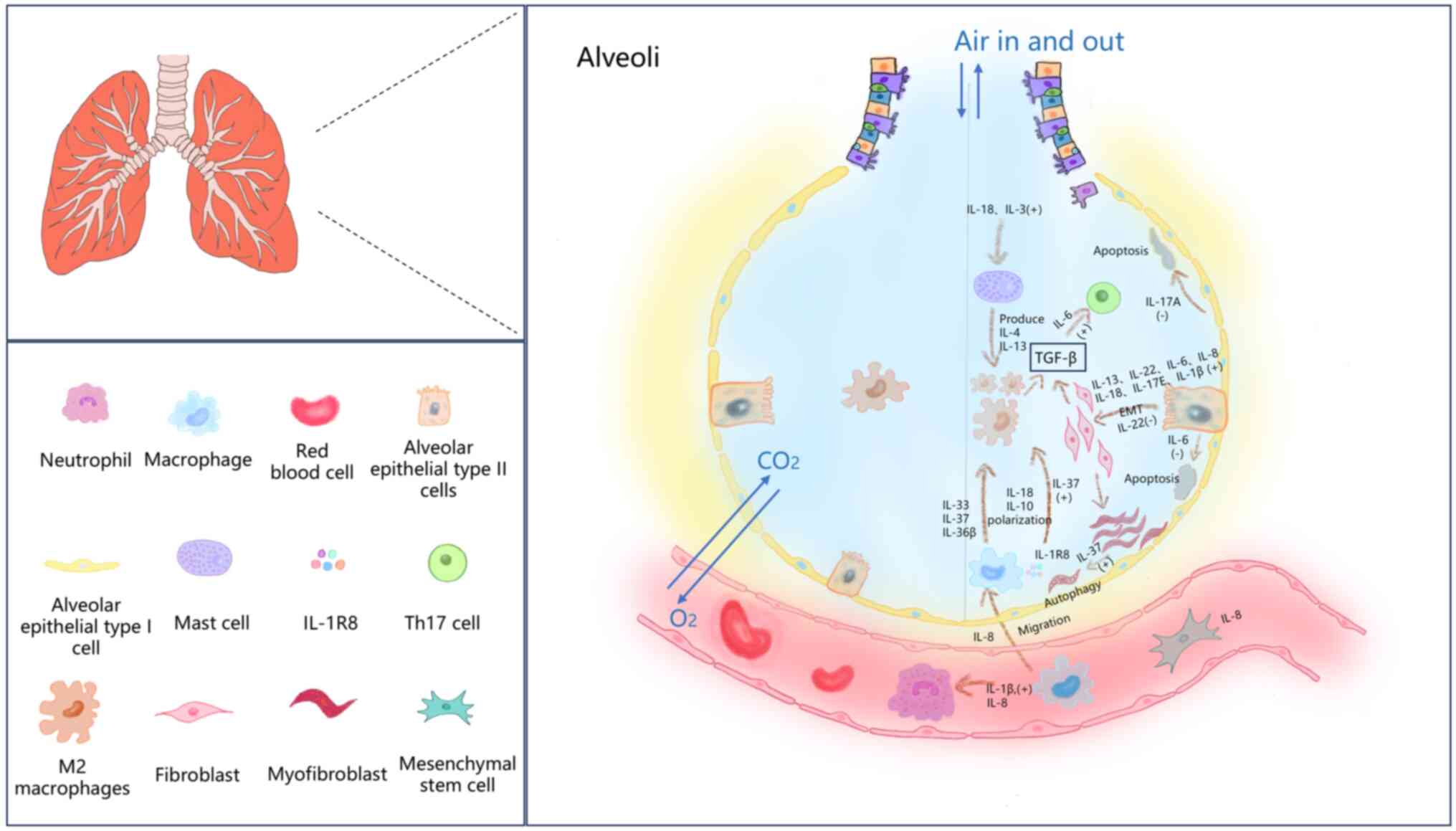
Pulmonary fibrosis (PF) is a severe and irreversible
consequence of various respiratory diseases, such as alveolitis and
interstitial pneumonitis, which is characterized by excessive
accumulation of extracellular matrix (ECM) and fibroblast
proliferation (1). These
pathological processes are accompanied by inflammatory responses
and significant damage to the lung architecture (2,3).
Subsequent to the injury of alveolar epithelial cells (AECs),
multiple interconnected downstream profibrotic pathways are
activated, and subsequent aberrant repair mechanisms can lead to
harmful scarring in pulmonary tissues and severe impairment of lung
function (4,5). Inflammatory responses (6), oxidative stress (7,8) and
apoptosis (9) have been reported
as possible pathological mechanisms underlying PF.
Globally, the number of patients with PF is
increasing, which may be related to aging, increased awareness of
the disease as well as improved diagnostic techniques and tools
(10,11). The estimated incidence and
prevalence of PF are 0.09-1.30/10,000 patients and 0.33-4.51/10,000
patients, respectively (12).
Despite recent advances in the treatment of PF, the disease shows a
poor prognosis and current available therapeutic options can only
slow its progression (13).
ILs belong to a large family that can be subdivided
into numerous types and are critical mediators of PF pathogenesis
(14). Recent studies have
demonstrated that ILs contribute to PF development by modulating
various biological processes, including inflammation (24,25),
immune responses (26), autophagy
(27), cellular senescence
(28) and epithelial-mesenchymal
transition (EMT) (29,30), as illustrated in Fig. 1. Notably, different types of ILs
exert disparate effects on PF, as subsequently discussed in the
present review.
IL-6 can stimulate fibrosis by initiating chronic
inflammation and activating the TGF-β pathway (43), whereas TGF-β1 is at present, the
most potent profibrotic cytokine (44). In bleomycin (BLM)-induced mice with
PF, the expression of fibrosis-related cytokine IL-6 and
myofibroblast marker collagen type I in the lymphoid fibroblast
supernatant, serum, blood and bronchoalveolar lavage fluid induced
by TGF-β1 were significantly increased (45).
IL-6 promotes PF as a multifunctional cytokine
involved in the inflammatory response and fibrosis. IL-6 induces
the maturation of T and B lymphocytes and participates in acute
inflammatory responses (46). IL-6
also induces collagen deposition and ECM accumulation and
stimulates fibroblast proliferation, thereby promoting the
development of fibrosis (45).
Clinical studies demonstrated an association between serum IL-6
levels and lung function impairment in patients with PF (47,48).
The detection of serum IL-6 levels in patients with PF could
provide a basis for the clinical judgment of respiratory function
(47). Moreover, results of a
phase III clinical trial of an IL-6 receptor antagonist has
indicated that IL-6 is a driver of PF, and inhibition of IL-6 could
help restore lung function (49).
To the best of our knowledge, there are no clinical studies that
directly focus on IL-6 and PF, but the aforementioned findings
indicate that targeting IL-6 may be a promising therapeutic
approach against PF.
Fibrotic mesenchymal progenitor cells (MPCs) in lung
tissue are the original cells of PF fibroblasts (43). IL-8 derived from MPCs facilitates
the proliferation, differentiation and migration of MPCs and
induces macrophage migration to fibroblast foci via the receptor
CXCR1/2(54). Li et al
(55) demonstrated that IL-8
production is induced in airway epithelial cells through the NF-κB
pathway, and the induced IL-8 expression is not only involved in
neutrophil recruitment in airway inflammation in lung diseases, but
also associated with airway fibrosis and remodeling by provoking
the proliferation and migration of lung fibroblasts and mesenchymal
stem cells.
The main subtypes of the IL-17 family are IL-17A to
IL-17F, which bind to the IL-17 receptor (IL-17R) complex and
consist of two chains: i) IL-17RA; and ii) IL-17RC (71). IL-17RA is expressed in almost every
cell type, including epithelial and endothelial cells and
fibroblasts (72,73). IL-17A expression levels are
positively correlated with inflammation scores of skin and lung as
well as skin fibrosis in PF mice and IL-17A inhibits autophagy in
AECs (69). IL-17RC acts on
numerous cell types, including CD4+ T cells, macrophages and
neutrophils, and plays important roles in lung inflammation
(74-76).
IL-17E, often referred to as IL-25, is secreted by T2, epithelial,
endothelial and T cells, alveolar macrophages, eosinophils and
basophils, all of which are closely associated with the
inflammatory response (77). In
lung tissues from patients with PF, the levels of IL-17E and its
receptor IL-17RB are increased, suggesting that they drive PF by
mediating EMT in AECs and by recruiting and activating lung
fibroblasts (29,30).
IL-36β activates M2 macrophages, and in bone marrow
dendritic cells, IL-36 stimulation induces the production of
proinflammatory cytokines (such as IL-1β, IL-6, IL-12, IL-23 and
TNF) (105,106). The IL-36 receptor is expressed
predominantly by initial CD4+ T cells, and promotes differentiation
towards Th1 and Th17 cells, and facilitates their proliferation and
inflammatory cytokine production (107).
IL-10, an inhibitory factor of human cytokine
synthesis, is an anti-inflammatory cytokine (108,109). IL-10 is mainly produced by
lymphocytes, macrophages and mast cells and has immunomodulatory
effects such as the inhibition of monocytes/macrophages, Th1 cell
function and enhancement of B lymphocyte function (110). IL-10 inhibits the expression of
inflammatory factors, such as TNF-α, IL-6 and IL-1, through
activating macrophages and can exert an immunostimulatory effect in
numerous cell types (111).
IL-10 inhibits the activation, migration and
adhesion of inflammatory cells by downregulating the expression of
the major histocompatibility antigen II on the surface of
monocytes, decreasing its antigen-presenting effect and
downregulating the activity of T lymphocytes (112,113). At the same time, IL-10 can reduce
inflammatory cytokines, such as IL-2, INF-γ, TNF-α and CSF-GM, and
thus attenuate the inflammatory response (114). Moreover, IL-10 inhibits the
expression of cytokines, such as TNF-α, IL-1β and IL-8, and the
expression of adhesion molecules (115).
The Th1/Th2 imbalance is crucial in the pathogenesis
of PF, with Th1-type cells represented by IFN-γ, which may promote
the repair of normal tissue structures, and Th2-type cytokines
represented by IL-4, which may cause excessive damage repair, ECM
deposition and fibrosis (122).
When the balance of Th1/Th2 type cytokines shifts towards Th2 type
cytokines, it can lead to the development of fibrosis (123). IL-12 induces the proliferation
and differentiation of Th1 cells to produce Th1-type cytokines, and
inhibits the proliferation and differentiation of Th2 cells
(124).
IL-37 and Smad3 are involved in the process of
alveolitis and PF, and the IL-37b-Smad3 complex can suppress the
phosphorylation of c-Jun N-terminal kinase and mitogen-activated
protein kinase (MAPK), which is associated with IL-1-induced
proinflammatory transcription factor AP-1(133). Inhibition of MAPK phosphorylation
can downregulate the expression of Th2-type factors in peripheral
blood mononuclear cells (133).
IL-38 is a potential inhibitor of IL-1 and the
Toll-like receptor family with potent anti-inflammatory effects
(134). The anti-inflammatory
effects of IL-38 are related to the inhibition of inflammatory
signaling pathways in target cells, suppression of T lymphocyte
function and reduction in the secretion of inflammatory factors,
including IL-6, TNF, CCL5 and CXCL10(135). IL-38 expression attenuates
BLM-induced inflammatory and fibrotic injury in the lungs and
reduces the production of proinflammatory and profibrotic cytokines
(136).
Moreover, IL-38 has been reported to inhibit
inflammatory processes by antagonizing IL-36R, similar to IL-36Ra
or IL-1Ra (137). Furthermore, in
a mouse model of BLM-induced PF, overexpression of IL-38 attenuated
lung inflammation and fibrosis, reduced the production of
inflammatory factors such as IL-1β, IL-6, IL-17A and TNF-α, and
promoted the expression of the anti-inflammatory cytokine IL-1Ra
(138).
IL-4 is a pleiotropic cytokine that is primarily
produced by activated T lymphocytes, mast cells, basophils and
eosinophils cells (139). IL-4
exerts immunomodulatory effects on B lymphocytes, T and mast cells,
macrophages and hematopoietic cells, and also exerts anti- and
pro-inflammatory effects (61).
On the one hand, IL-4 attenuates PF by inhibiting
macrophage infiltration, M2 polarization and collagen deposition,
and the pharmacological treatment enhances IL-4-induced autophagy
in macrophages and lung tissue in BLM-treated rats (27). On the other hand, IL-4 increases in
the serum of patients with PF and in bronchoalveolar lavage fluid
of mice after BLM intoxication (140). The primary role of IL-4 includes
the induction of Th2 responses and the alternative activation of
dendritic cell stimulation to present antigens to other immune
cells and macrophages (141). In
addition, the synergistic effect of IL-24 and IL-4 promotes M2-type
polarization of macrophages, which further promotes the development
of PF (140). However, M2
macrophages can have an anti-inflammatory and pro-wound healing
phenotype; when this process is dysregulated, the overactivation of
these responses can lead to the development of fibrosis (142). Notably, a previous study
confirmed that the development of fibrosis is closely related to
the production of Th2-type cytokines, mainly IL-4-mediated
macrophage activation, fibroblast proliferation and
differentiation, and ECM deposition, ultimately leading to PF
formation (143).
Notably, IL-22 was also reported to be a
proinflammatory and profibrotic factor (163). IL-22 is capable of activating
collagen production and deposition by stimulating fibroblast
proliferation and secretion of profibrotic cytokines, which promote
subepithelial fibrosis and lead to the remodeling of asthmatic
airways (164).
ILs are a group of cytokines originating from
diverse cell types and are known for their diverse effects. ILs can
exert either antifibrotic or profibrotic effects and rarely exhibit
dual actions by influencing the structure and functionality of
various cell types involved in PF predominantly by targeting
fibroblasts, macrophages and epithelial cells, which are closely
associated with PF progression. The majority of the studies
described in the present review still have some controversies.
Therefore, a full understanding of ILs and their association with
PF remains to be elucidated.
Not applicable.
Funding: This work was finally supported by Research Funds of
Center for Xin'an Medicine and Modernization of Traditional Chinese
Medicine, Institute of Health and Medicine (grant no.
2023CXMMTCM007), Municipal Science and Technology Project of Wuhu
Science and Technology Bureau (grant no. 2023yf090), Natural
Science Foundation of the Higher Education Institutions of Anhui
Province (grant no. 2023AH051751), Key Science and Technology
Program of Wuhu City (grant no. 2022jc33) and Key Natural Science
Research Project of Anhui Provincial Department of Education (grant
no. KJ2021A0858).
Not applicable.
SN and KZ contributed to the study conception and
design. Material preparation, and data collection and analysis were
performed by YH, XS, KZ and SN. The draft of the manuscript was
written by YH, and all authors commented on previous versions of
the manuscript. All authors read and approved the final version of
the manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Ding DL, Shen XB, Yu LZ, Zheng YY, Liu Y,
Wang W, Liu L, Zhao ZT, Nian SH and Liu LM: Timosaponin BII
inhibits TGF-β mediated epithelial-mesenchymal transition through
Smad-dependent pathway during pulmonary fibrosis. Phytother Res.
37:2787–2799. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Zhao T, Zhou Z, Wan H, Feng T, Hu X, Li X,
Zhao S, Li H, Hou J, Li W, et al: Otilonium bromide ameliorates
pulmonary fibrosis in mice through activating phosphatase PPM1A.
Acta Pharmacol Sin: August 19, 2024 (Epub ahead of print).
|
|
3
|
Tu JY, Chen XY, Li CY, Liu CF, Huang YB,
Wang X, Liang H and Yuan XL: Nintedanib mitigates radiation-induced
pulmonary fibrosis by suppressing epithelial cell inflammatory
response and inhibiting fibroblast-to-myofibroblast transition. Int
J Biol Sci. 20:3353–3371. 2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Confalonieri P, Volpe MC, Jacob J,
Maiocchi S, Salton F, Ruaro B, Confalonieri M and Braga L:
Regeneration or repair? The role of alveolar epithelial cells in
the pathogenesis of idiopathic pulmonary fibrosis (IPF). Cells.
11(2095)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kinoshita T and Goto T: Molecular
mechanisms of pulmonary fibrogenesis and its progression to lung
cancer: A review. Int J Mol Sci. 20(1461)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pu Z, Sui B, Wang X, Wang W, Li L and Xie
H: The effects and mechanisms of the anti-COVID-19 traditional
Chinese medicine, Dehydroandrographolide from Andrographis
paniculata (Burm.f.) Wall, on acute lung injury by the inhibition
of NLRP3-mediated pyroptosis. Phytomedicine.
114(154753)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang H, Hua C, Yang X, Fan X, Song H, Peng
L and Ci X: Pterostilbene prevents LPS-induced early pulmonary
fibrosis by suppressing oxidative stress, inflammation and
apoptosis in vivo. Food Funct. 11:4471–4484. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Otoupalova E, Smith S, Cheng GJ and
Thannickal VJ: Oxidative stress in pulmonary fibrosis. Compr
Physiol. 10:509–547. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Valenca SS, Dong BE, Gordon EM, Sun RC and
Waters CM: ASK1 regulates bleomycin-induced pulmonary fibrosis. Am
J Respir Cell Mol Biol. 66:484–496. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tremayne P and John Clark S: Idiopathic
pulmonary fibrosis: A more common condition than you may think. Br
J Nurs. 30:359–366. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hoyer N, Prior TS, Bendstrup E, Wilcke T
and Shaker SB: Risk factors for diagnostic delay in idiopathic
pulmonary fibrosis. Respir Res. 20(103)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Maher TM, Bendstrup E, Dron L, Langley J,
Smith G, Khalid JM, Patel H and Kreuter M: Global incidence and
prevalence of idiopathic pulmonary fibrosis. Respir Res.
22(197)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Glassberg MK: Overview of idiopathic
pulmonary fibrosis, evidence-based guidelines, and recent
developments in the treatment landscape. Am J Manag Care. 25 (11
Suppl):S195–S203. 2019.PubMed/NCBI
|
|
14
|
Agostini C and Gurrieri C:
Chemokine/cytokine cocktail in idiopathic pulmonary fibrosis. Proc
Am Thorac Soc. 3:357–363. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li H, Li Q and Hao Z, Zhang L, Zheng X,
Zhu L, Huo Y, Tian H, He L and Hao Z: A recombinant IL-1β vaccine
attenuates bleomycin-induced pulmonary fibrosis in mice. Vaccine.
42:3774–3788. 2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Park SJ, Ryu HW, Kim JH, Hahn HJ, Jang HJ,
Ko SK, Oh SR and Lee HJ: Daphnetin alleviates bleomycin-induced
pulmonary fibrosis through inhibition of epithelial-to-mesenchymal
transition and IL-17A. Cells. 12(2795)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu X, Dai W and Li C: Interleukins in the
treatment of melanoma. Chin Med J (Engl). 135:393–399.
2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gritsenko A, Diaz-Pino R and
López-Castejón G: NLRP3 inflammasome triggers interleukin-37
release from human monocytes. Eur J Immunol. 52:1141–1157.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fukaura R and Akiyama M: Targeting IL-36
in inflammatory skin diseases. Biodrugs. 37:279–293.
2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bequignon E, Mangin D, Bécaud J, Pasquier
J, Angely C, Bottier M, Escudier E, Isabey D, Filoche M, Louis B,
et al: Pathogenesis of chronic rhinosinusitis with nasal polyps:
Role of IL-6 in airway epithelial cell dysfunction. J Transl Med.
18(136)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mesas-Fernández A, Bodner E, Hilke FJ,
Meier K, Ghoreschi K and Solimani F: Interleukin-21 in autoimmune
and inflammatory skin diseases. Eur J Immunol.
53(e2250075)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Akdis M, Burgler S, Crameri R, Eiwegger T,
Fujita H, Gomez E, Klunker S, Meyer N, O'Mahony L, Palomares O, et
al: Interleukins, from 1 to 37, and interferon-γ: Receptors,
functions, and roles in diseases. J Allergy Clin Immunol.
127:701–721.e1-e70. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li Y, Yin H, Yuan H, Wang E, Wang C, Li H,
Geng X, Zhang Y and Bai J: IL-10 deficiency aggravates cell
senescence and accelerates BLM-induced pulmonary fibrosis in aged
mice via PTEN/AKT/ERK pathway. BMC Pulm Med. 24(443)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fattakhov N, Ngo A, Torices S, Joseph JA,
Okoro A, Moore C, Naranjo O, Becker S and Toborek M: Cenicriviroc
prevents dysregulation of astrocyte/endothelial cross talk induced
by ischemia and HIV-1 via inhibiting the NLRP3 inflammasome and
pyroptosis. Am J Physiol Cell Physiol. 326:C487–C504.
2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen P, Zhou J, Ruan AM, Ma YF and Wang
QF: Paeoniflorin, the Main monomer component of paeonia lactiflora,
exhibits anti-inflammatory properties in osteoarthritis synovial
inflammation. Chin J Integr Med. 30:433–442. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xiong Y, Cui X, Zhou Y, Chai G, Jiang X,
Ge G, Wang Y, Sun H, Che H, Nie Y and Zhao P: Dehydrocostus lactone
inhibits BLM-induced pulmonary fibrosis and inflammation in mice
via the JNK and p38 MAPK-mediated NF-κB signaling pathways. Int
Immunopharmacol. 98(107780)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yan L, Hou C, Liu J, Wang Y, Zeng C, Yu J,
Zhou T, Zhou Q, Duan S and Xiong W: Local administration of
liposomal-based Plekhf1 gene therapy attenuates pulmonary fibrosis
by modulating macrophage polarization. Sci China Life Sci.
66:2571–2586. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang LM, Zhang Y, Fei C, Zhang J, Wang L,
Yi ZW and Gao G: Neutralization of IL-18 by IL-18 binding protein
ameliorates bleomycin-induced pulmonary fibrosis via inhibition of
epithelial-mesenchymal transition. Biochem Biophys Res Commun.
508:660–666. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu X, Luo S, Li B, Dai H and Zhang J:
IL-25 contributes to lung fibrosis by directly acting on alveolar
epithelial cells and fibroblasts. Exp Biol Med (Maywood).
244:770–780. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nie YJ, Wu SH, Xuan YH and Yan G: Role of
IL-17 family cytokines in the progression of IPF from inflammation
to fibrosis. Mil Med Res. 9(21)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Boersma B, Jiskoot W, Lowe P and Bourquin
C: The interleukin-1 cytokine family members: Role in cancer
pathogenesis and potential therapeutic applications in cancer
immunotherapy. Cytokine Growth Factor Rev. 62:1–14. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Garlanda C and Mantovani A: Interleukin-1
in tumor progression, therapy, and prevention. Cancer Cell.
39:1023–1027. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dinarello CA: Interleukin-1 in the
pathogenesis and treatment of inflammatory diseases. Blood.
117:3720–3732. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dinarello CA: Interleukin-1. Cytokine
Growth Factor Rev. 8:253–265. 1997.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Calverley PM, Sethi S, Dawson M, Ward CK,
Finch DK, Penney M, Newbold P and van der Merwe R: A randomised,
placebo-controlled trial of anti-interleukin-1 receptor 1
monoclonal antibody MEDI8968 in chronic obstructive pulmonary
disease. Respir Res. 18(153)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Grönberg C, Rattik S, Tran-Manh C, Zhou X,
Rius Rigau A, Li YN, Györfi AH, Dickel N, Kunz M, Kreuter A, et al:
Combined inhibition of IL-1, IL-33 and IL-36 signalling by
targeting IL1RAP ameliorates skin and lung fibrosis in preclinical
models of systemic sclerosis. Ann Rheum Dis. 83:1156–1168.
2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang YX, Zhang XT, Li HJ, Zhou TF, Zhou
AC, Zhong ZL, Liu YH, Yuan LL, Zhu HY, Luan D and Tong JC:
Antidepressant-like effects of helicid on a chronic unpredictable
mild stress-induced depression rat model: Inhibiting the
IKK/IκBα/NF-κB pathway through NCALD to reduce inflammation. Int
Immunopharmacol. 93(107165)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li W, Zhao X, Yu TT, Hao W and Wang GG:
Knockout of PKC θ gene attenuates oleic acid-induced acute lung
injury via reduction of inflammation and oxidative stress. Iran J
Basic Med Sci. 24:986–991. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Witzenrath M and Kuebler WM: The
lung-brain axis in ventilator-induced brain injury: Enter IL-6. Am
J Respir Cell Mol Biol. 65:339–340. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jones BE, Maerz MD and Buckner JH: IL-6: A
cytokine at the crossroads of autoimmunity. Curr Opin Immunol.
55:9–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Le TTT, Karmouty-Quintana H, Melicoff E,
Le TTT, Weng T, Chen NY, Pedroza M, Zhou Y, Davies J, Philip K, et
al: Blockade of IL-6 trans signaling attenuates pulmonary fibrosis.
J Immunol. 193:3755–3768. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhao FZ, Sang XQ, Zhu Y and Yang J: Effect
and mechanism of IL-6 induced by M2 macrophages on the lung
fibroblasts activation. Acta Pharmaceutica Sinica. 55:892–897.
2020.
|
|
43
|
Yang LB, Herrera J, Gilbertsen AJ, Xia H,
Smith K, Benyumov A, Bitterman PB and Henke CA: IL-8 mediates
idiopathic pulmonary fibrosis mesenchymal progenitor cell
fibrogenicity. Am J Physiol Lung Cell Mol Physiol. 314:L127–L136.
2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shochet GE, Brook E, Bardenstein-Wald B
and Shitrit D: TGF-β pathway activation by idiopathic pulmonary
fibrosis (IPF) fibroblast derived soluble factors is mediated by
IL-6 trans-signaling. Respir Res. 12(56)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu Y, Lu F, Kang L, Wang Z and Wang Y:
Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice
by regulating Nrf2/Bach1 equilibrium. BMC Pulm Med.
17(63)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cebi M and Yilmaz Y: Immune system
dysregulation in the pathogenesis of non-alcoholic steatohepatitis:
Unveiling the critical role of T and B lymphocytes. Front Immunol.
15(1445634)2024.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Read J, Reid AT, Thomson C, Plit M, Mejia
R, Knight DA, Lize M, Kasmi KE, Grainge CL, Stahl H and Schuliga M:
Alveolar epithelial cells of lung fibrosis patients are susceptible
to severe virus-induced injury. Clin Sci (Lond). 138:537–554.
2024.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jøntvedt Jørgensen M, Holter JC,
Christensen EE, Schjalm C, Tonby K, Pischke SE, Jenum S, Skeie LG,
Nur S, Lind A, et al: Increased interleukin-6 and macrophage
chemoattractant protein-1 are associated with respiratory failure
in COVID-19. Sci Rep. 10(21697)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Khanna D, Lin CJF, Furst DE, Goldin J, Kim
G, Kuwana M, Allanore Y, Matucci-Cerinic M, Distler O, Shima Y, et
al: Tocilizumab in systemic sclerosis: A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Respir Med. 8:963–974.
2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sagaram M, Frimodig J, Jayanty D, Hu H,
Royer AJ, Bruner R, Kong M, Schwandt ML and Vatsalya V: One-month
assessment of Th-cell axis related inflammatory cytokines, IL-17
and IL-22 and their role in alcohol-associated liver disease. Front
Immunol. 14(1202267)2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kosmopoulos M, Christofides A, Drekolias
D, Zavras PD, Gargalionis AN and Piperi C: Critical role of IL-8
targeting in gliomas. Curr Med Chem. 25:1954–1967. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
W.Y. B: Changes of IL-2R, IL-6, IL-8, and
TNF-α in diffuse large B-cell lymphoma and their significance. J
Clin Hematol. 36:33–38. 2023.
|
|
53
|
Papiris SA, Tomos IP, Karakatsani A,
Spathis A, Korbila I, Analitis A, Kolilekas L, Kagouridis K,
Loukides S, Karakitsos P and Manali ED: High levels of IL-6 and
IL-8 characterize early-on idiopathic pulmonary fibrosis acute
exacerbations. Cytokine. 102:168–172. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yang L, Xia H, Gilbertsen A, Smith K,
Racila E, Bitterman PB and Henke CA: IL-8 concurrently promotes
idiopathic pulmonary fibrosis mesenchymal progenitor cell
senescence and PD-L1 expression enabling escape from immune cell
surveillance. Am J Physiol Lung Cell Mol Physiol. 324:L849–L862.
2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Li Y, Su G, Zhong Y, Xiong Z, Huang T,
Quan J, Huang J, Wen X, Luo C, Zheng W, et al: HB-EGF-induced IL-8
secretion from airway epithelium leads to lung fibroblast
proliferation and migration. BMC Pulm Med. 21(347)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kato A: Immunopathology of chronic
rhinosinusitis. Allergol Int. 64:121–130. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Moonwiriyakit A, Yimnual C, Noitem R,
Dinsuwannakol S, Sontikun J, Kaewin S, Worakajit N,
Soontornniyomkij V and Muanprasat C: GPR120/FFAR4 stimulation
attenuates airway remodeling and suppresses IL-4- and IL-13-induced
airway epithelial injury via inhibition of STAT6 and Akt. Biomed
Pharmacother. 168(115774)2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Iwaszko M, Biały S and Bogunia-Kubik K:
Significance of interleukin (IL)-4 and IL-13 in inflammatory
arthritis. Cells. 10(3000)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Husna SMN, Shukri NM, Ashari NSM and Wong
KK: IL-4/IL-13 axis as therapeutic targets in allergic rhinitis and
asthma. PeerJ. 10(e13444)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Bonser LR, Eckalbar WL, Rodriguez L, Shen
J, Koh KD, Ghias K, Zlock LT, Christenson S, Woodruff PG,
Finkbeiner WE and Erle DJ: The type 2 asthma mediator IL-13
inhibits severe acute respiratory syndrome coronavirus 2 infection
of bronchial epithelium. Am J Respir Cell Mol Biol. 66:391–401.
2022.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Le Floc'h A, Allinne J, Nagashima K, Scott
G, Birchard D, Asrat S, Bai Y, Lim WK, Martin J, Huang T, et al:
Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody,
is required to broadly inhibit type 2 inflammation. Allergy.
75:1188–1204. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Passalacqua G, Mincarini M, Colombo D,
Troisi G, Ferrari M, Bagnasco D, Balbi F, Riccio A and Canonica GW:
IL-13 and idiopathic pulmonary fibrosis: Possible links and new
therapeutic strategies. Pulm Pharmacol Ther. 45:95–100.
2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Bhatt SP, Rabe KF, Hanania NA, Vogelmeier
CF, Cole J, Bafadhel M, Christenson SA, Papi A, Singh D, Laws E, et
al: Dupilumab for COPD with type 2 inflammation indicated by
eosinophil counts. N Engl J Med. 389:205–214. 2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Maher TM, Costabel U, Glassberg MK, Kondoh
Y, Ogura T, Scholand MB, Kardatzke D, Howard M, Olsson J, Neighbors
M, et al: Phase 2 trial to assess lebrikizumab in patients with
idiopathic pulmonary fibrosis. Eur Respir J.
57(1902442)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Shaikh SB, Prabhu A and Bhandary YP:
Interleukin-17A: A potential therapeutic target in chronic lung
diseases. Endocr Metab Immune Disord Drug Targets. 19:921–928.
2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Berry SPDG, Dossou C, Kashif A,
Sharifinejad N, Azizi G, Hamedifar H, Sabzvari A and Zian Z: The
role of IL-17 and anti-IL-17 agents in the immunopathogenesis and
management of autoimmune and inflammatory diseases. Int
Immunopharmacol. 102(108402)2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Yang Z, Zhang J, Zhu Y, Zhang C, Li G, Liu
S, Du J, Han Y and You B: IL-17A induces valvular endothelial
inflammation and aggravates calcific aortic valve disease. Biochem
Biophys Res Commun. 672:145–153. 2023.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Luo J, An X, Yao Y, Erb C, Ferguson A,
Kolls JK, Fan S and Chen K: Epigenetic regulation of IL-17-induced
chemokines in lung epithelial cells. Mediators Inflamm.
2019(9050965)2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Lei L, Zhao C, Qin F, He ZY, Wang X and
Zhong XN: Th17 cells and IL-17 promote the skin and lung
inflammation and fibrosis process in a bleomycin-induced murine
model of systemic sclerosis. Clin Exp Rheumatol. 34 (Suppl
100):S14–S22. 2016.PubMed/NCBI
|
|
70
|
Gouda MM and Bhandary YP: Acute lung
injury: IL-17A-mediated inflammatory pathway and its regulation by
curcumin. Inflammation. 42:1160–1169. 2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Roos AB, Mori M, Gura HK, Lorentz A,
Bjermer L, Hoffmann HJ, Erjefält JS and Stampfli MR: Increased
IL-17RA and IL-17RC in end-stage COPD and the contribution to mast
cell secretion of FGF-2 and VEGF. Respir Res. 18(48)2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Miossec P and Kolls JK: Targeting IL-17
and TH17 cells in chronic inflammation. Nat Rev Drug Discov.
11:763–776. 2012.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Rex DAB, Dagamajalu S, Gouda MM, Suchitha
GP, Chanderasekaran J, Raju R, Prasad TSK and Bhandary YP: A
comprehensive network map of IL-17A signaling pathway. J Cell
Commun Signal. 17:209–215. 2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Schmidt T, Luebbe J, Kilian C, Riedel JH,
Hiekmann S, Asada N, Ginsberg P, Robben L, Song N, Kaffke A, et al:
IL-17 receptor C signaling controls CD4+ TH17
immune responses and tissue injury in immune-mediated kidney
diseases. J Am Soc Nephrol. 32:3081–3098. 2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
He F, Yu X, Zhang J, Cui J, Tang L, Zou S,
Pu J and Ran P: Biomass-related PM2.5 induced
inflammatory microenvironment via IL-17F/IL-17RC axis. Environ
Pollut. 342(123048)2024.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Ni Q, Li G, Chen Y, Bao C, Wang T, Li Y,
Ruan X, Wang H and Sun W: LECs regulate neutrophil clearance
through IL-17RC/CMTM4/NF-κB axis at sites of inflammation or
infection. Mucosal Immunol. 17:723–738. 2024.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Park SJ, Hahn HJ, Oh SR and Lee HJ:
Theophylline attenuates BLM-induced pulmonary fibrosis by
inhibiting Th17 differentiation. Int J Mol Sci.
24(1019)2023.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Maxwell JR, Yadav R, Rossi RJ, Ruby CE,
Weinberg AD, Aguila HL and Vella AT: IL-18 bridges innate and
adaptive immunity through IFN-gamma and the CD134 pathway. J
Immunol. 177:234–245. 2006.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Shao XF, Li B, Shen J, Wang QF, Chen SS,
Jiang XC and Qiang D: Ghrelin alleviates traumatic brain
injury-induced acute lung injury through pyroptosis/NF-κB pathway.
Int Immunopharmacol. 79(106175)2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Kobori T, Hamasaki S, Kitaura A, Yamazaki
Y, Nishinaka T, Niwa A, Nakao S, Wake H, Mori S, Yoshino T, et al:
Interleukin-18 amplifies macrophage polarization and morphological
alteration, leading to excessive angiogenesis. Front Immunol.
9(334)2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Cai G, Lu Y, Zhong W, Wang T, Li Y, Ruan
X, Chen H, Sun L, Guan Z, Li G, et al: Piezo1-mediated M2
macrophage mechanotransduction enhances bone formation through
secretion and activation of transforming growth factor-β1. Cell
Prolif. 56(e13440)2023.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Zhang C, Zhu X, Hua Y, Zhao Q, Wang K,
Zhen L, Wang G, Lü J, Luo A, Cho WC, et al: YY1 mediates
TGF-β1-induced EMT and pro-fibrogenesis in alveolar epithelial
cells. Respir Res. 20(249)2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Ibi M, Horie S, Kyakumoto S, Chosa N,
Yoshida M, Kamo M, Ohtsuka M and Ishisaki A: Cell-cell interactions
between monocytes/macrophages and synoviocyte-like cells promote
inflammatory cell infiltration mediated by augmentation of MCP-1
production in temporomandibular joint. Biosci Rep.
38(BSR20171217)2018.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Wu L, Pu L and Zhuang Z: miR-155-5p/FOXO3a
promotes pulmonary fibrosis in rats by mediating NLRP3 inflammasome
activation. Immunopharmacol Immunotoxicol. 45:257–267.
2023.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Schinocca C, Rizzo C, Fasano S, Grasso G,
La Barbera L, Ciccia F and Guggino G: Role of the IL-23/IL-17
pathway in rheumatic diseases: An overview. Front Immunol.
12(637829)2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Zhang Q, Tong L, Wang B, Wang T and Ma H:
Diagnostic value of serum levels of IL-22, IL-23, and il-17 for
idiopathic pulmonary fibrosis associated with lung cancer. Ther
Clin Risk Manag. 18:429–437. 2022.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Bhattacharya G, Sengupta S, Jha R, Shaw
SK, Jogdand GM, Barink PK, Padhan P, Parida JR and Devadas S:
IL-21/23 axis modulates inflammatory cytokines and RANKL expression
in RA CD4+ T cells via p-Akt1 signaling. Front Immunol.
14(1235514)2023.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Loo WJ, Turchin I, Prajapati VH, Gooderham
MJ, Grewal P, Hong CH, Sauder M, Vender RB, Maari C and Papp KA:
Clinical implications of targeting the JAK-STAT pathway in
psoriatic disease: Emphasis on the TYK2 pathway. J Cutan Med Surg.
27 (1 Suppl):3S–24S. 2023.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Vuyyuru SK, Shackelton LM, Hanzel J, Ma C,
Jairath V and Feagan BG: Targeting IL-23 for IBD: Rationale and
progress to date. Drugs. 83:873–891. 2023.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Ogishi M, Arias AA, Yang R, Han JE, Zhang
P, Rinchai D, Halpern J, Mulwa J, Keating N, Chrabieh M, et al:
Impaired IL-23-dependent induction of IFN-γ underlies mycobacterial
disease in patients with inherited TYK2 deficiency. J Exp Med.
219(e20220094)2022.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Senoo S, Taniguchi A, Itano J, Oda N,
Morichika D, Fujii U, Guo L, Sunami R, Kanehiro A, Tokioka F, et
al: Essential role of IL-23 in the development of acute
exacerbation of pulmonary fibrosis. Am J Physiol Lung Cell Mol
Physiol. 321:L925–L940. 2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Sanada S, Hakuno D, Higgins LJ, Schreiter
ER, McKenzie ANJ and Lee RT: IL-33 and ST2 comprise a critical
biomechanically induced and cardioprotective signaling system. J
Clin Invest. 117:1538–1549. 2007.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Piyadasa H, Lloyd D, Lee AHY, Altieri A,
Hemshekhar M, Osawa N, Basu S, Blimkie T, Falsafi R, Halayko AJ, et
al: Characterization of immune responses and the lung transcriptome
in a murine model of IL-33 challenge. Biochim Biophys Acta Mol
Basis Dis. 1866(165950)2020.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Drake LY and Kita H: IL-33: Biological
properties, functions, and roles in airway disease. Immunol Rev.
278:173–184. 2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Chen YL, Gutowska-Owsiak D, Hardman CS,
Westmoreland M, MacKenzie T, Cifuentes L, Waithe D, Lloyd-Lavery A,
Marquette A, Londei M and Ogg C: Proof-of-concept clinical trial of
etokimab shows a key role for IL-33 in atopic dermatitis
pathogenesis. Sci Transl Med. 11(eaax2945)2019.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Kosloski MP, Kalliolias GD, Xu CR, Harel
S, Lai CH, Zheng W, Davis JD and Kamal MA: Pharmacokinetics and
pharmacodynamics of itepekimab in healthy adults and patients with
asthma: Phase I first-in-human and first-in-patient trials. Clin
Transl Sci. 15:384–395. 2022.PubMed/NCBI View Article : Google Scholar
|
|
97
|
She YX, Yu QY and Tang XX: Role of
interleukins in the pathogenesis of pulmonary fibrosis. Cell Death
Discov. 7(52)2021.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Jayalatha AS, Hesse L, Ketelaar ME,
Koppelman GH and Nawijn MC: The central role of IL-33/IL-1RL1
pathway in asthma: From pathogenesis to intervention. Pharmacol
Ther. 225(107847)2021.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Nechama M, Kwon J, Wei S, Kyi AT, Welner
RS, Ben-Dov IZ, Arredouani MS, Asara JM, Chen CH, Tsai CY, et al:
The IL-33-PIN1-IRAK-M axis is critical for type 2 immunity in
IL-33-induced allergic airway inflammation. Nat Commun.
9(1603)2018.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Reid F, Singh D, Albayaty M, Moate R,
Jimenez E, Sadiq MW, Howe D, Gavala M, Killick H, Williams A, et
al: A randomized phase I study of the anti-interleukin-33 antibody
Tozorakimab in healthy adults and patients with chronic obstructive
pulmonary disease. Clin Pharmacol Ther. 115:565–575.
2024.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Catalan-Dibene J, McIntyre LL and Zlotnik
A: Interleukin 30 to interleukin 40. J Interferon Cytokine Res.
38:423–439. 2018.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Borthwick LA: The IL-1 cytokine family and
its role in inflammation and fibrosis in the lung. Semin
Immunopathol. 38:517–534. 2016.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Andoh A and Nishida A: Pro- and
anti-inflammatory roles of interleukin (IL)-33, IL-36, and IL-38 in
inflammatory bowel disease. J Gastroenterol. 58:69–78.
2023.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Aoyagi T, Newstead MW, Zeng XY, Kunkel SL,
Kaku M and Standiford TJ: IL-36 receptor deletion attenuates lung
injury and decreases mortality in murine influenza pneumonia.
Mucosal Immunol. 10:1043–1055. 2017.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Vigne S, Palmer G, Lamacchia C, Martin P,
Talabot-Ayer D, Rodriguez E, Ronchi F, Sallusto F, Dinh H, Sims JE
and Gabay C: IL-36R ligands are potent regulators of dendritic and
T cells. Blood. 118:5813–5823. 2011.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Cao J, Liu JH, Wise SG, Fan J, Bao S and
Zheng GS: The role of IL-36 and 37 in hepatocellular carcinoma.
Front Immunol. 15(1281121)2024.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Elias M, Zhao S, Le HT, Wang J, Neurath
MF, Neufert C, Fiocchi C and Rieder F: IL-36 in chronic
inflammation and fibrosis-bridging the gap? J Clin Invest.
131(144336)2021.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Montero-Blay A, Blanco JD, Rodriguez-Arce
I, Lastrucci C, Piñero-Lambea C, Lluch-Senar M and Serrano L:
Bacterial expression of a designed single-chain IL-10 prevents
severe lung inflammation. Mol Syst Biol. 19(e11037)2023.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Huaux F: Interpreting immunoregulation in
lung fibrosis: A new branch of the immune model. Front Immunol.
12(690375)2021.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Gabryšová L, Howes A, Saraiva M and
O'Garra A: The regulation of IL-10 expression. Curr Top Microbiol
Immunol. 380:157–190. 2014.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Saraiva M, Vieira P and O'garra A: Biology
and therapeutic potential of interleukin-10. J Exp Med.
217(e20190418)2020.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Chlastáková A, Kaščáková B, Kotál J,
Langhansová H, Kotsyfakis M, Kutá Smatanová I, Tirloni L and
Chmelař J: Iripin-1, a new anti-inflammatory tick serpin, inhibits
leukocyte recruitment in vivo while altering the levels of
chemokines and adhesion molecules. Front Immunol.
14(1116324)2023.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Neumann C, Scheffold A and Rutz S:
Functions and regulation of T cell-derived interleukin-10. Semin
Immunol. 44(101344)2019.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Zhang N, Li P, Lin H, Shuo T, Ping F, Su L
and Chen G: IL-10 ameliorates PM2.5-induced lung injury by
activating the AMPK/SIRT1/PGC-1α pathway. Environ Toxicol
Pharmacol. 86(103659)2021.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Jia Q, Wen J, Yang Q, Liu S, Zhang J, Wang
T and Cheng Y: Lonicera japonica Thunb extract ameliorates
lipopolysaccharide-induced acute lung injury associated with
luteolin-mediated suppression of NF-κB signaling pathway. J Inflamm
(Lond). 20(44)2023.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Boonyatecha N, Sangphech N, Wongchana W,
Kueanjinda P and Palaga T: Involvement of Notch signaling pathway
in regulating IL-12 expression via c-Rel in activated macrophages.
Mol Immunol. 51:255–262. 2012.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Utsunomiya T, Mimura-Kimura Y, Yamamoto T,
Aoe K, Oishi K, Kamei H, Matsunaga K, Yano M and Mimura Y: Cytokine
adsorption to polymyxin B-immobilized fiber: An in vitro study.
Blood Purif. 50:230–237. 2021.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Zhou L, Tian H, Wang Q, Xiong W, Zhou X
and Yan J: Effect of Qingfei Huaxian Decoction combined with
prednisone acetate on serum inflammatory factors and pulmonary
function of patients with idiopathic pulmonary fibrosis. Am J
Transl Res. 14:5905–5914. 2022.PubMed/NCBI
|
|
119
|
Kotani T, Masutani R, Suzuka T, Oda K,
Makino S and Ii M: Anti-inflammatory and anti-fibrotic effects of
intravenous adipose-derived stem cell transplantation in a mouse
model of bleomycin-induced interstitial pneumonia. Sci Rep.
7(14608)2017.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Bao L, Hao CF, Liu SN, Zhang L, Wang J,
Wang D, Li YP and Yao W: Dendritic cells trigger imbalance of
Th1/Th2 cells in silica dust exposure rat model via MHC-II, CD80,
CD86 and IL-12. RSC Adv. 8:26108–26115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Keane MP, Belperio JA, Burdick MD and
Strieter RM: IL-12 attenuates bleomycin-induced pulmonary fibrosis.
Am J Physiol Lung Cell Mol Physiol. 281:L92–L97. 2001.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Nie Y, Yang B, Hu J, Zhang L and Ma Z:
Bruceine D ameliorates the balance of Th1/Th2 in a mouse model of
ovalbumin-induced allergic asthma via inhibiting the NOTCH pathway.
Allergol Immunopathol (Madr). 49:73–79. 2021.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Kikuchi N, Ishii Y, Morishima Y, Yageta Y,
Haraguchi N, Itoh K, Yamamoto M and Hizawa N: Nrf2 protects against
pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2
balance. Respir Res. 11(31)2010.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Huaux F, Lardot C, Arras M, Delos M, Many
MC, Coutelier JP, Buchet JP, Renauld JC and Lison D: Lung fibrosis
induced by silica particles in NMRI mice is associated with an
upregulation of the p40 subunit of interleukin-12 and Th-2
manifestations. Am J Respir Cell Mol Biol. 20:561–672.
1999.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Wang XY, Liu DY, Zhang XH, Yang LM, Xia ZY
and Zhang QF: Exosomes from adipose-derived mesenchymal stem cells
alleviate sepsis-induced lung injury in mice by inhibiting the
secretion of IL-27 in macrophages. Cell Death Discov.
8(18)2022.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Chen Y, Zhu M, Hu J, He S, Li S, Liu B and
Yang J: IL-27 alleviates airway inflammation and airway
hyperresponsiveness in asthmatic mice by targeting the CD39/ATP
axis of dendritic cells. Inflammation. 47:807–821. 2024.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Dong Z, Zhao X, Tai W, Lei W, Wang Y, Li Z
and Zhang T: IL-27 attenuates the TGF-β1-induced proliferation,
differentiation and collagen synthesis in lung fibroblasts. Life
Sci. 146:24–33. 2016.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Dong Z, Tai W, Lei W, Wang Y, Li Z and
Zhang T: IL-27 inhibits the TGF-β1-induced epithelial-mesenchymal
transition in alveolar epithelial cells. BMC Cell Biol.
17(7)2016.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Ting L, Feng Y, Zhou Y, Tong Z and Dong Z:
IL-27 induces autophagy through regulation of the DNMT1/lncRNA
MEG3/ERK/p38 axis to reduce pulmonary fibrosis. Respir Res.
24(67)2023.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Riehl DR, Sharma A, Roewe J, Murke F,
Ruppert C, Eming SA, Bopp T, Kleinert H, Radsak MP, Colucci G, et
al: Externalized histones fuel pulmonary fibrosis via a
platelet-macrophage circuit of TGFβ1 and IL-27. Proc Natl Acad Sci
USA. 120(e2215421120)2023.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Dinarello CA and Bufler P: Interleukin-37.
Semin Immunol. 25:466–468. 2013.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Kim SK, Choe JY, Kim JW, Park KY and Kim
B: Anti-inflammatory effect of atorvastatin and rosuvastatin on
monosodium urate-induced inflammation through IL-37/Smad3-complex
activation in an in vitro study using THP-1 macrophages.
Pharmaceuticals (Basel). 17(883)2024.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Luo C, Shu Y, Luo J, Liu D, Huang DS, Han
Y, Chen C, Li YC, Zou JM, Qin J, et al: Intracellular IL-37b
interacts with Smad3 to suppress multiple signaling pathways and
the metastatic phenotype of tumor cells. Oncogene. 36:2889–2899.
2017.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Conti P, Caraffa A, Gallenga CE, Ross R,
Kritas SK, Frydas I, Younes A, Di Emidio P, Ronconi G and Pandolfi
F: Powerful anti-inflammatory action of luteolin: Potential
increase with IL-38. Biofactors. 47:165–169. 2021.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Diaz-Barreiro A, Huard A and Palmer G:
Multifaceted roles of IL-38 in inflammation and cancer. Cytokine.
151(155808)2022.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Chen W, Xi S, Ke Y and Lei Y: The emerging
role of IL-38 in diseases: A comprehensive review. Immun Inflamm
Dis. 11(e991)2023.PubMed/NCBI View Article : Google Scholar
|
|
137
|
van de Veerdonk FL, de Graaf DM, Joosten
LA and Dinarello CA: Biology of IL-38 and its role in disease.
Immunol Rev. 281:191–196. 2018.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Xu Z, Yuan X, Gao Q, Li Y and Li M:
Interleukin-38 overexpression prevents bleomycin-induced mouse
pulmonary fibrosis. Naunyn Schmiedebergs Arch Pharmacol.
394:391–399. 2021.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Kelly-Welch A, Hanson EM and Keegan AD:
Interleukin-4 (IL-4) pathway. Sci STKE. 2005(cm9)2005.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Rao LZ, Wang Y, Zhang L, Wu G, Zhang L,
Wang FX, Chen LM, Sun F, Jia S, Zhang S, et al: IL-24 deficiency
protects mice against bleomycin-induced pulmonary fibrosis by
repressing IL-4-induced M2 program in macrophages. Cell Death
Differ. 28:1270–1283. 2021.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Sterclova M, Kishore A, Sikorova K,
Skibova J, Petrek M and Vasakova M: Effect of genotype on the
disease course in idiopathic pulmonary fibrosis despite
antifibrotic treatment. Biomed Rep. 15(87)2021.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Singh B, Kasam RK, Sontake V, Wynn TA and
Madala SK: Repetitive intradermal bleomycin injections evoke
T-helper cell 2 cytokine-driven pulmonary fibrosis. Am J Physiol
Lung Cell Mol Physiol. 313:L796–L806. 2017.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Mattoo H, Bangari DS, Cummings S, Humulock
Z, Habiel D, Xu EY, Pate N, Resnick R, Savova V, Qian G, et al:
Molecular features and stages of pulmonary fibrosis driven by type
2 inflammation. Am J Respir Cell Mol Biol. 69:404–421.
2023.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Khansalar S, Faghih Z, Barani S, Kalani M,
Ataollahi MR, Mohammadi Z, Namdari S and Kalantar K: IFN-γ, IL-17,
IL-22+ CD4+ subset in patients with hepatitis
C virus and correlation with clinical factor. Am J Clin Exp
Immunol. 13:43–52. 2024.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Liang M, Wang J, Chu H, Zhu X, He H, Liu
Q, Qiu J, Zhou X, Guan M, Xue Y, et al: Interleukin-22 inhibits
bleomycin-induced pulmonary fibrosis. Mediators Inflamm.
2013(209179)2013.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Bao A, Ma E, Cornman H, Kambala A,
Manjunath J, Kollhoff AL, Imo BU, Kwatra MM and Kwatra SG:
Dupilumab therapy modulates circulating inflammatory mediators in
patients with prurigo nodularis. JID Innov.
4(100281)2024.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Liu J, Huang Y, Liu N, Qiu H, Zhang X, Liu
X, He M, Chen M and Huang S: The imbalance of pulmonary Th17/Treg
cells in BALB/c suckling mice infected with respiratory syncytial
virus-mediated intestinal immune damage and gut microbiota changes.
Microbiol Spectr. 12(e0328323)2024.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Li C, Liu M, Deng L, Luo D, Ma R and Lu Q:
Oxyberberine ameliorates TNBS-induced colitis in rats through
suppressing inflammation and oxidative stress via Keap1/Nrf2/NF-κB
signaling pathways. Phytomedicine. 116(154899)2023.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Zhang J, Wang W, Liang S, Zhou X, Rekha
RS, Gudmundsson GH, Bergman P, Ai Q, Mai K and Wan M: Butyrate
induces STAT3/HIF-1α/IL-22 signaling via GPCR and HDAC3 inhibition
to activate autophagy in head kidney macrophages from turbot
(Scophthalmus maximus L.). Fish Shellfish Immunol.
143(109214)2023.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Sajiir H, Keshvari S, Wong KY, Borg DJ,
Steyn FJ, Fercher C, Taylor K, Taylor B, Barnard RT, Müller A, et
al: Liver and pancreatic-targeted interleukin-22 as a therapeutic
for metabolic dysfunction-associated steatohepatitis. Nat Commun.
15(4528)2024.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Kamata K, Hara A, Minaga K, Yoshikawa T,
Kurimoto M, Sekai I, Okai N, Omaru N, Masuta Y, Otsuka Y, et al:
Activation of the aryl hydrocarbon receptor inhibits the
development of experimental autoimmune pancreatitis through
IL-22-mediated signaling pathways. Clin Exp Immunol. 212:171–183.
2023.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Zhang Z, Chakawa MB, Galeas-Pena M,
Frydman JA, Allen MJ, Jones M and Pociask D: IL-22 binding protein
controls IL-22-driven bleomycin-induced lung injury. Am J Pathol.
194:338–352. 2024.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Goulart A, Boko MMM, Martins NS, Gembre
AF, de Oliveira RS, Palma-Albornoz SP, Bertolini T, Ribolla PEM,
Ramalho LNZ, Fraga-Silva TFC and Bonato VLD: IL-22 is deleterious
along with IL-17 in allergic asthma but is not detrimental in the
comorbidity asthma and acute pneumonia. Int J Mol Sci.
24(10418)2023.PubMed/NCBI View Article : Google Scholar
|
|
154
|
He G, Lang Y, Zhao S, Wang X and Ouyang Y:
Advances in the role of interleukin-22 in airway remodeling in
asthma. J Pract Med. 38:2491–2494. 2022.
|
|
155
|
Nikoopour E, Bellemore SM and Singh B:
IL-22, cell regeneration and autoimmunity. Cytokine. 74:35–42.
2015.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Zindl CL, Wilson CG, Chadha AS, Duck LW,
Cai B, Harbour SN, Nagaoka-Kamata Y, Hatton RD, Gao M, Figge DA and
Weaver CT: Distal colonocytes targeted by C. rodentium recruit
T-cell help for barrier defence. Nature. 629:669–678.
2024.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Whittington HA, Armstrong L, Uppington KM
and Millar AB: Interleukin-22: A potential immunomodulatory
molecule in the lung. Am J Respir Cell Mol Biol. 31:220–226.
2004.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Zhang C, Tang S, Zong X, Duan L and Wang
YF: Anti- IL-22 neutralizing antibodies decrease inflammation
lesions and reduce mortality in enterovirus 71-infected mice. Cell
Mol Biol (Noisy-le-grand). 69:254–258. 2023.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Qu Z, Dou W, Zhang K, Duan L, Zhou D and
Yin S: IL-22 inhibits bleomycin-induced pulmonary fibrosis in
association with inhibition of IL-17A in mice. Arthritis Res Ther.
24(280)2022.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Gu P, Wang D, Zhang J, Wang X, Chen Z, Gu
L, Liu M, Meng F, Yang J, Cai H, et al: Protective function of
interleukin-22 in pulmonary fibrosis. Clin Transl Med.
11(e509)2021.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Fang S, Ju DW, Lin Y and Chen W: The role
of interleukin-22 in lung health and its therapeutic potential for
COVID-19. Front Immunol. 13(951107)2022.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Fang Q, Xie J, Zong J, Zhou Y, Zhou Q, Yin
S, Cao L, Yin H and Zhou D: Expression and diagnostic value of
interleukin-22 in rheumatoid arthritis-associated interstitial lung
disease. Int Immunopharmacol. 134(112173)2024.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Wu Y, Min J, Ge C, Shu JP, Tian D, Yuan Y
and Zhou D: Interleukin 22 in liver injury, inflammation and
cancer. Int J Biol Sci. 16(2405)2020.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Beppu AK, Zhao J, Yao C, Carraro G,
Israely E, Coelho AL, Drake K, Hogaboam CM, Parks WC, Kolls JK and
Stripp BR: Epithelial plasticity and innate immune activation
promote lung tissue remodeling following respiratory viral
infection. Nat Commun. 14(5814)2023.PubMed/NCBI View Article : Google Scholar
|