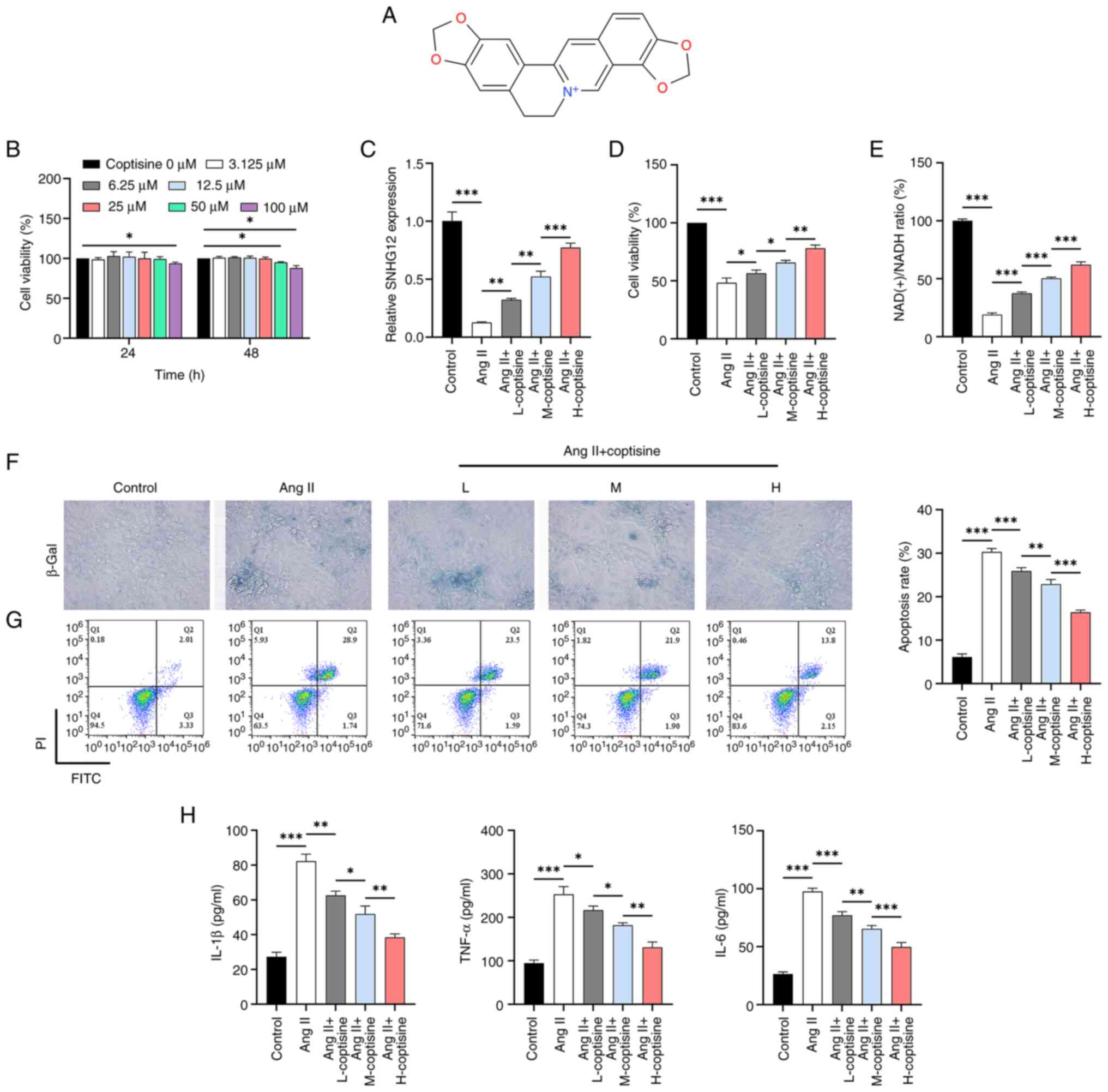
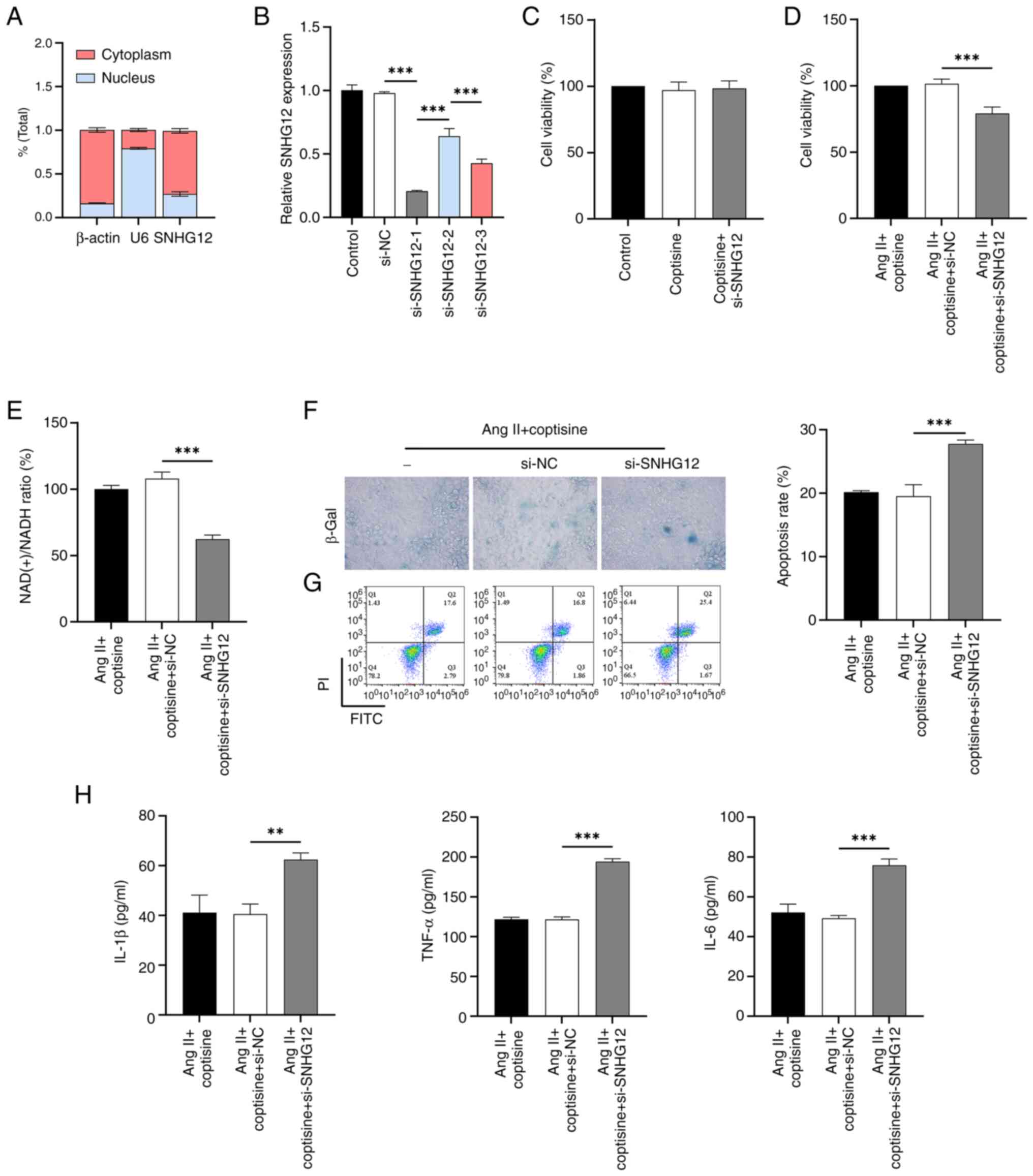
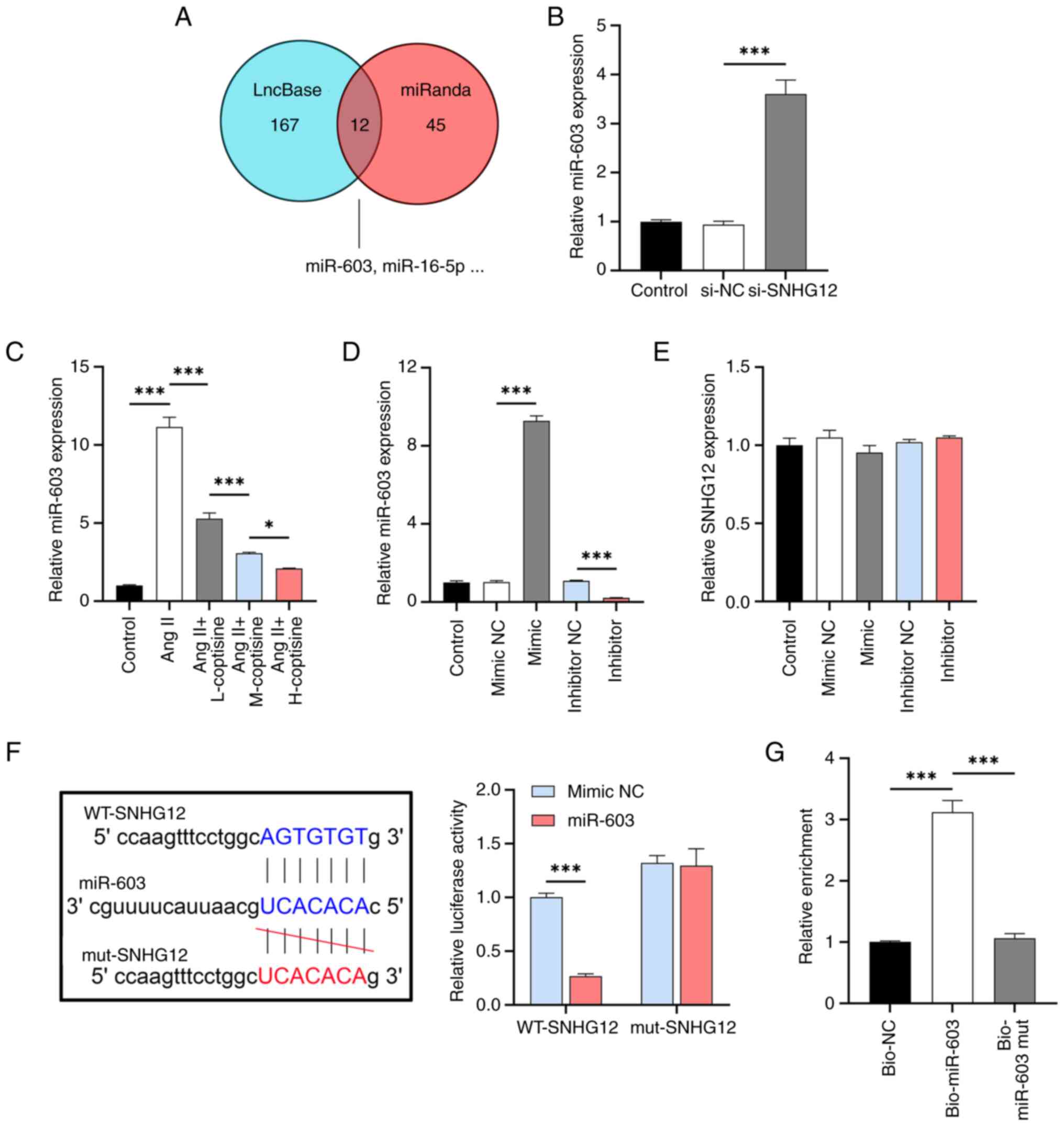
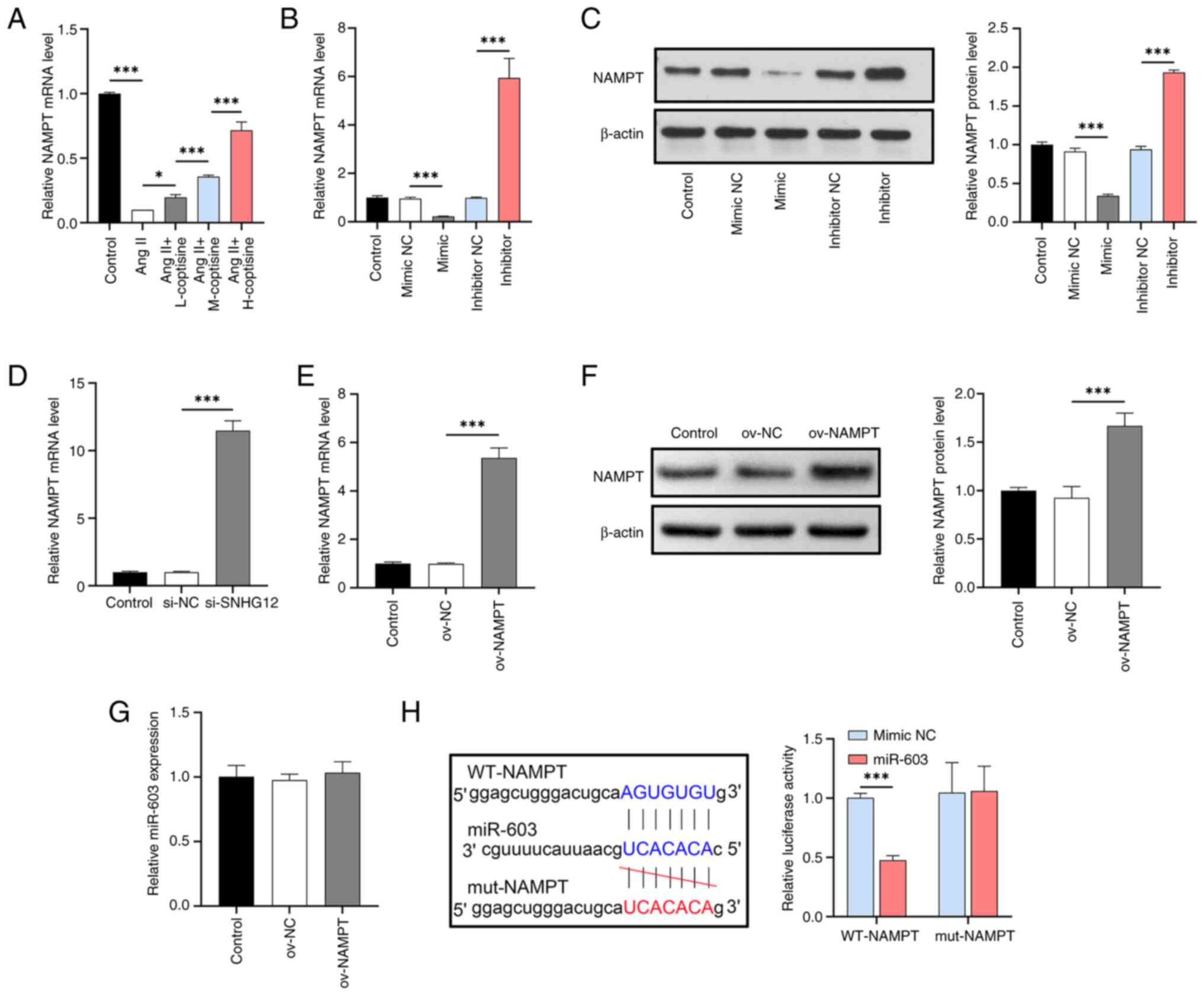
|
1
|
Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ
and Han M: Inflammation and atherosclerosis: Signaling pathways and
therapeutic intervention. Signal Transduct Target Ther.
7(131)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Libby P: The changing landscape of
atherosclerosis. Nature. 592:524–533. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Suzuki K, Susaki EA and Nagaoka I:
Lipopolysaccharides and cellular senescence: Involvement in
atherosclerosis. Int J Mol Sci. 23(11148)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
You Y, Sun X, Xiao J, Chen Y, Chen X, Pang
J, Mi J, Tang Y, Liu Q and Ling W: Inhibition of
S-adenosylhomocysteine hydrolase induces endothelial senescence via
hTERT downregulation. Atherosclerosis. 353:1–10. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen L, Qu H, Guo M, Zhang Y, Cui Y, Yang
Q, Bai R and Shi D: ANRIL and atherosclerosis. J Clin Pharm Ther.
45:240–248. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu Y, Gong S, Li K, Wu G, Zheng X, Zheng
J, Lu X, Zhang L, Li J, Su Z, et al: Coptisine protects against
hyperuricemic nephropathy through alleviating inflammation,
oxidative stress and mitochondrial apoptosis via PI3K/Akt signaling
pathway. Biomed Pharmacother. 156(113941)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim SY, Hwangbo H, Kim MY, Ji SY, Lee H,
Kim GY, Kwon CY, Leem SH, Hong SH, Cheong J and Choi YH: Coptisine
induces autophagic cell death through down-regulation of
PI3K/Akt/mTOR signaling pathway and up-regulation of ROS-mediated
mitochondrial dysfunction in hepatocellular carcinoma Hep3B cells.
Arch Biochem Biophys. 697(108688)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Suzuki H, Tanabe H, Mizukami H and Inoue
M: Differential gene expression in rat vascular smooth muscle cells
following treatment with coptisine exerts a selective
antiproliferative effect. J Nat Prod. 74:634–638. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Suzuki H, Tanabe H, Mizukami H and Inoue
M: Selective regulation of multidrug resistance protein in vascular
smooth muscle cells by the isoquinoline alkaloid coptisine. Biol
Pharm Bull. 33:677–682. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu Z, Feng W, Shen Q, Yu N, Yu K, Wang S,
Chen Z, Shioda S and Guo Y: Rhizoma coptidis and berberine as a
natural drug to combat aging and aging-related diseases via
anti-oxidation and AMPK activation. Aging Dis. 8:760–777.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cao Q, Wu J, Wang X and Song C: Noncoding
RNAs in vascular aging. Oxid Med Cell Longev.
2020(7914957)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu XH, Deng WY, Chen JJ, Xu XD, Liu XX,
Chen L, Shi MW, Liu QX, Tao M and Ren K: LncRNA kcnq1ot1 promotes
lipid accumulation and accelerates atherosclerosis via functioning
as a ceRNA through the miR-452-3p/HDAC3/ABCA1 axis. Cell Death Dis.
11(1043)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Haemmig S, Yang D, Sun X, Das D, Ghaffari
S, Molinaro R, Chen L, Deng Y, Freeman D, Moullan N, et al: Long
noncoding RNA SNHG12 integrates a DNA-PK-mediated DNA damage
response and vascular senescence. Sci Transl Med.
12(eaaw1868)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ramakrishnan V, Xu B, Akers J, Nguyen T,
Ma J, Dhawan S, Ning J, Mao Y, Hua W, Kokkoli E, et al:
Radiation-induced extracellular vesicle (EV) release of miR-603
promotes IGF1-mediated stem cell state in glioblastomas.
EBioMedicine. 55(102736)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lu J, Wang L, Chen W, Wang Y, Zhen S, Chen
H, Cheng J, Zhou Y, Li X and Zhao L: miR-603 targeted hexokinase-2
to inhibit the malignancy of ovarian cancer cells. Arch Biochem
Biophys. 661:1–9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Garten A, Schuster S, Penke M, Gorski T,
de Giorgis T and Kiess W: Physiological and pathophysiological
roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 11:535–546.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Abdellatif M, Sedej S and Kroemer G:
NAD+ metabolism in cardiac health, aging, and disease.
Circulation. 144:1795–1817. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cao M, Zhao Q, Sun X, Qian H, Lyu S, Chen
R, Xia H and Yuan W: Sirtuin 3: Emerging therapeutic target for
cardiovascular diseases. Free Radic Biol Med. 180:63–74.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yuan L, Yang J, Li Y, Yuan L, Liu F, Yuan
Y and Tang X: Matrine alleviates cisplatin-induced acute kidney
injury by inhibiting mitochondrial dysfunction and inflammation via
SIRT3/OPA1 pathway. J Cell Mol Med. 26:3702–3715. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu S, Li L, Wu J, An S, Fang H, Han Y,
Huang Q, Chen Z and Zeng Z: Melatonin attenuates sepsis-induced
small-intestine injury by upregulating SIRT3-mediated
oxidative-stress inhibition, mitochondrial protection, and
autophagy induction. Front Immunol. 12(625627)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang Q, Liu XM, Hu Q, Liu ZR, Liu ZY,
Zhang HG, Huang YL, Chen QH, Wang WX and Zhang XK: Dexmedetomidine
inhibits mitochondria damage and apoptosis of enteric glial cells
in experimental intestinal ischemia/reperfusion injury via
SIRT3-dependent PINK1/HDAC3/p53 pathway. J Transl Med.
19(463)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Barjaktarovic Z, Kempf SJ, Sriharshan A,
Merl-Pham J, Atkinson MJ and Tapio S: Ionizing radiation induces
immediate protein acetylation changes in human cardiac
microvascular endothelial cells. J Radiat Res. 56:623–632.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Navas LE and Carnero A: NAD+
metabolism, stemness, the immune response, and cancer. Signal
Transduct Target Ther. 6(2)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
O'Donoghue ML, Giugliano RP, Wiviott SD,
Atar D, Keech A, Kuder JF, Im K, Murphy SA, Flores-Arredondo JH,
López JAG, et al: Long-term evolocumab in patients with established
atherosclerotic cardiovascular disease. Circulation. 146:1109–1119.
2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bytyçi I, Penson PE, Mikhailidis DP, Wong
ND, Hernandez AV, Sahebkar A, Thompson PD, Mazidi M, Rysz J, Pella
D, et al: Prevalence of statin intolerance: A meta-analysis. Eur
Heart J. 43:3213–3223. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang X, Qin Y, Ruan W, Wan X, Lv C, He L,
Lu L and Guo X: Targeting inflammation-associated AMPK//Mfn-2/MAPKs
signaling pathways by baicalein exerts anti-atherosclerotic action.
Phytother Res. 35:4442–4455. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Dörffel Y, Franz S, Pruss A, Neumann G,
Rohde W, Burmester GR and Scholze J: Preactivated monocytes from
hypertensive patients as a factor for atherosclerosis?
Atherosclerosis. 157:151–160. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu B, Piao X, Niu W, Zhang Q, Ma C, Wu T,
Gu Q, Cui T and Li S: Kuijieyuan decoction improved intestinal
barrier injury of ulcerative colitis by affecting TLR4-dependent
PI3K/AKT/NF-κB oxidative and inflammatory signaling and gut
microbiota. Front Pharmacol. 11(1036)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang HG, Wang TP, Hu SA, Hu CZ, Jiang CH
and He Q: Long non-coding RNA SNHG12, a new therapeutic target,
regulates miR-199a-5p/Klotho to promote the growth and metastasis
of intrahepatic cholangiocarcinoma cells. Front Med (Lausanne).
8(680378)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Qian W, Zheng ZQ, Nie JG, Liu LJ, Meng XZ,
Sun H, Xiao FM and Kang T: LncRNA SNHG12 alleviates hypertensive
vascular endothelial injury through miR-25-3p/SIRT6 pathway. J
Leukoc Biol. 110:651–661. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Covarrubias AJ, Perrone R, Grozio A and
Verdin E: NAD+ metabolism and its roles in cellular
processes during ageing. Nat Rev Mol Cell Biol. 22:119–141.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang L, Shen J, Liu C, Kuang Z, Tang Y,
Qian Z, Guan M, Yang Y, Zhan Y, Li N and Li X: Nicotine rebalances
NAD+ homeostasis and improves aging-related symptoms in
male mice by enhancing NAMPT activity. Nat Commun.
14(900)2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gong H, Chen H, Xiao P, Huang N, Han X,
Zhang J, Yang Y, Li T, Zhao T, Tai H, et al: miR-146a impedes the
anti-aging effect of AMPK via NAMPT suppression and
NAD+/SIRT inactivation. Signal Transduct Target Ther.
7(66)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Diao Z, Ji Q, Wu Z, Zhang W, Cai Y, Wang
Z, Hu J, Liu Z, Wang Q, Bi S, et al: SIRT3 consolidates
heterochromatin and counteracts senescence. Nucleic Acids Res.
49:4203–4219. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nahálková J: Focus on molecular functions
of anti-aging deacetylase SIRT3. Biochemistry (Mosc). 87:21–34.
2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yan P, Li Z, Xiong J, Geng Z, Wei W, Zhang
Y, Wu G, Zhuang T, Tian X, Liu Z, et al: LARP7 ameliorates cellular
senescence and aging by allosterically enhancing SIRT1 deacetylase
activity. Cell Rep. 37(110038)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Guo RW, Yang LX, Li MQ, Liu B and Wang XM:
Angiotensin II induces NF-kappa B activation in HUVEC via the
p38MAPK pathway. Peptides. 27:3269–3275. 2006.PubMed/NCBI View Article : Google Scholar
|