Introduction
Postmenopausal osteoporosis (PMOP) is a common chronic bone disease characterized by excessive bone loss and the deterioration of bone microstructure due to estrogen deficiency (1). Estrogen deficiency also leads to chronic inflammation with elevated levels of proinflammatory mediators, which contribute to the development of osteoporosis (OP) (2).
To date, anti-osteoporotic drugs have been widely used in the clinical treatment of PMOP (3). Bisphosphonate is the first-line medications for OP treatment, however, adverse events like atypical femoral fractures and osteonecrosis of the jaw are concerning (4). Therefore, it is demanded to develop novel drugs with higher efficacy and milder undesired effect (3). In Asian countries, Traditional Chinese Medicine (TCM) has been extensively used to prevent and treat various diseases including OP due to its superiority on safety and effectiveness (5-8). Cornus officinalis (CO) is a common medicine in TCM that nourishes the liver and kidney and exerts protective effects against diseases such as aching lumbus and knees, dizziness, spermatorrhea and so on (9). A previous study has revealed that the master transcription factors of osteoclast differentiation can be inhibited by CO treatment (10). Loganin is a major bioactive iridoid glycoside derived from CO (11). Li et al (12) found that loganin directly enhances the function of osteoblasts and prolongs their survival, indirectly inhibits the function of osteoclasts and reduces the number of osteoclasts. However, the mechanism of action of loganin on PMOP has yet to be fully elucidated. The present study aimed to illustrate the pharmacological mechanisms of loganin in treating PMOP through network pharmacology, molecular docking and in vivo validation.
Materials and methods
Target acquisition of loganin and osteoclast-related genes
The active components of loganin were investigated by a combined searching and collection in the online databases including the Traditional Chinese Medicine Systems Pharmacology database (TCMSP; https://old.tcmsp-e.com/tcmsp.php) (13), the Swiss Target Prediction databases (http://www.swisstargetprediction.ch) (14), the Similarity Ensemble Approach platform (SEA; https://sea.bkslab.org/) (14) and STITCH platform (http://stitch.embl.de/) (15). The names of loganin targets were standardized by the UniProt database (https://www.uniprot.gov/) (16). Both ‘osteoclastogenesis’ and ‘osteoclast differentiation’ were the key words to search for targets related to osteoclast from three online public databases, including the Online Mendelian Inheritance in Man (OMIM; http://www.omim.org/), the GeneCards database (https://www.genecards.org/) and the TTD database (https://db.idrblab.net/) (17). A Venn diagram was constructed on an online website (https://bioinfogp.cnb.csic.es/tools/venny/index.html) to identify the overlapping target genes between loganin and osteoclast-related genes for further bioinformatic analyses (1).
Construction and analysis of a protein-protein interaction (PPI) network
With the parameters of ‘confidence score >0.4’ and ‘Homo sapiens’, the overlapping genes between loganin and osteoclast-related were analysed in STRING (https://string-db.org/) to build a PPI network. Subsequently, the gene-gene connection data was exported from STRING database, and further analysed their interconnection network by using the visualized software Cytoscape (version 3.9.1) (1). Larger protein targets with more linked nodes had higher degrees, participated in more biological functions and were more likely to be therapeutic targets (17).
Enrichment analysis of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways
For the target genes of loganin and osteoclast-related genes, the Metascape data platform (https://metascape.org/) was used for GO functional annotations and KEGG pathway enrichment analysis (18). ‘Homo species’ was selected on the Metascape data platform to analyze biological processes, cellular components, molecular functions and KEGG signalling pathways, and bioinformatics online platforms (https://www.bioinformatics.com.cn/) were used to visualize the results (18).
Molecular docking
The mol2 files of 3D chemical structures of loganin were downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov). The structure of the target proteins was obtained from the RCSB Protein Data Bank (https://www.rcsb.org) (1). AutoDockVina v.1.2.2 software was then used to perform molecular docking between loganin and the target proteins (1). Finally, PyMoL v.2.5 was used to analyze and visualize the binding mode and interactions of candidate active ingredients and key target proteins (1).
Animals and materials
Loganin (≥98% of purity) was purchased from Chengdu Must Bio-Technology Co., Ltd. A total of 28 healthy, SPF grade (weight, 17-19 g), 7-week-old C57BL/6J female mice, were obtained from Guangdong Medical Laboratory Animal Center. The mice were maintained at 21±1.5˚C in a 12/12-h light-dark cycle with standard food pellets and free access to tap water. After acclimation for 1 week, all mice were randomly assigned into 4 groups (n=7 per group): SHAM group, OVX group, OVX + Loganin-L (5 mg/kg) group and OVX + Loganin-H (20 mg/kg) group (19). The mice in loganin treated groups received the aforementioned concentrations of loganin, while the mice in OVX and Sham groups received intragastric administration of equal volume distilled water for 12 weeks. The animal protocol was approved by the Ethics Committee of The First Affiliated Hospital of Guangdong Pharmaceutical University (approval no. 00300814; Guangzhou, China), and was in adherence to the Guide for the Care and Use of Laboratory Animals (20).
Microcomputed tomography (Micro-CT) analysis and ELISA tests
The left distal femurs of mice were scanned by Micro-CT (SCANCO uCT-100 detector; SCANCO Medical AG), and the bone trabecular in the area 0.8 mm above 0.5 mm from the growth plate were selected as the region of interest (ROI) for 3D reconstruction. Bone mineral density (BMD), bone volume/tissue volume (BV/TV), trabecular number (Tb.N), and trabecular separation (Tb.Sp) of the ROI were measured using analysis the Evaluation V.6.5-3 software (SCANCO Medical AG).
Samples of serum were collected by eyeball extraction. The levels of C-terminal telopeptide (CTX), tartrate-resistant acid phosphatase (TRAP), procollagen type I intact n-terminal pro-peptide (P1NP), TNF-α, IL-6 and IL-10 were measured by ELISA according to the manufacturer's instructions. The ELISA kits (cat. no. 20220711M) were obtained from Jiangsu Meimian Industrial Co., Ltd.
Statistical analysis
Data are presented as the mean ± SD. SPSS software was used for statistical analysis (version 25.0; IBM Corp.). The one-Way ANOVA test was used for the data with normal distribution, and the Kruskal-Wallis test was used to analyze data that did not meet the assumptions of the ANOVA. The LSD test was used when data met normality and homogeneity of variance. Tamhane's T2 test was used when data met normality but not homogeneity of variance. P<0.05 was considered to indicate a statistically significant difference.
Results
Prediction of compound-diseases targets
After integration and deduplication of data derived from TCMSP, SwissTarget Prediction, SEA and STITCH databases, 106 targets of loganin were retrieved. A total of 1,152 targets related to osteoclastogenesis and osteoclast differentiation were captured from the OMIM, GeneCards and TTD databases, and crosschecked with the identified herbal component targets, generating 31 potential targets of loganin as shown in Fig. 1A.
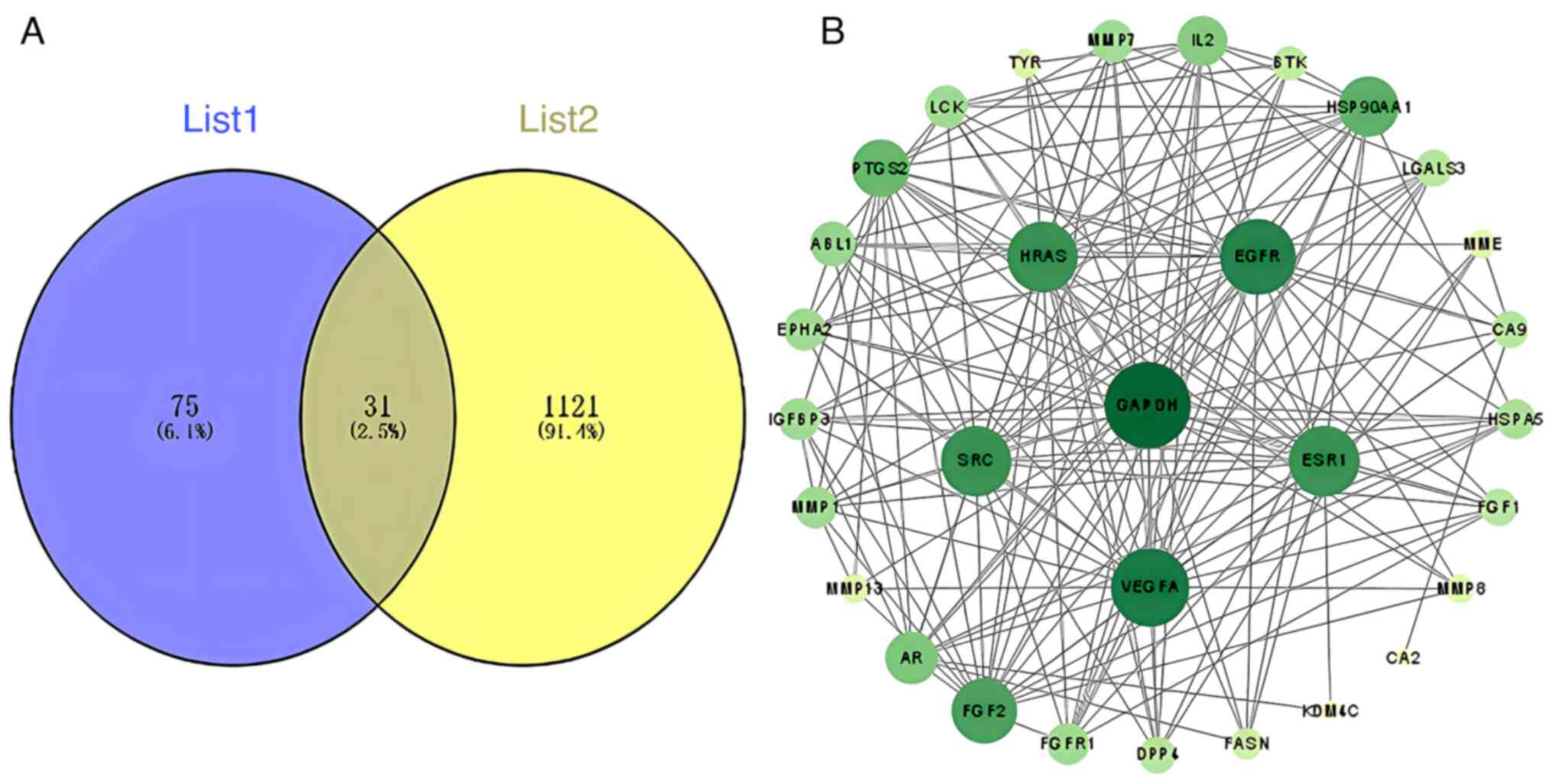 |
Figure 1
(A) The Venn diagram overlapping loganin's component targets (List 1) and osteoclast-related genes (List 2). (B) Protein-protein interaction network of the overlapping genes between loganin and osteoclasts was constructed basing on the STRING database. Nodes represent different protein hits, and edges represent interactions between nodes. The darker the color, the higher the node degree.
|
Construction of PPI network
Common targets shared by loganin and osteoclast differentiation were connected through STRING-based PPI analysis, resulting in a complex network diagram consisting of 31 nodes and 182 edges (Fig. 1B). The node degree is a critical evaluation parameter in PPI network, in which a higher node degree represents a higher weight in the network. Basing on the node degree, the top 10 core targets in the PPI network were GAPDH, VEGFA, EGFR, ESR1, HRAS, SRC, FGF2, HSP90AA1, PTGS2 and IL-2.
GO function and KEGG pathway enrichment analyses
GO and KEGG analyses were employed as important genetic tools to identify key biological pathways and processes that are associated with loganin's potential effect on OP treatment. The GO analysis revealed that loganin's target genes on osteoclast were mainly involved in cell migration, cell proliferation and peptidyl-tyrosine phosphorylation (Fig. 2A). On the other hand, the KEGG analysis indicated that loganin is likely to affect the Rap1, IL-17, NF-κB and HIF-1 signaling pathways (Fig. 2B).
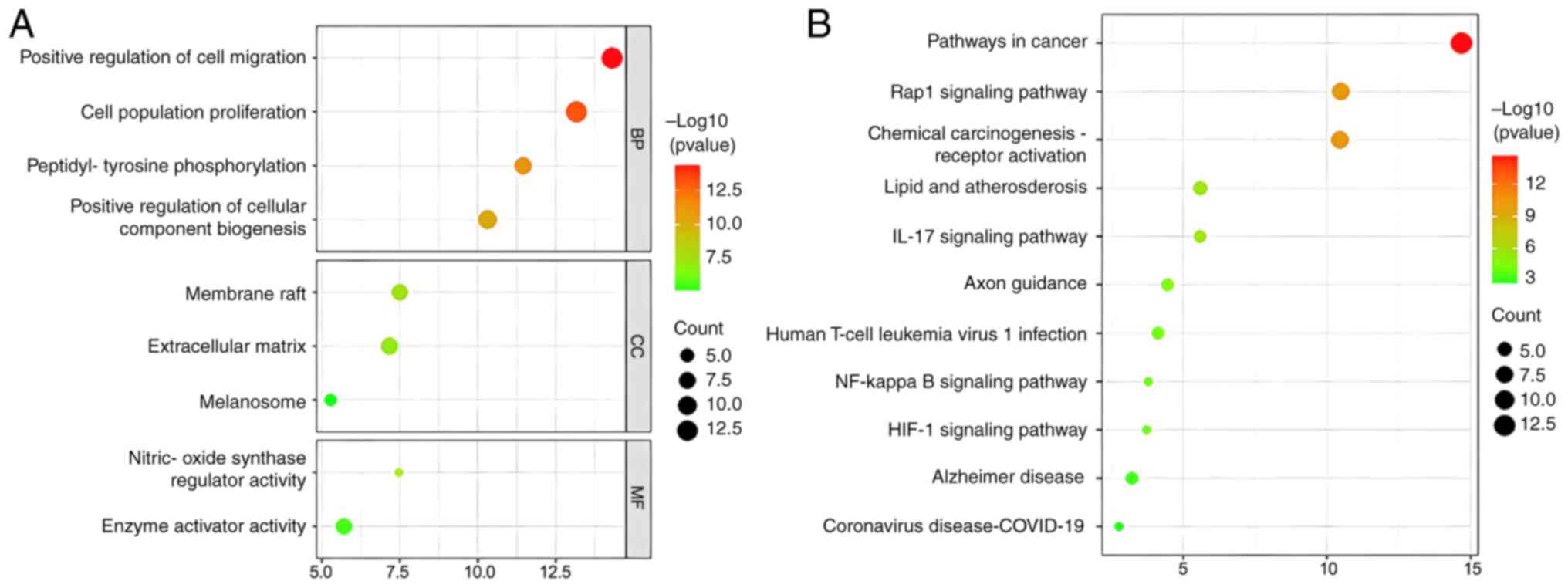 |
Figure 2
(A) The GO enrichment analysis for the overlapping genes between loganin and osteoclasts associated with molecular functions, biological processes and cellular components. The x-axis showing the significant enrichment in the genes counts. The y-axis showing the categories in the GO of the target genes. (B) The Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis of overlapping genes between loganin and osteoclasts. GO, Gene Ontology.
|
Molecular docking
In addition, molecular docking was utilized to evaluate the interactions between loganin and core target proteins. It is generally considered a strong binding affinity between the target and the protein when the binding energy is <-5.0 kcal/mol, and a highest affinity when the binding energy is <-7.0 kcal/mol (21). The binding energy of loganin to the core target proteins was between -5.0 and -7.0 kcal/mol (Table I), indicating that loganin potentially performs activity via actively interacting with the 10 core target proteins, as visualized in Fig. 3.
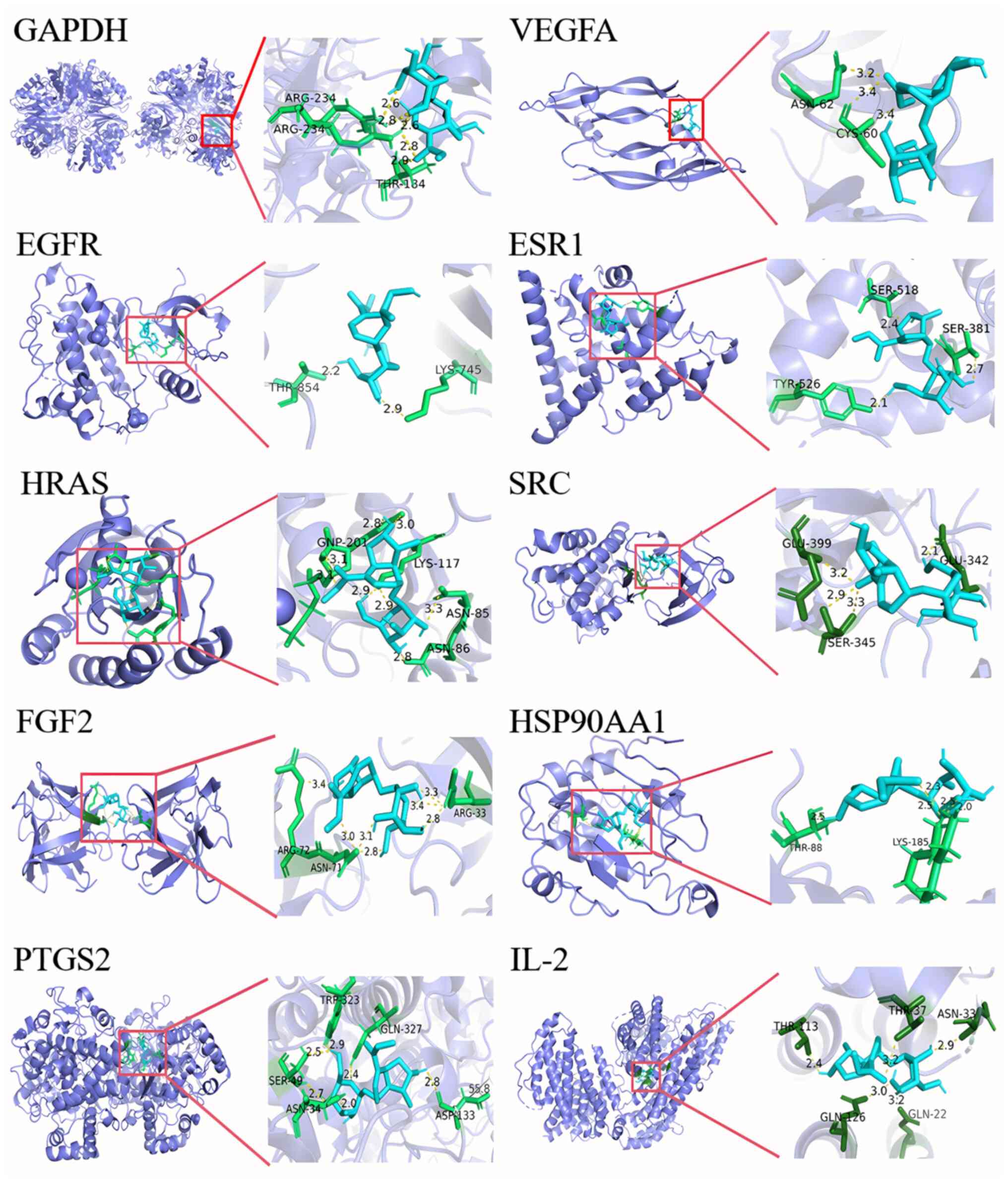 |
Figure 3
Molecular docking of loganin to the 10 core targets. The blue sticks represent the ligand, the green sticks represent the interactive amino acid residues and the yellow dotted lines represent the formed hydrogen bonds.
|
 |
Table I
Docking results of loganin and core target proteins.
|
Table I
Docking results of loganin and core target proteins.
| No. |
Targets |
PDB ID |
Affinity (kcal/mol) |
| 1 |
GAPDH |
6YND |
-7.3 |
| 2 |
VEGFA |
1MKK |
-6.5 |
| 3 |
EGFR |
7SI1 |
-6.6 |
| 4 |
ESR1 |
7R62 |
-6.9 |
| 5 |
HRAS |
7JIG |
-5.2 |
| 6 |
SRC |
6ATE |
-5.7 |
| 7 |
FGF2 |
5X1O |
-6.3 |
| 8 |
HSP90AA1 |
7S8Y |
-7.3 |
| 9 |
PTGS2 |
5IKR |
-7.3 |
| 10 |
IL-2 |
3QB1 |
-7.4 |
Effect of loganin on bone loss in OVX mice
To validate loganin's in vivo effect, an OVX mouse model was created and the bone volume and the microstructure of the left femurs were examined by Micro-CT (Fig. 4A). Compared with the SHAM group, the trabecular bone in OVX group was sparsely fractured, discontinuous, and the number of bone trabeculae was significantly reduced, which was dose-dependently rescued by 12-week loganin treatment manifested by increased bone volume and improved bone microstructure. The bone parameters, including BMD, BV/TV and Tb.N, were reduced significantly in OVX mice compared with the SHAM group, while Tb.Sp was increased significantly (P<0.05). The trend was partially reversed by loganin treatment in a dose-dependent manner (P<0.05) (Fig. 4B-E). These results demonstrated that loganin treatment can effectively release the osteoporotic symptoms and improve bone quality.
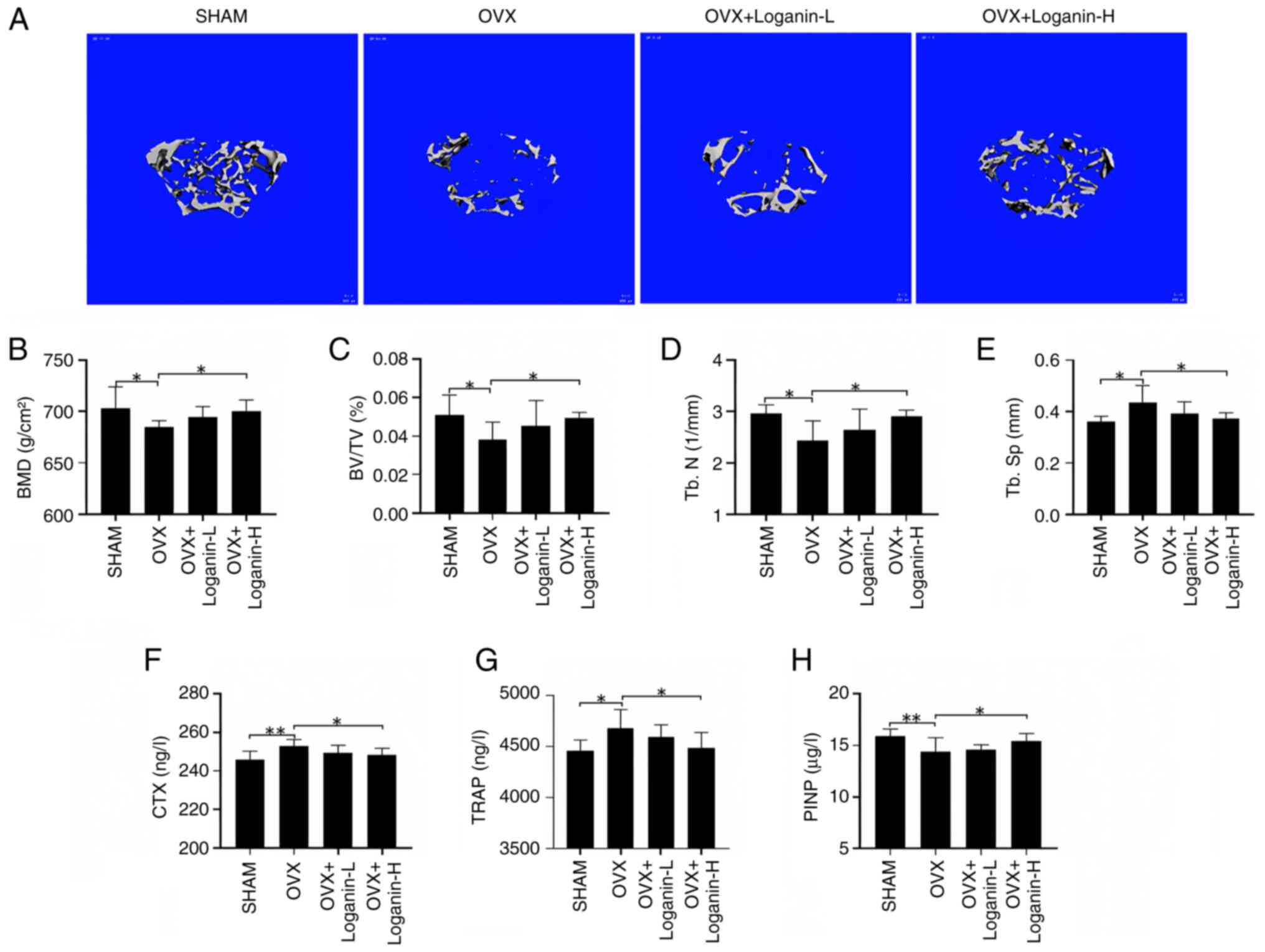 |
Figure 4
The protective effect of loganin on OVX-induced bone loss. (A) Representative micro-CT images indicated that the bone loss was suppressed by loganin treatment (scale bar, 100 µm). (B-E) Micro-CT quantitative parameters for bone microstructure, including BMD, BV/TV, Tb.N and Tb.Sp. (F-H) Serum levels of bone metabolism markers. Results were expressed as the mean ± SD (n=7). *P<0.05 and **P<0.01. OVX, ovariectomized; micro-CT, microcomputed tomography; L, low dose; H, high dose; BMD, bone mineral density; BV/TV, bone volume/tissue volume; Tb.N, trabecular number; Tb.Sp, trabecular separation; CTX, C-terminal telopeptide; TRAP, tartrate-resistant acid phosphatase; P1NP, procollagen type I intact n-terminal pro-peptide.
|
To further assess the efficacy of loganin, serum collected from the in vivo experiments were tested for bone resorption biomarkers CTX, TRAP and bone formation biomarkers P1NP (Fig. 4F-G). The serum levels of CTX and TRAP in OVX were significantly elevated compared with the SHAM group (P<0.05 and P<0.01). The increase of osteoclastic markers in OVX mice was reversed by loganin treatment (P<0.05). By contrast, the serum level of P1NP in OVX mice was significantly downregulated compared with the SHAM group (P<0.01), in which the decreased trend was reversed by loganin treatment (P<0.05). Collectively, loganin presented strong effect on the protection of excessive bone loss in OVX mice.
Effect of loganin on the serum levels of inflammatory cytokines in OVX mice
To explore the effects of loganin on inflammation, the serum levels of inflammatory cytokines were examined (Fig. 5A-C). In the OVX group, the serum levels of TNF-α and IL-6 increased, while IL-10 decreased (P<0.01 and P<0.05). Administration of loganin suppressed the increased levels of TNF-α and IL-6 and promoted the secretion of IL-10 in a dose-dependent manner (P<0.05). These findings indicated that loganin can inhibit the inflammatory response induced by estrogen deficiency.
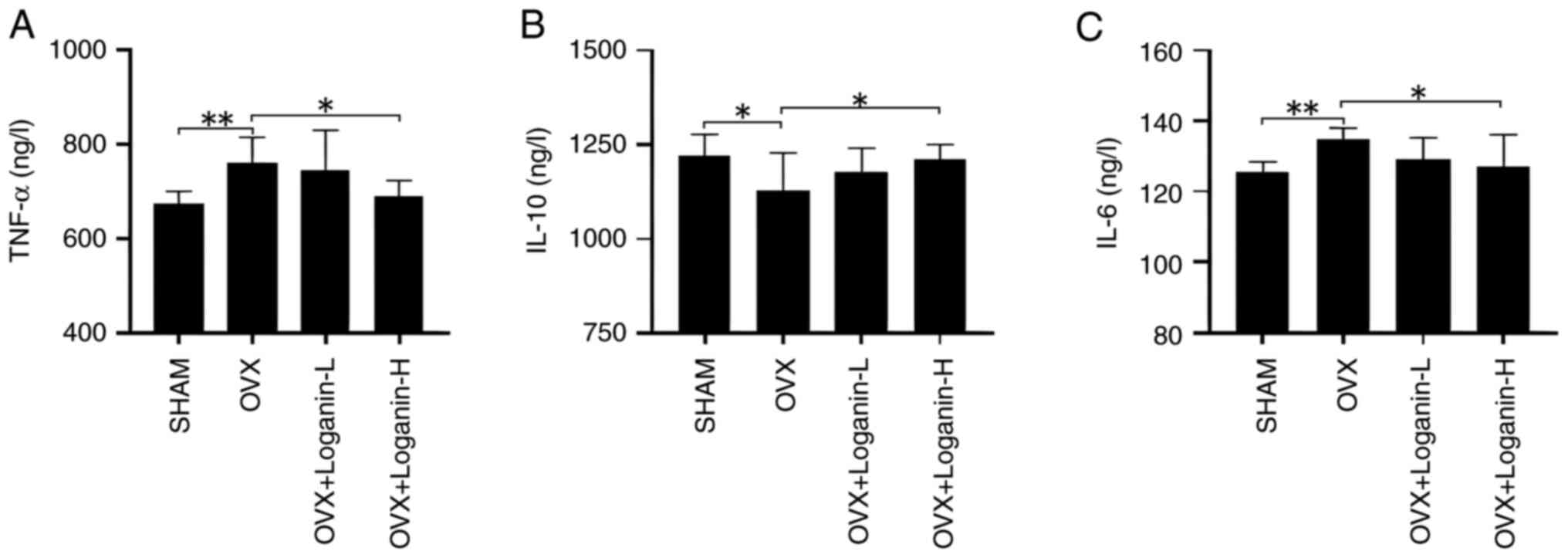 |
Figure 5
Effect of loganin on inflammatory cytokines in vivo. (A) TNF-α. (B) IL-10. (C) IL-6. Results were expressed as the mean ± SD (n=7). *P<0.05 and **P<0.01. OVX, ovariectomized; L, low dose; H, high dose.
|
Discussion
PMOP caused by estrogen deficiency, is a common chronic bone disease that majorly occurs in women >50 years old (1), which leads to imbalanced bone metabolism where bone resorption exceeds bone formation (13). Due to the rapid aging of the global population, the number of individuals with PMOP is increasing, resulting in challenges to individual families and health care (22). Therefore, it is vital to develop novel drugs for the prevention and treatment of PMOP. A recent study revealed the anti-OP effect of loganin by regulating osteoblast and osteoclast differentiation (23). In the present study, the mechanism of action of loganin was explored via network pharmacology and molecular docking and its in vivo protective effect on OVX-induced bone loss was experimentally validated.
Network pharmacology provides an in-depth analysis on how different network factors interact with potential drug candidates, enabling to repurpose existing drugs for a variety of conditions (24). In the present study, a total of 106 herbal component targets of loganin were retrieved and crosschecked with 1,152 targets involved in osteoclastogenesis and osteoclast differentiation, generating 31 potential mechanistic targets of loganin on osteoclasts. To further gain the mechanistic insights of loganin's actions on osteoclast formation, GO and KEGG enrichment analyses were performed to cluster the 31 potential targets of loganin. GO analysis demonstrated that loganin is likely to affect bone turnover through regulating biological processes including migration, proliferation, nitric-oxide synthase regulator activity and enzyme activator activity in osteoclasts. From a different perspective, KEGG pathway analysis revealed that the genetic effect imposed by loganin on osteoclasts was enriched in some essential pathways, such as the IL-17, NF-κB and HIF-1 signaling pathways. IL-17 is a well-described mediator of bone resorption in inflammatory diseases, which stimulates osteoblasts to produce receptor activator of NF-κB ligand (RANKL) that fuels osteoclastogenesis (25,26). NF-κB is a well-known inflammatory signaling pathway that is implicated in the development of endocrine system illnesses, particularly OP (27). A recent study identified that loganin can decrease the levels of RANKL (21). Besides, loganin was shown to ameliorate cartilage degeneration and osteoarthritis development in an osteoarthritis mouse model through inhibition of NF-κB activity and pyroptosis in chondrocytes (28). The HIF-1 signaling pathway is a classic multifunctional signaling pathway that plays an important role in inflammatory responses and bone formation and absorption, which can regulate the regeneration process of bone and blood vessels through osteogenic and angiogenic coupling (29,30). These results collectively suggested that loganin influences PMOP through acting on multiple inflammation-related pathways. In total, 31 potential targets were further constructed into a PPI network where the topology properties of the network were analysed. According to the node degree value in the PPI network, the top 10 genes were GAPDH, VEGFA, EGFR, ESR1, HRAS, SRC, FGF2, HSP90AA1, PTGS2 and IL-2. By computing the molecular interactions, it was observed that loganin exhibited strong binding affinities to the top 10 hub targets derived from the PPI network, which further indicated that loganin can interact with the core targets gene-encoded protein receptors. Taken together, it is reasonable to assume that the anti-OP effect led by loganin is largely depending on the regulation of the top 10 core genes which highly relates to multiple biological processes and signaling pathways. Next, in vivo studies were conducted to complement the in silico analyses. Loganin treatment significantly overturned the excessive bone loss caused by estrogen deficiency, characterized by elevated femoral BMD, BV/TV and Tb.N and reduced number of femoral Tb.Sp. Similarly, osteoclastic markers TRAP and CTX in serum dose-dependently decreased following loganin treatment, whereas bone formation marker P1NP was upregulated. TRAP and CTX are strong metabolic markers that correspond with osteoclast activity, while P1NP is an indicator of the cumulative quantity of new bone production (31). These in vivo results suggested that loganin treatment suppressed osteoclastogenesis and promoted osteoblast formation, exhibiting protective effect against PMOP.
Moreover, inflammation, which plays an important role during the development of PMOP, has been identified as a potential risk factor for PMOP (1). Estrogen deficiency is found to contribute to OP progress through elevating the proinflammatory and pro-osteoclastic cytokines, such as IL-6 and TNF-α, resulting in hyperactivation of osteoclasts and imbalanced bone turnover (32,33). Additionally, Sapra et al (34) demonstrated that IL-10 is an anti-osteoclastogenic cytokine that maintains bone health by inhibiting osteoclastogenesis. In the present study, it was found that loganin reduced the secretion of pro-inflammatory cytokines TNF-α and IL-6 and increased the anti-inflammatory factor IL-10. The results together suggested that loganin has an inhibitory effect on inflammatory response, potentially alleviating the progression of PMOP. TNF-α alone cannot produce complete osteoclasts with resorption function (35,36). These limits of TNF-α in osteoclast differentiation are overcome by the presence of other inflammatory cytokines such as IL-6 (35-37). IL-6 can promote osteoclast differentiation in the presence of RANKL in vitro, but it cannot differentiate osteoclasts on its own without RANKL (35,38). IL-6 in the presence of TNF-α also generates functional osteoclasts in vitro, which is independent to RANK/RANKL signaling (35,37) (Fig. 6).
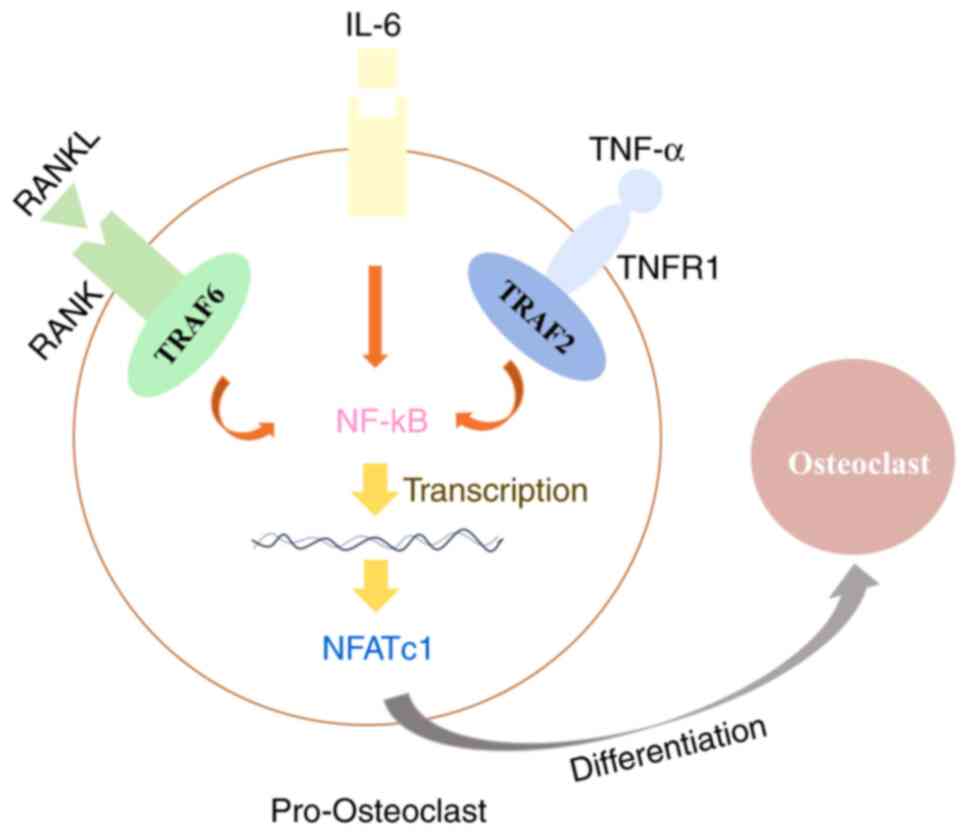 |
Figure 6
Schematic diagram summarizing the mechanism of the protective effect of loganin in ovariectomy-induced bone loss. TNF-α together with IL-6 can substitute for RANKL/RANK signaling through the activation of NF-kB and NFATc1, which are essential transcription factors for osteoclast differentiation. RANKL, receptor activator of NF-κB ligand.
|
In conclusion, the combination of network pharmacology and molecular docking revealed that loganin displayed anti-PMOP effect via acting on multi-targets and multiple inflammation-related signaling pathways on osteoclast differentiation. This is consistent to the in vivo results that loganin robustly attenuated OVX-induced bone loss and inflammatory response. The present study provided evidence for loganin as a novel potential therapy for PMOP, although more comprehensive and in-depth investigations are needed in the future.
Acknowledgements
The authors are grateful to Dr Heng Qiu (Department of Chemistry, University of Hong Kong, Hong Kong) for providing English editorial assistance.
Funding
The present study was supported by the Science and Technology Planning Project of Yunfu, Guangdong, China (grant no. 2020A090402) and the Guangdong Natural Science Foundation (grant no. 2023A1515010271).
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Authors' contributions
YX and XQ designed the current study. YX and XQ confirm the authenticity of all the raw data. YX and YZ wrote the manuscript and created the images. TZ and YZ conducted the network pharmacology and molecular docking analyses. JW, LT and YZ performed the OVX mice experiments. YX, XQ, TZ and LT analysed the in vivo data. QD and PS helped to design the experiment, analysed the network pharmacology data and in vivo data, provided reagents and materials, and reviewed and edited the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The experimental protocol of the present study was reviewed and approved (approval no. 00300814) by the Ethics Committee of The First Affiliated Hospital of Guangdong Pharmaceutical University (Guangzhou, China) according to the Guide for the Care and Use of Laboratory Animals. The study was conducted in accordance with the local legislation and institutional requirements.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Wang JB, Wang Y, Li LY, Cai SQ, Mao DD, Lou HK and Zhao J: Network pharmacology-based pharmacological mechanism prediction of Lycii Fructus against postmenopausal osteoporosis. Medicine (Baltimore). 102(e36292)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Iantomasi T, Romagnoli C, Palmini G, Donati S, Falsetti I, Miglietta F, Aurilia C, Marini F, Giusti F and Brandi ML: Oxidative stress and inflammation in osteoporosis: Molecular mechanisms involved and the relationship with microRNAs. Int J Mol Sci. 24(3772)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
An HQ, Chu C, Zhang Z, Zhang YH, Wei R, Wang B, Xu K, Li LH, Liu Y, Li G and Li X: Hyperoside alleviates postmenopausal osteoporosis via regulating miR-19a-5p/IL-17A axis. Am J Reprod Immunol. 90(e13709)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Noh JY, Yang Y and Jung HY: Molecular mechanisms and emerging therapeutics for osteoporosis. Int J Mol Sci. 21(7623)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou D, Zhang H, Xue X, Tao Y, Wang S, Ren X and Su J: Safety evaluation of natural drugs in chronic skeletal disorders: A literature review of clinical trials in the past 20 years. Front Pharmacol. 12(801287)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gu Y, Chen X, Wang Y, Liu Y, Zheng L, Li X, Wang R, Wang S, Li S, Chai Y, et al: Development of 3-mercaptopropyltrimethoxysilane (MPTS)-modified bone marrow mononuclear cell membrane chromatography for screening anti-osteoporosis components from Scutellariae Radix. Acta Pharm Sin B. 10:1856–1865. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yuan J, Maturavongsadit P, Zhou Z, Lv B, Lin Y, Yang J and Luckanagul JA: Hyaluronic acid-based hydrogels with tobacco mosaic virus containing cell adhesive peptide induce bone repair in normal and osteoporotic rats. Biomater Transl. 1:89–98. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu X, Zhang Z, Wang W, Yao H and Ma X: Therapeutic effect of cistanoside A on bone metabolism of ovariectomized mice. Molecules. 22(197)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huang J, Zhang Y, Dong L, Gao Q, Yin L, Quan H, Chen R, Fu X and Lin D: Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. J Ethnopharmacol. 1213:280–301. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim JY, Kim YK, Choi MK, Oh J, Kwak HB and Kim JJ: Effect of cornus officinalis on receptor activator of nuclear Factor-kappaB Ligand (RANKL)-induced Osteoclast Differentiation. J Bone Metab. 19:121–127. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu S, Shen H, Li J, Gong Y, Bao H, Zhang J, Hu L, Wang Z and Gong J: Loganin inhibits macrophage M1 polarization and modulates sirt1/NF-κB signaling pathway to attenuate ulcerative colitis. Bioengineered. 11:628–639. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li M, Wang W, Wang P, Yang K, Sun H and Wang X: The pharmacological effects of morroniside and loganin isolated from Liuweidihuang Wan, on MC3T3-E1 cells. Molecules. 15:7403–7314. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xia H, Liu J, Yang W, Liu M, Luo Y, Yang Z, Xie J, Zeng H, Xu R, Ling H, et al: Integrated strategy of network pharmacological prediction and experimental validation elucidate possible mechanism of Bu-Yang Herbs in treating postmenopausal osteoporosis via ESR1. Front Pharmacol. 12(654714)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bai X, Tang Y, Li Q, Chen Y, Liu D, Liu G, Fan X, Ma R, Wang S, Li L, et al: Network pharmacology integrated molecular docking reveals the bioactive components and potential targets of Morinda officinalis-Lycium barbarum coupled-herbs against oligoasthenozoospermia. Sci Rep. 11(2220)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen X, Wang J, Tang L, Ye Q, Dong Q, Li Z, Hu L, Ma C, Xu J and Sun P: The therapeutic effect of Fufang Zhenshu Tiaozhi (FTZ) on osteoclastogenesis and ovariectomized-induced bone loss: Evidence from network pharmacology, molecular docking and experimental validation. Aging (Albany NY). 14:5727–5748. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kong Y, Ma X, Zhang X, Wu L, Chen D, Su B, Liu D and Wang X: The potential mechanism of Fructus Ligustri Lucidi promoting osteogenetic differentiation of bone marrow mesenchymal stem cells based on network pharmacology, molecular docking and experimental identification. Bioengineered. 13:10640–10653. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang H, Zhou C, Zhang Z, Yao S, Bian Y, Fu F, Luo H, Li Y, Yan S, Ge Y, et al: Integration of Network Pharmacology and Experimental Validation to Explore the Pharmacological Mechanisms of Zhuanggu Busui Formula Against Osteoporosis. Front Endocrinol (Lausanne). 12(841668)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huang J, Wang X, Zheng J, Jia Q, Wang X, Xie Z and Ma H: Mechanisms underlying the therapeutic effects of isoflavones isolated from chickpea sprouts in treating osteoporosis based on network pharmacology. Biochem Biophys Res Commun. 671:26–37. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Park E, Lee CG, Yun SH, Hwang S, Jeon H, Kim J, Yeo S, Jeong H, Yun SH and Jeong SY: Ameliorative effects of loganin on arthritis in chondrocytes and destabilization of the medial meniscus-induced animal model. Pharmaceuticals (Basel). 14(135)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals: Guide for the Care and Use of Laboratory Animals. In: The National Academies Collection: Reports funded by National Institutes of Health. 8th edition. National Academy of Sciences (US), Washington, DC, 2011.
|
|
21
|
Xiao J, Shang W and Zhao Z, Jiang J, Chen J, Cai H, He J, Cai Z and Zhao Z: Pharmacodynamic material basis and potential mechanism study of spatholobi caulis in reversing osteoporosis. Evid Based Complement Alternat Med. 2023(3071147)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu Y, He Y, He B and Kong L: The Anti-osteoporosis Effects of Vitamin K in postmenopausal women. Curr Stem Cell Res Ther. 2:186–192. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee CG, Kim DW, Kim J, Uprety LP, Oh KI, Singh S, Yoo J, Jin HS, Choi TH, Park E and Jeong SY: Effects of loganin on bone formation and resorption in vitro and in vivo. Int J Mol Sci. 23(14128)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Isken I, Witayateeraporn W, Wirojwongchai T, Suraphan C, Pornputtapong N, Singharajkomron N, Nguyen HM and Pongrakhananon V: Identifying molecular targets of Aspiletrein-derived steroidal saponins in lung cancer using network pharmacology and molecular docking-based assessments. Sci Rep. 13(1545)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhu M, Guo Q, Kang H, Peng R, Dong Y, Zhang Y, Wang S, Liu H, Zhao H, Dong Z, et al: Inhibition of FAAH suppresses RANKL-induced osteoclastogenesis and attenuates ovariectomy-induced bone loss partially through repressing the IL17 pathway. FASEB J. 37(e22690)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Scheffler JM, Grahnemo L, Engdahl C, Drevinge C, Gustafsson KL, Corciulo C, Lawenius L, Iwakura Y, Sjögren K, Lagerquist MK, et al: Interleukin 17A: A Janus-faced regulator of osteoporosis. Sci Rep. 10(5692)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu Y, Yang Y, Wang L, Chen Y, Han X, Sun L, Chen H and Chen Q: Effect of Bifidobacterium on osteoclasts: TNF-α/NF-κB inflammatory signal pathway-mediated mechanism. Front Endocrinol (Lausanne). 14(1109296)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hu J, Zhou J, Wu J, Chen Q, Du W, Fu F, Yu H, Yao S, Jin H, Tong P, et al: Loganin ameliorates cartilage degeneration and osteoarthritis development in an osteoarthritis mouse model through inhibition of NF-κB activity and pyroptosis in chondrocytes. J Ethnopharmacol. 247(112261)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Brigati C, Banelli B, di Vinci A, Casciano I, Allemanni G, Forlani A, Borzì L and Romani M: Inflammation, HIF-1, and the epigenetics that follows. Mediators Inflamm. 2010(263914)2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhao J, Lin F, Liang G, Han Y, Xu N, Pan J, Luo M, Yang W and Zeng L: Exploration of the molecular mechanism of polygonati rhizoma in the treatment of osteoporosis based on network pharmacology and molecular docking. Front Endocrinol (Lausanne). 12(815891)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xue C, Luo H, Wang L, Deng Q, Kui W, Da W, Chen L, Liu S, Xue Y, Yang J, et al: Aconine attenuates osteoclast-mediated bone resorption and ferroptosis to improve osteoporosis via inhibiting NF-κB signaling. Front Endocrinol (Lausanne). 14(1234563)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Al-Daghri NM, Aziz I, Yakout S, Aljohani NJ, Al-Saleh Y, Amer OE, Sheshah E, Younis GZ and Al-Badr FBM: Inflammation as a contributing factor among postmenopausal Saudi women with osteoporosis. Medicine (Baltimore). 96(e5780)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mentzel J, Kynast T, Kohlmann J, Kirsten H, Blüher M, Simon JC, Kunz M and Saalbach A: Reduced serum levels of bone formation marker P1NP in psoriasis. Front Med (Lausanne). 8(730164)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sapra L, Bhardwaj A, Mishra PK, Garg B, Verma B, Mishra GC and Srivastava RK: Regulatory B Cells (Bregs) inhibit osteoclastogenesis and play a potential role in ameliorating ovariectomy-induced bone loss. Front Immunol. 12(691081)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jung YK, Kang YM and Han S: Osteoclasts in the inflammatory arthritis: Implications for pathologic osteolysis. Immune Netw. 19(e2)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, et al: Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 191:275–286. 2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
O'Brien W, Fissel BM, Maeda Y, Yan J, Ge X, Gravallese EM, Aliprantis AO and Charles JF: RANK-Independent osteoclast formation and bone erosion in inflammatory arthritis. Arthritis Rheumatol. 68:2889–2900. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Axmann R, Böhm C, Krönke G, Zwerina J, Smolen J and Schett G: Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 60:2747–2756. 2009.PubMed/NCBI View Article : Google Scholar
|




















