Introduction
Aging societies are progressing rapidly worldwide,
and the proportion of older people with cancer is increasing
(1). In Japan, which has one of
the most aged populations in the world, older patients aged >65
years account for >70% of gastrectomies performed on patients
with gastric cancer (2).
Postoperative complications have been reported to increase after
gastrectomy in older patients because they often have more
comorbidities and poorer physical and organ functions than younger
patients (3).
Among the complications after gastrectomy for
gastric cancer, infectious complications (ICs) are more likely to
be severe and prolong the hospital stay of patients (4). The Japanese national database
analysis reported that surgical site infections, such as an
anastomotic leak, pancreatic fistula, and intraabdominal abscess,
and remote infections, such as pneumonia and urinary tract
infections, are closely associated with postoperative mortality in
patients with gastric cancer, and that ICs, in general, require
attention (5). When treating
cancer in older persons, it is necessary to construct a treatment
strategy considering short-term risks such as postoperative
complications and the benefit of prolonged survival after surgery
(6). Endoscopic treatment with
expanded indications and surgery with reduced dissection have been
recently reported for high-risk patients (7,8).
Identifying high-risk patients for gastrectomy can be important in
determining which patients should undergo reduced treatment.
However, the risk of postoperative complications in older patients
with gastric cancer has not been well documented, especially
regarding risk factors for ICs.
This study aimed to examine the risk factors for
postoperative ICs in patients with gastric cancer aged ≥65 years
who underwent radical gastrectomy to determine whether only
preoperative or all perioperative factors can be used to identify
high-risk patients for ICs.
Materials and methods
Patients
This retrospective analysis involved 514 consecutive
individuals aged over 65 years, pathologically confirmed with stage
I-III primary gastric cancer, and who underwent curative
gastrectomy at Yamaguchi University Medical Hospital in Yamaguchi,
Japan, between January 2007 and December 2021. Ten participants
were excluded from the retrospective analysis due to either the
absence of preoperative computed tomography (CT; n=6) or the
presence of simultaneous double cancer (n=4), resulting in a total
of 504 included patients. Written informed consent was obtained
from all participants, who agreed to provide blood and tissue
samples for clinical examination and to be contacted during
follow-up. The Institutional Review Board of Yamaguchi University
Hospital approved this study (2022-032).
Perioperative parameters
All perioperative parameters were assessed and
calculated following the methodology outlined in the authors'
previous study (9). In summary,
demographic information (age and gender), comorbidity data
[modified frailty index (mFI) (10) and Charlson comorbidity index
(CCI)], and performance status (PS) data were extracted from
medical records. Body mass index (BMI) was the ratio of body weight
(kg) to the square of height (m2). Preoperative laboratory
assessments, including blood cell counts and serum albumin levels,
were routinely conducted within a 2-week timeframe preceding
surgery. The calculation of laboratory-related parameters was
performed as follows: prognostic nutritional index (PNI)=serum
albumin value (g/L)+0.005 x total lymphocyte count in peripheral
blood (per mm3); neutrophil-to-lymphocyte ratio (NLR)=neutrophil
count/lymphocyte count; and platelet-to-lymphocyte ratio
(PLR)=platelet count/lymphocyte count.
Multidetector CT (MDCT) scans were conducted on all
patients within 4 weeks prior to surgery. Body composition
parameters, such as visceral fat area (VFA) and skeletal muscle
area (SMA), were determined using MDCT and fat rate software (AZE
Virtual Place, Aze Ltd. Tokyo, Japan), as outlined in a prior study
(11). In brief, VFA was measured
as the fat area at the umbilical level on preoperative MDCT. The
SMA of the abdominal, psoas, and paraspinal muscle areas was
measured using axial slices at the third lumbar vertebra level. The
skeletal muscle index (SMI) was computed as SMA divided by height
squared. Preoperative lymph node metastasis (N factor) was
evaluated through preoperative CT, with metastatic nodes diagnosed
as having a short-axis diameter >10 mm or round nodes with a
short diameter of 5-9 mm. Histological types were categorized as
differentiated and undifferentiated. The depth of tumor invasion (T
factor), N factor, and stage were classified according to the Third
English edition of the Japanese Classification of Gastric Carcinoma
(12). Of the 504 patients,
preoperative CRP values were not measured in 79 patients, so the
category of preoperative CRP values has missing data for 79
cases.
Definitions of infectious and
noninfectious complications
ICs were defined as postoperative complications in
which infection by pathogens was suspected from culture, imaging,
or blood test findings. The main IC definitions are as follows:
anastomotic leakage (escape of a water-soluble contrast agent,
administered endoluminally, as seen on radiography), abdominal
abscess formation (collection of pus within the intraabdominal
space, confirmed by radiographic evidence or drainage), pancreatic
fistula (drainage of a measurable volume of fluid occurring on or
after the third postoperative day, with an amylase content
exceeding three times the serum amylase activity. This condition
requires medical intervention, such as antibiotics or drainage.)
(13), pneumonia (lung infection
identified through radiographic evidence and sputum culture),
urinary tract infections (infection of the urinary tract diagnosed
by urinary culture), and incisional surgical site infection
(infection of the superficial and deep incisional surgical site
diagnosed through culture).
Noninfectious complications (non-ICs) were defined
as postoperative complications in which the patient was suspected
of not being infected with a pathogen. The severity of ICs or
non-ICs was classified, according to the Clavien-Dindo (CD)
classification (14), as grade
0-V, and patients with grade II or higher were defined as having
ICs or non-ICs.
Surgical procedure
All patients underwent distal gastrectomy (DG),
total gastrectomy (TG), or proximal gastrectomy (PG) with D1, D1+,
or D2 lymphadenectomy, according to the Japanese guidelines. In
principle, lymph node dissection adhered to the guidelines as a
general practice; however, there were instances where the surgeon
chose to undertake a more limited dissection based on the overall
health condition of the patient. Consequently, this study aimed to
assess whether the lymph node dissection performed was in line with
established standards. Lymph node dissection carried out in
accordance with the recommended guidelines was categorized as
standard, while dissection falling below the prescribed range was
termed reduced lymph node dissection. DG reconstruction involved
the utilization of Billroth I, Billroth II, or Roux-en Y
techniques, TG reconstruction employed Roux-en Y, and PG
reconstruction utilized double tract reconstruction.
Statistical analysis
The optimal cutoff values for continuous variable
parameters, such as age, PS, mFI, CCI, PNI, NLR, PLR, BMI, VFA, and
SMI, were determined using receiver operating characteristic (ROC)
curves. Categorical variables are presented as numbers
(percentages), and continuous variables are presented as means ±
standard error. Categorical variables were analyzed using the
chi-squared or Fisher's exact tests, and continuous variables were
analyzed using the unpaired Student's t-test or Mann-Whitney test.
Multivariate analysis of the risk of developing infectious and
noninfectious complications were performed using logistic
regression, and the hazard ratios (HRs) and 95% confidence
intervals (CIs) were calculated. Statistical significance was set
at P<0.05. The confidence intervals for 10-fold cross-validated
area under the ROC curve (AUC) estimates were calculated by
dividing the 503 observations randomly into 10 folds, stratifying
by the event. We defined a function to fit a model on the training
data and to generate predicted values for the observations in the
validation fold, for a single iteration of the cross-validation
procedure. Then we applied this function across all folds to
generate predicted values for each validation fold (15). The 10-fold cross-validation
algorithm was computed using the R statistical programming language
(version 4.2.0). All statistical analyses except the 10-fold
cross-validation were performed using SPSS version 25.0 (SPSS
Corp., Armonk, NY, USA).
Results
Postoperative complications
Of the total patients, 165 (32.7%) developed
postoperative complications, of which 95 (18.8%) and 96 (19.0%)
developed ICs and non-ICs, respectively. Twenty-six patients (5.2%)
developed ICs and non-ICs. Serious grade ≥III complications of the
CD classification were observed in 48 (50.5%) IC and 18 (18.8%)
non-IC cases.
A total of 108 ICs occurred in the 95 patients. The
most common ICs were anastomotic leakage (n=29), pneumonia (n=26),
intraabdominal abscesses (n=23), and pancreatic fistulas (n=12).
Intraabdominal ICs (anastomotic leakage, intraabdominal abscess,
and pancreatic fistula) accounted for 59.2% of all ICs. In
contrast, 104 non-ICs were observed in 96 patients. The most common
non-ICs were delirium (n=53), delayed gastric emptying (n=12),
hemorrhage (n=10), and anastomotic stenosis (n=10). Details of
postoperative complications are shown in Table I.
 | Table IDetails of postoperative
complications in older patients. |
Table I
Details of postoperative
complications in older patients.
| | Grade |
|---|
| Type of
complication | All grades, n | II, n | III, n | IV, n | V, n |
|---|
| Infectious
complications (total events) | 108 | 60 | 40 | 8 | 0 |
|
Anastomotic
leakage | 29 | 12 | 16 | 1 | 0 |
|
Abdominal
abscess | 23 | 13 | 8 | 2 | 0 |
|
Pancreatic
fistula | 12 | 5 | 6 | 1 | 0 |
|
Pneumonia | 26 | 17 | 7 | 2 | 0 |
|
Urinary
tract infections | 3 | 3 | 0 | 0 | 0 |
|
Incisional
surgical site infection | 3 | 2 | 1 | 0 | 0 |
|
Perforation
of the digestive tube | 2 | 0 | 1 | 1 | 0 |
|
Enteritis | 2 | 2 | 0 | 0 | 0 |
|
Retrograde
infection of abdominal drain | 2 | 1 | 0 | 1 | 0 |
|
Vascular
catheter infection | 2 | 2 | 0 | 0 | 0 |
|
Splenic
infarction | 2 | 2 | 0 | 0 | 0 |
|
Infectious
pleural effusion | 1 | 0 | 1 | 0 | 0 |
|
Cholangitis | 1 | 1 | 0 | 0 | 0 |
| Non-infectious
complications (total events) | 104 | 87 | 15 | 1 | 1 |
|
Delirium | 53 | 53 | 0 | 0 | 0 |
|
Delayed
gastric emptying | 12 | 12 | 0 | 0 | 0 |
|
Hemorrhage | 10 | 4 | 4 | 1 | 1 |
|
Anastomotic
hemorrhage | 7 | 4 | 3 | 0 | 0 |
|
Intra-abdominal
hemorrhage | 2 | 0 | 1 | 1 | 0 |
|
Cerebral
hemorrhage | 1 | 0 | 0 | 0 | 1 |
|
Anastomotic
stenosis | 10 | 5 | 5 | 0 | 0 |
|
Ileus | 7 | 4 | 3 | 0 | 0 |
|
Ascites | 3 | 2 | 1 | 0 | 0 |
|
Arrhythmia | 3 | 3 | 0 | 0 | 0 |
|
Pleural
effusion | 3 | 1 | 2 | 0 | 0 |
|
Neurogenic
bladder | 2 | 2 | 0 | 0 | 0 |
|
Dermatitis | 1 | 1 | 0 | 0 | 0 |
Patient characteristics
The clinicopathological factors for all patients and
a comparison of clinicopathological factors between patients with
IC and those without IC are shown in Table II. The mean patient age was 74.8
(65-94) years, and 71.4% of the patients were male. Furthermore,
32.9% of patients had mFI scores ≥2, and 58.7% had comorbidities
with CCI scores ≥1. The pathological stages were I, II, and III in
66.4, 15.8, and 17.6% of patients, respectively. The operative
modes were DG, TG, and PG in 71.0, 24.8, and 4.2% of patients,
respectively, with laparoscopy in 73.8% and laparotomy in 26.2%.
Standard and reduced lymph node dissections were performed in 94.0
and 6.0% of the patients for guideline-based lymph node
dissection.
 | Table IIComparison of clinicopathological
factors between patients with and without ICs. |
Table II
Comparison of clinicopathological
factors between patients with and without ICs.
| Characteristic | All patients
(n=504) | IC group
(n=95) | Without-IC group
(n=409) | P-value |
|---|
| Age, years | 74.8±6.3 | 75.3±5.7 | 74.7±6.4 | 0.337 |
| Sex, n (%) | | | | 0.005a |
|
Male | 360 (71.4) | 79 (83.2) | 281 (68.7) | |
|
Female | 144 (28.6) | 16 (16.8) | 128 (31.3) | |
| PS, n (%) | | | | 0.241 |
|
0 | 431 (85.5) | 78 (82.1) | 353 (86.3) | |
|
1 | 59 (11.7) | 12 (12.6) | 47 (11.5) | |
|
≥2 | 14 (2.8) | 5 (5.3) | 9 (2.2) | |
| Modified frailty
index, n (%) | | | | 0.674 |
|
0 | 117 (23.2) | 21 (22.1) | 96 (23.5) | |
|
1 | 221 (43.8) | 38 (40.0) | 183 (44.7) | |
|
2 | 111 (22.0) | 23 (24.2) | 88 (21.5) | |
|
≥3 | 55 (10.9) | 13 (13.7) | 42 (10.3) | |
| Charlson
comorbidity index, n (%) | | | | 0.072 |
|
0 | 208 (41.3) | 37 (38.9) | 171 (41.8) | |
|
1 | 133 (26.4) | 21 (22.1) | 112 (27.4) | |
|
2 | 76 (15.1) | 12 (12.6) | 64 (15.6) | |
|
≥3 | 87 (17.3) | 25 (26.3) | 62 (15.2) | |
| Use of steroids, n
(%) | | | | 0.523 |
|
Negative | 488 (96.8) | 91 (95.8) | 397 (97.1) | |
|
Positive | 16 (3.2) | 4 (4.2) | 12 (2.9) | |
| Preoperative CRP,
mg/dl | 0.34±0.90 | 0.41±0.93 | 0.32±0.90 | 0.480 |
| PNI | 48.1±6.2 | 47.0±7.1 | 48.4±5.9 | 0.055 |
| NLR | 2.7±1.9 | 2.8±1.9 | 2.7±1.9 | 0.477 |
| PLR | 153.8±84.8 | 157.5±79.4 | 152.9±86.0 | 0.634 |
| BMI,
kg/m2 | 22.4±3.3 | 23.2±3.4 | 22.2±3.2 | 0.007a |
| VFA,
cm2 | 130.2±65.5 | 151.6±73.1 | 125.2±62.7 |
<0.001a |
| SMI,
cm2/m2 | 43.3±8.1 | 44.0±8.3 | 43.1±8.1 | 0.311 |
| Preoperative T
factor, n (%) | | | | 0.054 |
|
T1 | 309 (61.3) | 50 (52.6) | 259 (63.3) | |
|
T≥2 | 195 (38.7) | 45 (47.4) | 150 (36.7) | |
| Preoperative N
factor, n (%) | | | | 0.039a |
|
N0 | 395 (78.4) | 67 (70.5) | 328 (80.2) | |
|
N≥1 | 109 (21.6) | 28 (29.5) | 81 (19.8) | |
| Type of resection,
n (%) | | | | 0.009a |
|
Distal
gastrectomy | 358 (71.0) | 54 (56.8) | 304 (74.3) | |
|
Proximal
gastrectomy | 21 (4.2) | 7 (7.4) | 14 (3.4) | |
|
Total
gastrectomy | 125 (24.8) | 34 (35.8) | 91 (22.2) | |
| Approach, n
(%) | | | | 0.065 |
|
Open | 132 (26.2) | 32 (33.7) | 100 (24.4) | |
|
Laparoscopy | 372 (73.8) | 63 (66.3) | 309 (75.6) | |
| Extent of node
dissection, n (%) | | | | 0.055 |
|
D1/D1+ | 319 (63.3) | 52 (54.7) | 267 (65.3) | |
|
D2 | 185 (36.7) | 43 (45.3) | 142 (34.7) | |
| Node dissection
according to guidelines, n (%) | | | | 0.426 |
|
Standard | 474 (94.0) | 91 (95.8) | 383 (93.6) | |
|
Reduced | 30 (6.0) | 4 (4.2) | 26 (6.4) | |
| Operative duration,
min | 328.7±89.9 | 366.2±98.4 | 320.0±471.1 |
<0.001a |
| Operative blood
loss, ml | 250.9±337.8 | 392.7±471.1 | 217.9±289.4 | 0.001a |
| Pathological stage,
n (%) | | | | 0.003a |
|
I | 335 (66.5) | 55 (57.9) | 280 (68.5) | |
|
II | 80 (15.9) | 12 (12.6) | 68 (16.6) | |
|
III | 89 (17.7) | 28 (29.5) | 61 (14.9) | |
| Hospital stay,
days | 21.2±17.6 | 41.4±30.3 | 16.4±6.9 |
<0.001a |
Patients in the IC group were more likely to be male
and had preoperative lymph node metastasis, TG, pathological T3/T4,
pathological lymph node metastasis, pathological stage III, higher
VFA, longer operation times, and excessive intraoperative blood
loss than those in the non-IC group.
Diagnostic accuracy and cutoffs of
perioperative parameters
ROC curve analysis was performed to determine the
cutoff values and areas under the ROC curves (AUCs) of the
perioperative factors for ICs and non-ICs. The AUCs and optimal
cutoff values of each perioperative parameter for ICs are shown in
Fig. 1. The AUCs and optimal
cutoff values of each perioperative parameter for non-ICs are shown
in Fig. S1.
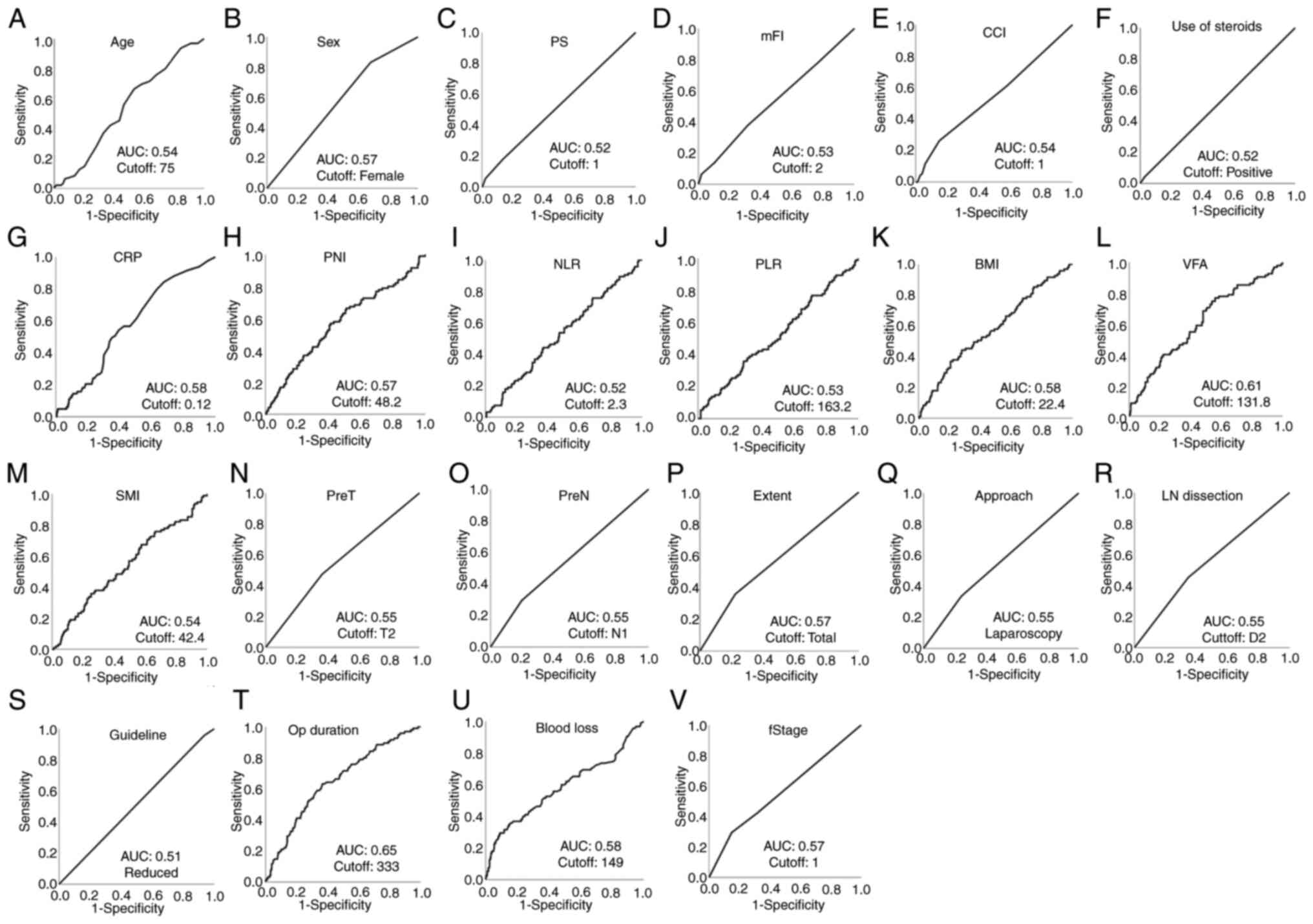 | Figure 1Receiver operating characteristic
curves of infectious complications for perioperative factors were
used to determine the cutoff values and AUCs. (A) Age, (B) sex, (C)
PS, (D) mFI, (E) CCI, (F) use of steroids, (G) preoperative CRP
levels, (H) PNI, (I) NLR, (J) PLR, (K) BMI, (L) VFA, (M) SMI, (N)
PreT, (O) PreN, (P) extent of resection, (Q) surgical approach, (R)
extent of LN dissection, (S) node dissection according to
guidelines, (T) Op duration, (U) blood loss and (V) fStage. AUC,
area under the receiver operating characteristic curve; BMI, body
mass index; CCI, Charlson comorbidity index; CRP, C-reactive
protein; fStage, final stage; LN dissection, lymph node dissection;
mFI, modified frailty index; NLR, neutrophil-to-lymphocyte ratio;
Op duration, operative duration; PLR, platelet-to-lymphocyte ratio;
PNI, prognostic nutritional index; PreN, preoperative N factor;
PreT, preoperative T factor; PS, performance status; SMI, skeletal
muscle index; VFA, visceral fat area. |
Perioperative risk factors for
ICs
Table III
summarizes the results of the univariate analysis for ICs after
categorizing all variables with cutoff values using the ROC curve.
Univariate analysis revealed that among the perioperative factors,
sex, PNI, VFA, preoperative N factor, the extent of resection,
operative duration, amount of blood loss, and pathological stage
were significantly associated with ICs. Table IV summarizes the results of the
multivariate analysis of independent preoperative factors and a
combination of all perioperative factors for ICs. Multivariate
analyses of only preoperative parameters with P<0.05 in the
univariate analyses revealed that sex, PNI, VFA, and TG were
independent risk factors for ICs (HR: 1.897, 95% CI: 1.044-3.448,
P=0.036; HR: 1.865, 95% CI: 1.153-3.019, P=0.011; HR: 1.673, 95%
CI: 1.040-2.697, P=0.034; and HR: 1.815, 95% CI: 1.098-3.000,
P=0.020, respectively). Multivariate analyses of all perioperative
parameters with P<0.05 in univariate analyses revealed that PNI
and operative duration were independent risk factors for ICs (HR:
1.961, 95% CI: 1.201-3.200, P=0.007; and HR: 2.555, 95% CI:
1.504-4.341, P=0.001, respectively).
 | Table IIIUnivariate analysis of risk factors
for infectious complications in older patients. |
Table III
Univariate analysis of risk factors
for infectious complications in older patients.
| | Infectious
complication | |
|---|
| Variables | Yes, n (%)
(n=95) | No, n (%)
(n=409) | P-value |
|---|
| Preoperative
factor | | | |
|
Age,
years | | | 0.101 |
|
<74 | 42 (44.2) | 219 (53.5) | |
|
>74 | 53 (55.8) | 190 (46.5) | |
|
Sex | | | 0.005a |
|
Male | 79 (83.2) | 281 (68.7) | |
|
Female | 16 (16.8) | 128 (31.3) | |
|
PS | | | 0.294 |
|
1 | 78 (82.1) | 353 (86.3) | |
|
≥2 | 17 (17.9) | 56 (13.7) | |
|
Modified
frailty index | | | 0.077 |
|
<2 | 71 (74.7) | 267 (65.3) | |
|
≥2 | 24 (25.3) | 142 (34.7) | |
|
Charlson
comorbidity index | | | 0.610 |
|
0 | 37 (38.9) | 171 (41.8) | |
|
≥1 | 58 (61.1) | 238 (58.2) | |
|
Use of
steroids | | | 0.523 |
|
No | 91 (95.8) | 397 (97.1) | |
|
Yes | 4 (4.2) | 12 (2.9) | |
|
CRP,
mg/dl | | | 0.052 |
|
≤0.12 | 36 (43.4) | 189 (55.3) | |
|
>0.12 | 47 (56.6) | 153 (44.7) | |
|
PNI | | | 0.012a |
|
≤48.24 | 54 (56.8) | 174 (42.5) | |
|
>48.24 | 41 (43.2) | 235 (57.5) | |
|
NLR | | | 0.339 |
|
≤2.3 | 45 (47.4) | 216 (52.8) | |
|
>2.3 | 50 (52.6) | 193 (47.2) | |
|
PLR | | | 0.842 |
|
≤136.23 | 48 (50.5) | 202 (49.4) | |
|
>136.23 | 47 (49.5) | 207 (50.6) | |
|
BMI,
kg/m2 | | | 0.175 |
|
≤22.38 | 44 (46.3) | 221 (54.0) | |
|
>22.38 | 51 (53.7) | 188 (46.0) | |
|
VFA,
cm2 | | | 0.017a |
|
≤131.75 | 41 (43.2) | 232 (56.7) | |
|
>131.75 | 54 (56.8) | 177 (43.3) | |
|
SMI,
cm2/m2 | | | 0.608 |
|
≤42.41 | 46 (48.4) | 210 (51.3) | |
|
>42.41 | 49 (51.6) | 199 (48.7) | |
|
Preoperative
T factor | | | 0.054 |
|
T1 | 50 (52.6) | 259 (63.3) | |
|
>T2 | 45 (47.4) | 150 (36.7) | |
|
Preoperative
N factor | | | 0.039a |
|
N0 | 67 (70.5) | 328 (80.2) | |
|
≥N1 | 28 (29.5) | 81 (19.8) | |
| Operation,
pathological, and postoperative factors | | | |
|
Extent of
resection | | | 0.006a |
|
Distal/proximal
gastrectomy | 61 (64.2) | 318 (77.8) | |
|
Total
gastrectomy | 34 (35.8) | 91 (22.2) | |
| Approach | | | 0.065 |
|
Open | 32 (33.7) | 100 (24.4) | |
|
Laparoscopy | 63 (66.3) | 309 (75.6) | |
|
Lymph node
dissection | | | 0.055 |
|
D1/D1+ | 52 (54.7) | 267 (65.3) | |
|
D2 | 43 (45.3) | 142 (34.7) | |
|
Node
dissection according to guidelines | | | 0.426 |
|
Standard | 91 (95.8) | 383 (93.6) | |
|
Reduced | 4 (4.2) | 26 (6.4) | |
|
Operative
duration, min | | |
<0.001a |
|
≤333 | 35 (36.8) | 257 (62.8) | |
|
>333 | 60 (63.2) | 152 (37.2) | |
|
Amount of
blood loss, ml | | | 0.047a |
|
≤149 | 42 (44.2) | 227 (55.5) | |
|
>149 | 53 (55.8) | 182 (44.5) | |
|
Pathological
stage | | | 0.001a |
|
I/II | 67 (70.5) | 348 (85.1) | |
|
III | 28 (29.5) | 61 (14.9) | |
 | Table IVMultivariate analysis of risk factors
for infectious complications in older patients. |
Table IV
Multivariate analysis of risk factors
for infectious complications in older patients.
| | Preoperative
factors and planned surgical procedures | All perioperative
factors |
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex | | 0.036a | | 0.070 |
|
Male | 1.897
(1.044-3.448) | | 1.775
(0.953-3.304) | |
|
Female | 1 | | 1 | |
| PNI | | 0.011a | | 0.009a |
|
≤48.24 | 1.865
(1.153-3.019) | | 1.929
(1.179-3.155) | |
|
>48.24 | 1 | | 1 | |
| VFA,
cm2 | | 0.034a | | 0.174 |
|
≤131.75 | 1 | | 1 | |
|
>131.75 | 1.673
(1.040-2.697) | | 1.408
(0.860-2.305) | |
| Preoperative N
factor | | 0.256 | | 0.942 |
|
N0 | 1 | | 1 | |
|
≥N1 | 1.320
(0.817-2.132) | | 1.024
(0.536-1.958) | |
| Extent of
resection | | 0.020a | | 0.498 |
|
Distal/proximal
gastrectomy | 1 | | 1 | |
|
Total
gastrectomy | 1.815
(1.098-3.000) | | 1.215
(0.692-2.131) | |
| Operative duration,
min | | | | 0.001a |
|
≤333 | n/a | | 1 | |
|
>333 | n/a | | 2.440
(1.430-4.118) | |
| Amount of blood
loss, ml | | | | 0.798 |
|
≤149 | n/a | | 1 | |
|
>149 | n/a | | 1.069
(0.643-1.777) | |
| Pathological
stage | | | | 0.107 |
|
I/II | n/a | | 1 | |
|
III | n/a | | 1.736
(0.888-3.396) | |
Preoperative risk factors for ICs
after laparoscopic and open gastrectomy
Table SI
summarizes the results of the univariate analysis for ICs in a
subgroup restricted to laparoscopic and open gastrectomy. In
univariate analysis, age, sex, PNI and VFA in laparoscopic
gastrectomy and preoperative T factor, extent of resection and
extent of lymph node dissection in open gastrectomy were extracted
as factors significantly associated with ICs. Table SII summarizes the results of the
multivariate analysis of independent preoperative factors for ICs.
In multivariate analyses, age, PNI and VFA in laparoscopic
gastrectomy were independent risk factors for ICs (HR: 1.841, 95%
CI: 1.021-3.3191, P=0.043; HR: 2.122, 95% CI: 1.180-3.815, P=0.012;
and HR: 1.984, 95% CI: 1.040-2.697, P=0.034, respectively), but no
statistically significant independent risk factors were identified
in open gastrectomy.
Perioperative risk factors for
non-ICs
Univariate and multivariate analyses were performed
to compare the risk factors for non-ICs and ICs. The results of
univariate analyses for non-ICs are summarized in Table SIII. Univariate analyses revealed
that, among the perioperative factors, age, mFI, surgical approach,
and node dissection according to the guidelines were significantly
associated with non-ICs. Supplementary Table SIV summarizes the results of the
multivariate analysis for non-ICs performed first with only
preoperative factors and then with all perioperative factors.
Multivariate analyses of only preoperative parameters with
P<0.05 in the univariate analyses revealed that age and mFI were
independent risk factors for non-ICs (HR: 1.744, 95% CI:
1.093-2.781, P=0.020; and HR: 1.616, 95% CI: 1.011-2.584, P=0.045,
respectively). Multivariate analyses of all perioperative
parameters with P<0.05 in the univariate analyses revealed that
failure to perform lymph node dissection according to guidelines
was an independent risk factor for non-ICs (HR: 3.019, 95% CI:
1.356-6.722, P=0.007).
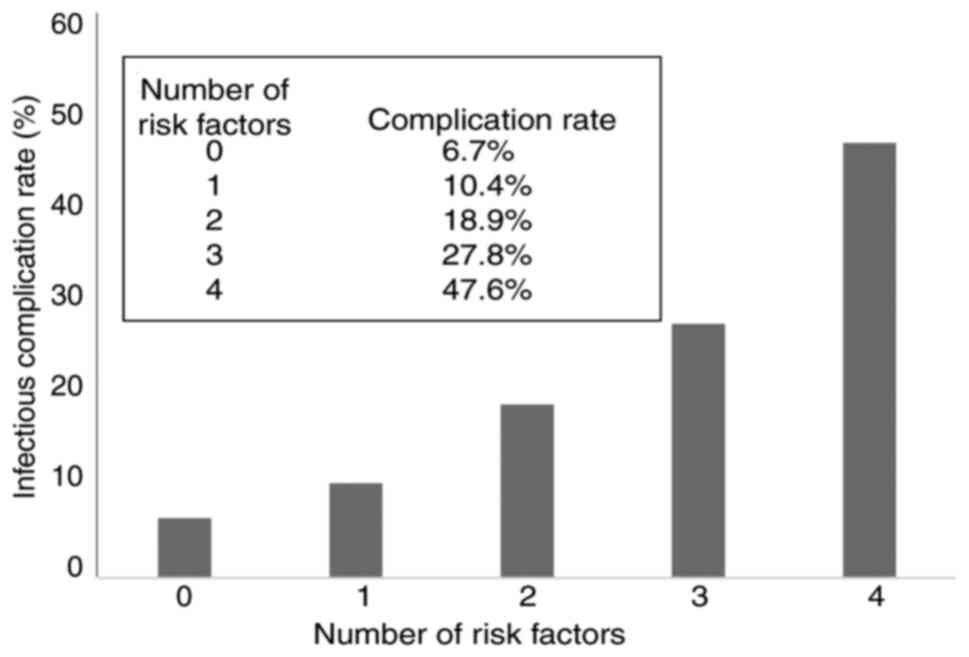
IC incidence rate according to the
number of positive preoperative IC risk factors
The incidence rate of ICs was stratified using the
number of positive preoperative risk factors for ICs detected by
multivariate analysis (sex, positive for males; PNI, positive for
scores <48.24; VFA, positive for values >131.75
cm2; and extent of gastrectomy, positive for TG). The
patients were divided into five categories according to the number
of risk factors as follows: i) risk factor 0 (no positive risk
factors; n=45), ii) risk factor 1 (one positive risk factor;
n=124), iii) risk factor 2 (two positive risk factors; n=206), iv)
risk factor 3 (three positive risk factors; n=108), and v) risk
factor 4 (four positive risk factors; n=21). The IC rates for
patients with 0, 1, 2, 3 and 4 risk factors were 6.7, 10.4, 18.9,
27.8, and 47.6%, respectively, showing a significant correlation
with the number of risk factors (P<0.001; Fig. 2). The AUC for the number of
preoperative risk factors of entire cohort was 0.653 (95% CI:
0.592-0.713, P=0.000), while the AUC for the number of preoperative
risk factors by 10-fold cross-validation was 0.551 (95% CI:
0.485-0.617, P=0.0638).
Discussion
This study focused on identifying risk factors for
ICs after gastrectomy for gastric cancer in older patients,
especially those that can be assessed preoperatively. The results
showed that preoperative factors such as male sex, low PNI, high
VFA, and TG were independent risk factors for postgastrectomy ICs
in older persons. Additionally, the higher the number of positive
preoperative risk factors, the higher the incidence of ICs.
Decision-making regarding cancer treatment in older
persons should consider short-term risks, such as postoperative
complications, and the benefits of surgery, such as prolonged
prognosis. In Japan, approximately 70% of radical gastrectomies for
gastric cancer are undergone by patients with stage I gastric
cancer (16). Hence, minimally
invasive treatment options such as ESD with expanded indications or
palliative local stomach resection may be options if patients with
a high risk for complications after gastrectomy are identified
preoperatively (7,8). ICs are likelier to be more severe and
lead to postoperative mortality than non-ICs (17). Furthermore, ICs are associated with
worse long-term prognoses after surgery, and preoperative
assessment of the risk of ICs after gastrectomy is valuable for
decision-making in older persons undergoing treatment (18).
The National Clinical Database (NCD) in surgery has
been established in Japan, and a prediction system for
postoperative complications based on preoperative factors in distal
gastrectomy and total gastrectomy has been reported and shown to be
useful (5,19). The risk factors for intraabdominal
ICs in patients with gastric cancer using the NCD were male sex,
high BMI, use of steroids, peripheral vascular disease, and TG
(20). Although this previous
study included all age groups, similar risk factors were possibly
identified in the present study as patients aged ≥65 years
accounted for 72% of the total study population. In contrast, the
NCD complication prediction model is based on registered clinical
data and does not include all factors that are known to be useful
in predicting ICs. VFA and PNI, which were independent risk factors
for ICs in our study, have been reported as risk factors for ICs
but are not included in the NCD analysis, so it is possible that
different risk factors are extracted in our study and the NCD
prediction model.
Very few reports have examined in detail the risk of
ICs after gastrectomy in older individuals. Liu et al
(21) analyzed the risk factors
for ICs after gastrectomy in patients aged >70 years. They
reported that preoperative weight loss ≥5%, CCI scores ≥3, and
preoperative C-reactive protein levels were risk factors. Their
study may have differed from the present study's data in that
two-thirds of the patients had stage >2 advanced cancer, they
did not analyze detailed body composition data such as VFA, and the
cutoff values were set independently.
In the present study, among the preoperative factors
for gastric cancer in older patients, male sex, low PNI, high VFA,
and TG were independent risk factors for ICs after gastrectomy. The
male sex is a well-known risk factor for pneumonia after
gastrectomy. It has also been reported as a risk factor for all
complications and ICs after gastrectomy (20,22).
PNI has been reported as a predictive indicator of postoperative
complications after gastric cancer surgery and has also been
reported as a risk factor for ICs after various abdominal surgical
procedures. It has also been shown that PNI is low in older
patients with postoperative complications after gastric cancer
surgery (23,24). VFAs have been reported to be
associated with postoperative complications of gastric cancer and
are a better predictor of postoperative complications than BMI
(25). High VFA values have been
reported to increase the incidence of surgical site infections
after gastrectomy (26). TG is
reported to be a risk factor for all postoperative complications
and ICs (20,22), and TG in older persons is reported
to be a risk for pneumonia (27).
However, it is unclear from this study alone whether
the risk factors identified are themselves the underlying causes of
ICs, and whether preoperative interventions for the risk factors
are effective in reducing ICs. PNI is associated with a patient's
nutritional status, and preoperative nutritional management for
gastric cancer patients has been shown to decrease postoperative
complications (28). Preoperative
exercise programs for obese patients have also shown promise in
reducing postoperative complications (29). However, whether these preoperative
interventions lead to a reduction in ICS in elderly patients needs
to be prospectively evaluated. A risk factor analysis of all
perioperative factors, including intraoperative factors, was also
performed. Low PNI and prolonged operative time were independent
risk factors for ICs. Procter et al (30) reported in the American College of
Surgeons National Surgical Quality Improvement Program database
that prolonged operative time generally increases the incidence of
ICs after general surgery. Wang et al (31) also reported that prolonged
operative time was a risk factor for complications after
gastrectomy performed by the same surgeon. Therefore, when
performing gastrectomy in older patients, it is important to
consider the surgical technique and device selection while
shortening the operative time.
To explore the risk factors for ICs, cutoff values
were set for all continuous variables to facilitate their use in
daily clinical practice. No appropriate method has been established
for determining cutoff values for each indicator, and different
cutoff values are often used in different reports. Hence, this
study determined cutoff values using an ROC curve analysis, which
is considered advantageous in terms of objectivity (32). Among the factors extracted from the
analysis of preoperative factors, PNI and VFA were continuous
variables. No significant measurement differences were observed
among the centers, and they may be used as standard cutoff values.
However, the operative time is expected to vary among surgeons and
institutions, and the cutoff may differ among institutions and
surgeons.
This study had several limitations. First, the main
limitations of this study was the use of a small amount of sample
data from a single institution. The identified risk factors must be
validated using prospective data from a larger population.
Furthermore, predicting the incidence of ICs by the number of
identified risk factors was not validated by cross-validation. The
results shown in Fig. 2 simply
show the number of risk factors and the incidence of ICs in the
cohort studied here, and should be validated in a different sample
population in the future. There is room for developing more
accurate criteria by assessing more cases with multiple
institutions to establish more precise criteria, and we aim to
conduct a prospective study to verify the validity of this study's
findings. Second, the analysis used factors from the preoperative
examination, which is usually performed before gastrectomy, but did
not include items from a detailed functional assessment of older
patients, such as a comprehensive geriatric assessment (CGA). A CGA
is reportedly useful in predicting postoperative complications and
may also be useful in predicting ICs after gastrectomy (33). Third, the definition of older
persons in this study was ≥65 years of age, according to the
internationally accepted WHO definition. However, reports on
short-term results after gastrectomy for older persons have
included various age definitions, such as ≥65, 70, 75, and 80 years
of age. This may lead to discrepancies in results depending on the
age definition (6,21,34,35).
We performed univariate and multivariate analyses of risk factors
also in the subgroups aged 75 years and older in the present study
(Tables SV and SVI). Independent multivariate risk
factors for ICs aged 75 years and older were men and high BMI,
which differed from risk factors for those aged 65 years and older.
To identify more accurate risk factors for ICS, it may be necessary
to subdivide the analysis by age groups, such as 65-74, 75-84, and
84-94, rather than categorizing the age definition of the elderly
as >65 or >75 years old.
In conclusion, male sex, low PNI, high VFA, and TG
are risk factors for ICs after gastrectomy in older patients with
gastric cancer. For patients with multiple risk factors, the
indications for gastrectomy should be carefully considered, and
close postoperative management should be performed.
Supplementary Material
Receiver operating characteristic
curves of noninfectious complications for perioperative factors to
determine the cutoff values and AUC. (A) Age, (B) sex, (C) PS, (D)
mFI, (E) CCI, (F) use of steroids, (G) preoperative CRP levels, (H)
PNI, (I) NLR, (J) PLR, (K) BMI, (L) VFA, (M) SMI, (N) PreT, (O)
PreN, (P) extent of resection, (Q) surgical approach, (R) extent of
LN dissection, (S) node dissection according to guidelines, (T) Op
duration, (U) blood loss and (V) fStage. AUC, area under the
receiver operating characteristic curve; BMI, body mass index; CCI,
Charlson comorbidity index; CRP, C-reactive protein; fStage, final
stage; LN dissection, lymph node dissection; mFI, modified frailty
index; NLR, neutrophil-to-lymphocyte ratio;Op duration, operative
duration;PLR, platelet-to-lymphocyte ratio; PNI, prognostic
nutritional index; PreN, preoperative N factor; PreT, preoperative
T factorT factor; PS, performance status; SMI, skeletal muscle
index; VFA, visceral fat area.
Univariate analysis of risk factors
for infectious complications in older patients after laparoscopic
and open gastrectomy.
Multivariate analysis of risk factors
for infectious complications in older patients after laparoscopic
and open gastrectomy.
Univariate analysis of risk factors
for non infectious complications in older patients.
Multivariate analysis of risk factors
for non infectious complications in older patients.
Univariate analysis of risk factors
for infectious complications in patients ≥75 years old.
Multivariate analysis of risk factors
for infectious complications in patients ≥75 years old.
Acknowledgements
The authors would like to thank Dr Shoichi Hazama
(Department of Surgery, Shunan Memorial Hospital, Shunan, Yamaguchi
744-0033, Japan), Dr Shigefumi Yoshino (Department of Surgery,
National Hospital Organization Kanmon Medical Center, Shimonoseki,
Yamaguchi 752-8510, Japan) and Professor Tatsuya Ioka (Oncology
Center, Yamaguchi University Hospital, Ube, Yamaguchi 755-8505,
Japan) for suggesting the topic studied in this paper. The authors
would also like to thank Dr Gen Kanesada (Department of
Gastroenterological, Breast and Endocrine Surgery, Yamaguchi
University Graduate School of Medicine, Ube, Yamaguchi 755-8505,
Japan) for sharing his dataset.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MI and STa conceived the presented idea. MI wrote
the manuscript. TY, CN, MN and YW contributed to data collection.
YS, YT, HT and YN performed analytical calculations. STo and HN
helped interpret the results. HN revised the manuscript critically
for important intellectual content and gave final approval of the
version to be published. All authors provided critical feedback,
and helped shape the research, analysis and manuscript. MI and CN
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Yamaguchi University Hospital (approval no.
2022-032; Ube, Japan). Written informed consent was obtained from
the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the human
development index (2008-2030): A population-based study. Lancet
Oncol. 13:790–801. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hasegawa H, Takahashi A, Kakeji Y, Ueno H,
Eguchi S, Endo I, Sasaki A, Takiguchi S, Takeuchi H, Hashimoto M,
et al: Surgical outcomes of gastroenterological surgery in Japan:
Report of the national clinical database 2011-2017. Ann
Gastroenterol Surg. 3:426–450. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang JY, Lee HJ, Kim TH, Huh YJ, Son YG,
Park JH, Ahn HS, Suh YS, Kong SH and Yang HK: Short- and long-term
outcomes after gastrectomy in elderly gastric cancer patients. Ann
Surg Oncol. 24:469–477. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim JH, Kim J, Lee WJ, Seong H, Choi H,
Ahn JY, Jeong SJ, Ku NS, Son T, Kim HI, et al: The incidence and
risk factors for surgical site infection in older adults after
gastric cancer surgery: A STROBE-compliant retrospective study.
Medicine (Baltimore). 98(e16739)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kunisaki C, Miyata H, Konno H, Saze Z,
Hirahara N, Kikuchi H, Wakabayashi G, Gotoh M and Mori M: Modeling
preoperative risk factors for potentially lethal morbidities using
a nationwide Japanese web-based database of patients undergoing
distal gastrectomy for gastric cancer. Gastric Cancer. 20:496–507.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nunobe S, Oda I, Ishikawa T, Akazawa K,
Katai H, Isobe Y, Miyashiro I, Tsujitani S, Ono H, Tanabe S, et al:
Surgical outcomes of elderly patients with stage I gastric cancer
from the nationwide registry of the Japanese gastric cancer
association. Gastric Cancer. 23:328–338. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sumiyoshi T, Kondo H, Fujii R, Minagawa T,
Fujie S, Kimura T, Ihara H, Yoshizaki N, Hirayama M, Oyamada Y and
Okushiba S: Short- and long-term outcomes of endoscopic submucosal
dissection for early gastric cancer in elderly patients aged 75
years and older. Gastric Cancer. 20:489–495. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhao PY, Ma ZF, Jiao YN, Yan Y, Li SY and
Du XH: Laparoscopic and endoscopic cooperative surgery for early
gastric cancer: Perspective for actual practice. Front Oncol.
12(969628)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Iida M, Takeda S, Nakashima C, Nishiyama
M, Watanabe Y, Suzuki N, Yoshino S, Nakagami Y, Tanabe T and Nagano
H: Risk factors for non-gastric-cancer-related death after
gastrectomy in elderly patients. Ann Gastroenterol Surg. 6:753–766.
2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lu J, Zheng HL, Li P, Xie JW, Wang JB, Lin
JX, Chen QY, Cao LL, Lin M, Tu RH, et al: High preoperative
modified frailty index has a negative impact on short- and
long-term outcomes of octogenarians with gastric cancer after
laparoscopic gastrectomy. Surg Endosc. 32:2193–2200.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Iida M, Takeda S, Nakagami Y, Kanekiyo S,
Nakashima C, Nishiyama M, Yoshida S, Suzuki N, Yoshino S and Nagano
H: The effect of the visceral fat area on the predictive accuracy
of C-reactive protein for infectious complications after
laparoscopy-assisted gastrectomy. Ann Gastroenterol Surg.
4:386–395. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Japanese Gastric Cancer Association.
Japanese classification of gastric carcinoma: 3rd english edition.
Gastric Cancer. 14:101–112. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bassi C, Dervenis C, Butturini G,
Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W
and Buchler M: International Study Group on Pancreatic Fistula
Definition. Postoperative pancreatic fistula: An international
study group (ISGPF) definition. Surgery. 138:8–13. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Clavien PA, Barkun J, de Oliveira ML,
Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J,
Slankamenac K, Bassi C, et al: The clavien-dindo classification of
surgical complications: Five-year experience. Ann Surg.
250:187–196. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
LeDell E, Petersen M and van der Laan M:
Computationally efficient confidence intervals for cross-validated
area under the ROC curve estimates. Electron J Stat. 9:1583–1607.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Isobe Y, Nashimoto A, Akazawa K, Oda I,
Hayashi K, Miyashiro I, Katai H, Tsujitani S, Kodera Y, Seto Y and
Kaminishi M: Gastric cancer treatment in Japan: 2008 annual report
of the JGCA nationwide registry. Gastric Cancer. 14:301–316.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kurita N, Miyata H, Gotoh M, Shimada M,
Imura S, Kimura W, Tomita N, Baba H, Kitagawa Y, Sugihara K and
Mori M: Risk model for distal gastrectomy when treating gastric
cancer on the basis of data from 33,917 Japanese patients collected
using a nationwide web-based data entry system. Ann Surg.
262:295–303. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tokunaga M, Tanizawa Y, Bando E, Kawamura
T and Terashima M: Poor survival rate in patients with
postoperative intra-abdominal infectious complications following
curative gastrectomy for gastric cancer. Ann Surg Oncol.
20:1575–1583. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kikuchi H, Miyata H, Konno H, Kamiya K,
Tomotaki A, Gotoh M, Wakabayashi G and Mori M: Development and
external validation of preoperative risk models for operative
morbidities after total gastrectomy using a Japanese web-based
nationwide registry. Gastric Cancer. 20:987–997. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fujiya K, Kumamaru H, Fujiwara Y, Miyata
H, Tsuburaya A, Kodera Y, Kitagawa Y, Konno H and Terashima M:
Preoperative risk factors for postoperative intra-abdominal
infectious complication after gastrectomy for gastric cancer using
a Japanese web-based nationwide database. Gastric Cancer.
24:205–213. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu X, Xue Z, Yu J, Li Z, Ma Z, Kang W, Ye
X and Jiang L: Risk factors for postoperative infectious
complications in elderly patients with gastric cancer. Cancer Manag
Res. 12:4391–4398. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guner A, Kim SY, Yu JE, Min IK, Roh YH,
Roh C, Seo WJ, Cho M, Choi S, Choi YY, et al: Parameters for
predicting surgical outcomes for gastric cancer patients: Simple is
better than complex. Ann Surg Oncol. 25:3239–3247. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Takama T, Okano K, Kondo A, Akamoto S,
Fujiwara M, Usuki H and Suzuki Y: Predictors of postoperative
complications in elderly and oldest old patients with gastric
cancer. Gastric Cancer. 18:653–661. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Watanabe M, Iwatsuki M, Iwagami S,
Ishimoto T, Baba Y and Baba H: Prognostic nutritional index
predicts outcomes of gastrectomy in the elderly. World J Surg.
36:1632–1639. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu Y, Guo D, Niu Z, Wang Y, Fu G, Zhou Y,
Xue Q, Jin X and Gong Z: Prediction of the risk of
laparoscopy-assisted gastrectomy by comparing visceral fat area and
body mass index. Gastroenterol Res Pract.
2018(1359626)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sugisawa N, Tokunaga M, Tanizawa Y, Bando
E, Kawamura T and Terashima M: Intra-abdominal infectious
complications following gastrectomy in patients with excessive
visceral fat. Gastric Cancer. 15:206–212. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Endo S, Yamatsuji T, Fujiwara Y, Higashida
M, Kubota H, Tanaka H, Ito Y, Okada T, Yoshiatsu K and Ueno T: The
comparison of prognoses between total and distal gastrectomy for
gastric cancer in elderly patients ≥80 years old. Surg Today.
53:569–577. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xu R, Chen XD and Ding Z: Perioperative
nutrition management for gastric cancer. Nutrition.
93(111492)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cho H, Yoshikawa T, Oba MS, Hirabayashi N,
Shirai J, Aoyama T, Hayashi T, Yamada T, Oba K, Morita S, et al:
Matched pair analysis to examine the effects of a planned
preoperative exercise program in early gastric cancer patients with
metabolic syndrome to reduce operative risk: The adjuvant exercise
for general elective surgery (AEGES) study group. Ann Surg Oncol.
21:2044–2050. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Procter LD, Davenport DL, Bernard AC and
Zwischenberger JB: General surgical operative duration is
associated with increased risk-adjusted infectious complication
rates and length of hospital stay. J Am Coll Surg. 210:60–65.e1-2.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang X, Yao Y, Qian H, Li H and Zhu X:
Longer operating time during gastrectomy has adverse effects on
short-term surgical outcomes. J Surg Res. 243:151–159.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Toyokawa T, Muguruma K, Tamura T, Sakurai
K, Amano R, Kubo N, Tanaka H, Yashiro M, Hirakawa K and Ohira M:
Comparison of the prognostic impact and combination of preoperative
inflammation-based and/or nutritional markers in patients with
stage II gastric cancer. Oncotarget. 9:29351–29364. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xue DD, Cheng Y, Wu M and Zhang Y:
Comprehensive geriatric assessment prediction of postoperative
complications in gastrointestinal cancer patients: A meta-analysis.
Clin Interv Aging. 13:723–736. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu D, Liang L, Liu L, Zhu Z, Liu S, Hu L,
He Y, Fang Y and Wan X: Short-term outcomes and prognosis of
laparoscopy-assisted total gastrectomy in elderly patients with
stomach cancer. Surg Endosc. 34:5428–5438. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lu J, Cao LL, Zheng CH, Li P, Xie JW, Wang
JB, Lin JX, Chen QY, Lin M, Tu RH and Huang CM: The preoperative
frailty versus inflammation-based prognostic score: Which is better
as an objective predictor for gastric cancer patients 80 years and
older? Ann Surg Oncol. 24:754–762. 2017.PubMed/NCBI View Article : Google Scholar
|