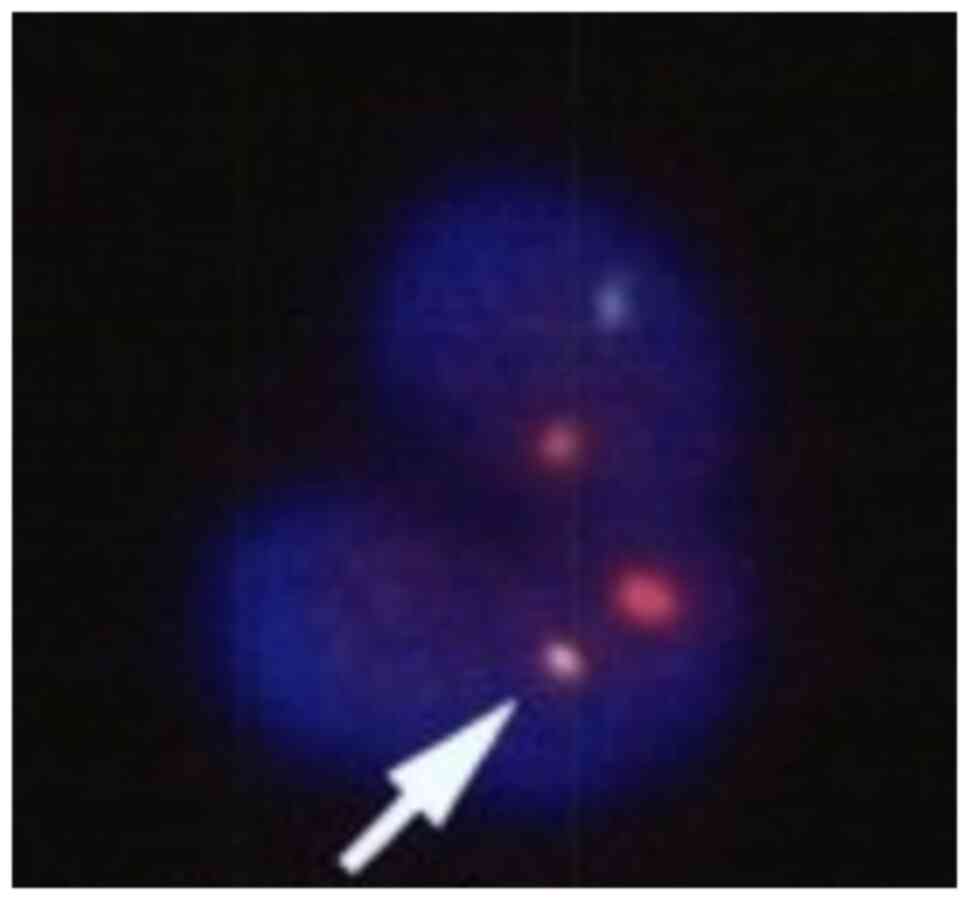
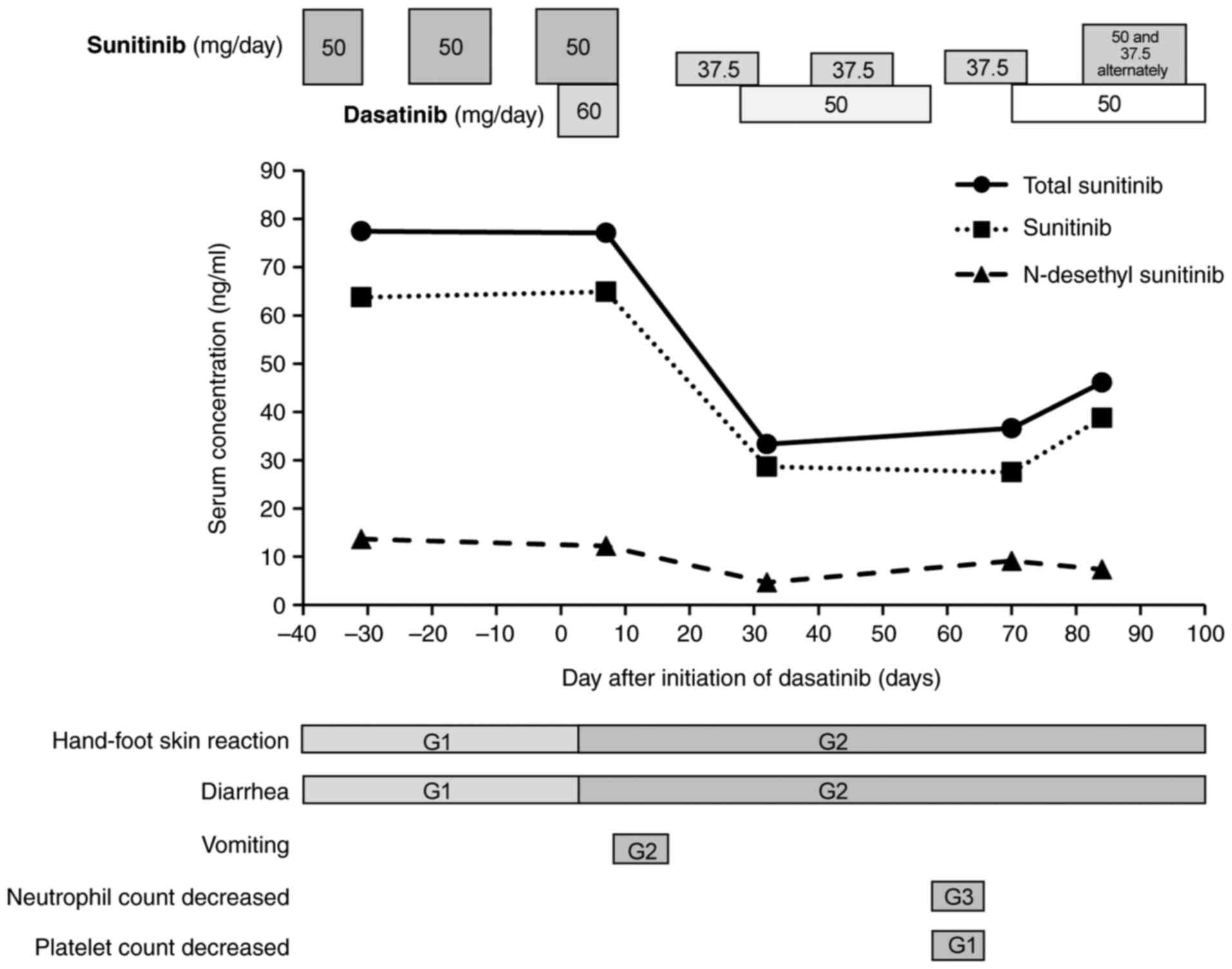
|
1
|
Verheijen RB, Yu H, Schellens JHM, Beijnen
JH, Steeghs N and Huitema ADR: Practical recommendations for
therapeutic drug monitoring of kinase inhibitors in oncology. Clin
Pharmacol Ther. 102:765–776. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Mueller-Schoell A, Groenland SL,
Scherf-Clavel O, van Dyk M, Huisinga W, Michelet R, Jaehde U,
Steeghs N, Huitema ADR and Kloft C: Therapeutic drug monitoring of
oral targeted antineoplastic drugs. Eur J Clin Pharmacol.
77:441–464. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Noda S, Otsuji T, Baba M, Yoshida T,
Kageyama S, Okamoto K, Okada Y, Kawauchi A, Onishi H, Hira D, et
al: Assessment of sunitinib-induced toxicities and clinical
outcomes based on therapeutic drug monitoring of sunitinib for
patients with renal cell carcinoma. Clin Genitourin Cancer.
13:350–358. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Numakura K, Fujiyama N, Takahashi M,
Igarashi R, Tsuruta H, Maeno A, Huang M, Saito M, Narita S, Inoue
T, et al: Clinical implications of pharmacokinetics of sunitinib
malate and N-desethyl-sunitinib plasma concentrations for treatment
outcome in metastatic renal cell carcinoma patients. Oncotarget.
9:25277–25284. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Takasaki S, Kawasaki Y, Kikuchi M, Tanaka
M, Suzuka M, Noda A, Sato Y, Yamashita S, Mitsuzuka K, Saito H, et
al: Relationships between sunitinib plasma concentration and
clinical outcomes in Japanese patients with metastatic renal cell
carcinoma. Int J Clin Oncol. 23:936–943. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Houk BE, Bello CL, Poland B, Rosen LS,
Demetri GD and Motzer RJ: Relationship between exposure to
sunitinib and efficacy and tolerability endpoints in patients with
cancer: Results of a pharmacokinetic/pharmacodynamic meta-analysis.
Cancer Chemother Pharmacol. 66:357–371. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mendel DB, Laird AD, Xin X, Louie SG,
Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, et
al: In vivo antitumor activity of SU11248, a novel tyrosine kinase
inhibitor targeting vascular endothelial growth factor and
platelet-derived growth factor receptors: Determination of a
pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res.
9:327–337. 2003.PubMed/NCBI
|
|
8
|
Faivre S, Delbaldo C, Vera K, Robert C,
Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, et
al: Safety, pharmacokinetic, and antitumor activity of SU11248, a
novel oral multitarget tyrosine kinase inhibitor, in patients with
cancer. J Clin Oncol. 24:25–35. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang J, Cui X, Cheng C, Wang Y, Sun W,
Huang CK, Chen RJ and Wang Z: Effects of CYP3A inhibitors
ketoconazole, voriconazole, and itraconazole on the
pharmacokinetics of sunitinib and its main metabolite in rats. Chem
Biol Interact. 338(109426)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Filppula AM, Neuvonen PJ and Backman JT:
In vitro assessment of time-dependent inhibitory effects on CYP2C8
and CYP3A activity by fourteen protein kinase inhibitors. Drug
Metab Dispos. 42:1202–1209. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li X, He Y, Ruiz CH, Koenig M, Cameron MD
and Vojkovsky T: Characterization of dasatinib and its structural
analogs as CYP3A4 mechanism-based inactivators and the proposed
bioactivation pathways. Drug Metab Dispos. 37:1242–1250.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Eley T, Luo FR, Agrawal S, Sanil A,
Manning J, Li T, Blackwood-Chirchir A and Bertz R: Phase I study of
the effect of gastric acid pH modulators on the bioavailability of
oral dasatinib in healthy subjects. J Clin Pharmacol. 49:700–709.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yago MR, Frymoyer A, Benet LZ, Smelick GS,
Frassetto LA, Ding X, Dean B, Salphati L, Budha N, Jin JY, et al:
The use of betaine HCl to enhance dasatinib absorption in healthy
volunteers with rabeprazole-induced hypochlorhydria. AAPS J.
16:1358–1365. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Takahashi N, Miura M, Niioka T and Sawada
K: Influence of H2-receptor antagonists and proton pump inhibitors
on dasatinib pharmacokinetics in Japanese leukemia patients. Cancer
Chemother Pharmacol. 69:999–1004. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hofmann J, Bartůněk A, Hauser T, Sedmak G,
Beránek J, Ryšánek P, Šíma M and Slanař O: Dasatinib anhydrate
containing oral formulation improves variability and
bioavailability in humans. Leukemia. 37:2486–2492. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lacouture ME, Reilly LM, Gerami P and
Guitart J: Hand foot skin reaction in cancer patients treated with
the multikinase inhibitors sorafenib and sunitinib. Ann Oncol.
19:1955–1961. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chu D, Lacouture ME, Fillos T and Wu S:
Risk of hand-foot skin reaction with sorafenib: A systematic review
and meta-analysis. Acta Oncol. 47:176–186. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Azad NS, Aragon-Ching JB, Dahut WL,
Gutierrez M, Figg WD, Jain L, Steinberg SM, Turner ML, Kohn EC and
Kong HH: Hand-foot skin reaction increases with cumulative
sorafenib dose and with combination anti-vascular endothelial
growth factor therapy. Clin Cancer Res. 15:1411–1416.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chai Y, Huang Y, Tang H, Tu X, He J, Wang
T, Zhang Q, Xiong F, Li D and Qiu Z: Role of stem cell growth
factor/c-Kit in the pathogenesis of irritable bowel syndrome. Exp
Ther Med. 13:1187–1193. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Al-Najjar F and Jarkowski A III: Treatment
of concurrent metastatic renal cell carcinoma and chronic
myelogenous leukemia-easier said than done? A case report. J Oncol
Pharm Pract. 17:436–439. 2011.PubMed/NCBI View Article : Google Scholar
|