 25 and 75%
quartiles and range of values. Statistical analyses were carried
using SPSS 19.0 version (IBM Corp.). Two-sided P<0.05 was
regarded as statistically significant.
Results
Baseline and final follow-up
characteristics
The number of BPA interventions performed was 195
(mean, 4 BPAs/patient), and the number of vessels treated was 392
(mean, 8 vessels/patient). The most frequent complications among
the patients who underwent BPA were hemoptysis in three cases
(6.4%) and reperfusion injury in one case (2.1%). However, no cases
of periinterventional AKI were observed.
The baseline characteristics of patients categorized
by GFR values are listed in Table
I. A significant difference was observed between the low and
high level GFR groups in terms of NT-proBNP levels (P=0.007). There
were no other significant differences identified in any other
variables at baseline. Characteristics at final follow-up for
patients categorized by GFR values are listed in Table II. NT-proBNP (P=0.046), Troponin I
(P<0.001) and right ventricle diameter (P=0.041) were
significantly lower in the GFR >53 group compared with the GFR
≤53 group. 6MWD (P=0.001) and TAPSE (P=0.045) was significantly
higher in the GFR >53 group compared with the GFR ≤53 group. As
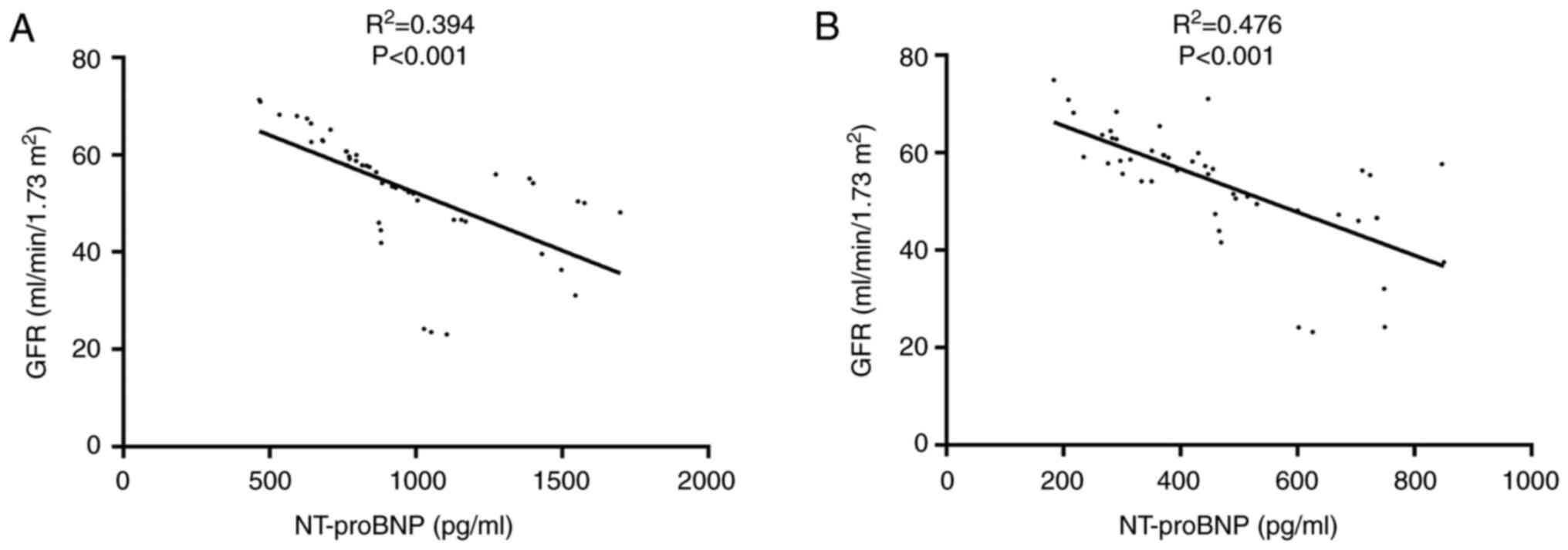
shown in Fig. 3, the GFR and
NT-proBNP levels were significantly correlated at baseline and
final follow-up (P<0.001).
 | Table IBaseline characteristics categorized
by GFR values. |
Table I
Baseline characteristics categorized
by GFR values.
| Characteristics | GFR >53
(n=26) | GFR ≤53 (n=21) | P-value |
|---|
| Age at first BPA,
years | 67.04±7.47 | 63.24±10.07 | 0.145 |
| Male, n (%) | 13 (50.00) | 12 (57.14) | 0.770 |
| BMI,
kg/m2 | 24.08±2.84 | 24.29±1.98 | 0.777 |
| 6MWD, m | 377.38±18.95 | 376.90±23.08 | 0.938 |
| Duration from PE to
CTEPH, months | 16.42±5.84 | 14.24±4.87 | 0.177 |
| Vital signs | | | |
|
Systolic
blood pressure, mmHg | 109.04±11.70 | 106.10±14.63 | 0.447 |
|
Heart rate,
beats/min | 94.19±5.10 | 95.33±4.89 | 0.441 |
|
Respiratory
rate, breaths/min | 22.15±3.55 | 22.52±5.62 | 0.795 |
| PE location | | | |
|
Unilateral,
right | 1 (3.85) | 0 (0.00) | 1.000 |
|
Unilateral,
left | 0 (0.00) | 0 (0.00) | 1.000 |
|
Central
only | 1 (3.85) | 2 (9.52) | 0.574 |
|
Bilateral
only | 14 (53.85) | 10 (47.62) | 0.772 |
|
Central plus
bilateral | 10 (38.46) | 9 (42.86) | 0.775 |
| Medical
history | | | |
|
Prostanoid | 9 (34.61) | 9 (42.86) | 0.763 |
|
Riociguat | 18 (69.23) | 17 (80.95) | 0.505 |
|
Sildenafil | 7 (26.92) | 5 (23.81) | 1.000 |
|
Endothelin
receptor blockers | 13 (50.00) | 15 (71.43) | 0.232 |
|
Diuretic | 18 (69.23) | 17 (80.95) | 0.505 |
| Laboratory
testing | | | |
|
D-dimers,
µg/l | 191.31±85.15 | 153.10±56.29 | 0.084 |
|
NT-proBNP,
pg/ml | 852.00±250.02 | 1091.38±331.25 | 0.007 |
|
Troponin I,
µg/l | 0.77±0.49 | 0.84±0.54 | 0.648 |
|
Serum
sodium, mmol/l | 142.22±2.60 | 142.21±0.35 | 0.993 |
| Echocardiographic
parameters | | | |
|
PASP,
mmHg | 77.08±10.91 | 78.33±11.01 | 0.698 |
|
PADP,
mmHg | 23.19±10.45 | 23.33±7.19 | 0.958 |
|
mPAP,
mmHg | 40.12±6.58 | 41.38±7.18 | 0.532 |
|
PVR, WU | 4.94±1.64 | 4.67±1.34 | 0.547 |
|
RV diameter,
mm | 37.98±7.08 | 39.57±7.80 | 0.468 |
|
RV
hypokinesis or dyskinesia | 10 (38.46) | 9 (42.86) | 0.775 |
|
TAPSE,
cm | 2.12±1.11 | 1.91±0.55 | 0.418 |
 | Table IICharacteristics at final follow-up
categorized by GFR values. |
Table II
Characteristics at final follow-up
categorized by GFR values.
|
Characteristics | GFR >53
(n=26) | GFR ≤53 (n=21) | P-value |
|---|
| 6MWD, m | 425.92±14.63 | 408.69±10.47 | 0.001 |
| Vital signs | | | |
|
Systolic
blood pressure, mmHg | 109.08±14.65 | 105.50±15.03 | 0.525 |
|
Heart rate,
beats/min | 85.46±3.53 | 85.25±7.96 | 0.925 |
|
Respiratory
rate, breaths/min | 19.08±1.80 | 18.38±5.12 | 0.615 |
| Serum values | | | |
|
WBC,
x109/l | 9.60±2.20 | 10.53±3.98 | 0.458 |
|
CRP,
mg/l | 5.42±3.02 | 7.19±2.74 | 0.111 |
|
D-dimers,
µg/l | 164.62±57.75 | 152.81±46.46 | 0.547 |
|
NT-proBNP,
pg/ml | 387.31±105.87 | 519.94±221.31 | 0.046 |
|
Troponin I,
µg/l | 0.26±0.19 | 0.89±0.29 | <0.001 |
|
Serum
sodium, mmol/l | 144.98±2.76 | 144.64±3.67 | 0.780 |
| Echocardiographic
parameters | | | |
|
PASP,
mmHg | 38.44±15.79 | 39.75±9.61 | 0.768 |
|
PADP,
mmHg | 17.04±9.27 | 17.88±8.52 | 0.773 |
|
mPAP,
mmHg | 24.56±11.31 | 25.19±3.49 | 0.831 |
|
PVR, WU | 2.68±1.63 | 3.46±1.78 | 0.155 |
|
RV diameter,
mm | 34.78±4.79 | 41.51±11.14 | 0.041 |
|
RV
hypokinesis | 13 (50.00) | 16 (76.19) | 0.80 |
|
TAPSE,
cm | 3.76±0.52 | 3.30±0.66 | 0.045 |
RHC
All 47 patients underwent RHC on admission for BPA
treatment and 41 patients underwent RHC at the final follow-up. The
changes in the hemodynamic characteristics determined by RHC from
baseline to final follow-up are shown in Fig. 4. Among all RHC hemodynamic
characteristics, pulmonary arterial oxygen saturation, cardiac
output and CI exhibited an increasing trend; pulmonary artery
systolic pressure, pulmonary artery diastolic pressure, right
atrial mean pressure and pulmonary vascular resistance exhibited a
decreasing trend; and the right ventricular mean pressure appeared
to be unchanged. Furthermore, the RHC results of the two groups did
not differ on admission (Fig. 5A
and B). At the final follow-up, 6
patients died before the RHC was performed, and the remaining
patients in the two groups did not exhibit any significant
differences in RHC hemodynamic characteristics (Fig. 5C and D).
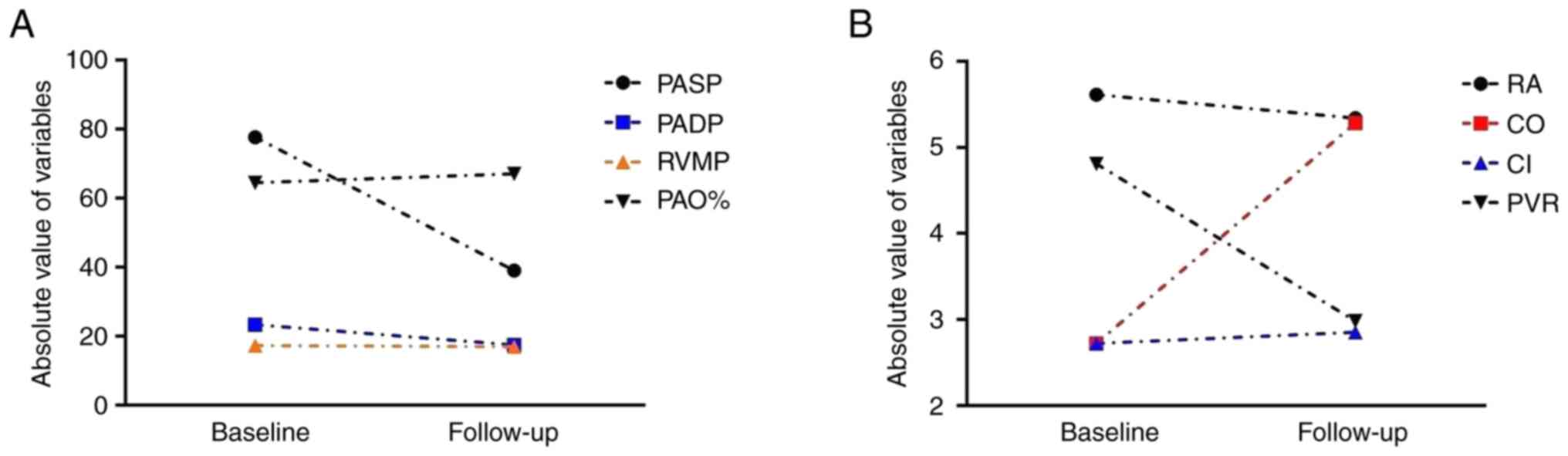 | Figure 4Changes of right heart catheterization
hemodynamic characteristics from baseline to follow-up. (A) PASP,
PADP, RVMP and PAO%, and (B) RA, CO, CI and PVR from baseline to
follow-up. PASP, pulmonary artery systolic pressure; PADP,
pulmonary artery diastolic pressure; RVMP, right ventricular mean
pressure; PAO%, pulmonary arterial oxygen saturation percentage;
RA, right atrial mean pressure; CO, cardiac output; CI, cardiac
index; PVR, pulmonary vascular resistance. |
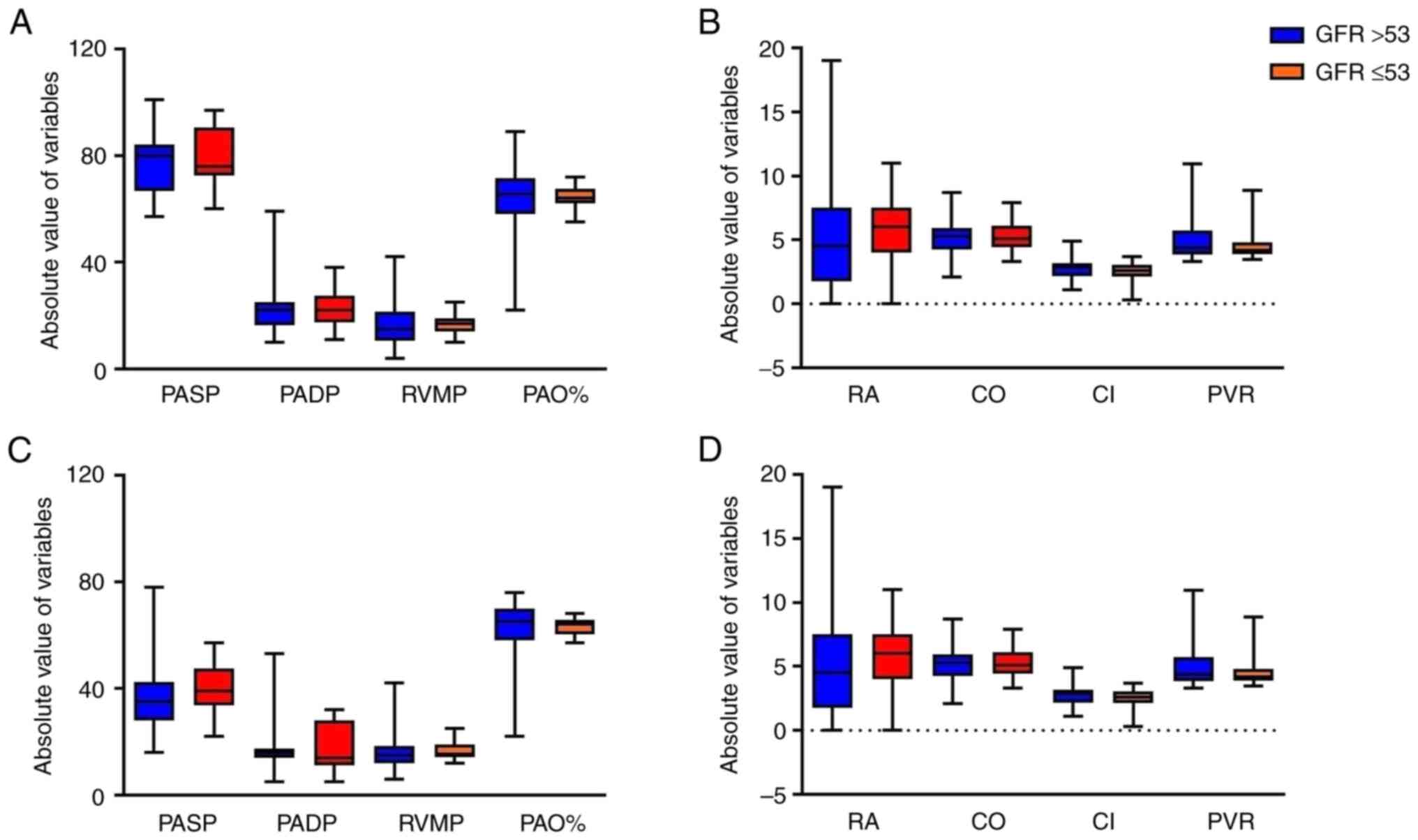 | Figure 5Comparison of right heart
catheterization hemodynamic characteristics between the two groups
according to GFR. (A) PASP, PADP, RVMP and PAO% and (B) RA, CO, CI
and PVR on admission. (C) PASP, PADP, RV and PAO% and (D) RA, CO,
CI and PVR at final follow-up. The data are presented as box plots
with a median bar, 25 and 75% quartiles and range of values. GFR,
glomerular filtration rate; PASP, pulmonary artery systolic
pressure; PADP, pulmonary artery diastolic pressure; RVMP, right
ventricular mean pressure; PAO%, pulmonary arterial oxygen
saturation percentage; RA, right atrial mean pressure; CO, cardiac
output; CI, cardiac index; PVR, pulmonary vascular resistance. |
Major adverse events at final
follow-up
As shown in Table
III, the proportion of MAEs in the GFR >53 group was
significantly lower than that in the GFR ≤53 group at final
follow-up (P=0.002). In the univariate logistic regression
analysis, MAEs were associated with the GFR (odds ratio, 0.730;
P=0.001) and NT-proBNP (odds ratio, 1.004; P=0.010) (Table IV). The GFR (odds ratio, 0.693;
P=0.008) was demonstrated to be independently associated with MAEs
at the final follow-up in the multivariate logistic regression
analysis (Table IV).
 | Table IIIMajor adverse events at final
follow-up. |
Table III
Major adverse events at final
follow-up.
| Events | GFR >53
(n=26) | GFR ≤53 (n=21) | P-value |
|---|
| MAEs | 12 (46.15) | 19 (90.48) | 0.002 |
| All-cause
mortality | 1 (3.85) | 5 (23.81) | 0.076 |
| Death from RHF | 1 (3.85) | 4 (16.80) | 0.158 |
| Rehospitalization
associated with RHF | 10 (38.46) | 10 (47.62) | 0.566 |
 | Table IVUnivariate and multivariate logistic
regression analysis of MAEs at final follow-up. |
Table IV
Univariate and multivariate logistic
regression analysis of MAEs at final follow-up.
| | Univariate | Multivariate |
|---|
| Variable | Odds ratio (95%
CI) | P-value | Odds ratio (95%
CI) | P-value |
|---|
| 6MWD | 0.975
(0.944-1.007) | 0.128 | - | - |
| Heart rate | 1.017
(0.900-1.148) | 0.729 | - | - |
| GFR | 0.730
(0.603-0.885) | 0.001 | 0.693
(0.529-0.908) | 0.008 |
| NT-proBNP | 1.004
(1.001-1.008) | 0.010 | 1.002
(0.999-1.006) | 0.222 |
Discussion
To the best of our knowledge, this is the first
study that has investigated the impact of different GFR levels on
the clinical and prognostic outcome of patients with CTEPH who have
undergone BPA. The results in the present study showed that MAEs
during follow-up can be independently predicted by renal function,
as indicated by GFR.
According to a previous study, even symptoms of mild
renal dysfunction with an estimated GFR of between 60 and 80
ml/min/1.73 m2 are closely associated with
cardiovascular disease, and all-cause mortality can be partly
attributed to CKD (23). GFR is an
important indicator used to assess the severity of RHF. RHF leads
to reductions in cardiac output and blood flow to the kidneys, and
the reduction in renal perfusion causes the GFR to decline. A
markedly reduced GFR indicates that RHF has progressed to an
advanced stage with critical underfilling of the arterial
circulation. Monitoring the GFR allows clinicians to classify the
degree of RHF as mild, moderate or severe. A progressive decline in
GFR serves as a warning of the exacerbation of RHF and systemic
congestion. Thus, monitoring GFR over time provides crucial
information about the status of right heart function and fluid
overload in patients with RHF (24). There is evidence to support the
notion that renal function is associated with outcome in patients
with pulmonary arterial hypertension (25). Venous congestion, caused by
PH-associated RHF, is generally considered to be one of the main
reasons for renal dysfunction (26). Insufficient cardiac output,
congestion of the retrograde veins and feedback from the secondary
neurohumoral system are suggested to be the major mechanisms
leading to malperfusion (27). The
procedure used to assess renal function in patients with CTEPH who
undergo BPA deserves further discussion.
It is currently considered that BPA should be
carried out in stages involving several sessions at intervals of
4-8 weeks (28). BPA can result in
a distinct reduction in reperfusion injury but has the
disadvantages of increasing the number of interventions, and the
utilization of iodine contrast agents and radiation. According to
the present research, following BPA, the renal function of patients
significantly improved and no periinterventional AKI was detected.
Three mechanisms for the improvement may be proposed as follows: i)
Following strict periprocedural renal protection guidelines,
including nephroprotective drugs, hydration and low-dose contrast
media prior to each BPA; ii) the BPA therapy effectively increases
circulating blood volume, and the appropriate perfusion of tissues
is preserved, improving renal function; iii) venous congestion,
known to be a major cause of worsening renal function in patients
with decompensated heart failure (29,30),
is reduced.
Previous studies have examined the frequent
coincidence of cardiac disease and renal dysfunction, including
chronic renal impairment as a comorbidity of PH. It is possible
that chronic increases in the right ventricular afterload may
ultimately RHF, resulting in backward failure and congestion of the
veins, further contributing to renal failure. This may ultimately
result is reduction of the estimated GFR (31). It has been reported that venous
congestion following a reduction in the circulating blood volume is
potentially the main cause of renal insufficiency (26). Based on the analysis of a cohort of
patients with pulmonary arterial hypertension, Bitker et al
(6) reported that CI and right
atrial pressure correlated with estimated GFR at baseline.
Following BPA therapy, the improvement of pulmonary hemodynamics
led to decreased pressure in the right heart, which was reflected
in the reduction of NT-proBNP concentration and venous congestion
and thereby improved renal function in two other studies (32,33).
Importantly, these findings indicate that there is a balance
between the advantage mediated by hemodynamic improvements,
possibly through improved venous congestion, and the disadvantage
of renal function impairment.
In addition to expanding the coronary and lung
circulation, BNP reduces myocardial oxygen consumption and
increases coronary blood flow, which prevents cardiac remodeling
(34,35). The present study revealed that the
serum levels of troponin I and NT-proBNP were significantly
decreased in the GFR >53 group at final follow-up. Thus, we
hypothesize that an elevated GFR is an indicator of improved
perfusion of the myocardium, limited myocardial impairment size,
ameliorated dysfunction of the heart and reduced ventricular
remodeling in patients with CTEPH who receive BPA. The findings
support this, as they suggest that all patients with CTEPH after
BPA may experience a significant improvement in renal function. In
the cohort in the present study, the GFR exhibited an independent
association with clinical prognosis at follow-up, which may be
interpreted as indicating the predominant role of venous congestion
in the context of renal function.
We hypothesize that patients with CTEPH and less
impairment of the kidney function at baseline may have greater
physiological reserves enabling them to endure the intraprocedural
hemodynamic shifts during BPA. Preserved renal perfusion and the
avoidance of AKI during BPA may portend improved postoperative
recovery of kidney function. This mitigates the risk of exacerbated
CKD progression, which has established associations with morbidity
and mortality. Therefore, less compromised baseline renal function
in patients with CTEPH undergoing BPA may confer protection against
perioperative renal decompensation, which may translate to more
optimal long-term outcomes. However, further research is required
to precisely define the relationship between pre-procedural renal
function, the risk of worsening kidney dysfunction with BPA, and
post-BPA prognosis.
The present study has certain limitations. Firstly,
a relatively small number of patients were included in the study.
Secondly, considering the retrospective nature of the study and its
single-center design, a significant referral bias may exist.
Thirdly, few data were collected on long-term events and follow-up,
and it is planned to collect more of such data in a future study.
In conclusion, the present study indicated that besides being
associated with right ventricular function, GFR is also a
prognostic marker in CTEPH treated with BPA.
Acknowledgements
Not applicable.
Funding
Funding: The study was funded by the National Natural Science
Foundation of China (grant no. 81970297).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YuZ and XW were responsible for the research concept
and study design. YuZ, YoZ, CL and XW acquired the data and
organized the study. YuZ performed data management and CL performed
the statistical analysis. YoZ and XW interpreted the data. CL and
XW supervised or provided mentorship. YuZ and XW confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Research
Ethics Committee of The Second Affiliated Hospital of Harbin
Medical University (approval nos. SYDWGZR-2010-152 and
SYDWGZR-2013-088) in compliance with the 1975 Declaration of
Helsinki. Each patient provided written informed consent for
inclusion in this retrospective study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Teerapuncharoen K and Bag R: Chronic
thromboembolic pulmonary hypertension. Lung. 200:283–299.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Galiè N, Humbert M, Vachiery J, Gibbs S,
Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A,
Beghetti M, et al: 2015 ESC/ERS guidelines for the diagnosis and
treatment of pulmonary hypertension. Rev Esp Cardiol (Engl Ed).
69(177)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Delcroix M, Lang I, Pepke-Zaba J, Jansa P,
D'Armini A, Snijder R, Bresser P, Torbicki A, Mellemkjaer S,
Lewczuk J, et al: Long-term outcome of patients with chronic
thromboembolic pulmonary hypertension: Results from an
international prospective registry. Circulation. 133:859–871.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pepke-Zaba J, Delcroix M, Lang I, Mayer E,
Jansa P, Ambroz D, Treacy C, D'Armini A, Morsolini M, Snijder R, et
al: Chronic thromboembolic pulmonary hypertension (cteph): Results
from an international prospective registry. Circulation.
124:1973–1981. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mizoguchi H, Ogawa A, Munemasa M, Mikouchi
H, Ito H and Matsubara H: Refined balloon pulmonary angioplasty for
inoperable patients with chronic thromboembolic pulmonary
hypertension. Circ Cardiovasc Interv. 5:748–755. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bitker L, Sens F, Payet C, Turquier S,
Duclos A, Cottin V and Juillard L: Presence of kidney disease as an
outcome predictor in patients with pulmonary arterial hypertension.
Am J Nephrol. 47:134–143. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shah S, Thenappan T, Rich S, Tian L,
Archer S and Gomberg-Maitland M: Association of serum creatinine
with abnormal hemodynamics and mortality in pulmonary arterial
hypertension. Circulation. 117:2475–2483. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Andreassen A, Ragnarsson A, Gude E, Geiran
O and Andersen R: Balloon pulmonary angioplasty in patients with
inoperable chronic thromboembolic pulmonary hypertension. Heart.
99:1415–1420. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Srisawasdi P, Vanavanan S,
Charoenpanichkit C and Kroll M: The effect of renal dysfunction on
bnp, nt-probnp, and their ratio. Am J Clin Pathol. 133:14–23.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Anwaruddin S, Lloyd-Jones DM, Baggish A,
Chen A, Krauser D, Tung R, Chae C and Januzzi JL Jr: Renal
function, congestive heart failure, and amino-terminal pro-brain
natriuretic peptide measurement: Results from the ProBNP
Investigation of Dyspnea in the Emergency Department (PRIDE) study.
J Am Coll Cardiol. 47:91–97. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang Y, Gu X, Fan W, Fan Y, Li W and Fu X:
Effects of recombinant human brain natriuretic peptide on renal
function in patients with acute heart failure following myocardial
infarction. Am J Transl Res. 8:239–245. 2016.PubMed/NCBI
|
|
12
|
Ogo T, Shimokawahara H, Kinoshita H, Sakao
S, Abe K, Matoba S, Motoki H, Takama N, Ako J, Ikeda Y, et al:
Selexipag for the treatment of chronic thromboembolic pulmonary
hypertension. Eur Respir J. 60(2101694)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Humbert M, Kovacs G, Hoeper MM,
Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS,
Escribano-Subias P, Ferrari P, et al: 2022 esc/ers guidelines for
the diagnosis and treatment of pulmonary hypertension. Eur Respir
J. 61:2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wiedenroth C, Ghofrani H, Adameit M,
Breithecker A, Haas M, Kriechbaum S, Rieth A, Hamm C, Mayer E, Guth
S and Liebetrau C: Sequential treatment with riociguat and balloon
pulmonary angioplasty for patients with inoperable chronic
thromboembolic pulmonary hypertension. Pulm Circ.
8(2045894018783996)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Galiè N, Humbert M, Vachiery JL, Gibbs S,
Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A,
Beghetti M, et al: 2015 ESC/ERS guidelines for the diagnosis and
treatment of pulmonary hypertension: The joint task force for the
diagnosis and treatment of pulmonary hypertension of the european
society of cardiology (ESC) and the european respiratory society
(ERS): Endorsed by: Association for european paediatric and
congenital cardiology (AEPC), international society for heart and
lung transplantation (ISHLT). Eur Heart J. 37:67–119.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
McConnell MV, Solomon SD, Rayan ME, Come
PC, Goldhaber SZ and Lee RT: Regional right ventricular dysfunction
detected by echocardiography in acute pulmonary embolism. Am J
Cardiol. 78:469–473. 1996.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qu C, Feng W, Zhao Q, Liu Q, Luo X, Wang
G, Sun M, Yao Z, Sun Y, Hou S, et al: Effect of levosimendan on
acute decompensated right heart failure in patients with connective
tissue disease-associated pulmonary arterial hypertension. Front
Med. 9(778620)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Olsson K, Wiedenroth C, Kamp J,
Breithecker A, Fuge J, Krombach G, Haas M, Hamm C, Kramm T, Guth S,
et al: Balloon pulmonary angioplasty for inoperable patients with
chronic thromboembolic pulmonary hypertension: The initial german
experience. Eur Respir J. 49(1602409)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Smilde T, van Veldhuisen D, Navis G, Voors
A and Hillege H: Drawbacks and prognostic value of formulas
estimating renal function in patients with chronic heart failure
and systolic dysfunction. Circulation. 114:1572–1580.
2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
National Kidney Foundation. K/DOQI
clinical practice guidelines for chronic kidney disease:
Evaluation, classification, and stratification. Am J Kidney Dis.
39(2 Suppl 1):S1–S266. 2002.PubMed/NCBI
|
|
21
|
Mehta R, Kellum J, Shah S, Molitoris B,
Ronco C, Warnock D and Levin A: Acute kidney injury network: Report
of an initiative to improve outcomes in acute kidney injury. Crit
Care. 11(R31)2007.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Pistolesi V, Regolisti G, Morabito S,
Gandolfini I, Corrado S, Piotti G and Fiaccadori E: Contrast medium
induced acute kidney injury: A narrative review. J Nephrol.
31:797–812. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Manjunath G, Tighiouart H, Ibrahim H,
MacLeod B, Salem DN, Griffith JL, Coresh J, Levey AS and Sarnak MJ:
Level of kidney function as a risk factor for atherosclerotic
cardiovascular outcomes in the community. J Am Coll Cardiol.
41:47–55. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Damman K and Testani JM: The kidney in
heart failure: An update. Eur Heart J. 36:1437–1444.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Navaneethan SD, Wehbe E, Heresi GA, Gaur
V, Minai OA, Arrigain S, Nally JV Jr, Schold JD, Rahman M and Dweik
RA: Presence and outcomes of kidney disease in patients with
pulmonary hypertension. Clin J Am Soc Nephrol. 9:855–863.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Damman K, van Deursen VM, Navis G, Voors
AA, van Veldhuisen DJ and Hillege HL: Increased central venous
pressure is associated with impaired renal function and mortality
in a broad spectrum of patients with cardiovascular disease. J Am
Coll Cardiol. 53:582–588. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Felker GM, Adams KF, Konstam MA, O'Connor
CM and Gheorghiade M: The problem of decompensated heart failure:
Nomenclature, classification, and risk stratification. Am Heart J.
145:S18–S25. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Muller D and Liebetrau C: Percutaneous
treatment of chronic thromboembolic pulmonary hypertension (CTEPH).
EuroIntervention. 12 (Suppl X):X35–X43. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kimura M, Kataoka M, Kawakami T, Inohara
T, Takei M and Fukuda K: Balloon pulmonary angioplasty using
contrast agents improves impaired renal function in patients with
chronic thromboembolic pulmonary hypertension. Int J Cardiol.
188:41–42. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mullens W, Abrahams Z, Francis GS, Sokos
G, Taylor DO, Starling RC, Young JB and Tang WHW: Importance of
venous congestion for worsening of renal function in advanced
decompensated heart failure. J Am Coll Cardiol. 53:589–596.
2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Vonk-Noordegraaf A, Haddad F, Chin K,
Forfia P, Kawut S, Lumens J, Naeije R, Newman J, Oudiz R,
Provencher S, et al: Right heart adaptation to pulmonary arterial
hypertension: Physiology and pathobiology. J Am Coll Cardiol. 62
(Suppl 25):D22–D33. 2013.PubMed/NCBI
|
|
32
|
Kriechbaum S, Wiedenroth C, Hesse M,
Ajnwojner R, Keller T, Sebastian Wolter J, Haas M, Roller F,
Breithecker A, Rieth A, et al: Development of renal function during
staged balloon pulmonary angioplasty for inoperable chronic
thromboembolic pulmonary hypertension. Scand J Clin Lab Invest.
79:268–275. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li N, Jin H, Song Z, Bai C, Cui Y and Gao
Y: Protective effect of recombinant human brain natriuretic peptide
on acute renal injury induced by endotoxin in canines. Cell Biochem
Biophys. 70:1317–1324. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Miao Z, Hou A, Zang H, Huang R, Zheng X,
Lin H, Wang W, Hou P, Xia F and Li Z: Effects of recombinant human
brain natriuretic peptide on the prognosis of patients with acute
anterior myocardial infarction undergoing primary percutaneous
coronary intervention: A prospective, multi-center, randomized
clinical trial. J Thorac Dis. 9:54–63. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen H, Martin F, Gibbons R, Schirger J,
Wright R, Schears R, Redfield M, Simari R, Lerman A, Cataliotti A
and Burnett JC Jr: Low-dose nesiritide in human anterior myocardial
infarction suppresses aldosterone and preserves ventricular
function and structure: A proof of concept study. Heart.
95:1315–1319. 2009.PubMed/NCBI View Article : Google Scholar
|