Introduction
Atherosclerosis (AS), an important factor in
cardiovascular disease, is characterized by the buildup of lipids,
immune cells and extracellular matrix elements in the vascular
intima, resulting in plaque development and vascular remodeling
(1,2). Senescence-associated secretory
phenotype, a proinflammatory phenotype that contributes to the
chronic inflammatory milieu within atherosclerotic plaques, has
emerged as a key factor in AS onset and progression (3,4).
Cellular senescence causes the malfunction of endothelial cells in
blood vessels (5). Targeting
cellular senescence could be a potential treatment strategy for AS
and its associated side effects.
Coptisine is a bioactive alkaloid compound that is
primarily found in various plant species, especially those in the
Coptis genus in the family Ranunculaceae, which have been
reported to exhibit various pharmacological effects, including
anti-inflammatory, anti-oxidant and anti-atherosclerotic properties
(6,7). Previous studies have demonstrated the
potential of coptisine to ameliorate endothelial dysfunction and
inhibit vascular smooth muscle cell proliferation (8,9).
Additionally, coptisine exerts protective effects against cellular
senescence (10). Although no
studies have confirmed the effect of coptisine on endothelial cell
senescence, such as in human umbilical vein endothelial cells
(HUVECs), which are commonly used in in vitro cell models of
AS in the presence of angiotensin II (Ang II), its antioxidative
and anti-inflammatory properties indicates its potential anti-aging
role in these cells. This finding warrants further in-depth
investigation of the underlying mechanisms.
Accumulating evidence suggests that long non-coding
RNAs (lncRNAs) and microRNAs (miRNAs) play crucial roles in
regulating cellular senescence and AS (11). Particularly, lncRNAs can function
as competing endogenous RNAs that sponge miRNAs, thereby modulating
the expression of miRNA target genes and influencing cellular
processes relevant to AS (12).
The lncRNA small nucleolar RNA host gene 12 (SNHG12) is
downregulated in AS and is an effective AS inhibitor (13). Studies on miR-603 have focused on
its effects on tumor growth (14,15).
However, because of its effects on inflammation (14), it was hypothesized that it may
regulate AS.
Nicotinamide phosphoribosyltransferase (NAMPT) is a
key enzyme involved in the biosynthesis of nicotinamide adenine
dinucleotide (NAD+), a crucial coenzyme in cellular energy
metabolism and redox reactions (16). Altered NAD(+) homeostasis has been
implicated in the development of age-related diseases, including AS
(17). Sirtuin 3 (SIRT3) is an
NAD+-dependent protein deacetylase that removes acetyl groups from
other proteins in the presence of the coenzyme NAD+ (18) and regulates cellular processes such
as inflammation, oxidative stress and apoptosis (19,20)
by deacetylating target proteins, including p53(21). The NAD(+)/SIRT3/p53 signaling axis
has been implicated in endothelial function regulation and AS
development (22); however, its
potential involvement in the protective effects of coptisine
remains to be elucidated.
In summary, the present study highlighted the
importance of coptisine as a potential therapeutic agent for
combating AS and its associated complications by targeting cellular
senescence. Identifying the SNHG12/miR-603/NAMPT axis as a critical
mediator of the anti-senescence effects of coptisine provides a
novel target for future therapeutic interventions and broadens our
understanding of the molecular mechanisms governing endothelial
cell function and dysfunction in AS.
Materials and methods
Cell culture, treatment and
transfection
HUVECs were purchased from ATCC and cultured in
endothelial cell growth medium (Procell Life Science &
Technology Co., Ltd.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.). Cells were maintained at
37˚C and 5% CO2. HUVECs were exposed to different
concentrations of coptisine (MilliporeSigma) dissolved in dimethyl
sulfoxide (MilliporeSigma) for 24 h at 37˚C. Coptisine at 0-100 µM
in HUVECs for 24 h at 37˚C was used to assay the effect of
coptisine itself on cell viability. HUVECs were pre-treated with
6.25 (low-dose, L), 12 (medium-dose, M) and 25 µM (high-dose, H)
coptisine for 6 h before Ang II treatment. After pre-treatment,
cells were exposed to 1 µM Ang II in the presence of coptisine for
an additional 24 or 48 h at 37˚C. SNHG12 small interfering
(si)RNAs, miR-603 mimic/inhibitor and NAMPT overexpression vector
(ov-NAMPT) constructed using the pCMV6-Entry backbone and their
negative controls (NCs) were from Guangzhou RiboBio Co., Ltd. and
were transfected into HUVECs using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 4 h and continued
in culture at 37˚C for 24 or 48 h after fresh medium change. The
si-SNHG12, miRNAs and their NC sequences used were as follows:
si-SNHG12-1, CTTTAAGATTCATGTTACATT; si-SNHG12-2,
GGGTAATGACAGTGATGAATT; si-SNHG12-3, GGATGACTGACTTAGTCTATT; si-NC,
TTGTACTACACAAAAGTACTG; miR-603 mimic, CACACACTGCAATTACTTTTGC; mimic
NC, ACTGTACCACAGTTTCCCTTAA; miR-603 inhibitor,
GCAAAAGTAATTGCAGTGTGTG; and inhibitor NC,
CAGTACTTTTGTGTAGTACAA.
Cell viability assay
Following the manufacturer's instructions, HUVECs
were cultured for 24 h and incubated for 60 min with 10 ml Cell
Counting Kit-8 (CCK8; Beijing Solarbio Science & Technology
Co., Ltd.) reagent every 24 h for 48 h at 37˚C. Optical density was
determined using a spectrophotometer (Thermo Fisher Scientific,
Inc.).
Senescence-associated β-galactosidase
(β-Gal) staining
A senescence-associated β-Gal staining kit (Beyotime
Institute of Biotechnology) was used to measure cellular senescence
in accordance with the manufacturer's instructions. HUVECs were
seeded in 6-well plates and treated as previously described.
Following treatment, cells were rinsed with phosphate-buffered
saline (PBS), fixed for 15 min with 4% paraformaldehyde at 21˚C and
then stained overnight at 37˚C. A light microscope (Olympus
Corporation) was used to count the blue-stained cells and calculate
the proportion of senescent cells.
Apoptosis detection
The Annexin V-FITC/propidium iodide (PI) apoptosis
detection kit (BD Biosciences) was used to measure apoptosis.
Following treatment, the HUVECs were rinsed with cold PBS and
resuspended in a binding buffer. The cells were treated with PI and
Annexin V-FITC for 15 min at 21˚C in the dark. Flow cytometry (BD
FACSCalibur™ flow cytometer; BD Biosciences) was used to
investigate apoptosis and the FlowJo software (version 10.6.2;
FlowJo LLC) was used to calculate the percentage of early + late
apoptotic cells.
NAD(+)/NADH (H for hydrogen)
analysis
Following the manufacturer's instructions, after
microglia were lysed using the extract, the NAD(+)/NADH ratio was
measured using an NAD(+) kit (Beyotime Institute of Biotechnology).
The extraction solution was used to construct a standard curve. The
sample and enzyme working solution were added to the 96-well plate,
incubated at 37˚C and the chromogenic agent was added for further
incubation. A spectrophotometer (UV-8000A; Shanghai Metash
Instruments Co., Ltd.) was used to measure absorbance at 450
nm.
Enzyme-linked immunosorbent assay
(ELISA)
Inflammatory markers, including interleukin-1 β
(IL-1β; cat. no. SLB50), IL-6 (cat. no. S6050) and tumor necrosis
factor-α (TNF-α; cat. no. STA00D) were measured using ELISA kits
(R&D Systems, Inc.) following the manufacturer's instructions.
After treatment, cell culture supernatants were collected and
centrifuged at 1,000 x g for 5 min at 21˚C to remove debris. The
supernatants were then used for ELISA and the absorbance was
measured at 450 nm using a microplate reader (BioTek Instruments,
Inc.). The concentrations of the inflammatory markers were
calculated based on standard curves.
Nucleocytoplasmic separation
experiments
HUVECs were harvested and washed with cold PBS. The
cells were then resuspended in a hypotonic buffer containing 10
Tris-HCl (pH 7.4), 10 NaCl and 3 mM MgCl2. After a
10-min incubation on ice, 10% NP-40 (MilliporeSigma) was added to
the cell suspension, followed by vortexing for 10 sec. The cell
lysate was centrifuged at 3,000 x g for 10 min at 4˚C. The
supernatant, which contained the cytoplasmic fraction, was
carefully transferred to a new tube. The pellet, which contains the
nuclear fraction, was washed with the hypotonic buffer to remove
any remaining cytoplasmic contaminants. Total RNA from both
the cytoplasmic and nuclear fractions was extracted using the
TRIzol® reagent (Thermo Fisher Scientific, Inc.),
following the manufacturer's instructions. Cytoplasmic and nuclear
RNA were isolated from HUVECs for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis using a cytoplasmic and nuclear RNA purification kit
(Norgen Biotek Corp.).
RT-qPCR assay
Total RNA was isolated from HUVECs
(1x106) using TRIzol reagent (Thermo Fisher Scientific,
Inc.), following the manufacturer's instructions. The RNA
concentration and purity were measured using a NanoDrop
spectrophotometer (Thermo Fisher Scientific) according to the
manufacturer's instructions. A High-Capacity complementary DNA
Reverse Transcription Kit (Applied Biosystems) was used to generate
complementary DNA according to the manufacturer's instructions. On
the Applied Biosystems 7500 Real-Time PCR System (Applied
Biosystems), RT-qPCR was carried out using the Power SYBR Green PCR
Master Mix (Applied Biosystems) according to the manufacturer's
instructions. PCR amplification was performed under the following
conditions: 95˚C for an initial 30 sec, followed by 40 cycles at
95˚ for 5 sec and 60˚C for 30 sec. The relative expression of
target genes was calculated using the 2-ΔΔCq method
(23) and normalized to the
expression of β-actin or U6 small nuclear RNA as internal controls.
The primer sequences used were as follows: SNHG12 forward,
5'-ACAGGCGGATAAAACGGTCC-3' and reverse, 5'-AGTACGCCGGGATCTCTGTA-3';
miR-603 forward, 5'-ACACTCCAGCTGGGCACACACTGCAATTAC-3' and reverse,
5'-CTCAACTGGTGTCGTGGA-3'; NAMPT forward, 5'-ACCATAACAGCTTGGGGGAA-3'
and reverse, 5'-CTGACCACAGATACAGGCAC-3'; β-actin forward,
5'-AGCGAGCATCCCCCAAAGTT-3' and reverse, 5'-GGGCACGAAGGCTCATCATT-3';
and U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'. These experiments were repeated three
times.
Binding site analysis and
dual-luciferase reporter gene assay
The LncBase v 3.0 (https://diana.e-ce.uth.gr/lncbasev3/interactions)
and miRanda v3.3a (http://www.microrna.org/) were used to analyze the
potential binding sites between lncRNA and miRNA, and themiRDB
(https://mirdb.org/) database was used to identify the
downstream targets of miRNA. Interactions between miR-603 with
NAMPT mRNA 3'UTR and SNHG12 were investigated using the
dual-luciferase reporter gene assay. HUVECs were co-transfected
with miR-603 mimic or mimic NC and either wild type (WT) or mutant
(mut) NAMPT 3'UTR or SNHG12 reporter plasmids using
Lipofectamine® 3000. The plasmids were synthesized by
Guangzhou RiboBio Co., Ltd., and inserted into
psiCHECK™-2 vector plasmids (Promega Corporation), and
pRL-SV40 reporter vector plasmids (Promega Corporation) were
acquired. After 48 h, the lysed cells were used to assess
luciferase activity using a Dual-Luciferase Reporter Assay System
(Promega Corporation) and GloMax Luminometer (Promega Corporation).
To increase transfection effectiveness, firefly luciferase activity
was normalized to Renilla luciferase activity.
RNA pull-down assay
The interaction between SNHG12 and miR-603 was
assessed using an RNA pull-down assay. Biotinylated miR-603 mimics
and biotinylated NC mimics were synthesized by Guangzhou RiboBio
Co., Ltd. The plasmid backbone employed was pcDNA3.1 (Guangzhou
RiboBio Co., Ltd.) used at a concentration of 100 ng/µl. HUVECs
were lysed and incubated (4˚C) with the biotinylated RNA probes for
2 h. Following this, 50 µl streptavidin agarose beads (Thermo
Fisher Scientific, Inc.) was added to the mixture and incubation
continued for an additional 2 h at 4˚C. The centrifugation steps
were conducted at 1,000 x g for 5 min at 4˚C, both after the
initial incubation and following the addition of the beads. After
these steps, the beads were washed and the bound RNAs were
extracted using the PureLink RNA Mini Kit (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The beads were washed and the bound RNAs were
extracted using TRIzol (Thermo Fisher Scientific, Inc.) for RT-qPCR
analysis.
Western blotting
HUVECs were lysed using RIPA buffer (Beyotime
Institute of Biotechnology). The protein concentration in the
sample was measured using a bicinchoninic acid kit (Beyotime
Institute of Biotechnology). Proteins (20 µg) were separated by
SDS-PAGE on 10% gels and transferred to polyvinylidene fluoride
membranes (MilliporeSigma), before being blocked with 5% bovine
serum albumin for 1 h at 21˚C (Thermo Fisher Scientific, Inc.) and
incubated with primary antibodies against NAMPT (1:1,000;
MA5-24108; Invitrogen), SIRT3 (1:1,000; ab217319; Abcam), p53
(1:1,000; ab32049; Abcam) and β-actin (1:1,000; ab8227; Abcam)
overnight at 4˚C. The membranes were washed and then incubated for
1 h at 21˚C with horseradish peroxidase-conjugated secondary
antibodies (1:5,000; ab97051; Abcam). An enhanced chemiluminescence
substrate was used to detect the protein bands and a ChemiDoc XRS+
imaging system (Bio-Rad Laboratories, Inc.) was used for
visualization. Gray values were quantified using ImageJ software
(National Institutes of Health). NAMPT, SIRT3 and p53 protein
levels were adjusted to β-actin as the internal reference.
Statistical analysis
All experiments were performed at least three times
and data were presented as mean ± standard deviation. Statistical
analyses were performed using GraphPad Prism 9 software (GraphPad
Software; Dotmatics). Comparisons between groups were conducted
using one-way analysis of variance, followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Investigation of the role of SNHG12
and coptisine concentrations
Initially, at different coptisine concentrations
(Fig. 1A), HUVECs showed a slight
decrease in activity start at a concentration of 50 µM (Fig. 1B). Concentrations of 6.25, 12 and
25 µM (48 h) were chosen for subsequent experiments because they
did not have a significant effect on cell viability. The results
showed a decrease in SNHG12 expression upon Ang II induction,
whereas coptisine treatment at different concentrations induced a
dose-dependent increase in SNHG12 expression (Fig. 1C). Meanwhile, Ang II induction
resulted in decreased cell viability (Fig. 1D) and NAD(+)/NADH ratio (Fig. 1E), as well as increased levels of
senescence (Fig. 1F), apoptosis
(Fig. 1G) and inflammation
(increased IL-1β, IL-6 and TNF-α; Fig.
1H). Treatment with coptisine attenuated the effects of Ang II
induction, with the improvements becoming more pronounced as
coptisine concentration increased.
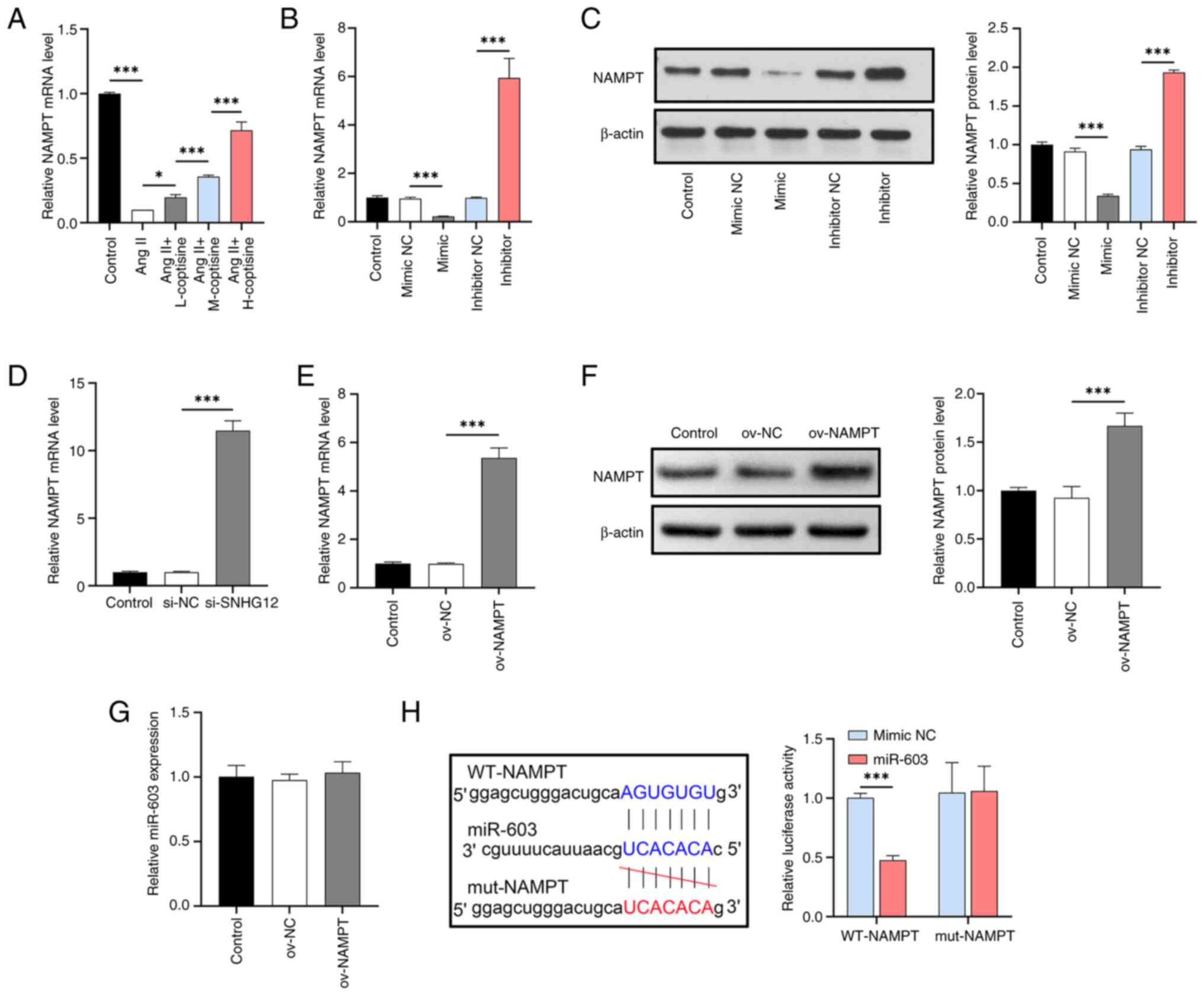
 | Figure 1Evaluation of the effects of SNHG12
and varying coptisine concentrations on HUVECs. (A) Chemical
structure of coptisine. (B) CCK8 assay analyzing the impact of
different doses of coptisine on the viability of HUVECs. (C)
RT-qPCR analysis evaluating the influence of various coptisine
doses on SNHG12 expression under Ang II induction. (D) CCK8 assay
exploring the effects of different coptisine doses on HUVEC
viability following Ang II induction. (E) NAD(+) assay kit used to
determine the NAD(+)/NADH ratio. (F) β-Gal assay analyzing the
effects of coptisine on cellular senescence in HUVECs induced by
Ang II. Magnification, x400. (G) Flow cytometry examining the
effect of coptisine on apoptosis in HUVECs induced by Ang II. (H)
ELISA investigation of the influence of coptisine on the
inflammatory cytokine levels (IL-1β, TNF-α and IL-6) in HUVECs
following Ang II induction. *P<0.05,
**P<0.01 and ***P<0.001. SNHG12, small
nucleolar RNA host gene 12; HUVECs, human umbilical vein
endothelial cells; CCK8, Cell Counting Kit-8; RT-qPCR, real-time
quantitative polymerase chain reaction; Ang II, angiotensin II;
NAD, nicotinamide adenine dinucleotide; β-Gal, β-galactosidase;
ELISA, enzyme-linked immunosorbent assay; IL, interleukin; TNF-α,
tumor necrosis factor-α; L, 6.25 µM; M,12 µM; H, 25 µM. |
Coptisine inhibits cell senescence and
apoptosis via the SNHG12 pathway
The results of nucleoplasmic segregation experiments
showed that, consistent with the positive control β-actin and in
contrast to the positive control U6, lncRNA SNHG12 was mainly
expressed in the cytoplasm of HUVECs (Fig. 2A). For silencing SNHG12 expression,
si-SNHG12-1 was selected for optimal inhibition efficiency
(Fig. 2B). It was found that
SNHG12 knockdown had no significant effect on the activity of
coptisine (25 µM)-treated HUVECs (Fig.
2C). Subsequent results showed that SNHG12 interference
counteracted the protective effects of coptisine against Ang
II-induced decreases in cell viability (Fig. 2D) and NAD(+)/NADH ratio (Fig. 2E), as well as increased senescence
(Fig. 2F), apoptosis (Fig. 2G) and inflammation (Fig. 2H). The maximum safe concentration
for coptisine dose selection was 25 µM.
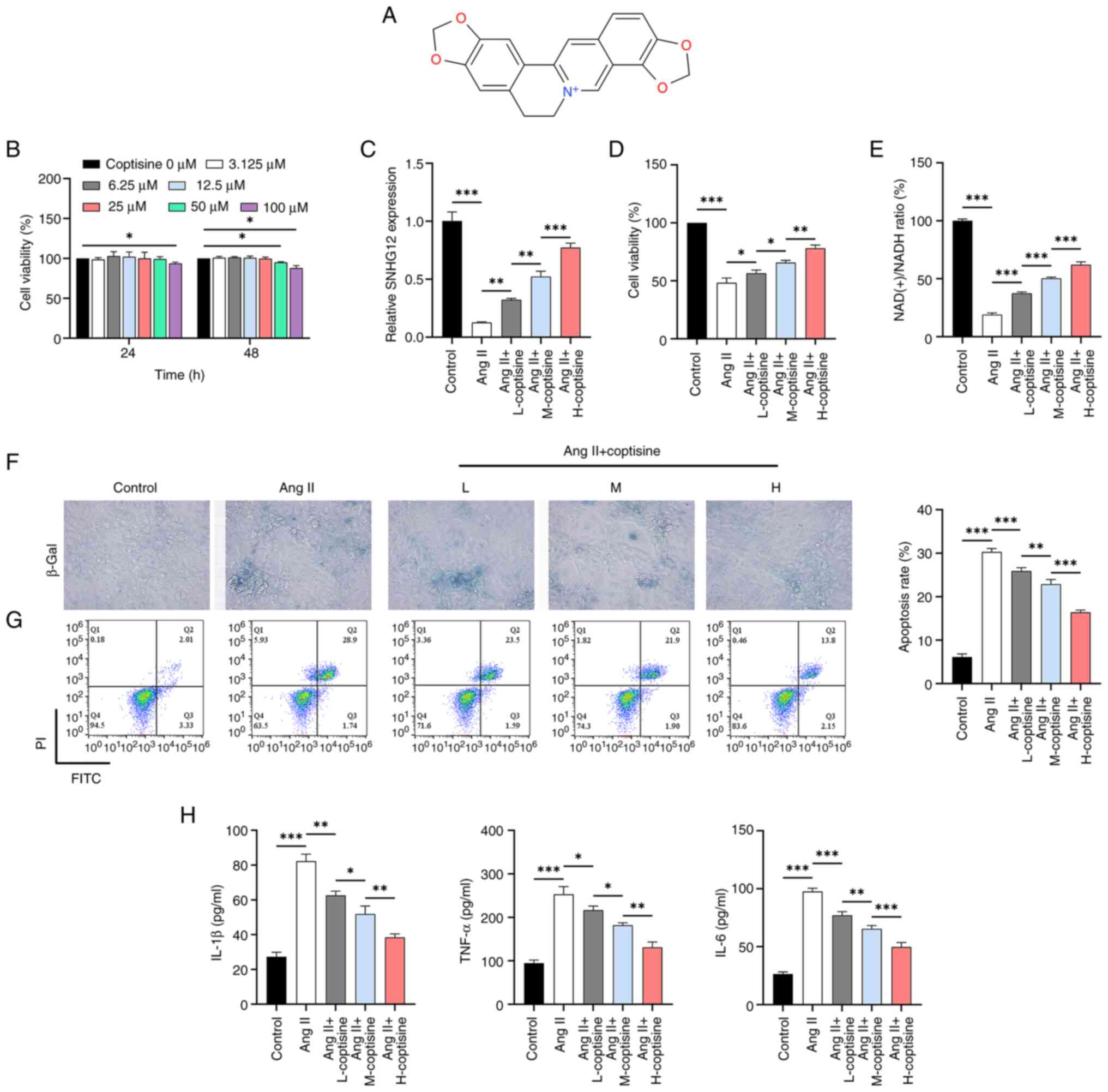
 | Figure 2SNHG12 pathway mediated
anti-senescence and anti-apoptosis effects of coptisine. (A)
Nuclear cytoplasmic separation experiment analyzing subcellular
localization of SNHG12. (B) RT-qPCR analysis of the inhibition
efficiency of three siRNAs targeting SNHG12. CCK8 assay assessing
the influence of si-SNHG12 on the viability of HUVECs induced by
(C) coptisine or/and (D) Ang II. (E) NAD(+) assay kit determining
the NAD(+)/NADH ratio in HUVECs induced by Ang II and treated with
si-SNHG12. (F) β-Gal assay exploring the effects of si-SNHG12 on
cellular senescence in HUVECs induced by Ang II. Magnification,
x400. (G) Flow cytometry analyzing the impact of si-SNHG12 on
apoptosis in HUVECs induced by Ang II. (H) ELISA investigation of
the influence of si-SNHG12 on inflammatory cytokine levels (IL-1β,
TNF-α and IL-6) in HUVECs induced by Ang II. **P<0.01
and ***P<0.001. SNHG12, small nucleolar RNA host gene
12; RT-qPCR, real-time quantitative polymerase chain reaction;
siRNA, short interfering RNA; HUVECs, human umbilical vein
endothelial cells; CCK8, Cell Counting Kit-8; Ang II, Angiotensin
II; β-Gal, β-galactosidase; NAD, nicotinamide adenine dinucleotide;
ELISA, enzyme-linked immunosorbent assay; IL, interleukin; TNF-α,
tumor necrosis factor-α. |
lncRNA SNHG12 acts as a molecular
sponge to absorb miR-603
To investigate the mechanism of action of SNHG12,
the target miRNAs of SNHG12 (Fig.
3A) were identified using the LncBase and miRanda databases and
12 miRNAs with potential binding sites to SNHG12 were identified. A
literature search revealed that only one miRNA, miR-603, has not
been reported in AS and its expression was increased by si-SNHG12
(Fig. 3B) and Ang II induction but
inhibited by coptisine in a dose-dependent manner (Fig. 3C). After validating the efficacy of
the miR-603 mimic/inhibitor in HUVECs (Fig. 3D), no significant effect of its
expression changes on lncRNA SNHG12 was observed (Fig. 3E). Furthermore, WT SNHG12
cotransfected with the miR-603 mimic showed significantly lower
fluorescence activity compared to the cotransfected mimic NC, as
determined by the dual luciferase reporter gene assay. By contrast,
the fluorescence intensity of the miR-603 mimic or mimic NC
cotransfected with mut SNHG12 did not change significantly
(Fig. 3F). RNA pull-down
experiments showed that SNHG12 was enriched in miR-603
precipitation compared to the control, further supporting their
interaction (Fig. 3G). These
results indicated that miR-603 was a direct SNHG12 target.
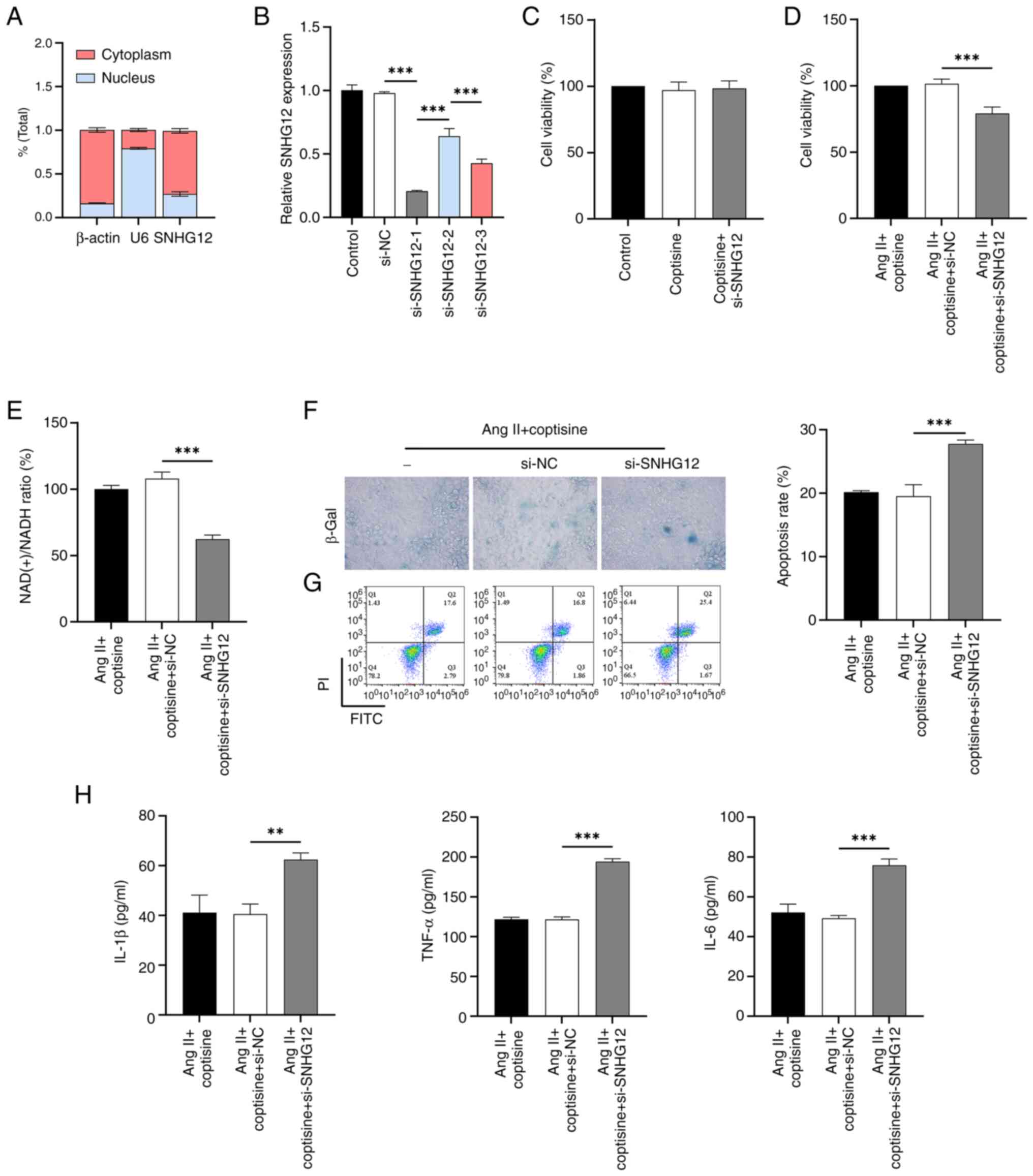
 | Figure 3LncRNA SNHG12 as a molecular sponge
for miR-603. (A) Joint analysis of LncBase and miRanda databases
for potential target miRNAs of lncRNA SNHG12. (B) RT-qPCR analysis
of the effect of si-SNHG12 on miR-603 expression. (C) RT-qPCR
analysis assessing the effects of coptisine on miR-603 expression
in HUVECs induced by Ang II. (D) RT-qPCR validation of miR-603
mimic/inhibitor in HUVECs. (E) RT-qPCR analysis of the effect of
miR-603 expression changes on SNHG12 expression. (F)
Dual-luciferase reporter gene assay determining the binding between
SNHG12 and miR-603. (G) RNA pull-down experiment analyzing SNHG12
enrichment in the bio-miR-603 group. *P<0.05 and
***P<0.001. lncRNA, long non-coding RNA; SNHG12,
small nucleolar RNA host gene 12; miR, microRNA; RT-qPCR, real-time
quantitative polymerase chain reaction; HUVECs, human umbilical
vein endothelial cells; bio, biotinylated; Ang II, Angiotensin II;
L, 6.25 µM; M,12 µM; H, 25 µM; mut, mutant; WT, wild type; NC,
negative control. |
NAMPT is a direct target of
miR-603
To further understand the mechanism of
SNHG12/miR-603, the miRDB database was used to identify the
downstream targets of miR-603. Among the mRNAs with potential
binding sites, NAMPT is a known age-related gene and its activation
significantly promotes the NAD(+)/NADH ratio (24). The present study showed that Ang II
induction reduced NAMPT expression, whereas treatment with
different coptisine concentrations resulted in a dose-dependent
increase in NAMPT expression (Fig.
4A). Additionally, NAMPT expression and protein levels were
negatively regulated by miR-603 (Fig.
4B and C) and its expression
was inhibited by si-SNHG12 (Fig.
4D). Subsequent dual-luciferase detection confirmed that NAMPT
was a direct target of miR-603 (Fig.
4E). Therefore, NAMPT was identified as an miR-603 target and
an NAMPT overexpression vector was constructed and validated for
gene and protein levels in HUVECs (Fig. 4F and G), with no significant effect on miR-603
(Fig. 4H).
SNHG12/miR-603/NAMPT is involved in
the protective mechanism of coptisine
As expected, coptisine significantly ameliorated Ang
II-induced decreases in cell viability (Fig. 5A) and NAD(+)/NADH ratio (Fig. 5B), as well as increased senescence
(Fig. 5C), apoptosis (Fig. 5D) and inflammation (Fig. 5E). This ameliorative effect was
enhanced by the miR-603 inhibitor but was partly neutralized by
si-SNHG12. Transfection with ov-NAMPT reversed the neutralizing
effect of si-SNHG12. Furthermore, unlike p53 protein levels, Ang II
decreased NAMPT and SIRT3 protein levels, which were partially
counteracted by coptisine. The miR-603 inhibitor enhanced the
effect of coptisine; however, this effect was partially abrogated
by the interference with SNHG12 expression; ov-NAMPT reversed these
effects (Fig. 5F).
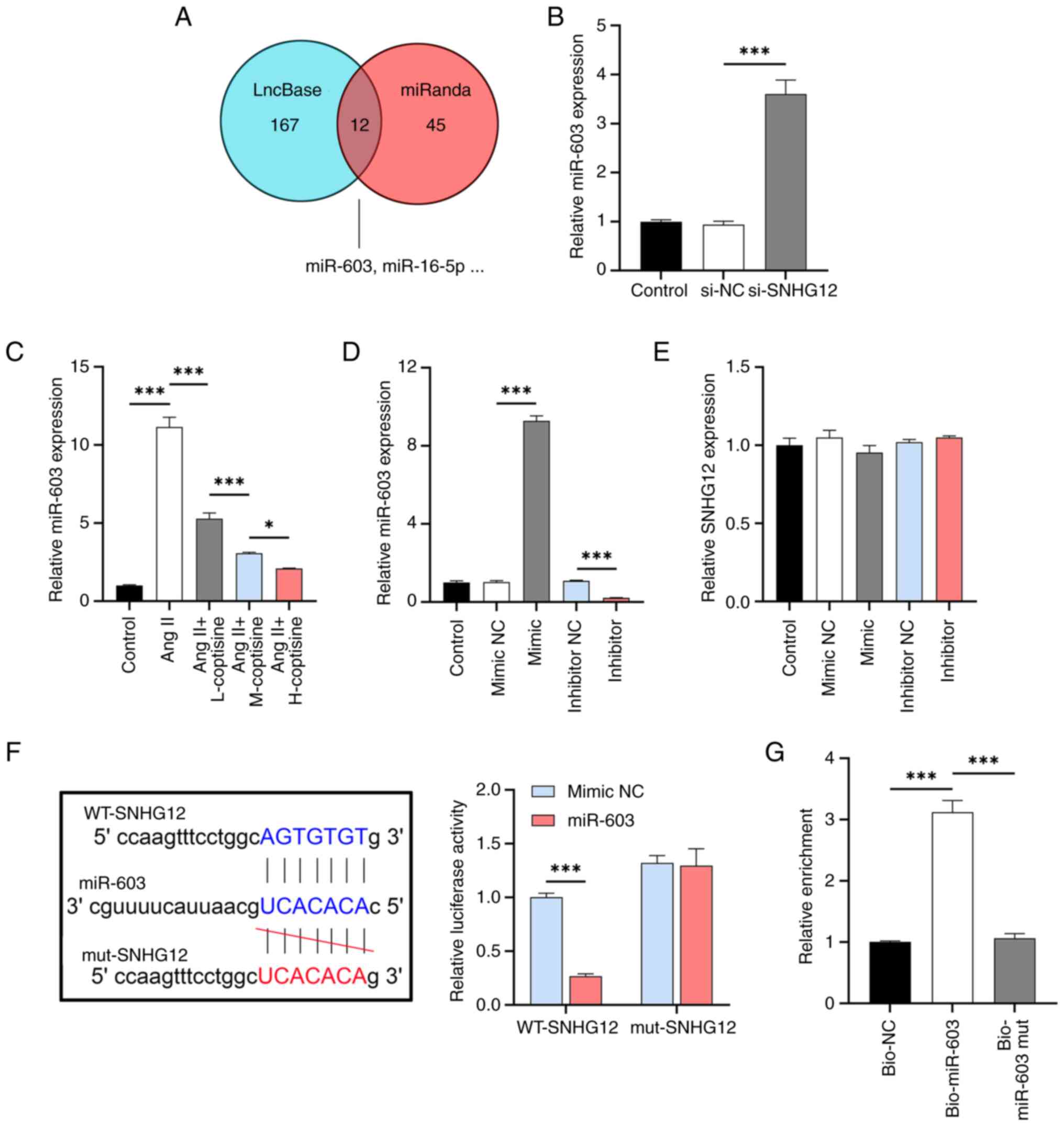
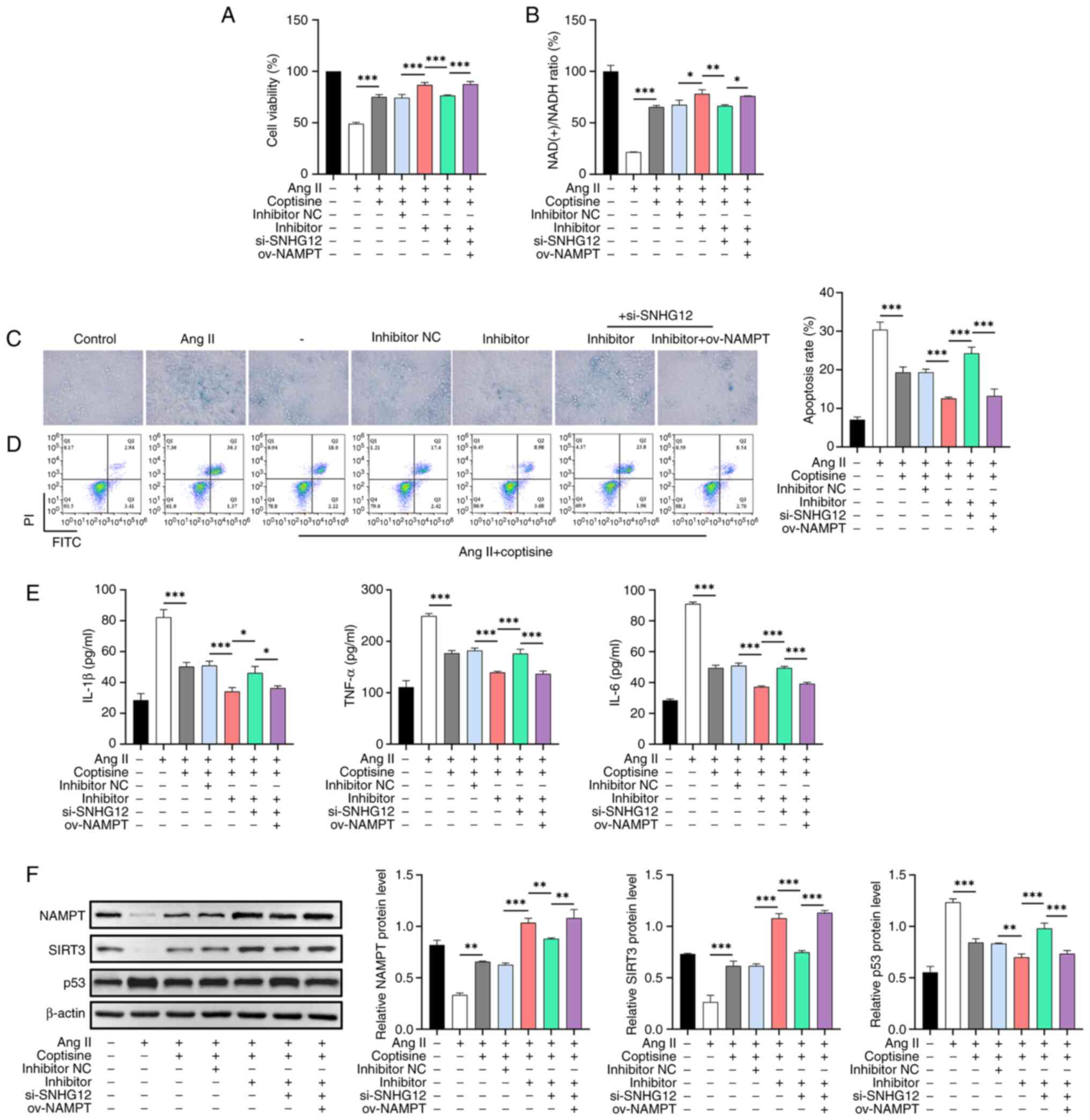 | Figure 5The role of the SNHG12/miR-603/NAMPT
axis in protective mechanism of coptisine. (A) CCK8 assay analyzing
the effect of the SNHG12/miR-603/NAMPT axis on the viability of
HUVECs induced by Ang II. (B) NAD(+) assay kit determining the
NAD(+)/NADH ratio in HUVECs induced by Ang II and influenced by the
SNHG12/miR-603/NAMPT axis. (C) β-Gal assay examining the effects of
the SNHG12/miR-603/NAMPT axis on cellular senescence in HUVECs
induced by Ang II. Magnification, x400. (D) Flow cytometry
analyzing the impact of the SNHG12/miR-603/NAMPT axis on apoptosis
in HUVECs induced by Ang II. (E) ELISA investigation of the
influence of the SNHG12/miR-603/NAMPT axis on the inflammatory
cytokine levels (IL-1β, TNF-α and IL-6) in HUVECs induced by Ang
II. (F) Western blot analysis assessing the effects of the
SNHG12/miR-603/NAMPT axis on the NAMPT, SIRT3 and p53 protein
levels in HUVECs induced by Ang II. *P<0.05,
**P<0.01 and ***P<0.001. SNHG12, small
nucleolar RNA host gene 12; miR, microRNA; NAMPT, Nicotinamide
phosphoribosyltransferase; ov, overexpression; CCK8, Cell Counting
Kit-8; HUVECs, human umbilical vein endothelial cells; Ang II,
Angiotensin II; NAD, nicotinamide adenine dinucleotide; β-Gal,
β-galactosidase; ELISA, enzyme-linked immunosorbent assay; IL,
interleukin; TNF-α, tumor necrosis factor-α; SIRT3, Sirtuin 3; p53,
protein 53; ov, overexpression; NC, negative control. |
Discussion
Currently, the primary treatment for AS involves
statins to lower cholesterol levels and slow disease progression.
However, potential side effects include muscle pain and liver and
kidney damage (25,26). Therefore, safer and more effective
therapeutic strategies are crucial. The present study aimed to
explore novel treatment strategies for ameliorating AS and their
underlying molecular mechanisms. It investigated the role of
coptisine in HUVECs by focusing on its effects on cell viability,
senescence, apoptosis and inflammation. The results showed that
coptisine treatment mitigated the detrimental effects of Ang II
induction in HUVECs by modulating the SNHG12/miR-603/NAMPT
signaling pathway.
Ang II acting on HUVECs is a commonly used in
vitro cellular model of AS (27,28)
and this model was used to explore the potential role and mechanism
of coptisine on AS and to demonstrate, for the first time to the
best of the authors' knowledge, the antioxidant, anti-inflammatory
and anti-aging effects of coptisine in HUVECs. These results were
consistent with those of previous studies on coptisine usefulness
(7,29). In parallel, SNHG12 expression
increased with increasing coptisine doses, suggesting that SNHG12
may play a role in the protective effects of coptisine. The present
study reported for the first time, to the best of the authors'
knowledge, that the lncRNA SNHG12 is a key coptisine mediator in AS
treatment because the depletion of SNHG12 eliminated the protective
effect of coptisine on HUVECs.
Consistent with previous reports (30,31),
SNHG12 was predominantly located in the cytoplasm of HUVECs,
suggesting that this non-coding RNA may regulate downstream targets
that act as molecular sponges to adsorb miRNAs. Dual-luciferase and
RNA pull-down assays were performed to confirm the interaction
between SNHG12 and miR-603 in HUVECs for the first time to the best
of the authors' knowledge. In addition, the age-related gene NAMPT,
which is involved in NAD(+) regulation, is a direct miR-603 target.
This finding is particularly important because NAD(+) and NADH are
interconverted in cells and a decrease in NAD(+) levels can lead to
mitochondrial dysfunction, increased cellular oxidative stress and
reduced DNA repair capacity, resulting in a lower NAD(+)/NADH ratio
(32). The dose-dependent increase
in NAMPT expression observed following coptisine treatment
suggested a potential mechanism through which NAMPT protected
HUVECs. Subsequent experiments showed that inhibiting miR-603
expression enhanced the protective effect of coptisine, whereas
simultaneous SNHG12 expression suppression counteracted the effect
of miR-603. However, these effects were reversed when NAMPT was
overexpressed, which is consistent with previous studies that
demonstrate NAMPT-induced cellular senescence reduction (33,34).
Thus, SNHG12 was found to promote NAMPT expression by inhibiting
miR-603, confirming that the SNHG12/miR-603/NAMPT axis was involved
in coptisine's protective effect on HUVECs against Ang II-induced
decrease in cell viability, increased senescence, apoptosis and
inflammation. This is the first evidence that miR-603 affects the
growth, apoptosis and cellular senescence of HUVECs.
SIRT3, a member of the sirtuin protein family,
exhibits deacetylase activity and requires NAD(+) as a cofactor
(35). Elevated NAD(+) levels can
enhance SIRT3 activity, leading to deacetylation of p53, a key
cellular senescence and apoptosis regulator (36,37).
Reduced p53 transcriptional activity inhibited cellular senescence
and apoptosis (37). The present
study showed that upregulating NAMPT expression mediated NAD(+)'s
accelerated synthesis, which promoted SIRT3 activation and p53
deacetylation. Therefore, the SNHG12/miR-603/NAMPT axis is involved
in coptisine's protective effects through accelerated NAD(+)
synthesis and SIRT3 activation, causing p53 deacetylation and
preventing senescence and apoptosis in HUVECs. This molecular axis
is activated in response to coptisine, which improves the function
of HUVECs.
However, the present study had several limitations.
First, the experiments were primarily based on in vitro cell
models. Future research should verify the therapeutic effects of
coptisine on AS animal models. Second, the experiments were
performed using HUVECs, which may not fully reflect the complex
environment of the vascular system in vivo. Third, AngII
induces NF-κB activation in HUVEC through the p38MAPK pathway
(38); thus, more validation is
needed to verify whether flavonoid alkaloids directly inhibit NF-κB
and thus reduce inflammation. Fourth, the use of HUVECs is largely
based on their wide availability and extensive use in preliminary
studies and more accurate characterization employing human coronary
artery endothelial cell lines might be preferable, especially in
conjunction with 3D culture. Fifth, the performance of HUVECs in
the presence of low doses of coptisine was only tested for cell
viability and perhaps insufficiently. Finally, clinical trials
investigating the treatment of AS using coptisine have not yet been
conducted. These issues require further investigation in future
research.
In conclusion, the present study contributed to the
growing body of evidence highlighting the potential of coptisine as
a therapeutic agent for AS and age-related vascular disorders.
Identifying the SNHG12/miR-603/NAMPT signaling pathway as a
critical mediator of the protective effects of coptisine provided a
novel target for future therapeutic interventions and expanded our
understanding of the molecular mechanisms underlying vascular
homeostasis and dysfunction.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Peak Plateau
Subject of Shanghai University of Traditional Chinese Medicine
(Special Project for Clinical Talents; grant no. 171319).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM and DL designed the study and developed the
methodology. XS and XX conducted the experiments. DL and JC
collected and interpreted data. JM and DL drafted the manuscript.
All authors read and approved the final manuscript. JM and DL
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ
and Han M: Inflammation and atherosclerosis: Signaling pathways and
therapeutic intervention. Signal Transduct Target Ther.
7(131)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Libby P: The changing landscape of
atherosclerosis. Nature. 592:524–533. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Suzuki K, Susaki EA and Nagaoka I:
Lipopolysaccharides and cellular senescence: Involvement in
atherosclerosis. Int J Mol Sci. 23(11148)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
You Y, Sun X, Xiao J, Chen Y, Chen X, Pang
J, Mi J, Tang Y, Liu Q and Ling W: Inhibition of
S-adenosylhomocysteine hydrolase induces endothelial senescence via
hTERT downregulation. Atherosclerosis. 353:1–10. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen L, Qu H, Guo M, Zhang Y, Cui Y, Yang
Q, Bai R and Shi D: ANRIL and atherosclerosis. J Clin Pharm Ther.
45:240–248. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu Y, Gong S, Li K, Wu G, Zheng X, Zheng
J, Lu X, Zhang L, Li J, Su Z, et al: Coptisine protects against
hyperuricemic nephropathy through alleviating inflammation,
oxidative stress and mitochondrial apoptosis via PI3K/Akt signaling
pathway. Biomed Pharmacother. 156(113941)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim SY, Hwangbo H, Kim MY, Ji SY, Lee H,
Kim GY, Kwon CY, Leem SH, Hong SH, Cheong J and Choi YH: Coptisine
induces autophagic cell death through down-regulation of
PI3K/Akt/mTOR signaling pathway and up-regulation of ROS-mediated
mitochondrial dysfunction in hepatocellular carcinoma Hep3B cells.
Arch Biochem Biophys. 697(108688)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Suzuki H, Tanabe H, Mizukami H and Inoue
M: Differential gene expression in rat vascular smooth muscle cells
following treatment with coptisine exerts a selective
antiproliferative effect. J Nat Prod. 74:634–638. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Suzuki H, Tanabe H, Mizukami H and Inoue
M: Selective regulation of multidrug resistance protein in vascular
smooth muscle cells by the isoquinoline alkaloid coptisine. Biol
Pharm Bull. 33:677–682. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu Z, Feng W, Shen Q, Yu N, Yu K, Wang S,
Chen Z, Shioda S and Guo Y: Rhizoma coptidis and berberine as a
natural drug to combat aging and aging-related diseases via
anti-oxidation and AMPK activation. Aging Dis. 8:760–777.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cao Q, Wu J, Wang X and Song C: Noncoding
RNAs in vascular aging. Oxid Med Cell Longev.
2020(7914957)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu XH, Deng WY, Chen JJ, Xu XD, Liu XX,
Chen L, Shi MW, Liu QX, Tao M and Ren K: LncRNA kcnq1ot1 promotes
lipid accumulation and accelerates atherosclerosis via functioning
as a ceRNA through the miR-452-3p/HDAC3/ABCA1 axis. Cell Death Dis.
11(1043)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Haemmig S, Yang D, Sun X, Das D, Ghaffari
S, Molinaro R, Chen L, Deng Y, Freeman D, Moullan N, et al: Long
noncoding RNA SNHG12 integrates a DNA-PK-mediated DNA damage
response and vascular senescence. Sci Transl Med.
12(eaaw1868)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ramakrishnan V, Xu B, Akers J, Nguyen T,
Ma J, Dhawan S, Ning J, Mao Y, Hua W, Kokkoli E, et al:
Radiation-induced extracellular vesicle (EV) release of miR-603
promotes IGF1-mediated stem cell state in glioblastomas.
EBioMedicine. 55(102736)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lu J, Wang L, Chen W, Wang Y, Zhen S, Chen
H, Cheng J, Zhou Y, Li X and Zhao L: miR-603 targeted hexokinase-2
to inhibit the malignancy of ovarian cancer cells. Arch Biochem
Biophys. 661:1–9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Garten A, Schuster S, Penke M, Gorski T,
de Giorgis T and Kiess W: Physiological and pathophysiological
roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 11:535–546.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Abdellatif M, Sedej S and Kroemer G:
NAD+ metabolism in cardiac health, aging, and disease.
Circulation. 144:1795–1817. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cao M, Zhao Q, Sun X, Qian H, Lyu S, Chen
R, Xia H and Yuan W: Sirtuin 3: Emerging therapeutic target for
cardiovascular diseases. Free Radic Biol Med. 180:63–74.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yuan L, Yang J, Li Y, Yuan L, Liu F, Yuan
Y and Tang X: Matrine alleviates cisplatin-induced acute kidney
injury by inhibiting mitochondrial dysfunction and inflammation via
SIRT3/OPA1 pathway. J Cell Mol Med. 26:3702–3715. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu S, Li L, Wu J, An S, Fang H, Han Y,
Huang Q, Chen Z and Zeng Z: Melatonin attenuates sepsis-induced
small-intestine injury by upregulating SIRT3-mediated
oxidative-stress inhibition, mitochondrial protection, and
autophagy induction. Front Immunol. 12(625627)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang Q, Liu XM, Hu Q, Liu ZR, Liu ZY,
Zhang HG, Huang YL, Chen QH, Wang WX and Zhang XK: Dexmedetomidine
inhibits mitochondria damage and apoptosis of enteric glial cells
in experimental intestinal ischemia/reperfusion injury via
SIRT3-dependent PINK1/HDAC3/p53 pathway. J Transl Med.
19(463)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Barjaktarovic Z, Kempf SJ, Sriharshan A,
Merl-Pham J, Atkinson MJ and Tapio S: Ionizing radiation induces
immediate protein acetylation changes in human cardiac
microvascular endothelial cells. J Radiat Res. 56:623–632.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Navas LE and Carnero A: NAD+
metabolism, stemness, the immune response, and cancer. Signal
Transduct Target Ther. 6(2)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
O'Donoghue ML, Giugliano RP, Wiviott SD,
Atar D, Keech A, Kuder JF, Im K, Murphy SA, Flores-Arredondo JH,
López JAG, et al: Long-term evolocumab in patients with established
atherosclerotic cardiovascular disease. Circulation. 146:1109–1119.
2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bytyçi I, Penson PE, Mikhailidis DP, Wong
ND, Hernandez AV, Sahebkar A, Thompson PD, Mazidi M, Rysz J, Pella
D, et al: Prevalence of statin intolerance: A meta-analysis. Eur
Heart J. 43:3213–3223. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang X, Qin Y, Ruan W, Wan X, Lv C, He L,
Lu L and Guo X: Targeting inflammation-associated AMPK//Mfn-2/MAPKs
signaling pathways by baicalein exerts anti-atherosclerotic action.
Phytother Res. 35:4442–4455. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Dörffel Y, Franz S, Pruss A, Neumann G,
Rohde W, Burmester GR and Scholze J: Preactivated monocytes from
hypertensive patients as a factor for atherosclerosis?
Atherosclerosis. 157:151–160. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu B, Piao X, Niu W, Zhang Q, Ma C, Wu T,
Gu Q, Cui T and Li S: Kuijieyuan decoction improved intestinal
barrier injury of ulcerative colitis by affecting TLR4-dependent
PI3K/AKT/NF-κB oxidative and inflammatory signaling and gut
microbiota. Front Pharmacol. 11(1036)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang HG, Wang TP, Hu SA, Hu CZ, Jiang CH
and He Q: Long non-coding RNA SNHG12, a new therapeutic target,
regulates miR-199a-5p/Klotho to promote the growth and metastasis
of intrahepatic cholangiocarcinoma cells. Front Med (Lausanne).
8(680378)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Qian W, Zheng ZQ, Nie JG, Liu LJ, Meng XZ,
Sun H, Xiao FM and Kang T: LncRNA SNHG12 alleviates hypertensive
vascular endothelial injury through miR-25-3p/SIRT6 pathway. J
Leukoc Biol. 110:651–661. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Covarrubias AJ, Perrone R, Grozio A and
Verdin E: NAD+ metabolism and its roles in cellular
processes during ageing. Nat Rev Mol Cell Biol. 22:119–141.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang L, Shen J, Liu C, Kuang Z, Tang Y,
Qian Z, Guan M, Yang Y, Zhan Y, Li N and Li X: Nicotine rebalances
NAD+ homeostasis and improves aging-related symptoms in
male mice by enhancing NAMPT activity. Nat Commun.
14(900)2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gong H, Chen H, Xiao P, Huang N, Han X,
Zhang J, Yang Y, Li T, Zhao T, Tai H, et al: miR-146a impedes the
anti-aging effect of AMPK via NAMPT suppression and
NAD+/SIRT inactivation. Signal Transduct Target Ther.
7(66)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Diao Z, Ji Q, Wu Z, Zhang W, Cai Y, Wang
Z, Hu J, Liu Z, Wang Q, Bi S, et al: SIRT3 consolidates
heterochromatin and counteracts senescence. Nucleic Acids Res.
49:4203–4219. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nahálková J: Focus on molecular functions
of anti-aging deacetylase SIRT3. Biochemistry (Mosc). 87:21–34.
2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yan P, Li Z, Xiong J, Geng Z, Wei W, Zhang
Y, Wu G, Zhuang T, Tian X, Liu Z, et al: LARP7 ameliorates cellular
senescence and aging by allosterically enhancing SIRT1 deacetylase
activity. Cell Rep. 37(110038)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Guo RW, Yang LX, Li MQ, Liu B and Wang XM:
Angiotensin II induces NF-kappa B activation in HUVEC via the
p38MAPK pathway. Peptides. 27:3269–3275. 2006.PubMed/NCBI View Article : Google Scholar
|