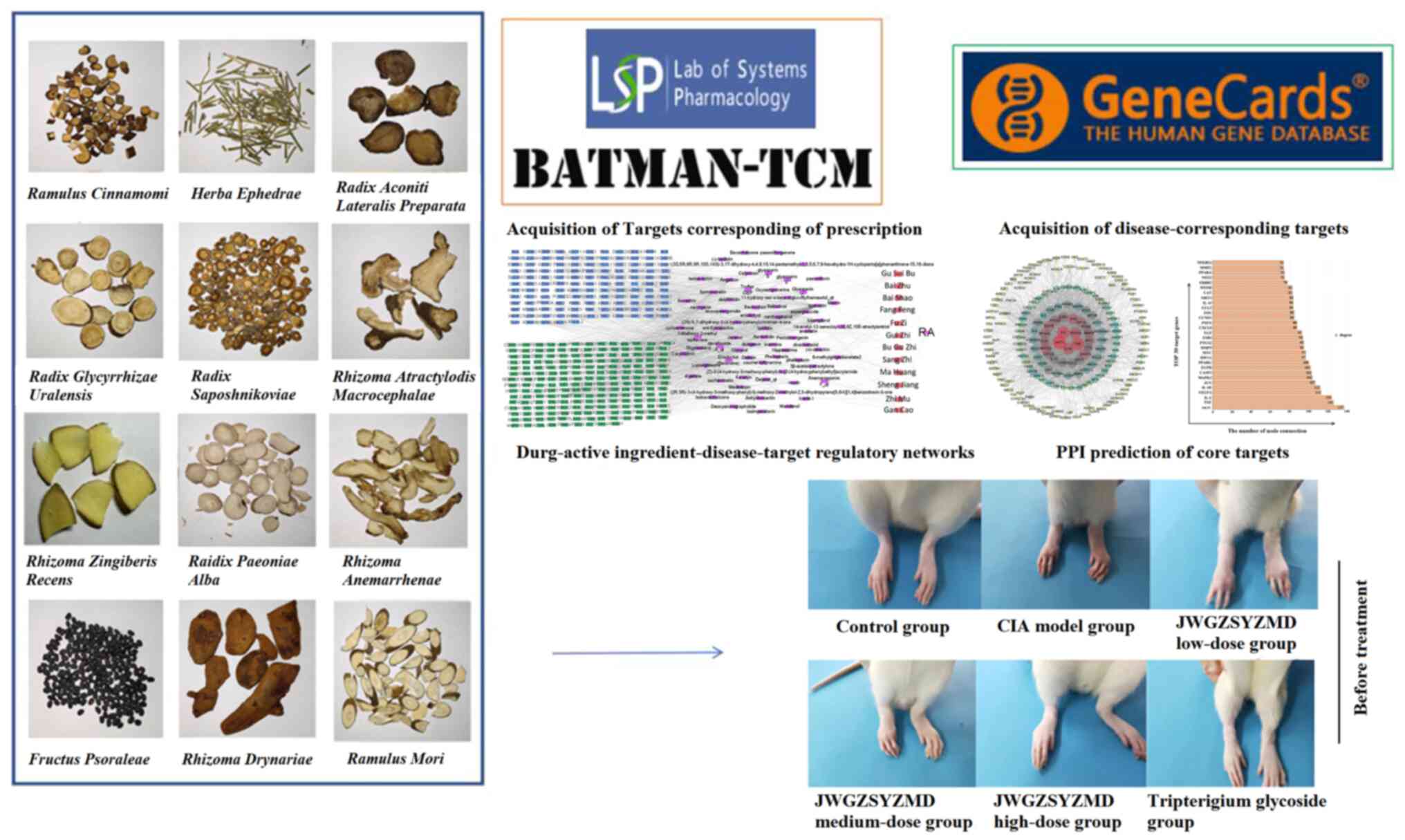
Introduction
Guizhishaoyaozhimu Decoction (GSZD) has been used as
a Traditional Chinese Medicine (TCM) since at least the Han Dynasty
period, for >2,000 years in China. It has mainly been used for
the treatment of anemofrigid-damp arthralgia (1). At present, it is commonly prescribed
in clinical practice, particularly for the treatment of rheumatoid
arthritis (RA) and other joint disease, such as osteoarthropathy
(2). It has been previously
reported to significantly alleviate pain and swelling in patients
whilst effectively promoting the recovery of joint function
(3). RA is an autoimmune disease
with symmetrical, invasive swelling and pain in the small joints,
such as the metacarpophalangeal joints (4). RA frequently affects multiple joints
in the same individual (5,6). It has been reported that 0.5-1% of
the global population is affected by RA (7). RA inflammation is associated with
overproduction of inflammatory factors, expressing high levels of
inflammatory factors, chemokines, and adhesion molecules (e.g.,
IL-6, TNF-α, etc.)causing synovial inflammation (8). It also promotes the formation and
maintenance of T and B cell survival and the formation of a
specific immune microenvironment (9). Yan et al previously reported
beneficial effects exerted by Jiawei Guizhishaoyaozhimu Decoction
(JWGZSYZMD), which is based on the original GSZD preparation
supplemented with ‘Bu Gu Zhi’ (Fructus Psoraleae) and ‘Gu
Sui Bu’ (Rhizoma Drynariae). These compounds have previously
been proposed as therapeutic agents for treating muscle and joint
pain and stiffness, regardless of the disease location, size of the
joint or the course of disease (10,11).
In addition, the use of ‘Sang Zhi’ (Ramulus Mori) before the
signs of arthritis-induced fever has been documented to confer
beneficial effects clinically (12,13).
In the present study, ‘Gu Sui Bu’, ‘Sang Zhi’ and
‘Bu Gu Zhi’ were added into the existing formulation of GSZD. A
collagen II-induced arthritis (CIA) model was established in rats
to observe the efficacy of JWGZSYZMD on joint inflammation. In
addition, its effects on IL-1, IL-6, IL-17 and TNF-α expression
levels were measured. The aim of the present study was to interpret
the effects and underlying mechanism of action of JWGZSYZMD in the
treatment of RA, in addition to establishing an active
component-disease target regulatory network to predict core targets
for RA therapy.
Materials and methods
Network pharmacological study.
Acquisition of targets corresponding to prescription
JWGZSYZMD was comprised of ‘Gui Zhi’ (Ramulus
Cinnamomi), ‘Bai Zhu’ (Rhizoma Atractylodis
Macrocephalae), ‘Gan Cao’ (Zhi; Radix Glycyrrhizae
Uralensis), ‘Ma Huang’ (Herba Ephedrae), ‘Bai Shao’
(Raidix Paeoniae Alba), ‘Zhi Mu’ (Rhizoma
Anemarrhenae), ‘Sheng Jiang’ (Rhizoma Zingiberis
Recens), ‘Fang Feng’ (Radix Saposhnikoviae), ‘Bu Gu Zhi’
(Fructus Psoraleae), ‘Fu Zi’ (Radix Aconiti Lateralis
Preparata), ‘Sang Zhi’ (Ramulus Mori) and ‘Gu Sui Bu’
(Rhizoma Drynariae) (Fig.
1). Traditional Chinese Medicine Systems Pharmacology Database
and Analysis Platform (TCMSP; tcmsp-e.com/)
was used to obtain ingredients and targets corresponding to these
aforementioned compounds. ‘Bu Gu Zhi’ is not included in TCMSP and
was instead analyzed using the Bioinformatics Analysis Tool of
Molecular Mechanism of Traditional Chinese Medicine (BATMAN-TCM,
http://bionet.ncpsb.org.cn/batman-tcm/index.php/Home/Index/index)
to obtain the ingredients and targets. Orally-applied TCM has to
overcome the obstacles of the absorption, distribution, metabolism
and excretion process for maximal efficacy, in which oral
bioavailability (OB) is an important pharmacokinetic parameters.
Substances with OB value ≥30% were considered to have a high OB.
Drug-likeness (DL) was used to estimate the pharmacological
properties of molecules and is useful for the rapid screening of
active substances. It has been previously reported that substances
with DL ≥0.18 would indicate a superior effect (14). Therefore, the screening criteria
for active ingredients in the present study were OB ≥30% and DL
≥0.18 in the TCMSP database, whereas in BATMAN-TCM the screening
criteria were score ≥20 and P<0.05 (15,16).
Acquisition of RA-associated drug targets.
The corresponding genes associated with RA were obtained from the
Genecards database (https://www.genecards.org/) using the term ‘Rheumatoid
Arthritis’ (17). The potential
drug targets were mapped against the genes corresponding to RA to
obtain potential mechanisms by which JWGZSYZMD regulated RA.
Construction of the drug-active component-target
regulatory network. Corresponding drug-active component-target
regulatory networks were constructed using the Cytoscape 3.7.2
software (cytoscape.org). Possible connections
between drug composition and active ingredients of JWGZSYZMD, in
addition to their potential regulatory targets in the context of
RA, were then visualized. According to the number of connecting
nodes and determination of the core based on network topology
parameters targets and the main active ingredients that exert
pharmacological effects, the potential active ingredients contained
within the JWGZSYZMD for regulating RA were screened.
Protein-protein interaction (PPI) for predicting
the core target of JWGZSYZMD in the treatment of RA.
Relationships among the targets of JWGZSYZMD in RA were then
assessed using the STRING database (https://cn.string-db.org/), where targets ranked in
the top 30 in terms of the number of connections were visualized
using histograms (18). In
addition, a network representing the specific relationships among
the targets were visualized using the Cytoscape software (18).
Path prediction of JWGZSYZMD in the treatment of
RA. Targets of JWGZSYZMD predicted to regulate RA were screened
for in Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways
using the STRING database, with the criteria of P<0.05(19). The top 10 ranked KEGG pathways were
then visualized using bubble plots after selecting the RA-related
pathways.
Molecular docking. In summary, 3D structures
of IL-1β, IL-6, IL-17A and TNF-α targets were retrieved from the
Protein Data Bank (PDB) protein structure database (http://www.rcsb.org/). The structures were then saved
in the ‘.pdb’ format after using the PyMol 2.4 (pymol.org/2) software to delete water molecules and
small molecule ligands. Using the Pubchem database (http://zinc.docking.org/), 2D structures of the top 30
ranked active ingredients were retrieved, before their minimum free
energies were calculated using Chem3D 6.6.0 (chemdoodle.com/3d/) software and saved in the ‘MOL2’
format. The aforementioned active ingredients and targets were then
input into AutoDockTools 1.5.6 (autodock.scripps.edu) software for hydrogenation and
format conversion, before being output into the ‘PDBQT’ format.
Previous studies have reported that the involvement of TNF-α and
IL-6 forms the core of RA pathogenesis (20,21).
However, IL-6 is also a regulatory factor in bone metabolism, in
which IL-17 and IL-1β also serve an important role (20,21).
Therefore, AutoDock Vina 1.1.2 (vina.scripps.edu) was used to perform molecular
docking for TNF-α, IL-6, IL-17 and IL-1β, where the minimum binding
energy of each potential active ingredient onto each of these
proteins was calculated. The three results which demonstrated the
most optimal molecular docking visualized using PyMol and Ligplot
2.2 (ebi.ac.uk/thornton-srv/software/LIGPLOT) software.
Experimental validation studies.
Experimental reagents
Bovine collagen II (cat. no. 20022; Chondrex, Inc.),
Freund's incomplete adjuvant (cat. no. 7002; Chondrex, Inc.),
tissue fixative solution (Beijing Jiuzhou Berlin Biotechnology Co.,
Ltd.), EDTA (cat. no. E8030; Beijing Solarbio Science &
Technology Co., Ltd.), rat IL-6 ELISA kit (cat. no. CRE0005;
Beijing 4A Biotech Co., Ltd.), rat IL-1β ELISA kit (cat. no.
CRE0006; Beijing 4A Biotech Co., Ltd.), rat IL-17A ELISA kit (cat.
no. CRE0021; Beijing 4A Biotech Co., Ltd.) and rat TNF-α ELISA kit
(cat. no. CRE0003; Beijing 4A Biotech Co., Ltd.) were
purchased.
Experimental drugs. JWGZSYZMD-related
medicinal materials were purchased from the Pharmacy of Beijing
University of Chinese Medicine and manufactured by Beijing Temages
Pharmaceutical Co., Ltd.. This decoction comprises 10 g ‘Gui Zhi’
(Ramulus Cinnamomi; Guangdong), 9 g ‘Gan Cao’ (Zhi;
Radix Glycyrrhizae Uralensis; Inner Mongolia), 12 g ‘Ma
Huang’ (Herba Ephedrae; Inner Mongolia), 12 g ‘Bai Zhu’
(Rhizoma Atractylodis Macrocephalae; Zhejiang), 9 g ‘Bai
Shao’ (Raidix Paeoniae Alba; Anhui), 12 g ‘Zhi Mu’
(Rhizoma Anemarrhenae; Inner Mongolia), 12 g ‘Sheng Jiang’
(Rhizoma Zingiberis Recens; Sichuan), 12 g ‘Fang Feng’
(Radix Saposhnikoviae; Jilin), 12 g ‘Bu Gu Zhi’ (Fructus
Psoraleae; Henan), 10 g ‘Fu Zi’ (Radix Aconiti Lateralis
Preparata; Sichuan), 12 g ‘Sang Zhi’ (Ramulus Mori;
Hebei) and 15 g ‘Gu Sui Bu’ (Rhizoma Drynariae; Guangdong).
Each sample stock contained a total of 137 g crude drug
preparation. In total, 2.283 g/kg crude drug is recommended, with
the assumption that the average human body weight is 60 kg. The
conversion factor of body surface area between humans and rats is
6.3(22). Therefore, the dose to
be administered to the rats was calculated to be 14.385 g/kg crude
drug preparation, which equated to that administered to the medium
dose group of animals. The high dose was double that of the medium
dose (28.770 g/kg) whereas the low dose was 50% of the medium dose
(7.193 g/kg). The JWGZSYZMD was soaked in 10 volumes of water for 1
h, before being decocted for 1 h with 100˚C distilled water
followed by filtration using a gauze. The aforementioned decoction
step was repeated twice. The resulting preparation was diluted with
distilled water to 0.7193, 1.4385 and 2.877 g/ml of crude drug for
the low-, medium- and high-dose groups, respectively.
The clinical dosage of tripterygium glycoside for
humans is 1.5 mg/kg (23) and the
dose administered to rats was calculated to be 9.45 mg/kg.
Tripterygium glycoside tablets (Zhejiang DND Pharmaceutical Co.,
Ltd.) were ground and diluted with distilled water to form a 0.945
mg/ml solution. Following preparation, the drug was stored in a
refrigerator at 4˚C for future use. This solution was mixed well
before use.
Experimental animals. The present study was
assessed and approved by the Sub-Committee of Experimental Animal
Ethics of Academic Committee of Beijing University of Traditional
Chinese Medicine (approval no. BUCM-4-2021090605-3104; Beijing,
China).
A total of 60 SPF male Sprague Dawley rats (age, 6
weeks; weight, 180-200 g) were purchased from Spefford (Beijing)
Biotechnology Co., Ltd. license number, SYXK (Jing) 2020-0033). The
experimental animals were housed in the animal room of China-Japan
Friendship Hospital (temperature, 23±2˚C; humidity, 65±5%; light,
12 h/day) with free access to clean drinking water and food during
rearing. Animal health and behavior were monitored on a daily
basis. A pathophysiological drop in body temperature was used as a
humane endpoint and no rats had to be euthanized before the end of
the experiment. Within 2 h of removal of ankle tissue, the rats
were euthanized by CO2 inhalation at 30% vol/min. Death
was confirmed by the lack of breathing, stiffness and dilated
pupils in the rats, after which the CO2 inhalation
ceased and the rats were observed for 2 min to verify death. After
euthanasia, the animals were stored in a designated freezer at
~-20˚C.
Establishment of CIA model. A total of 6 rats
were randomly assigned into the control group, whereas the other 54
rats were used to establish the CIA model by injecting bovine
collagen II/Freund's incomplete adjuvant at the tail root
(n=6/group). A bovine collagen II solution of 4 g/l was prepared
with 0.1 mol/l glacial acetic acid (Xilong Science Co., Ltd.) and
was added dropwise to the syringe. An equal volume of collagen II
acetate solution was mixed with incomplete Freund's adjuvant in an
ice bath until fully emulsified. Use a blender(IKA) to homogenize
was performed for 3 min and halted for 30 sec at each cycle, with
10 cycles performed in total until the emulsion dripped into the
water without diffusion. The homogenized collagen was then wrapped
in tinfoil and placed on ice ready for injection. The skin at the
base of the rat's tail was wiped using a cotton ball with alcohol,
before 200 µl of the collagen mixture was injected subcutaneously
at the right side of the base of the rat's tail. After 1 week, 100
µl of the collagen mixture was injected at the left side of the
base of the tail (24).
Drug-dosing interventions. Rats were assessed
1 week after the second injection using a joint score from 0-4 as
follows: i) 0, normal or no inflammation of the paws; ii) 1,
swelling or slight redness of the toe joints; iii) 2, redness and
swelling of the toe joints and paws; iv) 3, redness and swelling of
the entire paw below the ankle joint; and v) 4, severe redness and
swelling of the ankle joint with signs of joint deformation. Rats
with a total score of >4 on both sides were selected for further
study, resulting in 30 rats being randomly selected and divided
into the CIA model, JWGZSYZMD low-dose, JWGZSYZMD medium-dose,
JWGZSYZMD high-dose and tripterygium glycoside groups. Saline was
administered to the CIA model group, whereas the other groups were
given the corresponding drug at a dose of 1 ml/100 g body weight
for 21 consecutive days.
Histopathological analysis. After 21
consecutive days of treatment, rats were anesthetized by an
intraperitoneal injection of 30 mg/kg pentobarbital before the left
ankle joint was removed from the excess tissue and muscle. The
joint tissues were 0.3 cm thick and fixed with 10% formalin for 72
h at 25˚C, before being added to 10% EDTA for decalcification. The
10% EDTA solution was changed once a week for 8 consecutive weeks
until the completion of decalcification. After dehydration,
paraffin embedding, section to 5 µm and hematoxylin in 5 min and
eosin in 1 min (H&E) staining were performed in 35˚C and rat
joint pathology was observed using a light microscope.
Detection of synovial inflammatory
factors in rats by ELISA
The right ankle joint of the rat was dissected from
the medial side and the skin was dissected to open the ankle joint
cavity to obtain the synovial tissue. The synovium was separated
using ophthalmic scissors under a dissecting microscope and saline
was added for adequate grinding. The grinding solution was
centrifuged at 1,006.2 x g) for 10 min with 20˚C before the
supernatant was collected and stored at -80˚C for future use. The
expression levels of IL-1β, IL-6, IL-17A and TNF-α in each group
were detected using ELISA kits according to the manufacturer's
protocols.
Reverse transcription-quantitative
PCR
TRIzol® solution (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract the total RNA from the
synovial membrane of the ankle joint tissue. Reverse transcription
was performed using the All-in-One™ First-Strand cDNA Synthesis Kit
(cat. no. AORT-0050; GeneCopoeia, Inc.) in accordance with the
manufacturer's instructions. after qPCR in the ABI7500 system
(Thermo Fisher Scientific, Inc.). qPCR was performed using the
Platinum® SYBR® Green Realtime PCR Master Mix
(cat. no. QPK-201; Toyobo Life Science) as follows: Initial
denaturation at 95˚C for 30 sec, followed by 40 cycles of 95˚C for
10 sec, the annealing and extension temperatures are maintained at
60˚C for 30 sec. The 2-ΔΔCq method
(25) was used to quantify mRNA
expression. Primer sequences are listed in Table V.
 | Table VPrimers for reverse
transcription-quantitative PCR analysis. |
Table V
Primers for reverse
transcription-quantitative PCR analysis.
| Gene target | Sequence
(5'-3') |
|---|
| IL-1β | F:
CTCTGTGACTCGTGGGATGATG |
| | R:
CACTTGTTGGCTTATGTTCTGTCC |
| IL-6 | F:
AACGAAAGTCAACTCCATCTG |
| | R:
GGTATCCTCTGTGAAGTCTCC |
| TNF-α | F:
TGGCCCAGACCCTCACACTC |
| | R:
CTCCTGGTATGAAATGGCAAATC |
| IL-17A | F:
ACAGTGAAGGCAGCGGTACT |
| | R:
GCTCATAGTCCAGGGTGAAG |
| β-actin | F:
CACCCGCGAGTACAACCTTC |
| | R:
CCCATACCCACCATCACACC |
Statistical analysis
SPSS (version 26.0; IBM Corp.) and GraphPad prism
(version 8.0; Dotmatics) were used for statistical analysis. Data
normality was analyzed using the Shapiro-Wilk test. Protein
expression levels of IL-1β and TNF-α and mRNA expression levels of
IL-6, IL-1β and IL-17A are presented the mean ± standard deviation.
The joint scores of rats, protein expression of IL-17A and IL-6 and
mRNA expression levels of TNF-α were presented as the median
(interquartile range). Paired data of joint scores were analyzed
using Wilcoxon's signed-rank test followed by Bonferroni
correction. One-way ANOVA followed by Tukey's test was used for
statistical analysis of parametric data, whereas the Kruskal-Wallis
H test followed by Dunn's test for statistical analysis of
non-parametric data (Protein expression of IL-17A and IL-6, mRNA
expression of TNF-α) for two-group comparisons and two-group
comparisons for joint scores of rats (after treatment) subsequently
followed by Bonferroni correction. P<0.05 was considered to
indicate a statistically significant difference.
Results
Drugs and disease corresponding
targets
Through TCMSP and BATMAN-TCM database screening,
searches of ‘Gui Zhi’ (Ramulus Cinnamomi), ‘Bai Zhu’
(Rhizoma Atractylodis Macrocephalae), Gan Cao (Zhi; Radix
Glycyrrhizae Uralensis), ‘Ma Huang’ (Herba Ephedrae),
‘Bai Shao’ (Raidix Paeoniae Alba), ‘Zhi Mu’ (Rhizoma
Anemarrhenae), ‘Sheng Jiang’ (Rhizoma Zingiberis
Recens), ‘Fang Feng’ (Radix Saposhnikoviae), ‘Bu Gu Zhi’
(Fructus Psoraleae), ‘Fu Zi’ (Radix Aconiti Lateralis
Preparata), ‘Sang Zhi’ (Ramulus Mori) and ‘Gu Sui Bu’
(Rhizoma Drynariae) yielded a total of 69 potentially
effective active ingredients contained within JWGZSYZMD. In
addition, 4,792 RA-related gene targets were obtained through the
Genecards database, including 191 targets possibly affected by
JWGZSYZMD.
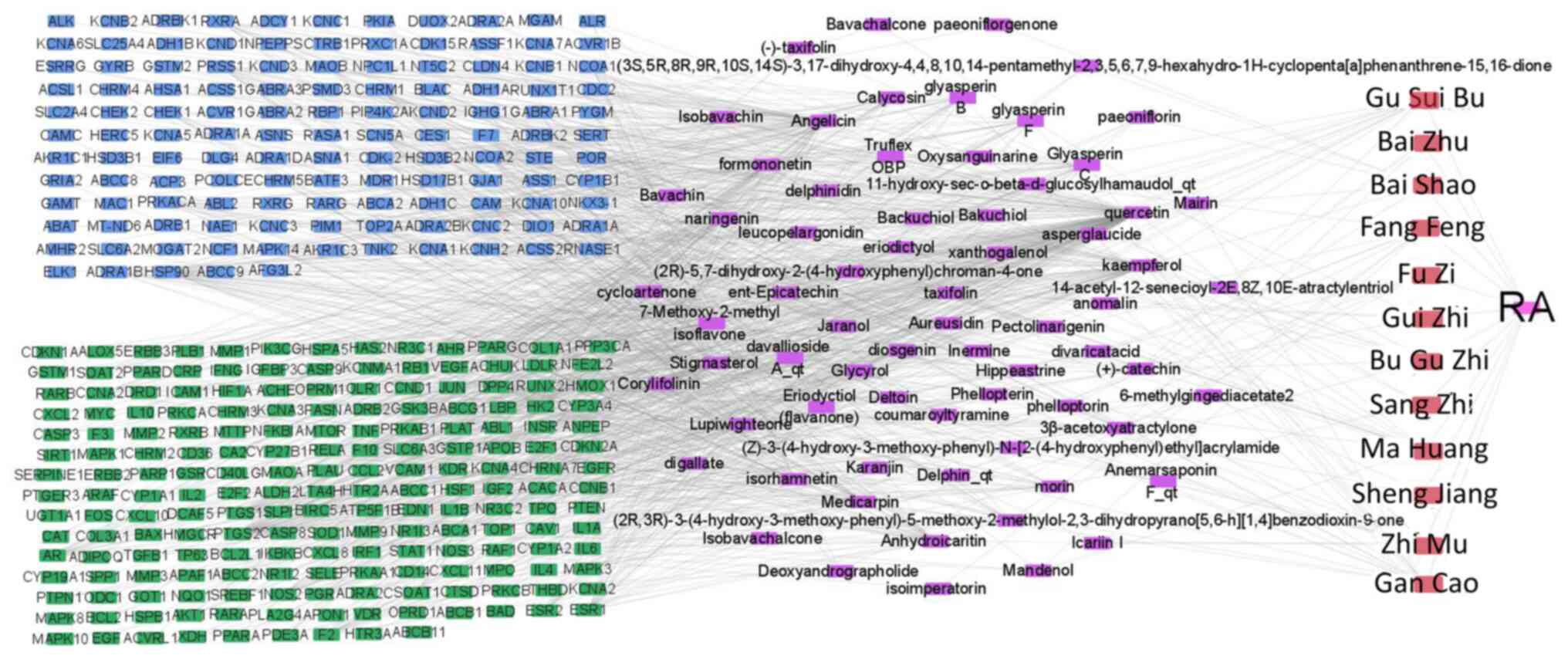
Drug-active component-disease target
regulatory networks
Cytoscape 3.7.2 software was used to construct the
‘drug-active ingredient-disease target’ network of JWGZSYZMD in the
regulation of RA (Fig. 2). The
number of node connections in the network was calculated and the
top 30 active ingredients with the highest number of targets were
selected to be potential active ingredients for RA treatment. These
were quercetin, angelicin, kaempferol, 7-methoxy-2-methyl
isoflavone, naringin, formononetin, isorhamnetin, icaritin
dehydrated, alfalfa toxin, stigmasterol, glyasperin C, calycosin,
glyasperin B, lupiwighteone, glyasperin F, Inermine, morin,
aureusidin, diosgenin, catechin, jaranol, taxifolin, xanthogalenol,
pectolinarigenin, phellopterin, glycyrol, hippeastrine, deltoin,
coumaroyltyramine and eriodictyol (Table I).
 | Table ITop 30 potential active components of
Jiawei Guizhishaoyaozhimu Decoction for the treatment of rheumatoid
arthritis. |
Table I
Top 30 potential active components of
Jiawei Guizhishaoyaozhimu Decoction for the treatment of rheumatoid
arthritis.
| Compound names | ID | Active
ingredient | Oral
bioavailability | Drug likeness | Number of
targets |
|---|
| Ma Huang (Herba
Ephedrae) | MOL000098 | Quercetin | 46.43 | 0.28 | 152 |
| Bu Gu Zhi
(Fructus Psoraleae)a | NA | Angelicin | NA | NA | 74 |
| Sang Zhi
(Ramulus Mori), Ma Huang (Herba Ephedrae), Bai
Shao (Raidix Paeoniae Alba), Gu Sui Bu (Rhizoma
Drynariae), Zhi Mu (Rhizoma Anemarrhenae) and Gan Cao
(Zhi; Radix Glycyrrhizae Uralensis) | MOL000422 | Kaempferol | 41.88 | 0.24 | 68 |
| Gan Cao (Zhi;
Radix Glycyrrhizae Uralensis) | MOL003896 | 7-Methoxy-2-methyl
isoflavone | 42.56 | 0.20 | 44 |
| Gu Sui Bu
(Rhizoma Drynariae), Ma Huang (Herba Ephedrae) and
Gan Cao (Zhi; Radix Glycyrrhizae Uralensis) | MOL004328 | Naringenin | 59.29 | 0.21 | 40 |
| Gan Cao (Zhi;
Radix Glycyrrhizae Uralensis) | MOL000392 | Formononetin | 69.67 | 0.21 | 39 |
| Gan Cao (Zhi;
Radix Glycyrrhizae Uralensis) | MOL000354 | Isorhamnetin | 49.60 | 0.31 | 38 |
| Zhi Mu (Rhizoma
Anemarrhenae) | MOL004373 |
Anhydroicaritin | 45.41 | 0.44 | 38 |
| Gan Cao (Zhi;
Radix Glycyrrhizae Uralensis) | MOL002565 | Medicarpin | 49.22 | 0.34 | 35 |
| Sheng Jiang
(Rhizoma Zingiberis Recens), Ma Huang (Herba
Ephedrae) and Gu Sui Bu (Rhizoma Drynariae) | MOL000449 | Stigmasterol | 43.83 | 0.76 | 35 |
| Gan Cao (Zhi;
Radix Glycyrrhizae Uralensis) | MOL004811 | Glyasperin C | 45.56 | 0.40 | 25 |
| Gan Cao (Zhi;
Radix Glycyrrhizae Uralensis) | MOL000417 | Calycosin | 47.75 | 0.24 | 23 |
| Gan Cao (Zhi;
Radix Glycyrrhizae Uralensis) | MOL004808 | Glyasperin B | 65.22 | 0.44 | 22 |
| Gan Cao (Zhi;
Radix Glycyrrhizae Uralensis) | MOL003656 | Lupiwighteone | 51.64 | 0.37 | 22 |
| Gan Cao (Zhi;
Radix Glycyrrhizae Uralensis) | MOL004810 | Glyasperin F | 75.84 | 0.54 | 19 |
| Gan Cao (Zhi;
Radix Glycyrrhizae Uralensis) | MOL001484 | Inermine | 75.18 | 0.54 | 18 |
| Sang Zhi
(Ramulus Mori) | MOL000737 | Morin | 46.23 | 0.27 | 18 |
| Gu Sui Bu
(Rhizoma Drynariae) | MOL001978 | Aureusidin | 53.42 | 0.24 | 18 |
| Zhi Mu (Rhizoma
Anemarrhenae) | MOL000546 | Diosgenin | 80.88 | 0.81 | 17 |
| Gu Sui Bu
(Rhizoma Drynariae), Bai Shao (Raidix Paeoniae Alba),
Ma Huang (Herba Ephedrae) and Gui Zhi (Ramulus
Cinnamomi) | MOL000492 | Catechin | 54.83 | 0.24 | 15 |
| Gan Cao (Zhi;
Radix Glycyrrhizae Uralensis) | MOL000239 | Jaranol | 50.83 | 0.29 | 14 |
| Gui Zhi (Ramulus
Cinnamomi) and Ma Huang (Herba Ephedrae) | MOL004576 | Taxifolin | 57.84 | 0.27 | 14 |
| Gu Sui Bu
(Rhizoma Drynariae) | MOL009091 | Xanthogalenol | 41.08 | 0.32 | 14 |
| Ma Huang (Herba
Ephedrae) | MOL005842 |
Pectolinarigenin | 41.17 | 0.30 | 13 |
| Fang Feng (Radix
Saposhnikoviae) | MOL002644 | Phellopterin | 40.19 | 0.28 | 13 |
| Gan Cao (Zhi;
Radix Glycyrrhizae Uralensis) | MOL002311 | Glycyrol | 90.78 | 0.67 | 12 |
| Zhi Mu (Rhizoma
Anemarrhenae) | MOL004497 | Hippeastrine | 51.65 | 0.62 | 12 |
| Fu Zi (Rhizoma
Typhonii Gigantei) | MOL002392 | Deltoin | 46.69 | 0.37 | 12 |
| Zhi Mu (Rhizoma
Anemarrhenae) | MOL000631 |
Coumaroyltyramine | 112.90 | 0.20 | 11 |
| Gu Sui Bu
(Rhizoma Drynariae) and Ma Huang (Herba
Ephedrae) | MOL005190 | Eriodictyol | 71.79 | 0.24 | 11 |
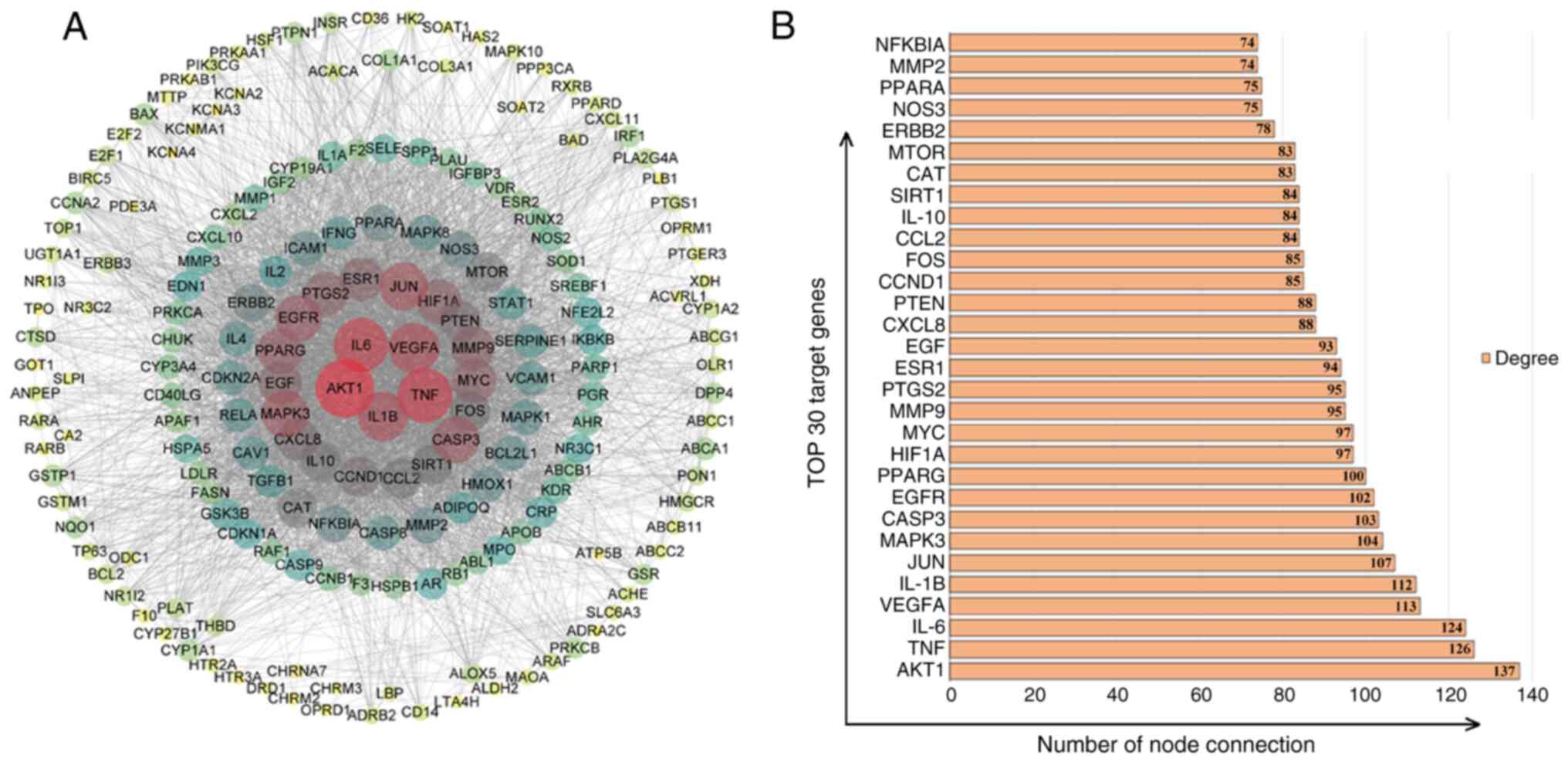
PPI predictions of core targets of
JWGZSYZMD during therapy of RA
The STRING database and Cytoscape were used to
analyze and visualize the targets of RA regulation by JWGZSYZMD.
The STRING database used PPI network (Fig. 3A). The top 30 targets were found to
mainly involve factors associated with inflammation (TNF, IL-6,
IL-1B, prostaglandin-endoperoxide synthase, IL-10 and IL-8), key
node proteins of inflammatory pathways (AKT1, JUN, MAPK3, nitric
oxide synthase 3 and MMP9), synovial angiogenesis processes [VEGFA
and hypoxia-inducible factor (HIF) 1A], apoptosis and cycle
regulation (caspase 3, Myc, cyclin D1, PTEN and Fos), hormone
regulation (EGFR, peroxisome proliferator-activated receptor γ,
estrogen receptor 1 and EGF) and chemokines (chemokine ligand 2)
(Fig. 3B).
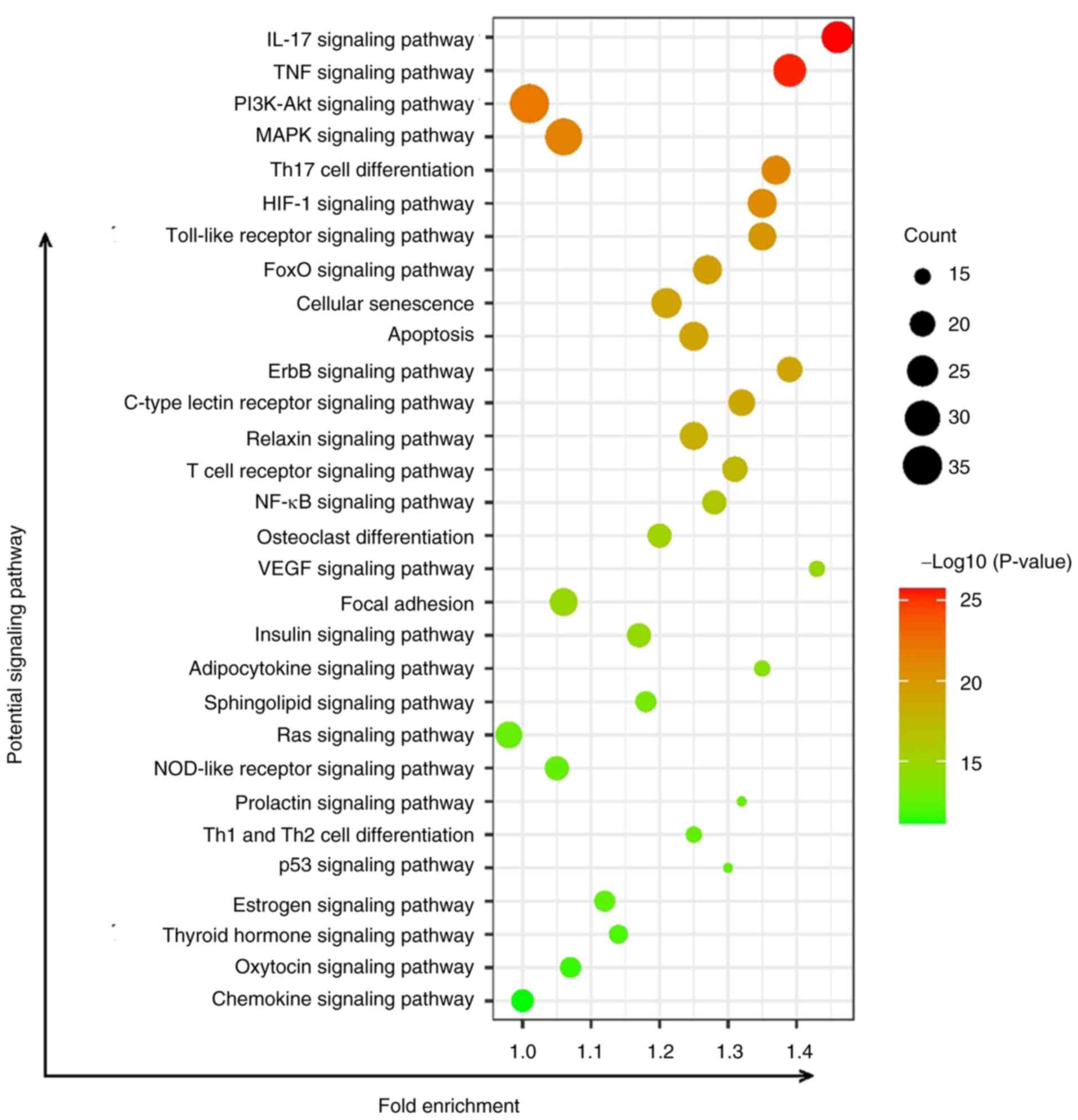
KEGG predictions of key pathways of
JWGZSYZMD during RA therapy
The pathways affected by JWGZSYZMD treatment of RA
mainly involved inflammation-related pathways (such as ‘IL-17
signaling pathway’, ‘TNF signaling pathway’, ‘PI3K/Akt signaling
pathway’, ‘MAPK signaling pathway’, ‘NF-κB signaling pathway’,
‘Toll-like receptor signaling pathway’ and ‘NOD-like receptor
signaling pathway’), T cell differentiation-related signaling
(‘Th17 cell differentiation’, ‘T cell receptor signaling pathway’
and ‘Th1 and Th2 cell differentiation’), neovascularization
signaling (‘HIF-1 signaling pathway’ and ‘VEGF signaling pathway’),
‘Osteoclast differentiation signaling’ and ‘Apoptosis’ (Fig. 4).
Molecular docking verification
The AutoDock Vina program was used to perform
molecular docking of the top 30 active ingredients onto IL-1β,
IL-6, IL-17A and TNF-α in the ‘drug-active ingredient-disease
target’ network, where the lowest binding energy was calculated.
The lower the binding energy, the stronger the binding force
between the chemical composition and the target. It is generally
considered that the binding energy of <-5.0 kJ/mol suggests good
binding activity, whilst <-7.0 kJ/mol would suggest strong
binding activity (26). In the
present study, the minimum binding energy for molecular docking
between active components and target sites was ≤-5.0 kJ/mol,
whereas 76 molecules demonstrated docking binding energy of ≤-7.0
kJ/mol (Table II). This finding
suggested that the binding ability between the potential active
components and target sites is viable. The three results with the
strongest binding activity found were IL-17 with diosgenin, TNF
with eriodictyol and IL-17 with glycyrol, which were then
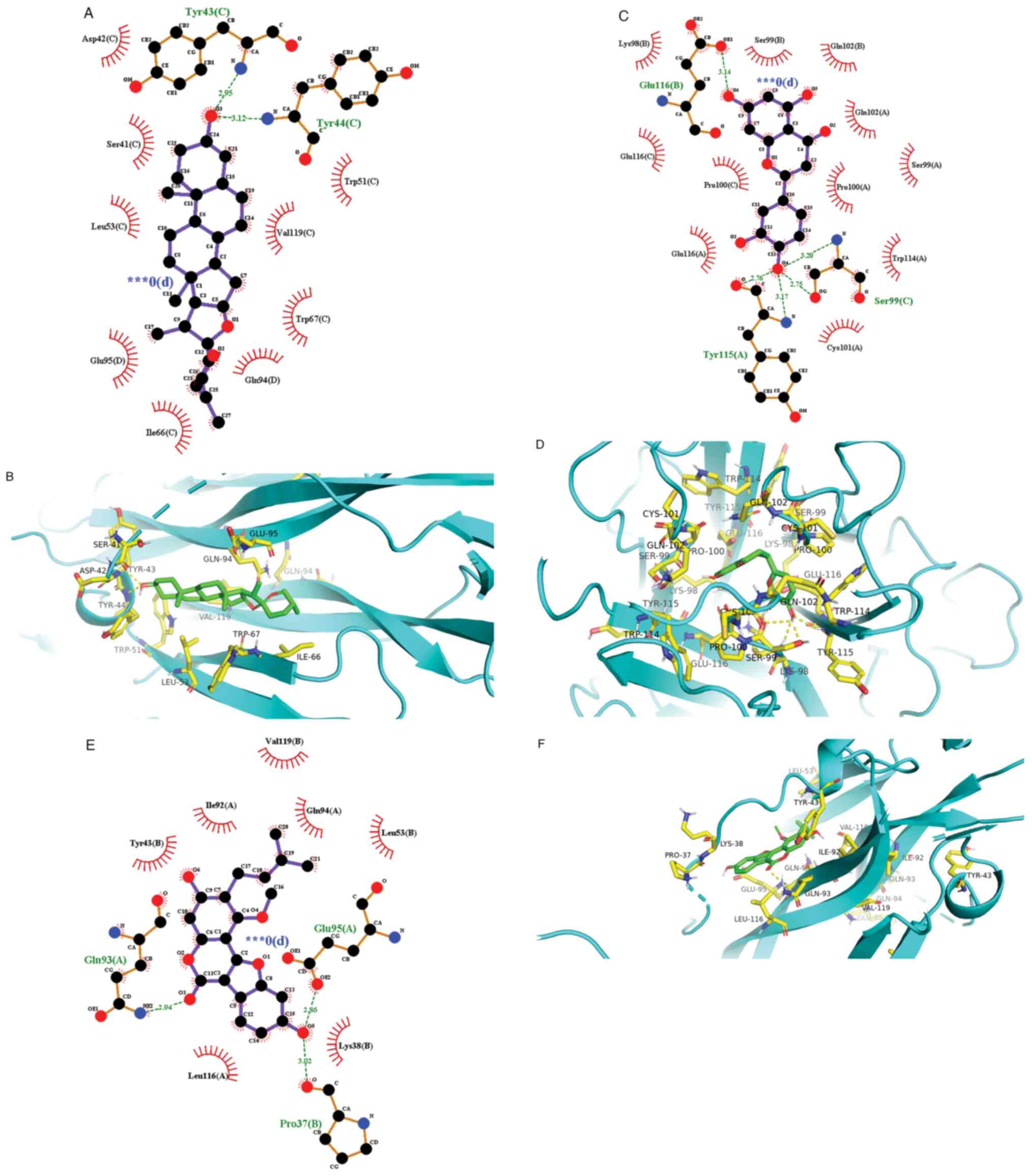
visualized using the Pymol and Ligplot software (Fig. 5) (27).
 | Table IIMolecular docking results of the
active ingredients of Jiawei Guizhishaoyaozhimu Decoction to
inflammatory cytokines. |
Table II
Molecular docking results of the
active ingredients of Jiawei Guizhishaoyaozhimu Decoction to
inflammatory cytokines.
| Active
ingredient | Binding energy to
IL-1β (kcal/mol) | Binding energy to
IL-6, kcal/mol) | Binding energy to
IL-17 (kcal/mol) | Binding energy to
TNF-α (kcal/mol) |
|---|
| Quercetin | -7.3 | -6.8 | -7.9 | -8.8 |
| Angelicin | -5.8 | -6.1 | -6.7 | -7.2 |
| Kaempferol | -7.2 | -6.7 | -7.6 | -8.6 |
| 7-Methoxy-2-methyl
isoflavone | -6.2 | -6.2 | -8.0 | -8.3 |
| Naringenin | -7.3 | -6.9 | -8.2 | -8.3 |
| Formononetin | -6.5 | -6.8 | -7.7 | -8.4 |
| Isorhamnetin | -7.0 | -7.0 | -7.8 | -8.0 |
|
Anhydroicaritin | -6.9 | -7.1 | -8.3 | -6.6 |
| Medicarpin | -6.8 | -6.5 | -7.4 | -8.3 |
| Stigmasterol | -7.2 | -6.6 | -8.3 | -6.5 |
| Glyasperin C | -7.0 | -6.7 | -7.9 | -5.8 |
| Calycosin | -6.6 | -6.6 | -8.0 | -6.5 |
| Glyasperin B | -6.5 | -6.5 | -8.6 | -5.9 |
| Lupiwighteone | -7.1 | -6.8 | -7.9 | -6.6 |
| Glyasperin F | -7.5 | -7.1 | -8.7 | -6.7 |
| Inermine | -7.1 | -7.0 | -8.3 | -7.0 |
| Morin | -7.2 | -6.7 | -7.7 | -8.6 |
| Aureusidin | -7.3 | -7.2 | -8.4 | -8.5 |
| Diosgenin | -8.0 | -7.5 | -10.3 | -7.4 |
| Catechin | -6.6 | -7.2 | -7.4 | -6.9 |
| Jaranol | -6.2 | -6.5 | -7.7 | -7.6 |
| Taxifolin | -7.0 | -7.3 | -7.6 | -7.6 |
| Xanthogalenol | -6.5 | -6.1 | -7.7 | -6.0 |
|
Pectolinarigenin | -6.6 | -6.8 | -7.6 | -7.7 |
| Phellopterin | -5.8 | -6.6 | -7.4 | -8.1 |
| Glycyrol | -7.2 | -6.8 | -8.9 | -6.7 |
| Hippeastrine | -7.0 | -7.4 | -8.4 | -8.3 |
| Deltoin | -7.4 | -6.0 | -7.5 | -7.3 |
|
Coumaroyltyramine | -5.8 | -6.3 | -8.0 | -7.9 |
| Eriodictyol | -7.0 | -7.3 | -7.9 | -9.0 |
Effect of JWGZSYZMD on joint score of
rats
The joint score of rats in the five treatment groups
was measured and was defined as the baseline. No statistically
significant differences were found between groups (Table III). After 21 days of the drug
treatments, the joint scores of JWGZSYZMD high-dose and the
tripterygium glycoside groups significantly decreased joint score
compared with that in the CIA model group (P<0.05). By contrast,
rats in the control group exhibited a joint score of 0 throughout
the experiment. There no statistically significant difference in
the joint scores of rats treated with low-, medium- or high-dose
JWGZSYZMD.
 | Table IIIJoint scores of CIA rats before and
after treatment with JWGZSYZMD. |
Table III
Joint scores of CIA rats before and
after treatment with JWGZSYZMD.
| Treatment
group | Joint score before
treatment | Joint score after
treatment | Z-score | P-value |
|---|
| Control | 0.00 (0.00,
0.00) | 0.00 (0.00,
0.00) | 0.000 | 1.000 |
| CIA model | 6.50 (6.00,
8.00) | 7.50 (6.75,
8.00) | -1.134 | >0.999 |
| JWGZSYZMD
low-dose | 7.00 (5.00,
8.00) | 6.00 (5.00,
7.00) | -1.633 | 0.612 |
| JWGZSYZMD
medium-dose | 7.00 (6.00,
7.25) | 5.50 (5.00,
6.25) | -1.633 | 0.612 |
| JWGZSYZMD
high-dose | 6.00 (5.75,
7.25) | 5.00 (5.00,
6.00)a | -1.604 | 0.654 |
| Tripterygium
glycoside | 7.00 (5.75,
8.00) | 5.00 (4.00,
6.00)a | -1.841 | 0.396 |
| H | 1.100 | 14.179 | | |
| P-value | 0.894 | 0.007 | | |
Effect of JWGZSYZMD on pathology in
rats
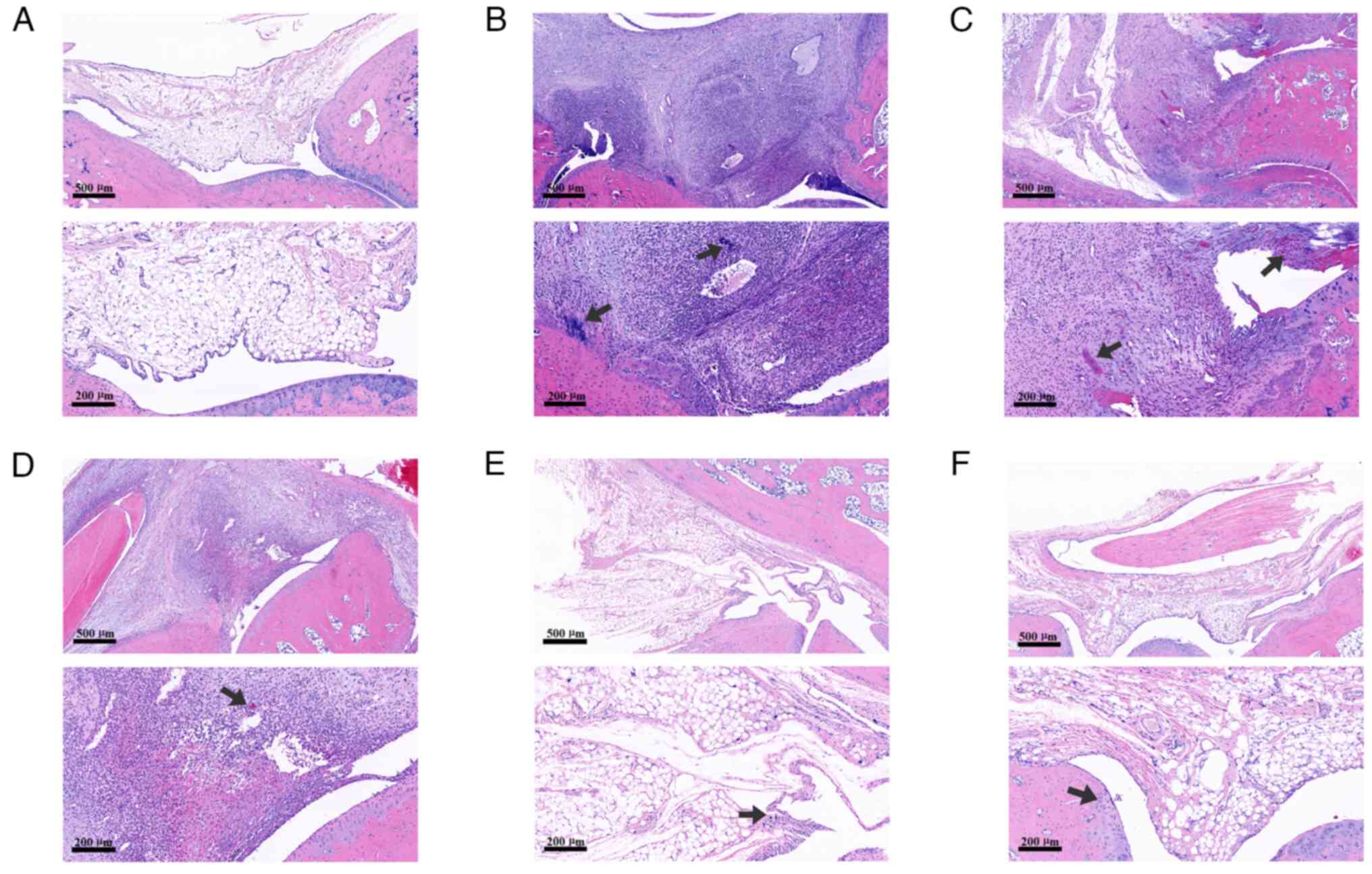
The staining results demonstrated that the joint
cavity structure of rats in the control group was normal, as no
infiltration of monocytes or bone erosion was observed in the
synovium. However, a large number of inflammatory cells were be
observed in the synovium of rats in the CIA model group, where
cartilage erosion could also be seen. By contrast, synovial
inflammatory cell infiltration and cartilage erosion were markedly
milder in the low-dose and medium-dose groups of JWGZSYZMD compared
with those in the CIA model group. Only a small amount of
inflammatory cell infiltration was observed in the high-dose group
of JWGZSYZMD and the tripterygium glycoside group (Fig. 6).
Effects of JWGZSYZMD on the mRNA
expression levels of synovial inflammatory factors in rats
The mRNA expression levels of the inflammatory
factors IL-6, TNF-α, IL-1β and IL-17A in the CIA model rats were
significantly increased compared with those in the control group
(P<0.05; Fig. 7). The
expression levels of IL-6 and TNF-α in the tripterygium glycoside
group were significantly increased compared with those in the
control group (P<0.05). The expression levels of IL-1β, TNF-α
and IL-6 in the low-, medium- and high-dose JWGZSYZMD groups and
IL-17A in the low- and medium-dose JWGZSYZMD groups were
significantly increased compared with those in the control group
(P<0.05). The expression levels of TNF-α mRNA in the low-,
medium- and high-dose JWGZSYZMD groups were significantly lower
compared with that in the CIA model group (P<0.05). The
expression levels of IL-1β in the low-, medium- and high-dose
JWGZSYZMD groups and IL-6 and IL-17A in the medium- and high-dose
JWGZSYZMD groups were significantly lower compared with that in the
CIA model group (P<0.05). The expression levels of IL-1β, IL-6
and IL-17A in the high-dose JWGZSYZMD group were significantly
lower compared with that in the low-dose JWGZSYZMD group
(P<0.05). The expression levels of IL-1β and IL-6 in the
high-dose JWGZSYZMD group were decreased compared with that in the
medium-dose JWGZSYZMD group (P<0.05). A dose-dependent effect
was observed with increasing concentrations of JWGZSYZMD treatment.
The expression levels of IL-6, TNF-α, IL-1β and IL-17A in the
tripterygium glycoside group was significantly decreased compared
with that in the CIA model group (P<0.05). The expression levels
of IL-1β, IL-6, IL-17A and TNF-α in the tripterygium glycoside
group were significantly lower compared with that in the low-dose
JWGZSYZMD group (P<0.05). The expression levels of IL-1β, IL-6
and TNF-α in the tripterygium glycoside group were decreased
compared with that in the medium-dose JWGZSYZMD group
(P<0.05).
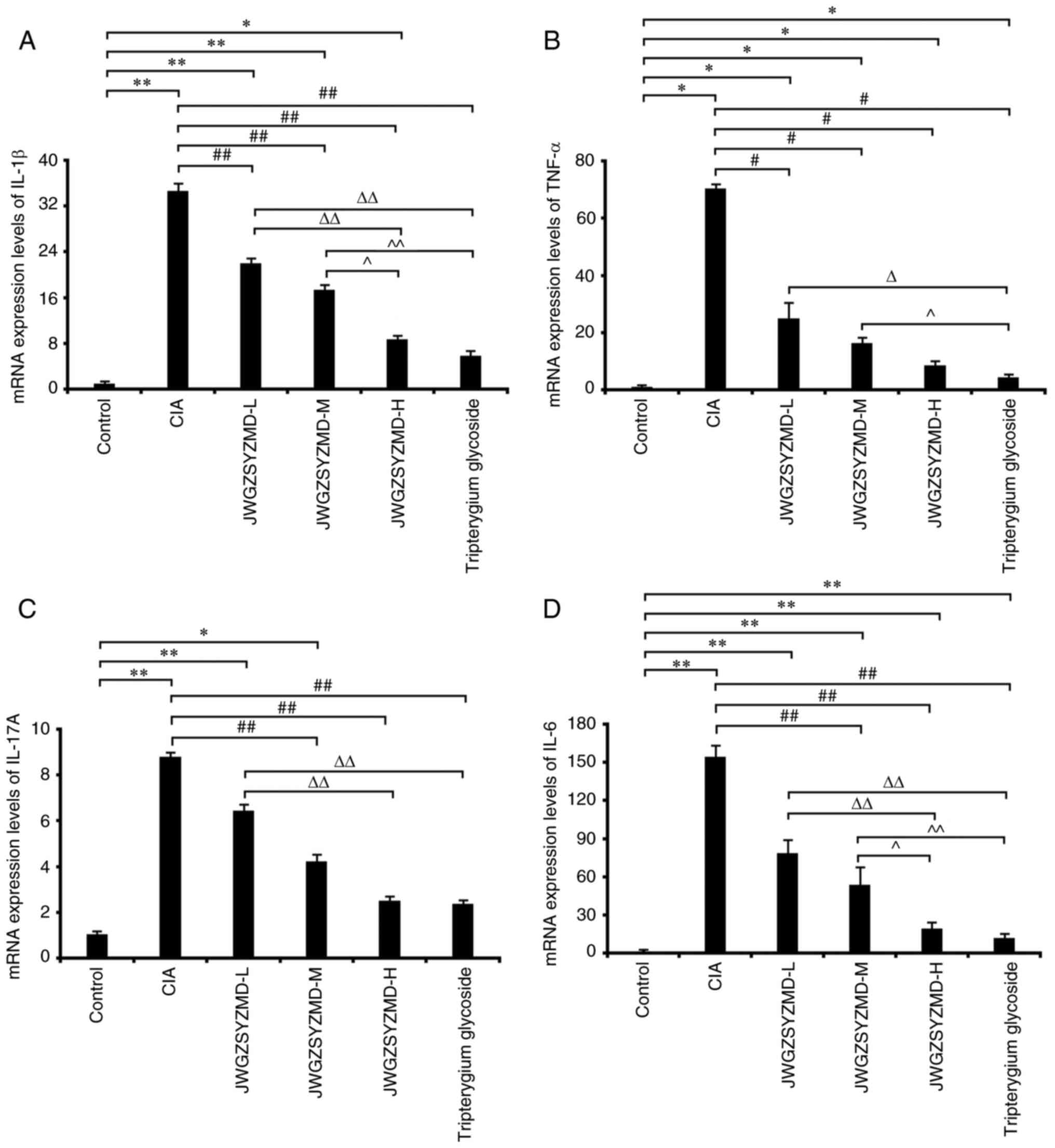 | Figure 7mRNA expression levels of synovial
inflammatory factors in rats treated with JWGZSYZMD. Expression
levels of (A) IL-6, (B) TNF-α, (C) IL-1β and (D) IL-17A in the
synovium of rats in each treatment group. One-way ANOVA followed by
Tukey's test was used to analyze the expression levels of IL-6,
IL-1β and IL-17A, whereas the Kruskal-Wallis H test followed by
Dunn's test was used to analyze the expression levels of TNF-α.
*P<0.05, **P<0.01 vs. Control Group.
#P<0.05, ##P<0.01 vs. CIA model Group.
ΔP<0.05, ΔΔP<0.01 vs. JWGZSYZMD-L.
^P<0.05, ^^P<0.01 vs. JWGZSYZMD-M. JWGZSYZMD, Jiawei
Guizhishaoyaozhimu Decoction; L, low; M, medium; H, high; CIA,
collagen II-induced arthritis. |
Effects of JWGZSYZMD on the protein
expression levels of synovial inflammatory factors in rats
The protein expression levels of synovial
inflammatory factors IL-6, TNF-α, IL-1β and IL-17A were analyzed by
ELISA. The results suggested that the protein expression levels of
these factors in the CIA model rats, all JWGZSYZMD treatment groups
and the tripterygium glycoside group were significantly compared
with those in the control group (P<0.01; Table IV). The protein expression levels
of IL-1β and IL-6 in the high-dose JWGZSYZMD group and IL-17
protein expression levels in the medium-dose JWGZSYZMD group were
significantly lower compared with those in the CIA model group
(P<0.05). The protein expression levels of TNF-α in the medium-
and high-dose JWGZSYZMD group and IL-17A protein expression levels
in the high-dose JWGZSYZMD group were significantly lower compared
with those in the CIA model group (P<0.01). The protein
expression levels of IL-17A in the medium-dose JWGZSYZMD group and
IL-6 in the high-dose JWGZSYZMD group were significantly lower
compared with those in the low-dose JWGZSYZMD group (P<0.05).
The protein expression levels of TNF-α and IL-17A protein
expression in the high-dose JWGZSYZMD group were significantly
lower compared with those in the low-dose JWGZSYZMD group
(P<0.01). A dose-dependent effect of JWGZSYZMD on protein
expression levels of the synovial inflammatory factors was
observed. The protein expression levels of IL-6, TNF-α, IL-1β and
IL-17A in the tripterygium glycoside group was significantly lower
compared with those in the CIA model group (P<0.01). The protein
expression levels of TNF-α, IL-6, IL-1β and IL-17 in the
tripterygium glycoside group were significantly lower compared with
those in the low-dose JWGZSYZMD group (P<0.05). The protein
expression levels of IL-17, IL-6 and TNF-α in the tripterygium
glycoside group were significantly lower compared with those in the
medium-dose JWGZSYZMD group (P<0.05).
 | Table IVProtein expression levels of
synovitis inflammatory factors IL-6, TNF-α, IL-1β and IL-17A in CIA
mice treated with JWGZSYZMD. |
Table IV
Protein expression levels of
synovitis inflammatory factors IL-6, TNF-α, IL-1β and IL-17A in CIA
mice treated with JWGZSYZMD.
| | Inflammatory factor
(pg/ml) |
|---|
| Treatment
group | IL-1β, mean ±
SD | TNF-α, mean ±
SD | IL-17A, median (Q1,
Q3) | IL-6, median (Q1,
Q3) |
|---|
| Control | 3.118±1.507 | 2.596±1.226 | 1.346 (0.782,
1.852) | 2.332 (1.982,
6.891) |
| CIA model |
78.560±24.079a |
160.328±36.997a | 31.827 (22.443,
36.443)a | 466.148 (225.123,
594.994)a |
| JWGZSYZMD
low-dose |
60.644±11.951a |
122.052±33.630a | 23.722 (20.789,
28.861)a | 350.171 (265.022,
383.308)a |
| JWGZSYZMD
medium-dose |
45.612±23.427a |
82.914±18.868a,bb | 16.872 (15.448,
21.348)a,b,c | 239.062 (129.033,
300.533)a |
| JWGZSYZMD
high-dose |
34.719±12.894a,b |
48.809±23.509a,bb,cc | 15.732 (8.170,
16.700)a,bb,cc | 166.018 (82.241,
240.509)a,b,c |
| Tripterygium
glycoside |
26.067±4.203a,bb,c |
27.925±16.740a,bb,cc,dd | 11.848 (5.066,
15.653)a,bb,c,d | 90.638 (63.794,
131.868)a,bb,cc,d |
| F | 55.916 | 52.278 | | |
| H | | | 29.075 | 27.223 |
| P-value |
2.6653x10-14 |
6.5479x10-14 | 0.000022 | 0.000052 |
Discussion
RA is a heterogeneous, systemic and chronic
autoimmune disease that is characterized by the non-infectious
inflammation of joints and periarticular tissues (28,29).
The majority of patients with RA also have extra-articular
multisystem involvement, which can further aggravate the condition
(28,29). An effective, integrated Traditional
Chinese and Western medicinal treatment that can alleviate the
inflammation whilst also preventing bone destruction is necessary
for treatment of this disease. If the body is weak, or feels
seasonal changes, it may cause this disease, RA has been previously
attributed by the TCM research field to ‘arthralgia syndrome’,
where this disease is called ‘Wang Bi’ (30). As this condition is recalcitrant,
protracted and refractory and pain traverses the characteristics of
multiple joints throughout the body, it is also considered to be
different from general arthralgia syndrome (30). RA is typically classified as a
special type of arthralgia syndrome, which is similar to ‘Li Jie’,
a different joint disease, mentioned in the ‘Synopsis of the Golden
Chamber - Concurrent Treatment of Stroke Syndrome’ (31). According to the aforementioned
synopsis, RA is ‘a disease involving multiple joints, making them
unable to flex or extend’, ‘swollen, painful joints with limited
function’. The Guizhi Shaoyao Zhimu decoction was set by Zhang
Zhongjing, a doctor of TCM, for rheumatism affecting multiple
joints. This medicine contained nine components and is a common
prescription in China for treating RA (1-3).
The main manifestations of RA are the symmetrical
polyarthritis of the joints, including hands, wrists and feet,
although it can also involve joints in the knees and hips. RA can
also exhibit a number of extra-articular manifestations. Among its
joint manifestations, swelling and deformation are common symptoms
(32). The inflammatory response
induced by the CIA model used in the present study was reported to
induce polyperipheral arthritis, mainly in the feet, ankle and knee
joints, which causes local redness, swelling and deformity of the
joints (33). Pathological
manifestations include proliferative synovitis, articular cartilage
destruction, bone erosion, pannus formation and inflammatory cell
infiltration. The clinical manifestations, laboratory parameters
and immune and pathological changes are similar to those of human
RA (34). Therefore, using the CIA
rat model, the therapeutic effects of the drug on joint
inflammation were assessed by drug intervention followed by the
regular observation of changes in the joints of the rats. A number
of studies have reported the treatment of clinical RA with Guizhi
Shaoyao Zhimu Decoction (12,13,35),
however, to the best of our knowledge, there are currently no
reports on its mechanism of action, which limits the application of
this treatment for RA.
In the present study, the network relationship of
‘drug-active ingredient-disease target’ was constructed through the
network pharmacology, where the potential active ingredients
contained within JWGZSYZMD for the treatment of RA were analyzed.
Among those list were quercetin, angelicin, kaempferol,
7-methoxy-2-methyl isoflavone, naringin, formononetin,
isorhamnetin, icaritin, alfalfa toxin and stigmasterol. Quercetin
is a subclass of flavonoids with reported anti-inflammatory and
antioxidant effects (36,37). Hashemi et al (38) previously reported that quercetin
could reduce the production of inflammatory cytokines and IL-17 by
inhibiting the Toll-like receptor 4/MAPK signaling pathway, thereby
reducing the polarization of T helper 17 (Th17) cells and
inhibiting the inflammatory response. In addition, core targets of
JWGZSYZMD found in the therapy of RA in the present study include
AKT1, TNF, IL-6, VEGFA and IL-1B, following analysis of the PPI
network. These aforementioned core targets also underwent KEGG
pathway enrichment analysis. The KEGG results indicated that the
core targets were mainly enriched in inflammatory related pathways,
such as IL-17, TNF, PI3K/Akt and MAPK. During RA synovial
inflammation, MAPK signaling forms one of the core mechanisms
mediating the occurrence of inflammation. MAPK signaling in
synovial cells is mainly activated by TNF-α and IL-1, resulting in
the production of cytokines to promote the inflammatory response
(39,40). The major family members of MAPK
signaling include p38 MAPK, ERK1/2 and JNK, all of which are
present in phosphorylated forms in synovial tissue of RA patients.
(41). Another study previously
reported that p38 MAPK can regulate IL-17 signaling upstream of
proinflammatory and mediator cytokines (42).
In the present study, the joint scores of the model
group were increased after collagen II treatment, whilst those in
the JWGZSYZMD treatment groups were decreased compared with those
in the model group after collagen II treatment. In terms of
pathology, the synovium in the CIA model group exhibited notable
inflammatory cell infiltration, where the cartilage showed synovial
erosion. By contrast, the degree of synovial inflammatory cell
infiltration and cartilage erosion in the low- and medium-dose
groups of JWGZSYZMD were markedly milder compared with those in the
CIA model group. In addition, only a small quantity of inflammatory
cells could be observed in the high-dose group of JWGZSYZMD. The
pathophysiological features after JWGZSYZMD treatment were found to
be alleviated at a high-dose compared with those in the model
group. A similar result was observed with the tripterygium
glycoside treatment group. This suggests that this decoction can
relieve joint swelling and deformation in the CIA model animals,
even though there were no significant differences in joint scores
between dose groups. The results of the present study could be
influenced by the small sample of rats in each group. However,
high-doses of JWGZSYZMD decreased the expression of IL-1β, IL-6,
IL-17 and TNF-α in the synovium of model animals, which suggests
that this treatment could serve a role in relieving joint
inflammation by controlling expression of these factors. IL-1β is
one of the most extensively studied subtypes of IL-1β, which is a
typical proinflammatory cytokine. It is mainly produced by
inflammatory and immune cells and serves an important role in the
inflammatory response, bone resorption and cartilage destruction. A
previous study reported that IL-1β can serve a positive feedback
role in the regulation of NF-κB activation through a series of
cascade reactions to aggravate RA (43). IL-1β, after being secreted by the
synovial tissue, can activate osteoclasts and chondrocytes to
induce bone destruction and cartilage destruction or promote Th17
cell differentiation, which facilitate the pathogenesis of RA
(44). TNF-α is produced by
activated macrophages, monocytes and T lymphocytes, which can
inhibit the synthesis of bone collagen, stimulate the production of
prostaglandins and collagenases by fibroblasts and chondrocytes.
This in turn stimulates the secretion of MMPs by chondrocytes and
induces the differentiation of peripheral blood mononuclear cells
into osteoclasts, leading to cartilage destruction, aggravation of
the inflammatory response and, consequently, RA pathogenesis
(45-47).
IL-17A is a cytokine produced by CD4+Th17 cells and γδ T
cells, which can not only sustain the malignant inflammatory cycle
of the joint tissue to promote the occurrence and development of RA
but can also promote the production of TNF-α by infiltrating
leukocytes (48). The combination
of these two processes can cooperatively activate synovial
fibroblasts to produce IL-6(48).
IL-6 is a key factor in the pathogenesis of RA, contributing to the
production of IgM and IgG rheumatoid factors and citrullinated
peptide antibodies, which promote the development of synovitis and
joint destruction (49,50).
Previous research has reported that GSZD can reduce
the levels of pro-inflammatory factors TNF-α, IL-1β, IL-17 and
receptor activator for NF-κB ligand (RANKL), thus inhibiting the
recruitment and differentiation of osteoclast precursors. GSZD may
also increase the secretion of osteoclastogenesis inhibitory factor
(OPG), thereby regulating the ratio of RANKL/OPG that could promote
the protection of bone to improve the injury of cartilage and
synovium in CIA rats (51). In the
present study, ‘Gu Sui Bu’ (Rhizoma Drynariae), ‘Bu Gu Zhi’
(Fructus Psoraleae) and ‘Sang Zhi’ (Ramulus Mori) were added to the
original medicinal preparation. Previous studies have reported that
these components also serve a positive role in the protection of
bone destruction in RA. Bavachinin (derived from ‘Bu Gu Zhi’) may
inhibit the proliferation and migration of MH7A cells and
production of inflammatory factors and apoptosis by acting on the
PI3K/AKT signaling pathway (52).
In addition, ‘Gu Sui Bu’ flavones can significantly inhibit the
loss of bone mass around the inflammatory joints of CIA rats, which
can promote bone reconstruction (53). Low dose ‘Sang Zhi’ could inhibit
the expression of the HIF-1α/VEGF/MMP signaling pathway, thereby
reducing the inflammatory response and neovascularization of toes
in rats, which inhibited the destruction of bone and cartilage
(54). The new Chinese medicine
presented in the present study was shown to protect the joints and
enhances the curative effect of the original preparation.
Therefore, future studies will focus on analyzing the mechanisms
related to bone destruction and protection of cartilage.
Results from the present study demonstrated that
JWGZSYZMD reduced the expression levels of IL-1β, IL-6, IL-17 and
TNF-α in synovium of rats with CIA, which suggests that this
treatment may potentially alleviate joint inflammation by
inhibiting or downregulating the expression of these inflammatory
factors. A wide array of potential active ingredients contained
within JWGZSYZMD could bind to core factors involved in RA, such as
TNF and IL-6, which may explain the potential therapeutic
characteristics of this treatment. The main tools used for studying
the molecular docking of chemical components to targets were online
databases, therefore, there are some limitations associated with
these methods, such as the delayed updates of databases and
incomplete inclusion degrees. Further verification should be
performed on the basis of continuous updates associated with these
databases. Future research should aim to clarify the specific
mechanism of action of JWGZSYZMD for the treatment of RA to provide
a more precise theoretical basis for the clinical treatment of RA.
For example, future experiments could compare the efficacy of
glycyrol, eriodictyol and diosgenin for the treatment of RA.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the 2020 New Teacher
Launch Project for The Beijing University of Traditional Chinese
Medicine (grant no. 2020-JYB-XJSJJ-065).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and WJ designed the study, performed the
experiments and wrote the paper. YJ and KF analyzed data. XZ and YX
calibrated the data and revised the paper. All authors read and
approved the final version of the manuscript. YC and WJ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Medical and
Experimental Animal Ethics Committee of The Beijing University of
Traditional Chinese Medicine (approval no.
BUCM-4-2021090605-3104).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zheng AP and Zheng XH: Evolution and
information analysis of classical formulas Guizhi Shaoyao
Zhimutang. Chinese J Experimental Traditional Medical Formulae.
29:174–184. 2023.
|
|
2
|
Daily JW, Zhang T, Cao S and Park S:
Efficacy and safety of GuiZhi-ShaoYao-ZhiMu decoction for treating
rheumatoid arthritis: A systematic review and meta-analysis of
randomized clinical trials. J Altern Complement Med. 23:756–770.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yuan N: Effect of GuizhiShaoyaoZhimu
Decoction on rheumatoid arthritis. Cardiovascular Disease. J Integr
Trad Chin Western Med. 6:163–164. 2018.(In Chinese).
|
|
4
|
Chinese Rheumatology Association. 2018
Chinese guideline for the diagnosis and treatment of rheumatoid
arthritis. Chin J Intern Med. 57:242–251. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
England BR, Thiele GM, Anderson DR and
Mikuls TR: Increased cardiovascular risk in rheumatoid arthritis:
Mechanisms and implications. BMJ. 361(k1036)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu W, Fan Y, Tian C, Jin Y, Du S, Zeng P
and Wang A: Deciphering the molecular targets and mechanisms of
HGWD in the treatment of rheumatoid arthritis via network
pharmacology and molecular docking. Evid Based Complement Alternat
Med. 2020(7151634)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huang J, Fu X, Chen X, Li Z, Huang Y and
Liang C: Promising therapeutic targets for treatment of rheumatoid
arthritis. Front Immunol. 12(686155)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bradfield PF, Amft N, Vernon-Wilson E,
Exley AE, Parsonage G, Rainger GE, Nash GB, Thomas AM, Simmons DL,
Salmon M and Buckley CD: Rheumatoid fibroblast-like synoviocytes
overexpress the chemokine stromal cell-derived factor 1(CXCL12),
which supports distinct patterns and rates of CD4+and CD8+T cell
migration within synovial tissue. Arthritis Rheum. 48:2472–2482.
2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Filer A, Parsonage G, Smith E, Osborne C,
Thomas AMC, Curnow SJ, Ed Rainger G, Raza K, Nash GB, Lord J, et
al: Differential survival of leukocyte subsets mediated by
synovial, bone marrow, and skin fibroblasts:Site-specific versus
activation-depend-ent survival of T cells and neutrophils.
Arthritis Rheum. 54:2096–2108. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zheng DN, Hu LF, Kong WP and Yan XP: An
analysis of Professor Yan Xiaoping's experience in treating
rheumatoid arthritis by tonifying the kidney and strengthening the
bones. J China-Japan Friendship Hospital. 34:116–118. 2020.(In
Chinese).
|
|
11
|
Xu Y, et al: Effects of
kidney-supplementing cold-dispelling Zhi Wang Tang decoction on
inflammation, bone erosion and Wnt/β-catenin pathway in rats with
colla-gen-induced arthritis, 2020, 43(04), 289-295.
|
|
12
|
Jing WX and Yan XP: YAN Xiao-ping's
experience in the diagnosis and treatment of rheumatism discussing
by ‘combine and harmony’. China Journal of Traditional Chinese
Medicine and Pharmacy,. 2021, 36(8):4.
|
|
13
|
Wang WR, Shi LC, Liu S, et al: Professor
YAN Xiaoping's experience in treating zhoubi(palindromic
rheumatism). Tianjin Journal of Traditional Chinese Medicine. 2021,
38(12): 1505-1508.
|
|
14
|
Guo W, Huang J, Wang N, Tan HY, Cheung F,
Chen F and Feng Y: Integrating Network pharmacology and
pharmacological evaluation for deciphering the action mechanism of
herbal formula Zuojin Pill in suppressing hepatocellular carcinoma.
Front Pharmacol. 10(1185)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li HL and Chen C and Chen C: Network
pharmacology-based approach to investigate the mechanism of
Huang-Lian-Jie-Du-Decoction for treatment of type 2 diabetes
mellitus. Traditional Medicine Research. 6(36)2021.
|
|
16
|
Li D, Fan H, Dong J, Sun C, Su Y, Liu J
and Gu Y: Based on BATMAN-TCM to explore the molecular mechanism of
Xihuang Pill regulating immune function to treat breast
precancerous lesions. Breast Cancer (Dove Med Press). 13:725–742.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang J, Zhou Y and Ma Z: Multi-target
mechanism of Tripteryguim wilfordii Hook for treatment of
ankylosing spondylitis based on network pharmacology and molecular
docking. Ann Med. 53:1090–1098. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47(D1):D607–D613.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen L, Zhang YH, Lu G, Huang T and Cai
YD: Analysis of cancer-related lncRNAs using gene ontology and KEGG
paths. Artif Intell Med. 76:27–36. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kondo N, Kuroda T and Kobayashi D:
Cytokine networks in the pathogenesis of rheumatoid arthritis. Int
J Mol Sci. 22(10922)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Takeuchi T, Yoshida H and Tanaka S: Role
of interleukin-6 in bone destruction and bone repair in rheumatoid
arthritis. Autoimmun Rev. 20(102884)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu SY, Bian R and Chen X: Experimental
methodology of pharmacology. People's Medical Publishing House,
2002.
|
|
23
|
Ding Y, Ma T and Yang XQ: Effects of high
dose glycosides of Tripterygium wilfordii Hook. f on the fertility
of young rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 32:61–63.
2012.PubMed/NCBI(In Chinese).
|
|
24
|
Liu C, He L, Wang J, Wang Q, Sun C, Li Y,
Jia K, Wang J, Xu T, Ming R, et al: Anti-angiogenic effect of
Shikonin in rheumatoid arthritis by downregulating PI3K/AKT and
MAPKs signal paths. J Ethnopharmacol. 260(113039)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lu JD, Ge XY, Wang HL, et al: Effect of
Guizhi Shaoyao Zhimu Decoction combined with Methotrexate in the
treatment of cold-damp blockage syndrome of rheumatoid arthritis.
China Medical Herald,2021,8(18):4.
|
|
27
|
Hsin KY, Ghosh S and Kitano H: Combining
machine learning systems and multiple docking simulation packages
to improve docking prediction reliability for network pharmacology.
PLoS One. 8(e83922)2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Z, Huang J, Xie D, He D, Lu A and
Liang C: Toward overcoming treatment failure in rheumatoid
arthritis. Front Immunol. 12(755844)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu L, Wang D, Liu MY, Yu H, Chen Q, Wu Y,
Bao R, Zhang Y and Wang T: The development from hyperuricemia to
gout: Key mechanisms and natural products for treatment. Acupunct
Herb Med. 2:25–32. 2022.
|
|
30
|
Jiao SD and Wang WG: Study on Disease Name
Wangbi and Its Treatment Discipline. J Zhejiang Chin Med Univ.
33(5)2009.
|
|
31
|
Sun Y, Ma WQ and Qu XP: Implications of
Gui Zhi Pao Yao Zhi Materia Medica Soup in ‘The Essentials of the
Golden Chamber’ for the treatment of rheumatoid arthritis by the
method of ‘Supporting Positive and Dispelling Evil’. Global
Traditional Chinese Medicine, 2019, 12(3), 427-429.
|
|
32
|
Fraenkel L, Bathon JM, England BR, St
Clair EW, Arayssi T, Carandang K, Deane KD, Genovese M, Huston KK,
Kerr G, et al: 2021 American college of rheumatology guideline for
the treatment of rheumatoid arthritis. Arthritis Care Res
(Hoboken). 73:924–939. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Harris HE, Liljeström M and Klareskog L:
Characteristics of synovial fluid effusion in collagen-induced
arthritis (CIA) in the DA rat; a comparison of histology and
antibody reactivities in an experimental chronic arthritis model
and rheumatoid arthritis (RA). Clin Exp Immunol. 107:480–484.
1997.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kim YH and Kang JS: Effect of methotrexate
on collagen-induced arthritis assessed by micro-computed tomography
and histopathological examination in female rats. Biomol Ther
(Seoul). 23:195–200. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu ZD and Shi LP: Effect of Jiawei
Guizhishaoyaozhimu Decoction on ESR, CRP and RF in Patients with
Active Rheumatoid Arthritis. Shaanxi J Trad Chin Med. 40:82–84.
2019.(In Chinese).
|
|
36
|
Singh P, Arif Y, Bajguz A and Hayat S: The
role of quercetin in plants. Plant Physiol Biochem. 166:10–19.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Marunaka Y, Marunaka R, Sun H, Yamamoto T,
Kanamura N, Inui T and Taruno A: Actions of quercetin, a
polyphenol, on blood pressure. Molecules. 22(209)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hashemi AM, Kahnamouii SS, Aghajani H,
Frozannia K, Pournasrollah A, Sadegh R, Esmaeeli H, Ghadimi Y and
Razmpa E: Quercetin decreases Th17 production by down-regulation of
MAPK-TLR4 signaling path on T cells in dental pulpitis. J Dent
(Shiraz). 19:259–264. 2018.PubMed/NCBI
|
|
39
|
Xu ZH, Li X, Lin Y, et al: Guizhi Shaoyao
Zhimu decoction combined with methotrexate in the treatment of
rheumatoid arthritis: A meta-analysis. Hunan Journal of Traditional
Chinese Medicine,2020,36(8):5.
|
|
40
|
Gao F, Bao SY and Fu Q: Research progress
of traditional Chinese medicine regulating MAPK signal path in the
treatment of rheumatoid arthritis. Information on Traditional
Chinese Medicine. 2022, 39(1):75-79, ISTIC, 2022:Inner Mongolia
Autonomous Region Science and Technology Planning project.
|
|
41
|
Lu M, Wang Y and Zhan X: The MAPK
pathway-based drug therapeutic targets in pituitary adenomas. Front
Endocrinol (Lausanne). 10(330)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu S, Ma H, Zhang H, Deng C and Xin P:
Recent advances on signaling pathways and their inhibitors in
rheumatoid arthritis. Clin Immunol. 230(108793)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shi YJ and Shen J: Effect of Guizhi
Shaoyao Zhimu Decoction on Serum Inflammatory Factors, OPG and
RANKL Levels in Patients with Rheumatoid Arthritis of
Wind-cold-dampness Arthralgia Type. J Sichuan Trad Chin Med.
36:106–109. 2018.(In Chinese).
|
|
44
|
Zeng Q, Wang CF and Zhu H: Treating 96
cases of rheumatoid arthritis with the Guizhi Shaoyao Zhimu
decoction. Clin J Chin Med. 10:77–78. 2018.(In Chinese).
|
|
45
|
Carter SD, Barnes A and Gilmore WH: Canine
rheumatoid arthritis and inflammatory cytokines. Vet Immunol
Immunopathol. 69:201–214. 1999.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Murakami T, Nakaminami Y, Takahata Y, Hata
K and Nishimura R: Activation and function of NLRP3 inflammasome in
bone and joint-related diseases. Int J Mol Sci.
23(5365)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Slowikowski K, Nguyen HN, Noss EH, Simmons
DP, Mizoguchi F, Watts GFM, Gurish MF, Brenner MB and Raychaudhuri
S: CUX1 and IκBζ (NFKBIZ) mediate the synergistic inflammatory
response to TNF and IL-17A in stromal fibroblasts. Proc Natl Acad
Sci USA. 117:5532–5541. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shi H, Wang DT, Wu RG, et al: Role of
TNF-α-mediated NF-κB signal path in angiogenesis of rheumatoid
arthritis. Medical Recapitulate, 2012,18(15):2397-2400.
|
|
49
|
Chen HM and Wang YL: Role and regulation
of osteoclasts in the pathological changes of bone destruction
caused by rheumatoid arthritis. Chin J Osteop. 22(6)2016.(In
Chinese).
|
|
50
|
Yuan S, Li X, Lin A and Larsson SC:
Interleukins and rheumatoid arthritis: Bi-directional Mendelian
randomization investigation. Semin Arthritis Rheum.
53(151958)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Shujun W: Experimental Study on Bone
Protective Effect and Mechanism of Guizhi-Shaoyao-Zhimu Decoction
in Rheumatoid Arthritis (unpublished PhD thesis). Chengdu
University of Traditional Chinese Medical, 2022.
|
|
52
|
Deng H, Jiang J, Shu J, Huang M, Zhang QL,
Wu LJ and Sun WK: Bavachinin ameliorates rheumatoid arthritis
inflammation via PPARG/PI3K/AKT signaling pathway. Inflammation.
46:1981–1996. 2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Xiaojun G, Jun X, Lianbo X, Chao L, Huali
G, Xinxing H, Ningli L and Danjun MA: Therapeutic effect of
drynaria total flavonoids on the bone destruction of CIA rat.
Journal of Clinical Medicine in Practice. 17:13–17. 2013.
|
|
54
|
Qing D, Fei H, DQingqiao H, et al: Study
of Ramulus Mori on bone destruction in adjuvant-induced arthritis
rats by regulating HIF-1α/VEGF/MMPs signaling pathway. Journal of
Hunan University of Chinese Medicine. 42:1096–1104. 2022.
|