Introduction
Pruritus, commonly referred to as itching, is a
complex sensory phenomenon characterized by an irresistible urge to
scratch in response to stimuli affecting the skin or mucous
membranes (1). As a prevalent and
often debilitating symptom, pruritus is associated with a wide
range of dermatological, systemic, and neurological disorders,
significantly impairing the quality of life for affected
individuals (2). Itching can
present as either localized or generalized and varies in intensity
from mild to severe, depending on the underlying etiology (3). The pathogenesis of pruritus is
multifactorial, involving a combination of intrinsic and extrinsic
factors. Intrinsic factors include chronic infections, circulatory
disorders, endocrine and metabolic dysfunctions, and genetic
predispositions to allergic reactions, while extrinsic triggers
encompass environmental agents such as food allergens, airborne
particles, chemical irritants, and animal-derived allergens
(4,5).
The mechanisms underlying pruritus are broadly
categorized into histamine-dependent and histamine-independent
pathways. Histamine, a well-known pruritogen, is primarily released
by mast cells and is predominantly associated with acute itching
(6). Mast cell activation,
mediated by IgE, lipopolysaccharides (LPS), cytokines, and
chemokines, leads to degranulation and the subsequent release of
itch-inducing mediators, including histamine, interleukin-4 (IL-4),
and interleukin-31 (IL-31) (7).
Compound 48/80, a synthetic mast cell activator, has been widely
used in experimental studies to investigate histamine-mediated itch
pathways due to its ability to induce mast cell degranulation and
histamine release via the histamine-1 receptor (H1R) (8,9). In
contrast, chronic itching is often histamine-independent and is
associated with severe or recurrent pathological conditions
(10). Non-histaminergic
pruriceptive neurons can be activated by various endogenous and
exogenous pruritogens, such as proteases, cytokines, chemokines,
and amines, which interact with specific receptors on sensory
neurons (11). Recent advances in
understanding the neural circuitry of itch have highlighted the
role of gastrin-releasing peptide (GRP) and its receptor (GRPR) in
the spinal dorsal horn. GRP, released from primary itch afferents,
activates GRPR-expressing neurons in lamina I of the dorsal horn,
playing a critical role in itch signal transmission (12-14).
Chloroquine, an antimalarial drug, is a notable example of a
histamine-independent pruritogen that induces severe itching
through the GRP/GRPR signaling pathway, independent of histamine
(15,16). This property makes chloroquine a
valuable tool for studying histamine-independent itch
mechanisms.
Diospyros lotus, a perennial woody plant of
the Ebenaceae family, is native to various regions in Asia,
including Korea (17). Extracts
from D. lotus leaves (DLE) are rich in polyphenolic
compounds, including gallic acid, caffeic acid, chlorogenic acid,
myricetin-3-O-galactoside, myricitrin (MC), astragalin, quercetin,
and myricetin, with myricitrin being the most abundant, at
approximately 86 µg/mg (18,19).
DLE has demonstrated a range of pharmacological properties,
including anti-obesity, anti-photoaging, and hepatoprotective
effects, attributed to its high polyphenol content (19,20).
Additionally, DLE has shown anti-pruritic effects in various animal
models, including atopic dermatitis induced by
2,4-dinitrofluorobenzene (DNFB) and house dust mite antigens, as
well as acute itch models induced by compound 48/80 and chloroquine
(21,22). While existing research has
primarily focused on the cutaneous effects of DLE, its potential
impact on the central nervous system remains underexplored.
Given this context, the present study aims to
investigate the effects of DLE and its major constituent,
myricitrin, on both histamine-dependent and histamine-independent
itch pathways in ICR mice. Specifically, we will evaluate their
efficacy in alleviating pruritus induced by compound 48/80
(histamine-dependent) and chloroquine (histamine-independent),
thereby providing insights into the potential mechanisms of action
of DLE and MC in alleviating pruritus.
Materials and methods
Plant material collection and
preparation
Fresh leaves of D. lotus were collected in
June 2022 from Cheonjam Mountain, Jeonju-si, Jeollabuk-do, Republic
of Korea. The plant species was identified and authenticated by
Professor Hong-Jun Kim from the College of Oriental Medicine at
Woosuk University, Jeonbuk, Republic of Korea. A voucher specimen
(#2022-06-04) was deposited in the Department of Health Management,
College of Medical Science, Jeonju University, for future
reference. The collected leaves were thoroughly washed with
distilled water to remove impurities and dried in a
well-ventilated, shaded area to prevent degradation of bioactive
compounds. Dried leaves (100 g) were extracted with 2 l of 70%
(v/v) ethanol at room temperature for 48 h. The extract was
filtered twice through a 0.5-µm membrane filter (ADVANTEC),
concentrated under vacuum (EYELA Rotary evaporator N-1100, EYELA),
and freeze-dried to obtain a powdered form of D. lotus leaf
extract (DLE).
Reagents and chemicals
Toluidine blue (198161), chloroquine diphosphate
(C-271), and compound 48/80 (C2313) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Myricitrin (M2361) was obtained
from Tokyo Chemical Industry (Tokyo, Japan). A histamine ELISA kit
(ENZ-KIT140A-0001) was procured from Enzo Life Sciences
(Farmingdale, NY, USA). Primary antibodies against GRPR
(sc-398549), IL-31RA (sc-515465), p-STAT3 (sc-81523), STAT3
(sc-8019), m-IgGκ BP-HRP (sc-516102) and β-actin (sc-8432) were
acquired from Santa Cruz Biotechnology (Dallas, TX, USA). An IL-31
ELISA kit (ab213872) was sourced from Abcam (Cambridge, UK). RIPA
buffer (89900), goat anti-mouse IgG Alexa Fluor 488 secondary
antibody (A-11001) and goat anti-rabbit IgG HRP secondary antibody
(31460) were purchased from Thermo Fisher Scientific (Waltham, MA,
USA). WestGlow FEMTO (BWF0100) from Biomax (Guri, Korea).
Animals and experimental design
Male ICR mice (4 weeks old, specific-pathogen-free)
were purchased from Orient Bio Inc. (Gwangju, South Korea). The
mice were housed under controlled environmental conditions
(temperature: 22±2˚C, humidity: 50-60%, 12-h light/dark cycle) with
ad libitum access to a standard laboratory diet and water.
The present study was approved by Jeonju University Institutional
Animal Care and Use Committee. The health status of the animals was
monitored daily, and humane experimental endpoints were established
as a 20% or greater loss of body weight, decreased appetite for
more than two consecutive days, dyspnea, increased heart rate,
self-mutilation, jaundice, persistent diarrhea or vomiting, or a
decreased response to external stimuli. No mortality or euthanasia
occurred during the study. After a 1-week acclimatization period,
the dorsal hair of the mice was shaved using an electric clipper.
The mice were randomly divided into seven groups (n=5 per group):
i) Normal control (saline), ii) compound 48/80 alone, iii) compound
48/80 + 200 mg/kg DLE, iv) compound 48/80 + 20 mg/kg MC, v)
chloroquine alone, vi) chloroquine + 200 mg/kg DLE, and vii)
chloroquine + 20 mg/kg MC. The dose of DLE (200 mg/kg) was selected
based on previous studies (18-22)
that evaluated its physiological activities, including anti-itch,
anti-inflammatory, and other bioactive properties. These studies
identified 200 mg/kg as an effective concentration. A previous
study reported that DLE contains approximately 86 µg of MC per mg
of extract (18). Based on this,
administering 200 mg/kg of DLE corresponds to an estimated MC
intake of approximately 17 mg/kg. To account for variability in MC
content and measurement, the MC dose was set at 20 mg/kg. At the
end of the experiment, mice were anesthetized with a 2-6%
isoflurane for induction and maintained at a 1-3% concentration.
Blood samples (600-800 µl per animal, collected once) were
collected from the orbital venous plexus. Subsequently, mice were
euthanized through cervical dislocation and, after confirming the
absence of respiratory and heartbeat, the dorsal skin and vertebral
tissues were harvested and either 4% paraformaldehyde preserve in
formalin or deep frozen at -80˚C for further analysis.
Scratching behavior analysis
One day prior to the experiment, the dorsal hair of
the mice was shaved using a hair clipper. A total of 1 h before the
administration of compound 48/80 or chloroquine, mice in groups 1
and 2 received oral saline, while mice in groups 3 to 7 were orally
administered either DLE or MC. Scratching behavior was
video-recorded for 30 min immediately after the injection of
compound 48/80 or chloroquine. The recordings were analyzed in a
double-blind manner by independent researchers to minimize bias.
After the behavioral assessment, blood and dorsal skin samples were
collected for histopathological analysis, and vertebral tissues
were isolated for immunofluorescence staining.
Histopathological examination
Dorsal skin tissues were fixed in 4%
paraformaldehyde for 24 h at 4˚C, followed by washing in
phosphate-buffered saline (PBS) with five changes (30 min each).
Tissues were dehydrated in a graded ethanol series (60% to 100%)
for 30 min per concentration, cleared in xylene (two changes, 2 h
each), and embedded in paraffin (three changes, 1 h each).
Paraffin-embedded tissues were sectioned at 5 µm thickness using a
microtome (Leica, Wetzlar, Germany). Tissue sections were stained
with toluidine blue to evaluate mast cell infiltration and
degranulation.
Immunofluorescence staining
A segment of the 4-5th lumbar spine was fixed in 4%
paraformaldehyde for 4 h at room temperature and then incubated in
PBS containing 30% sucrose at 4˚C overnight. Tissues were sectioned
at 30 µm thickness using a Cryotome (Amos Scientific, Clayton
South, Australia). Sections were washed three times in PBS (10 min
each) and incubated for 1 h in PBS containing 0.3% Triton X-100 and
2% Bovine Serum Albumin (BSA). Tissues were then incubated
overnight at 4˚C with primary antibodies against GRPR (1:100),
IL-31RA (1:100), and p-STAT3 (1:50). After five washes in PBS,
sections were incubated with FITC-conjugated goat anti-rat IgG
secondary antibodies for 2 h at room temperature. Following
additional washes, tissues were mounted with DAPI-containing
mounting medium and visualized under a ZEISS fluorescence
microscope (Oberkochen, Germany).
Protein extraction and western
blotting
Segments of the 4th-5th lumbar spinal cord or dorsal
skin were homogenized in RIPA buffer containing protease and
phosphatase inhibitors. The homogenate was centrifuged at 12,000 x
g for 15 min at 4˚C, and the supernatant was collected to remove
debris. Protein concentration was determined using the Bradford
assay, and 50 µg of protein was loaded per lane. Samples were
separated by SDS-PAGE on a 12% or 7.5% gel and transferred onto a
polyvinylidene fluoride (PVDF) membrane. Membranes were blocked
with 5% skim milk at room temperature for 1 h and then washed five
times with TBST (5 min per wash). Membranes were incubated
overnight at 4˚C with the following primary antibodies: GRPR
(1:500), IL-31RA (1:500), STAT3 (1:1,000), p-STAT3 (1:500), IL-31
(1:2,000), and β-actin (1:500). After five washes with TBST,
membranes were incubated with HRP-conjugated secondary antibodies
in 5% skim milk for 2 h at room temperature. Following five
additional washes with TBST, protein bands were visualized using
the ALLIANCE LD4 imaging system (Uvitec, Cambridge, UK) with
WestGlow FEMTO chemiluminescent reagent. Band densities were
analyzed using ImageJ 1.53e (National Institutes of Health), with
β-actin used as the loading control.
Statistical analysis
All statistical analysis was performed using SPSS
version 26.0 (IBM, Armonk, NY, USA). Data are presented as the mean
± standard deviation (n=5). Statistical analysis was performed
using one-way ANOVA followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Antipruritic effects of DLE and MC on
compound 48/80 or chloroquine-induced pruritus
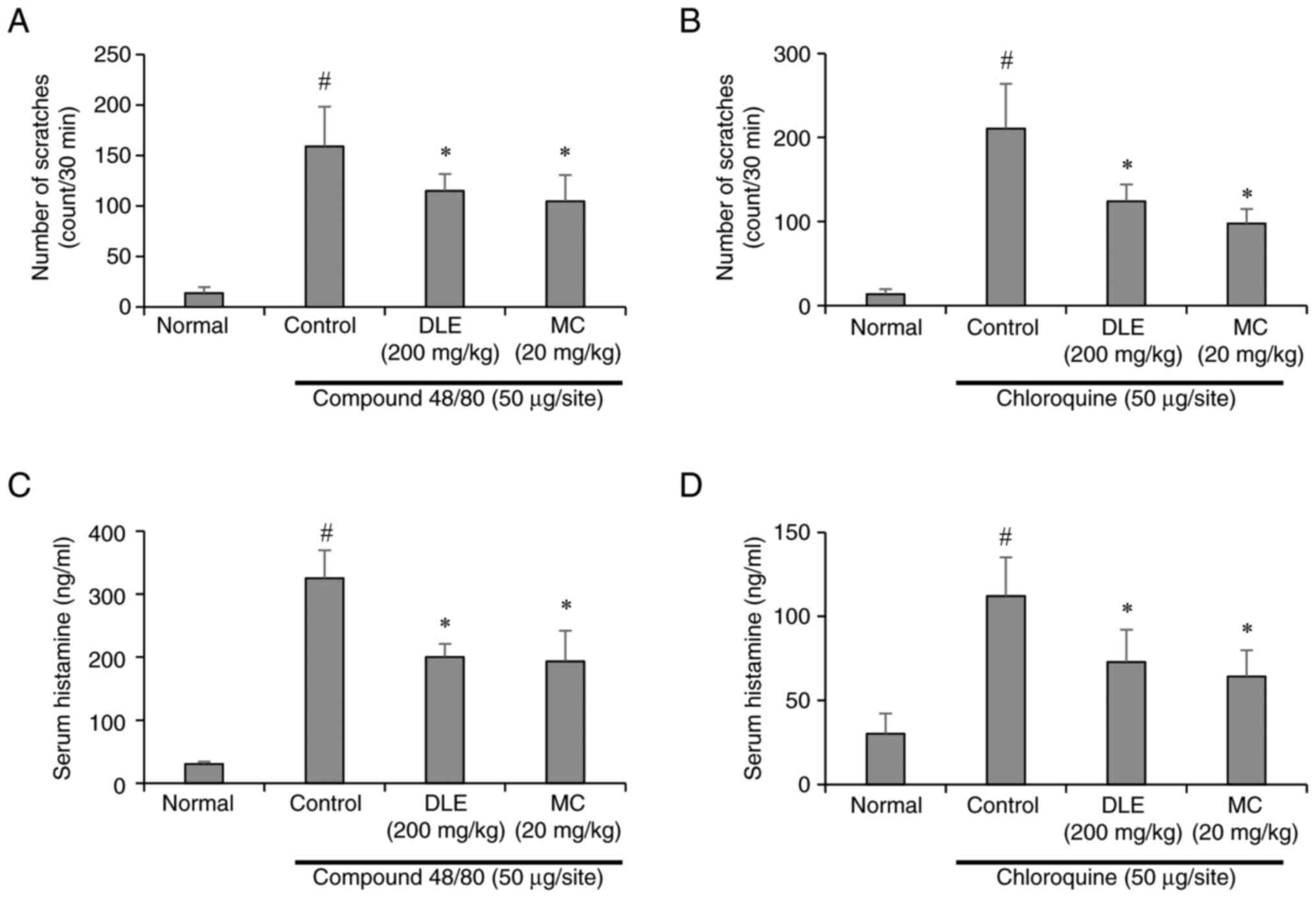
To evaluate the antipruritic effects of DLE and its
major constituent, MC, scratching behavior was assessed in ICR mice
following subcutaneous injection of compound 48/80 or chloroquine.
Both pruritogens significantly increased scratching behavior
compared to the normal control group, indicating successful
induction of itch. However, pretreatment with DLE (200 mg/kg) or MC
(20 mg/kg) significantly attenuated scratching behavior in both
compound 48/80- and chloroquine-induced models (Fig. 1A and B). These findings suggest that DLE and MC
exhibit significant antipruritic properties against both
histamine-dependent and histamine-independent itch pathways. To
further elucidate the mechanisms underlying these effects, serum
histamine levels were measured using ELISA. Compound 48/80, a known
mast cell degranulator, induced a marked increase in serum
histamine levels, consistent with its histamine-dependent pruritic
action. In contrast, chloroquine, which operates through
histamine-independent pathways, caused a comparatively smaller
increase in histamine levels. Notably, administration of DLE and MC
significantly reduced serum histamine levels in both models
(Fig. 1C and D), indicating their ability to modulate
mast cell activity and histamine release.
Effects of DLE and MC on mast cell
infiltration in compound 48/80- or chloroquine-induced
pruritus
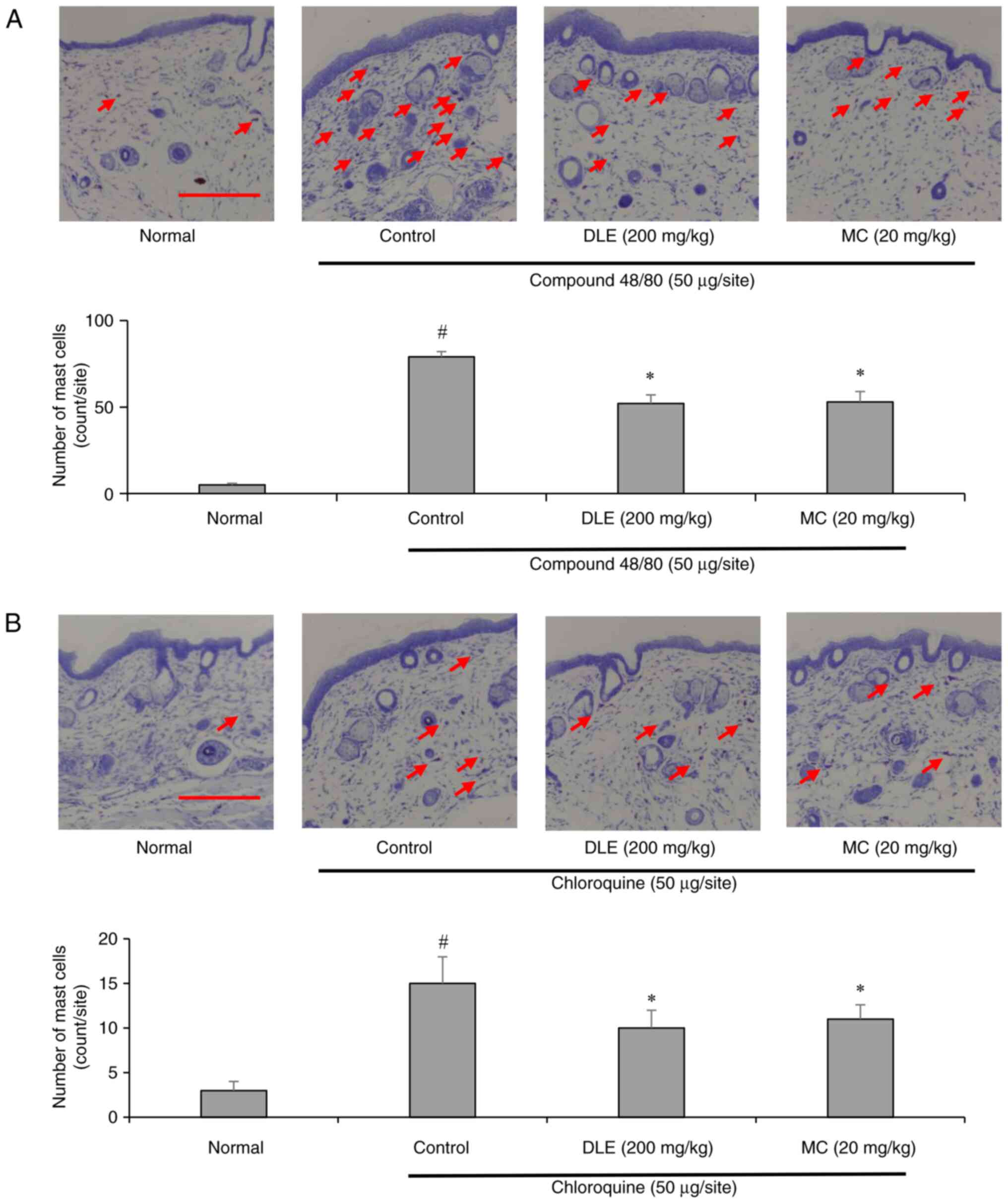
Mast cells play a pivotal role in itch pathogenesis,
particularly in histamine-dependent pruritus (23). To assess the impact of DLE and MC
on mast cell infiltration, toluidine blue staining was performed on
dorsal skin tissues. Compound 48/80 injection resulted in a
significant increase in mast cell infiltration, whereas chloroquine
induced a milder but still notable increase. Treatment with DLE and
MC markedly reduced mast cell infiltration in both models (Fig. 2A and B), suggesting that these compounds
inhibit mast cell recruitment and activation, thereby mitigating
pruritus.
Effects of DLE and MC on spinal cord
GRPR expression
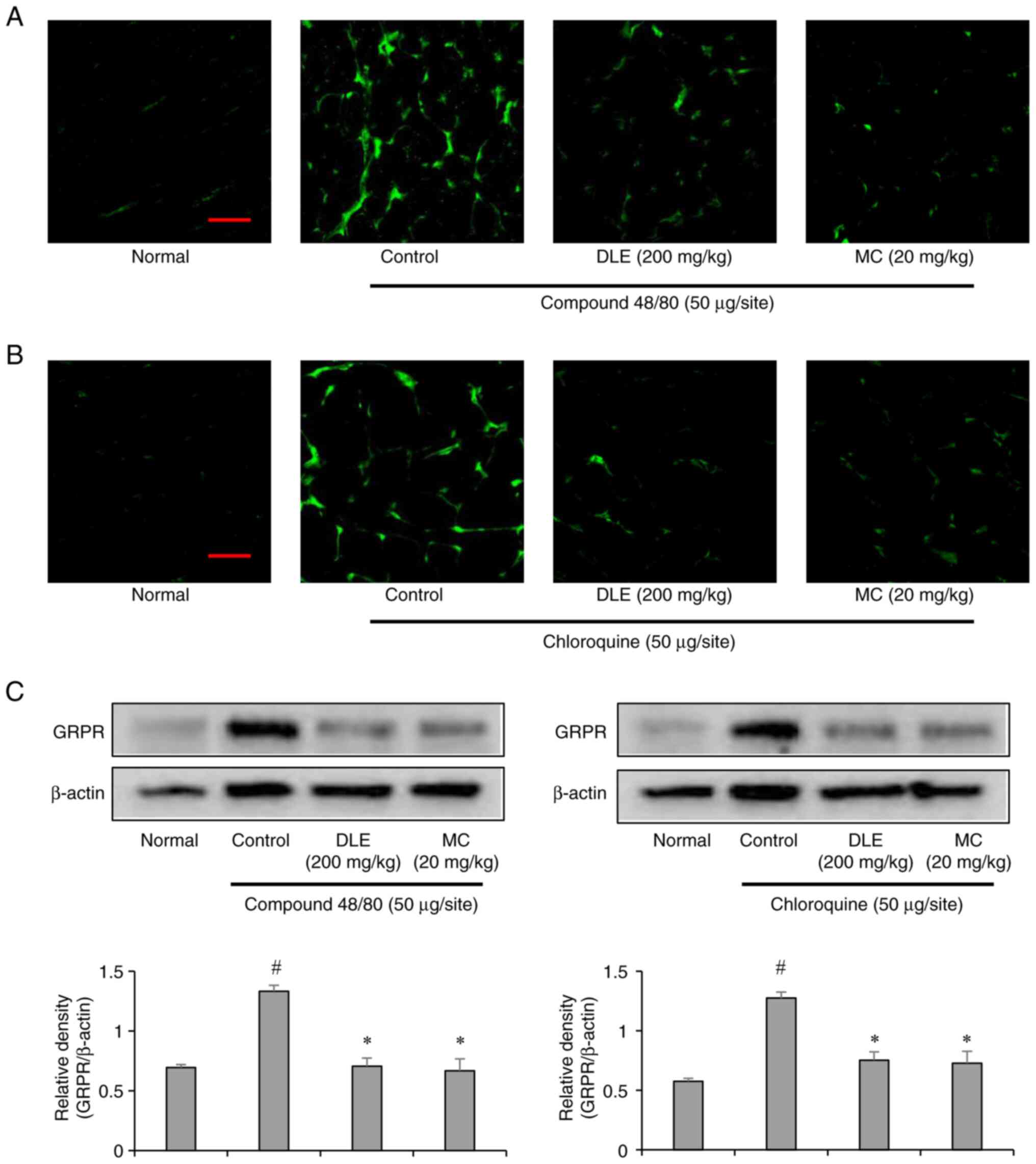
Gastrin-releasing peptide receptor (GRPR) is a key
neurotransmitter involved in itch signal transmission within the
spinal cord (24).
Immunofluorescence staining of the lumbar spinal cord (L4-L5)
revealed elevated GRPR expression in both compound 48/80- and
chloroquine-induced groups, consistent with their pruritogenic
effects. However, pretreatment with DLE and MC significantly
suppressed GRPR expression in both models (Fig. 3A-C). These results suggest that DLE
and MC modulate central itch signaling pathways by downregulating
GRPR expression, thereby reducing itch sensation.
Effects of DLE and MC on spinal cord
IL-31RA expression
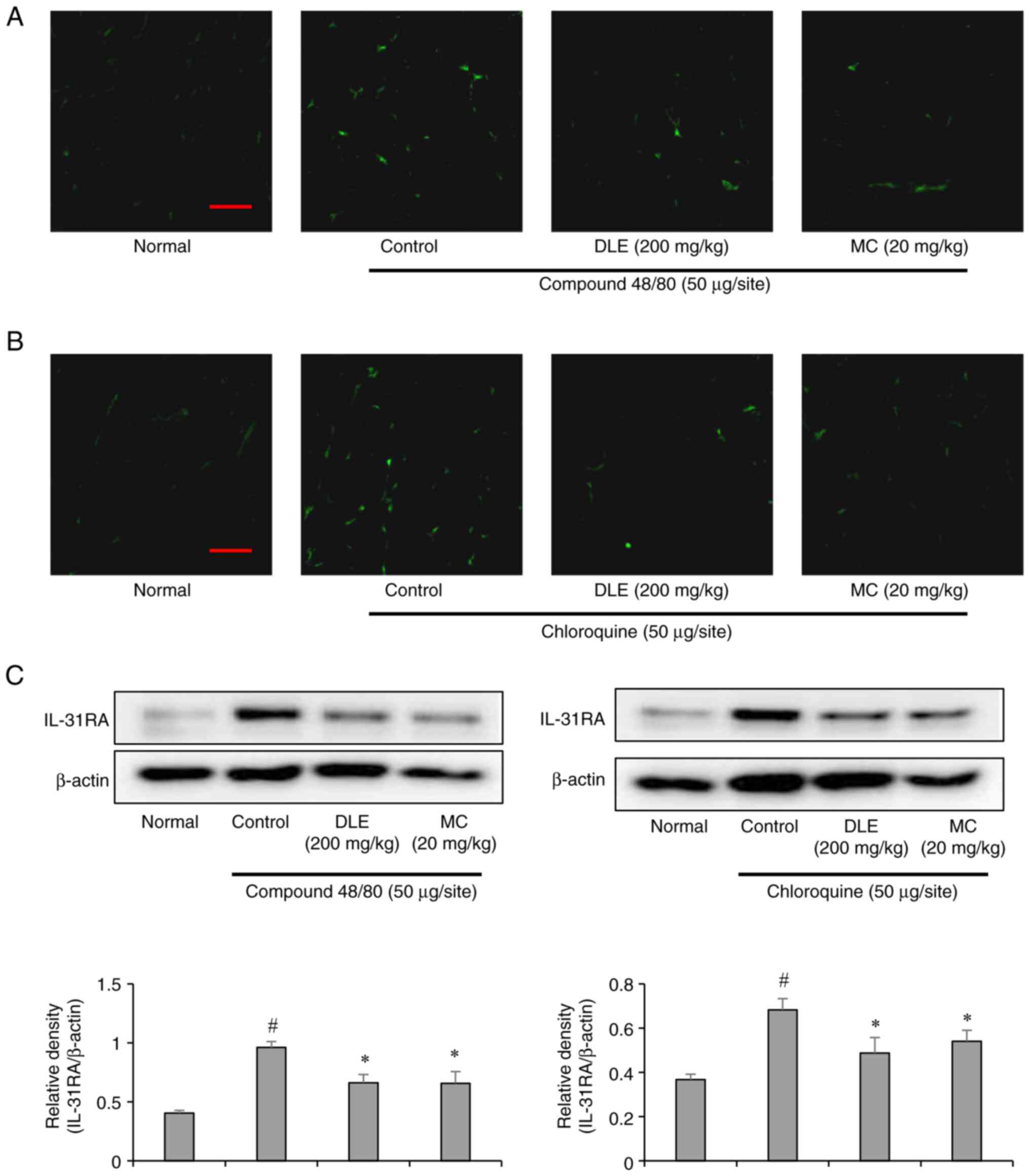
Interleukin-31 receptor A (IL-31RA) is a critical
mediator of chronic itch and neuroimmune interactions (25). Immunofluorescence analysis
demonstrated increased IL-31RA expression in the spinal cord of
mice injected with compound 48/80 or chloroquine. However,
treatment with DLE and MC significantly attenuated IL-31RA
expression in both groups (Fig.
4A-C). These findings indicate that DLE and MC may alleviate
pruritus by modulating IL-31 signaling pathways, which are
implicated in chronic itch conditions.
Effects of DLE and MC on spinal cord
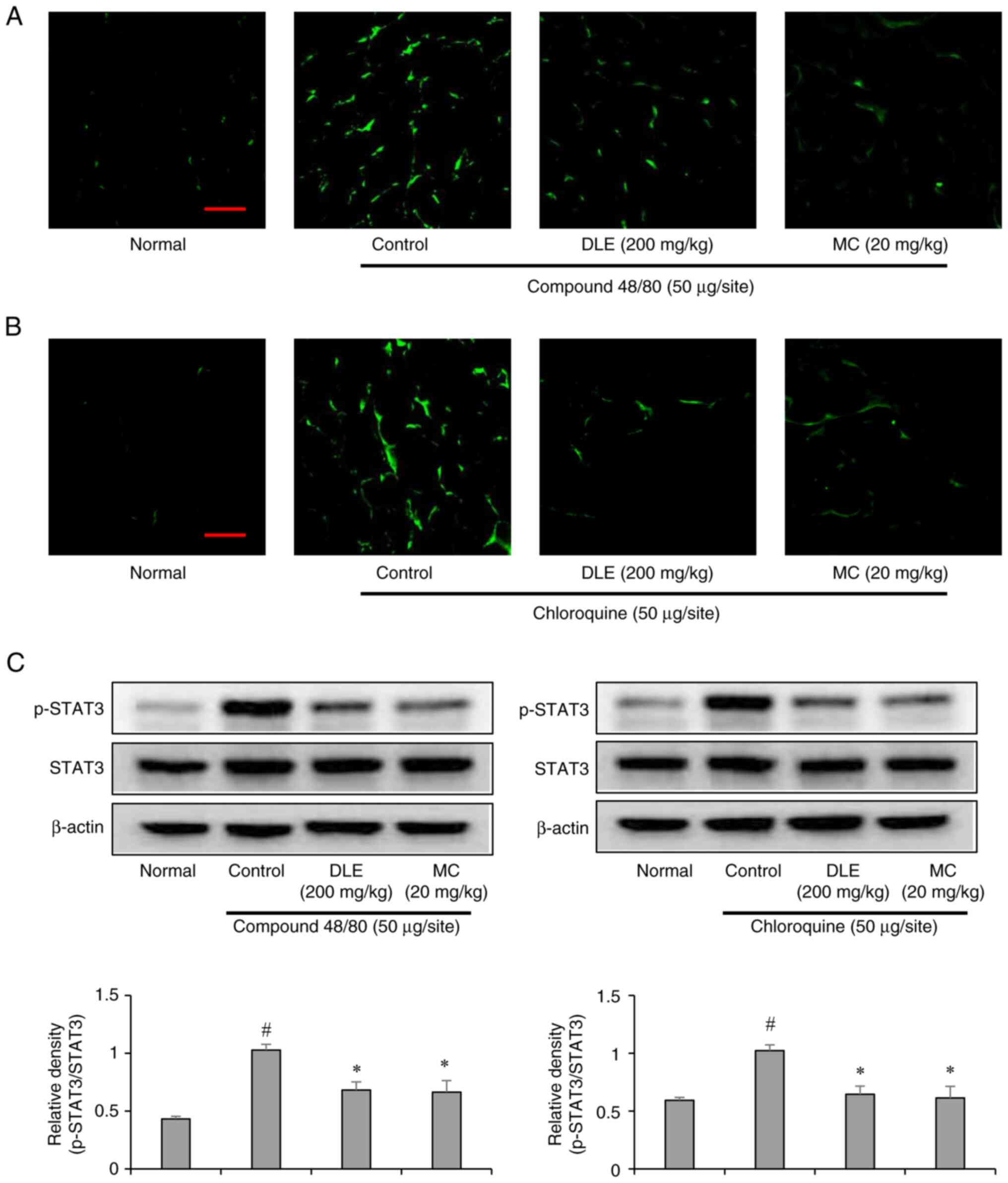
STAT3 activation
Signal transducer and activator of transcription 3
(STAT3) is a transcription factor involved in itch-related
inflammatory signaling (26).
Immunofluorescence staining revealed increased STAT3 activation in
the spinal cord of pruritus-induced mice. In contrast, DLE and MC
treatment significantly reduced STAT3 phosphorylation in both
compound 48/80- and chloroquine-induced models (Fig. 5A-C). This suggests that DLE and MC
exert antipruritic effects by inhibiting STAT3-mediated
inflammatory signaling in the central nervous system.
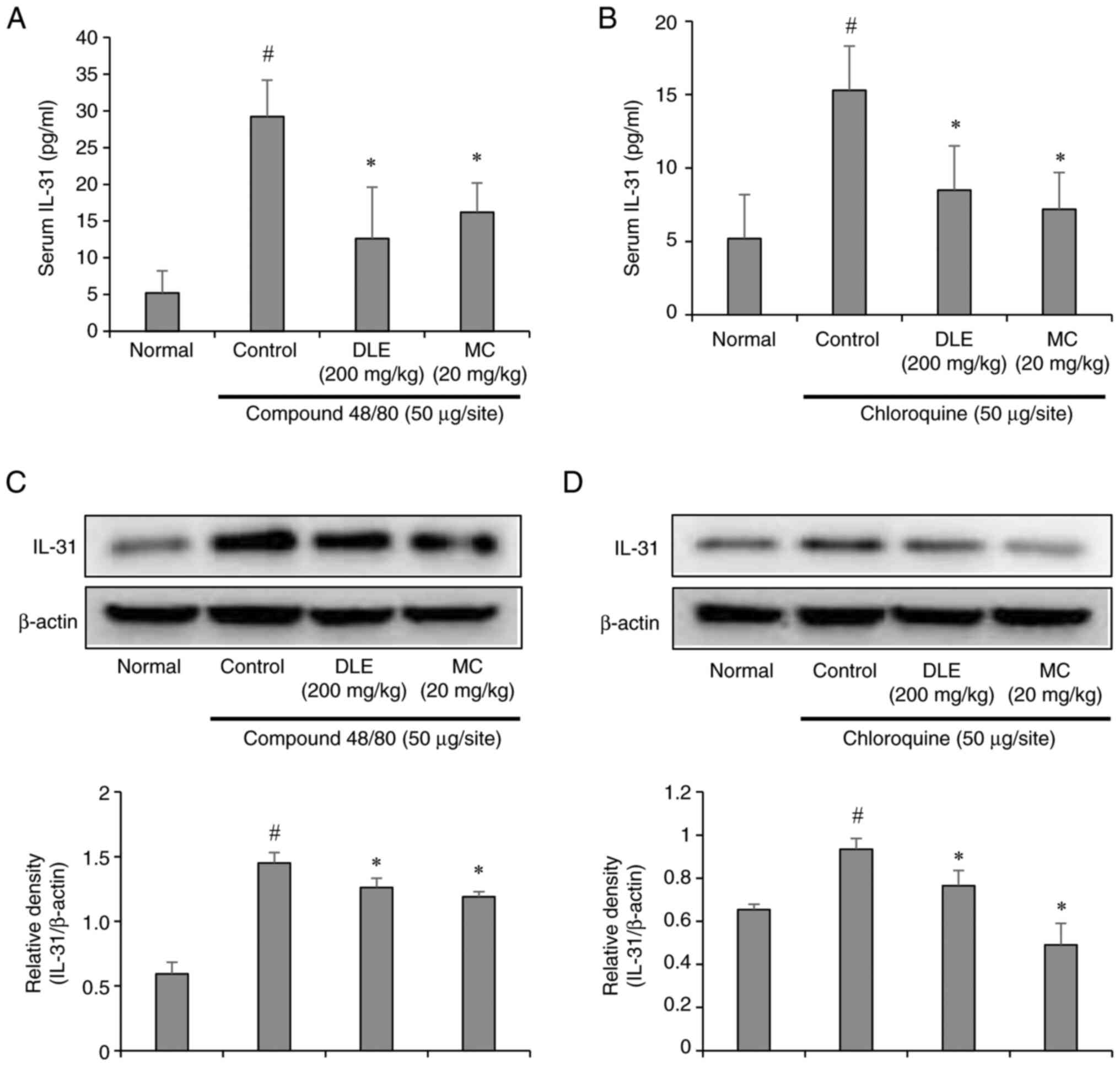
Effects of DLE and MC on IL-31
expression
Interleukin-31 (IL-31) is a pruritogenic cytokine
associated with chronic itch and inflammatory skin diseases
(25). ELISA and Western blot
analyses demonstrated elevated IL-31 levels in the serum and skin
of mice injected with compound 48/80 or chloroquine. Compound 48/80
induced a more pronounced increase in IL-31 than chloroquine,
consistent with its stronger histamine-dependent effects. However,
pretreatment with DLE and MC significantly reduced IL-31 expression
and secretion in both models (Fig.
6A-D). These results highlight the ability of DLE and MC to
suppress IL-31 production, further supporting their role in
alleviating pruritus.
Discussion
Pruritus, or itching, is a complex and multifaceted
sensory phenomenon that significantly impacts the quality of life
for affected individuals. It arises from a variety of
dermatological, systemic, and neurological conditions and can be
classified into histamine-dependent (6) and histamine-independent pathways
(15). Histamine-dependent
itching, mediated primarily by mast cell degranulation and
histamine release, is often associated with acute pruritus
(5). In contrast,
histamine-independent itching, involving mediators such as
gastrin-releasing peptide (GRP), interleukin-31 (IL-31), and signal
transducer and activator of transcription 3 (STAT3), is frequently
linked to chronic and refractory itch conditions (27). Understanding the mechanisms
underlying these pathways is crucial for developing effective
antipruritic therapies. In this study, we investigated the
antipruritic effects of D. lotus leaf extract (DLE) and its
major constituent, myricitrin (MC), in both histamine-dependent and
histamine-independent itch models. Our findings suggest that DLE
and MC exert significant antipruritic effects through modulation of
peripheral and central itch signaling pathways.
The results of this study revealed that DLE and MC
significantly reduced scratching behavior in ICR mice induced with
compound 48/80 (a histamine-dependent pruritogen) and chloroquine
(a histamine-independent pruritogen). These findings align with
previous studies demonstrating the antipruritic properties of
plant-derived polyphenols, which are known to exhibit
anti-inflammatory, antioxidant, and mast cell-stabilizing effects
(28-30).
The reduction in scratching behavior observed in both models
suggests that DLE and MC target multiple itch pathways, making them
promising candidates for the treatment of diverse pruritic
conditions.
Histamine is a well-established mediator of acute
itching, and its release from mast cells is a hallmark of
histamine-dependent pruritus (6).
Our results showed that compound 48/80, a potent mast cell
degranulator, significantly increased serum histamine levels and
mast cell infiltration in the skin. In contrast, chloroquine, which
operates through histamine-independent mechanisms, induced a milder
increase in histamine levels. Treatment with DLE and MC markedly
reduced serum histamine levels and mast cell infiltration in both
models, indicating their ability to stabilize mast cells and
inhibit histamine release. These findings are consistent with
previous reports highlighting the mast cell-stabilizing effects of
polyphenolic compounds, which are abundant in DLE (31-33).
For instance, myricitrin has been shown to inhibit mast cell
degranulation by suppressing calcium influx and downstream
signaling pathways (34).
In addition to their peripheral effects, DLE and MC
demonstrated significant modulation of central itch signaling
pathways. GRP, a neuropeptide expressed in primary itch afferents,
plays a critical role in transmitting itch signals to the spinal
cord (27). Our immunofluorescence
staining results revealed elevated GRP expression in the spinal
cord of pruritus-induced mice, which was significantly attenuated
by DLE and MC treatment. This suggests that DLE and MC may inhibit
the transmission of itch signals at the spinal level, providing a
potential mechanism for their antipruritic effects.
IL-31 and its receptor, IL-31RA, are key mediators
of chronic itch and neuroimmune interactions. Elevated IL-31 levels
have been reported in various pruritic conditions, including atopic
dermatitis and chronic idiopathic pruritus (35). In this study, both compound 48/80
and chloroquine increased IL-31 expression in the serum and skin,
with compound 48/80 inducing a more pronounced effect. However,
treatment with DLE and MC significantly reduced IL-31 levels,
suggesting that these compounds may alleviate pruritus by
suppressing IL-31-mediated signaling. This effect may be linked to
previous studies demonstrating that DLE and MC suppress IL-31 by
inhibiting the NF-κB, MAPK, and JAK/STAT pathways at the cellular
level (36). Moreover, the
observed downregulation of IL-31RA expression in the spinal cord
following DLE and MC treatment underscores their potential to
modulate central itch pathways.
STAT3, a transcription factor involved in
inflammatory signaling, has been implicated in the pathogenesis of
chronic itch. Activation of STAT3 in the spinal cord contributes to
the amplification of itch signals and the development of chronic
pruritus (37). Notably,
persistent STAT3 activation has been shown to upregulate LCN2
expression, which, in turn, exacerbates itch through the GRP/GRPR
signaling pathway (26). Our
results demonstrated increased STAT3 activation in the spinal cord
of pruritus-induced mice, which was significantly reduced by DLE
and MC treatment. This effect may be linked to previous findings
demonstrating that DLE and MC suppress LCN2 expression by
inhibiting IP3R1 and STAT3 activation in astrocytes (22). Furthermore, our results align with
previous reports indicating that polyphenolic compounds effectively
inhibit STAT3 phosphorylation and downstream inflammatory signaling
(38,39).
The findings of this study have important
implications for the development of novel antipruritic therapies.
The ability of DLE and MC to target both peripheral and central
itch pathways suggests their potential efficacy in alleviating
pruritus. Their mast cell-stabilizing, histamine-lowering, and
cytokine-modulating properties position them as potential
candidates for the treatment of diverse pruritic conditions,
including atopic dermatitis, chronic idiopathic pruritus, and
drug-induced itching. Moreover, the natural origin of DLE and its
constituents, such as myricitrin, offers a safer and potentially
more tolerable alternative to synthetic antipruritic agents, which
are often associated with adverse effects.
One of the strengths of this study is the
comprehensive evaluation of both peripheral and central mechanisms
underlying the antipruritic effects of DLE and MC. By employing
histamine-dependent and histamine-independent itch models, we were
able to demonstrate the broad-spectrum efficacy of these compounds.
This study focused on MC, the most abundant polyphenol in DLE,
present at concentrations 4-100 times higher than other
polyphenolic compounds. Although other polyphenols in DLE may also
contribute to the anti-itch effect, MC is likely the primary active
ingredient due to its predominant concentration. Exploring the
synergistic effects of the other polyphenolic compounds in DLE and
their combined actions on itch will provide a deeper understanding
of the full spectrum of DLE's therapeutic properties. However, this
study has some limitations. First, the experiments were conducted
exclusively in animal models, and further research is needed to
validate these findings in human subjects. Second, while we
identified myricitrin as a major active constituent of DLE, the
potential contributions of other polyphenolic compounds to the
observed effects cannot be ruled out. Additionally, the lack of
dose-response studies and long-term toxicity studies limits our
ability to optimize the dosages of DLE and MC. Future studies
should focus on these evaluations to establish the safety profile
of DLE and MC for potential therapeutic use.
In conclusion, this study demonstrates that D.
lotus leaf extract (DLE) and its major constituent, myricitrin
(MC), exert potent antipruritic effects through multiple
mechanisms, including modulation of mast cell activity, histamine
release, and central itch signaling pathways. Their ability to
target both histamine-dependent and histamine-independent pruritus
makes them promising candidates for the development of novel
antipruritic therapies. Further research is warranted to explore
their efficacy in human clinical trials and to elucidate the
precise molecular mechanisms underlying their antipruritic
effects.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by Wonkwang University (grant
no. Wonkwang University-2024).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JS and BK made major contributions to the study
design and manuscript writing, and made substantial contributions
to the acquisition, analysis and interpretation of the data. JS
drafted the manuscript. SJ contributed to the study
conceptualization, data curation, manuscript review and editing. JS
and SJ confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by Jeonju University
Institutional Animal Care and Use Committee (approval no.
jjIACUC-20230602-2022-0402-C1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bourane S, Duan B, Koch SC, Dalet A, Britz
O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q and Goulding M:
Gate control of mechanical itch by a subpopulation of spinal cord
interneurons. Science. 350:550–554. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Krajnik M and Zylicz Z: Understanding
pruritus in systemic disease. J Pain Symptom Manage. 21:151–168.
2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ständer S, Zeidler C, Magnolo N, Raap U,
Mettang T, Kremer AE, Weisshaar E and Augustin M: Clinical
management of pruritus. J Dtsch Dermatol Ges. 13:101–116.
2015.PubMed/NCBI View Article : Google Scholar : (In English,
German).
|
|
4
|
Lyell A: The itching patient. A review of
the causes of pruritus. Scott Med J. 17:334–347. 1972.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Song J, Xian D, Yang L, Xiong X, Lai R and
Zhong J: Pruritus: Progress toward pathogenesis and treatment.
Biomed Res Int. 2018(9625936)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang F, Yang TLB and Kim BS: The return of
the mast cell: New roles in neuroimmune itch biology. J Invest
Dermatol. 140:945–951. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Amin K: The role of mast cells in allergic
inflammation. Respir Med. 106:9–14. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Haugen HF and Skrede S: Nucleotide
pyrophosphatase and phosphodiesterase I. Demonstration of activity
in normal serum, and an increase in cholestatic liver disease.
Scand J Gastroenterol. 11:121–127. 1976.PubMed/NCBI
|
|
9
|
Sugimoto Y, Umakoshi K, Nojiri N and Kamei
C: Effects of histamine H1 receptor antagonists on compound
48/80-induced scratching behavior in mice. Eur J Pharmacol.
351:1–5. 1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mießner H, Seidel J and Smith ESJ: In
vitro models for investigating itch. Front Mol Neurosci.
15(984126)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yosipovitch G, Rosen JD and Hashimoto T:
Itch: From mechanism to (novel) therapeutic approaches. J Allergy
Clin Immunol. 142:1375–1390. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ma Q: Labeled lines meet and talk:
Population coding of somatic sensations. J Clin Invest.
120:3773–3778. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Mishra SK and Hoon MA: Transmission of
pruriceptive signals. In: Pharmacology of Itch. Cowan A and
Yosipovitch G (eds) Vol 226. Springer Berlin Heidelberg, Berlin,
Heidelberg, pp151-162, 2015.
|
|
14
|
Sun YG and Chen ZF: A gastrin-releasing
peptide receptor mediates the itch sensation in the spinal cord.
Nature. 448:700–703. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu Q, Tang Z, Surdenikova L, Kim S, Patel
KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al: Sensory
neuron-specific GPCR Mrgprs are itch receptors mediating
chloroquine-induced pruritus. Cell. 139:1353–1365. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mnyika KS and Kihamia CM:
Chloroquine-induced pruritus: Its impact on chloroquine utilization
in malaria control in Dar es Salaam. J Trop Med Hyg. 94:27–31.
1991.PubMed/NCBI
|
|
17
|
Rauf A, Uddin G, Patel S, Khan A, Halim
SA, Bawazeer S, Ahmad K, Muhammad N and Mubarak MS: Diospyros, an
under-utilized, multi-purpose plant genus: A review. Biomed
Pharmacother. 91:714–730. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cho BO, Yin HH, Fang CZ, Kim SJ, Jeong SI
and Jang SI: Hepatoprotective effect of Diospyros lotus leaf
extract against acetaminophen-induced acute liver injury in mice.
Food Sci Biotechnol. 24:2205–2212. 2015.
|
|
19
|
Kim BM, Cho BO and Jang SI: Anti-obesity
effects of Diospyros lotus leaf extract in mice with
high-fat diet-induced obesity. Int J Mol Med. 43:603–613.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cho BO, Che DN, Shin JY, Kang HJ, Kim JH,
Kim HY, Cho WG and Jang SI: Ameliorative effects of Diospyros
lotus leaf extract against UVB-induced skin damage in BALB/c
mice. Biomed Pharmacother. 95:264–274. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cho BO, Che DN, Yin HH, Shin JY and Jang
SI: Diospyros lotus leaf and grapefruit stem extract
synergistically ameliorate atopic dermatitis-like skin lesion in
mice by suppressing infiltration of mast cells in skin lesions.
Biomed Pharmacother. 89:819–826. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shin JY, Cho BO, Park JH, Kang ES, Kim YS
and Jang SI: Diospyros lotus leaf extract and its main
component myricitrin regulate pruritus through the inhibition of
astrocyte activation. Exp Ther Med. 26(323)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kwiatkowska D and Reich A: Role of mast
cells in the pathogenesis of pruritus in mastocytosis. Acta Derm
Venereol. 101(adv00583)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Barry DM, Liu XT, Liu B, Liu XY, Gao F,
Zeng X, Liu J, Yang Q, Wilhelm S, Yin J, et al: Exploration of
sensory and spinal neurons expressing gastrin-releasing peptide in
itch and pain related behaviors. Nat Commun.
11(1397)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Datsi A, Steinhoff M, Ahmad F, Alam M and
Buddenkotte J: Interleukin-31: The ‘itchy’ cytokine in inflammation
and therapy. Allergy. 76:2982–2997. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shiratori-Hayashi M, Yamaguchi C, Eguchi
K, Shiraishi Y, Kohno K, Mikoshiba K, Inoue K, Nishida M and Tsuda
M: Astrocytic STAT3 activation and chronic itch require
IP3R1/TRPC-dependent Ca2+ signals in mice. J Allergy
Clin Immunol. 147:1341–1353. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Steinhoff M, Ahmad F, Pandey A, Datsi A,
AlHammadi A, Al-Khawaga S, Al-Malki A, Meng J, Alam M and
Buddenkotte J: Neuroimmune communication regulating pruritus in
atopic dermatitis. J Allergy Clin Immunol. 149:1875–1898.
2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Di Salvo E, Gangemi S, Genovese C, Cicero
N and Casciaro M: Polyphenols from mediterranean plants: Biological
activities for skin photoprotection in atopic dermatitis,
psoriasis, and chronic urticaria. Plants (Basel).
12(3579)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kaag S and Lorentz A: Effects of dietary
components on mast cells: Possible use as nutraceuticals for
allergies? Cells. 12(2602)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Perrelli A, Goitre L, Salzano AM, Moglia
A, Scaloni A and Retta SF: Biological activities, health benefits,
and therapeutic properties of avenanthramides: from skin protection
to prevention and treatment of cerebrovascular diseases. Oxid Med
Cell Longev. 2018(6015351)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mwakalukwa R, Ashour A, Amen Y, Niwa Y,
Tamrakar S, Miyamoto T and Shimizu K: Anti-allergic activity of
polyphenolic compounds isolated from olive mill wastes. J Funct
Foods. 58:207–217. 2019.
|
|
32
|
Ortiz-Cerda T, Argüelles-Arias F,
Macías-García L, Vázquez-Román V, Tapia G, Xie K, García-García MD,
Merinero M, García-Montes JM, Alcudia A, et al: Effects of
polyphenolic maqui (Aristotelia chilensis) extract on the
inhibition of NLRP3 inflammasome and activation of mast cells in a
mouse model of Crohn's disease-like colitis. Front Immunol.
14(1229767)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shaik Y, Caraffa A, Ronconi G, Lessiani G
and Conti P: Impact of polyphenols on mast cells with special
emphasis on the effect of quercetin and luteolin. Cent Eur J
Immunol. 43:476–481. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vo TS, Le TT, Kim S and Ngo D: The role of
myricetin from Rhodomyrtus tomentosa (Aiton) Hassk fruits on
downregulation of FcɛRI-mediated mast cell activation. J Food
Biochem. 44(e13143)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Trier AM and Kim BS: Cytokine modulation
of atopic itch. Curr Opin Immunol. 54:7–12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shin JY, Cho BO, Park JH, Kang ES, Kim JH,
Ha HY, Kim YS and Jang SI: Diospyros lotus leaf extract and
its main component myricitrin inhibit itch-related IL-6 and IL-31
by suppressing microglial inflammation and microglial-mediated
astrocyte activation. Mol Med Rep. 30(178)2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tsuda M: Spinal dorsal horn astrocytes:
New players in chronic itch. Allergol Int. 66:31–35.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Aziz MdA, Sarwar MdS, Akter T, Uddin MS,
Xun S, Zhu Y, Islam MS and Hongjie Z: Polyphenolic molecules
targeting STAT3 pathway for the treatment of cancer. Life Sci.
268(118999)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yin Q, Wang L, Yu H, Chen D, Zhu W and Sun
C: Pharmacological effects of polyphenol phytochemicals on the
JAK-STAT signaling pathway. Front Pharmacol.
12(716672)2021.PubMed/NCBI View Article : Google Scholar
|