Introduction
In patients with severe alveolar ridge resorption
and inadequate residual bone volume, bone augmentation is necessary
before it possible to introduce a dental implant. However, when
conventional bone augmentation techniques such as guided bone
regeneration (1), lateral bone
condensation (2) and bone
splitting (3) are unable to
achieve adequate bone augmentation for implantation, autogenous
bone grafting (1), which is
considered the gold standard for bone grafting, is necessary.
Despite its effectiveness, autogenous bone grafting presents
challenges, including the need for a separate donor site, limited
availability, substantial surgical trauma, slow postoperative
recovery, rapid bone resorption, potential graft exposure and
infection, and the risk of nerve damage (4-6).
In addition, the graft shape may not conform to that of the defect
area.
Coral hydroxyapatite (CHA) is used as a bone
substitute due to its exceptional biocompatibility,
osteoconductivity and non-immunogenicity (7). CHA powder has been used extensively
for various clinical applications and provides effective bone
augmentation (8-10).
However, reports on the clinical application of CHA blocks are
limited (11,12).
Computer-aided design/computer-aided manufacturing
(CAD/CAM) is being increasingly used in dentistry, particularly in
the production of dental crowns and restorative frameworks. This
technology also has the potential to be applied to the fabrication
of customized CHA bone blocks. The present case report describes a
patient for whom a customized CHA block for the augmentation of a
severe bone defect was fabricated using CAD/CAM. The clinical
efficacy of the CHA block was evaluated as an alternative to
autogenous bone grafts that avoids the need for a bone donor site
and reduces trauma and postoperative reactions.
Case report
Patient
In October 2021, a 21-year-old man presented at The
Affiliated Hospital of Qingdao University (Qingdao, China) due to
congenitally missing mandibular central incisors. The patient was
in good general health and denied any systemic disease or allergies
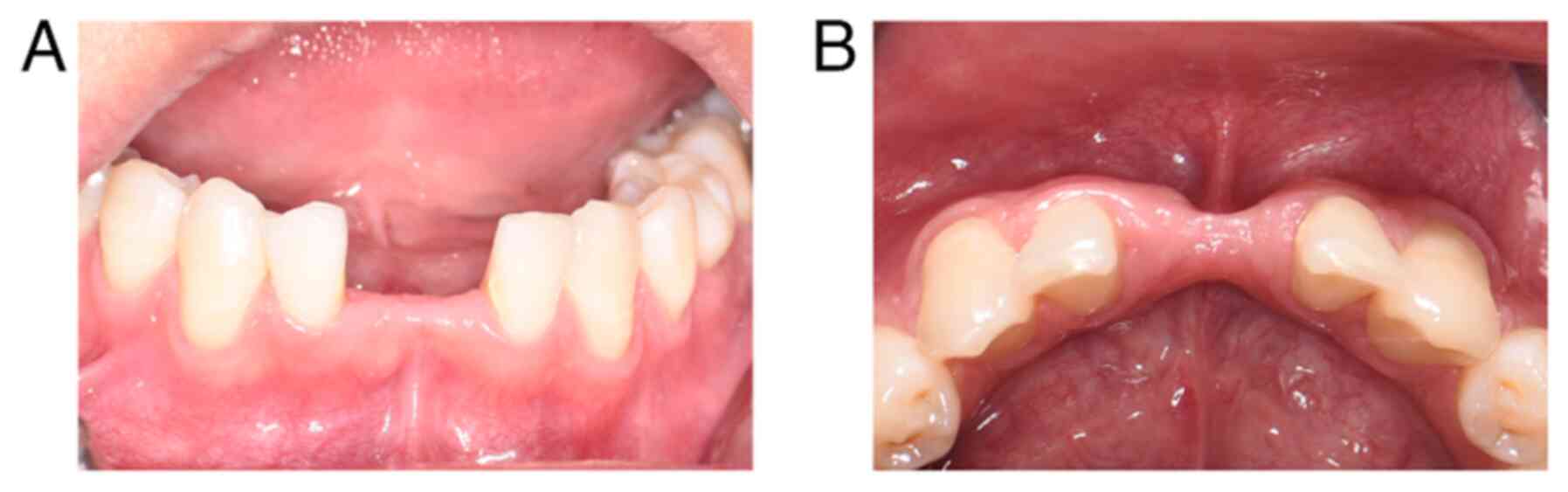
to drugs. Clinical examination revealed missing mandibular central
incisors, a significant labial concavity, and adequate keratinized
mucosa with a thin gingival biotype (13) (Fig.
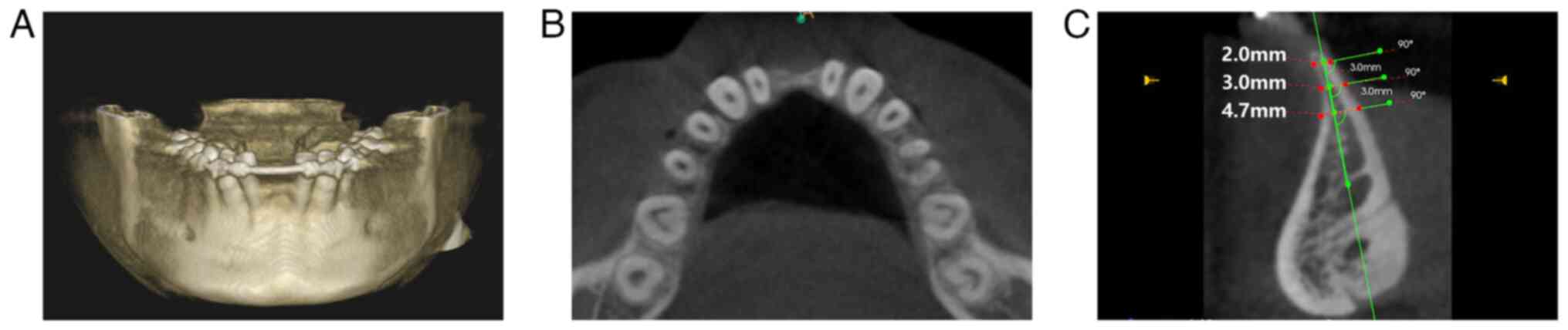
1). Cone-beam computed tomography (CBCT) performed using a CS
9300C system (Carestream Health, Inc.) revealed a knife-shaped
alveolar ridge with severe buccolingual width deficiency (2-3 mm)
and variable height (Fig. 2). The
timeline of the clinical procedures is shown in Table I.
 | Table ITimeline of the procedures. |
Table I
Timeline of the procedures.
| Timepoint | Procedure |
|---|
| Before surgery | Preoperative
preparation; CBCT and CHA block customization |
| 0 day | Augmentation surgery
to introduce the CHA block, immediately followed by CBCT |
| 10 days | Suture removal |
| 6 months | CBCT |
| 10 months | Implant surgery with
CBCT before and immediately after |
| 10 months and 10
days | Suture removal |
| 16 months | CBCT and second-stage
surgery |
| 17 months | Definitive
restoration |
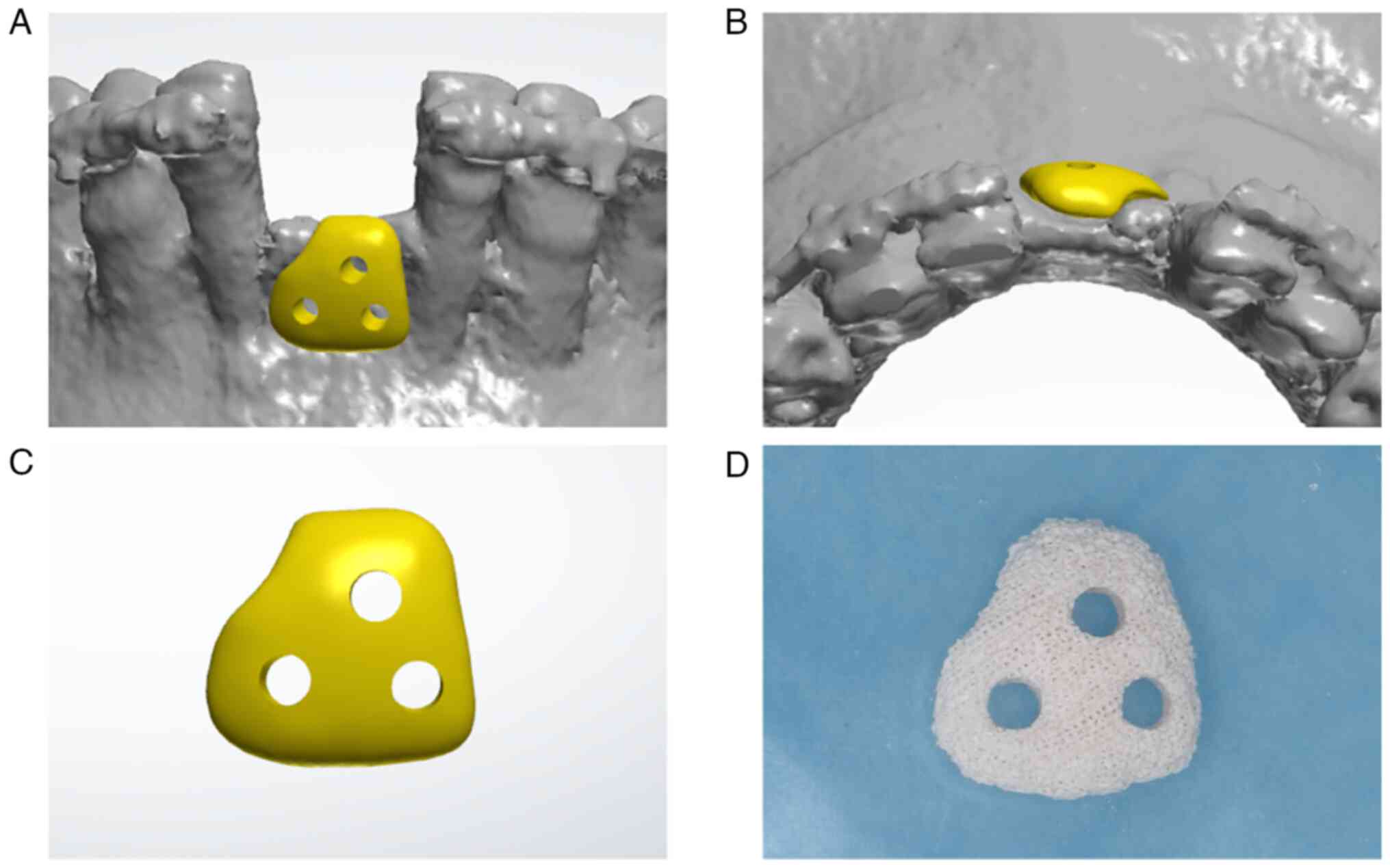
Customized CHA bone block
Preoperative CBCT data were exported in a Digital
Imaging and Communications in Medicine format, converted to
standard tessellation language (STL) using Mimics software (version
20.0; Materialise NV) and then imported into CAD software (3Shape
Dental System; version 2021; 3Shape A/S). A digital model of the
jaw was reconstructed in the 3Shape Dental System, and a bone block
was virtually designed to three-dimensionally fit the bone defect.
Additionally, holes were designed for block fixation. Following
finalization of the design, the STL data were exported to a
three-dimensional dental milling machine (DWX-4; Roland DG
Corporation) in which a raw CHA block (Bio-Osteon; Beijing YHJ
Science and Trade Co., Ltd.) was placed. After milling, the
customized CHA block was packaged, disinfected and sterilized
(Fig. 3).
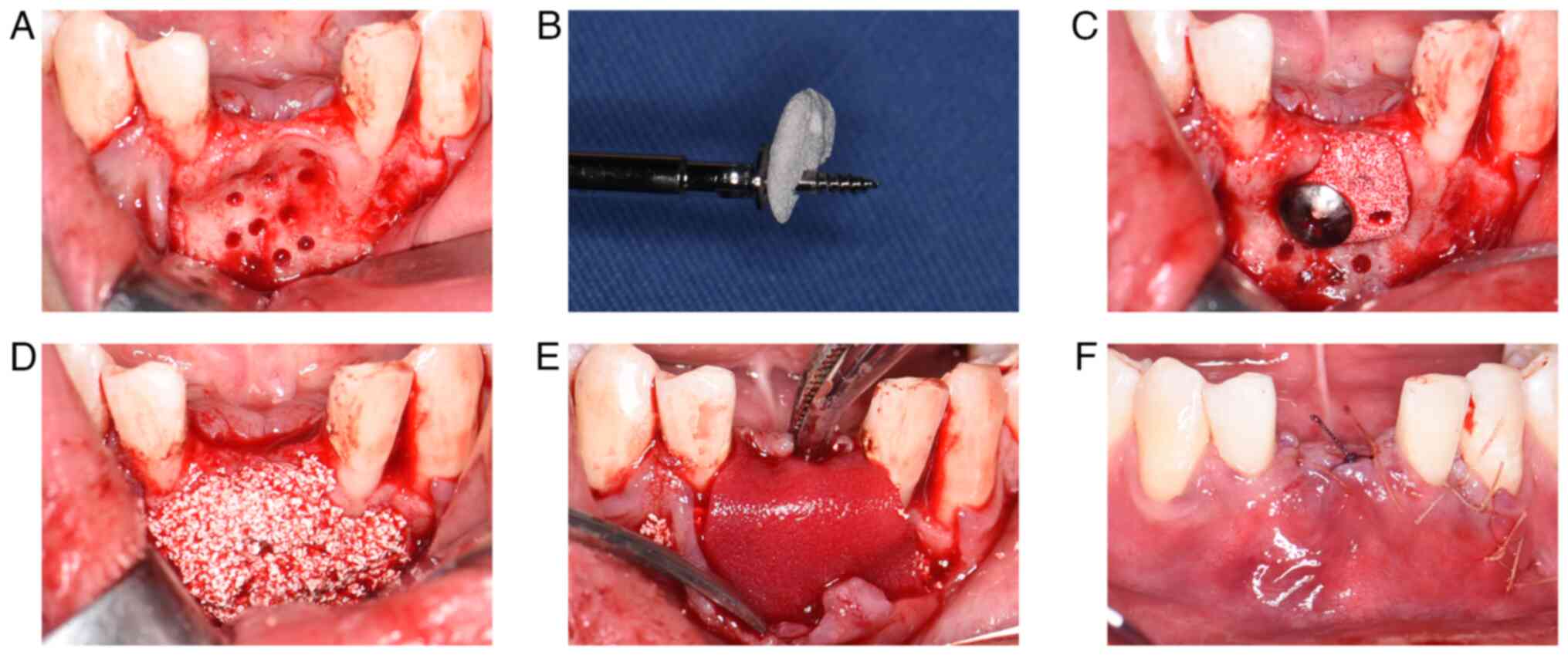
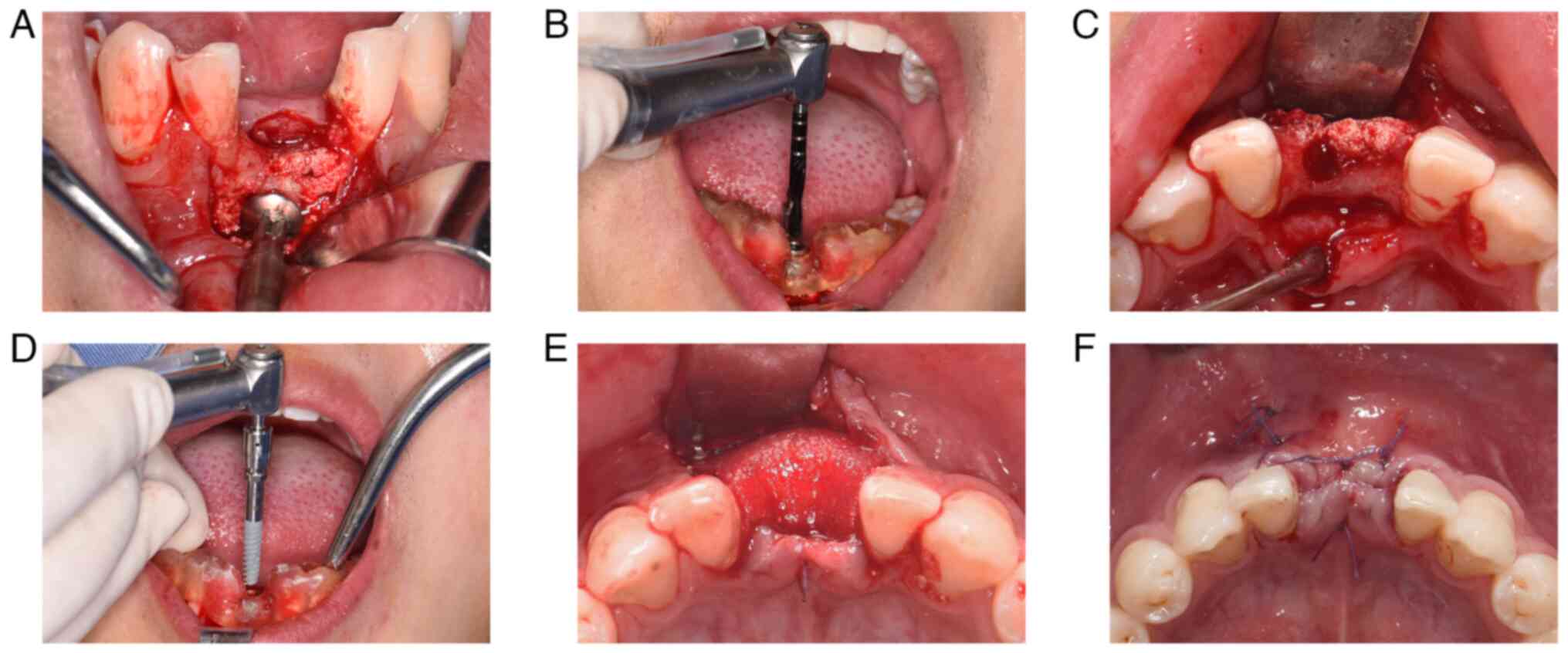
Augmentation surgery
A midcrestal incision was made under local
anesthesia, followed by a vertical-releasing incision mesial to the
left mandibular canine. A full-thickness flap was then elevated to
adequately expose the surgical site. Multiple cortical bone
perforations were performed using a 1-mm round bur to ensure
adequate vascularization. The customized CHA block was then placed
in the recipient area, and the fit was verified. The tenting screw
was screwed into the mandible by passing it through a pre-drilled
hole in the CHA block. The diameter of the head of the screw used
(~5 mm) was larger than that of a bone screw (~2 mm), which
provided greater stability. The CHA block was rigidly fixed to
promote blood absorption and integration into the host bone.
Residual voids were packed with CHA powder (Bio-Osteon) to
compensate for marginal deficiencies and achieve a smooth alveolar
contour. The graft was then covered with a resorbable collagen
membrane (Heal-All® membrane; Yantai Zhenghai Bio-tech
Co., Ltd.), and the flap was sutured under tension-free conditions
following periosteal releasing incisions and labial vestibular
extension (Fig. 4). The sutures
were removed 10 days after augmentation surgery, and wound healing
was assessed for any signs of infection or dehiscence. In addition,
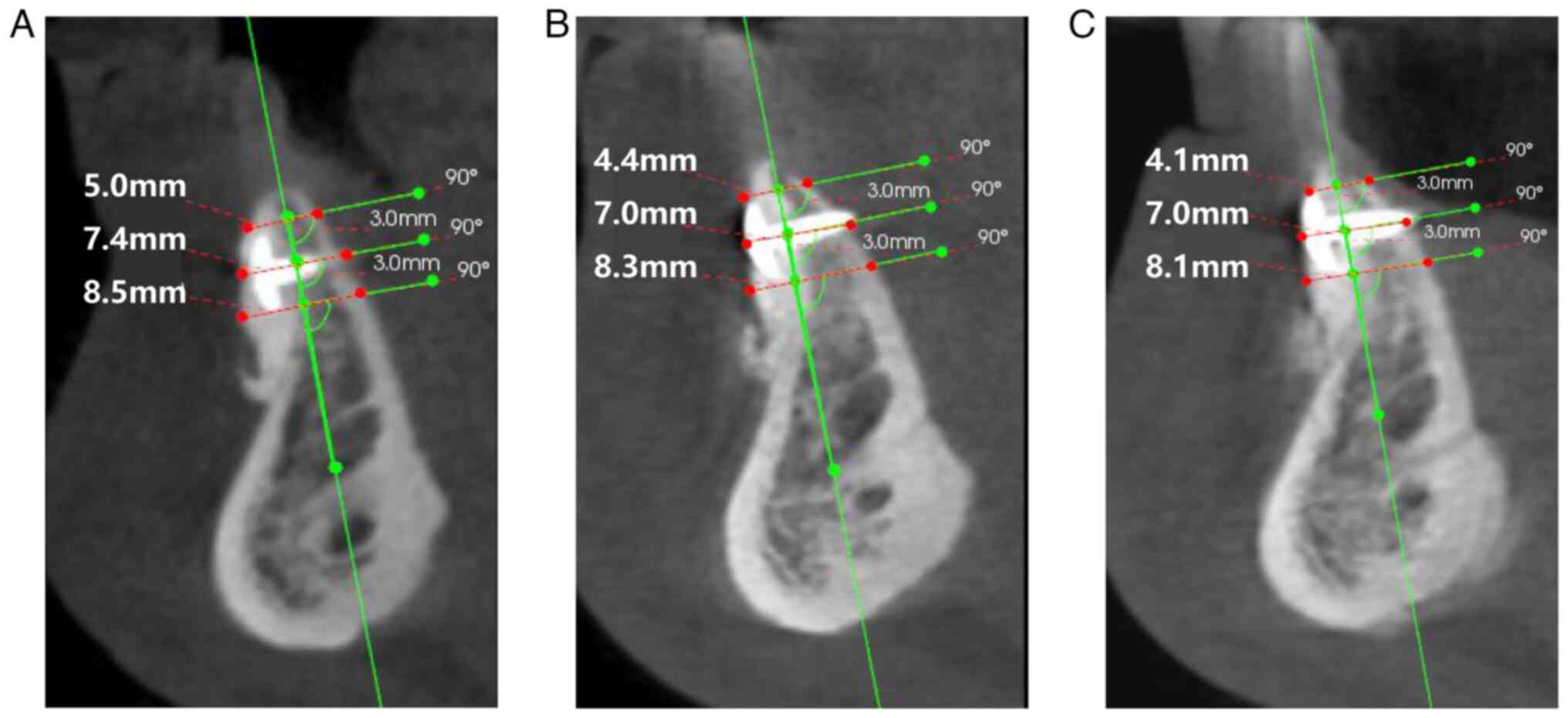
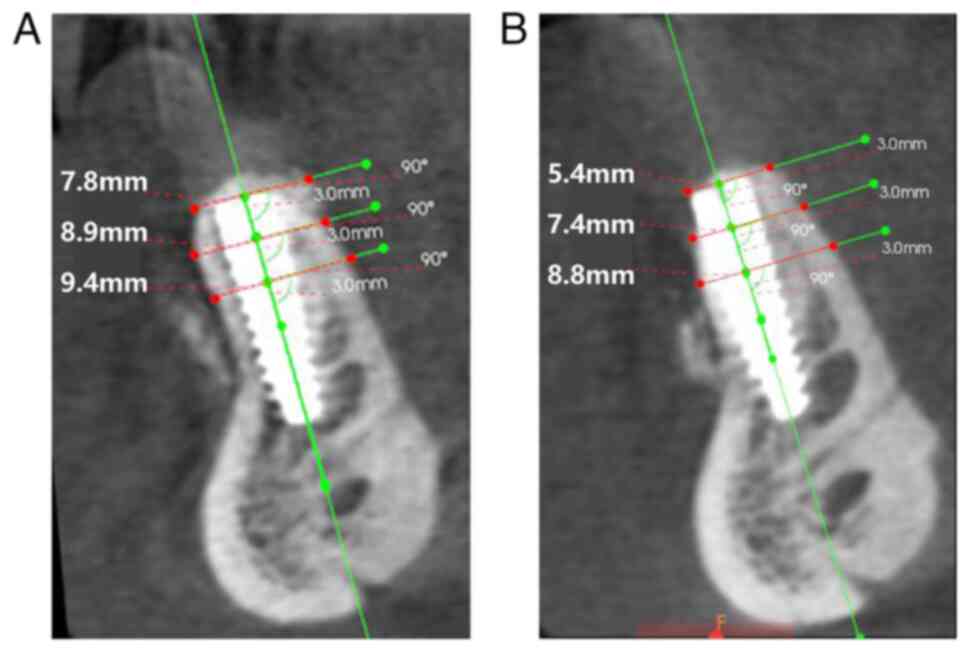
CBCT was performed immediately after, 6 months after and 10 months
after grafting surgery to evaluate the bone volume.
Implant placement
Re-entry was performed 10 months after bone
augmentation (Fig. 5). The
surgical site was adequately exposed under local anesthesia, the
tenting screw was removed and a 3.5x11.5 mm NobelActive®
implant (Nobel Biocare AB) was inserted. Guided bone regeneration
was then performed using bone powder and resorbable membrane. CBCT
was performed immediately after and 6 months after implant
placement. The second-stage surgery involving implant exposure and
healing abutment placement was performed 6 months after implant
placement, and the definitive restoration was completed with a
dental crown placement 1 month after the second-stage surgery
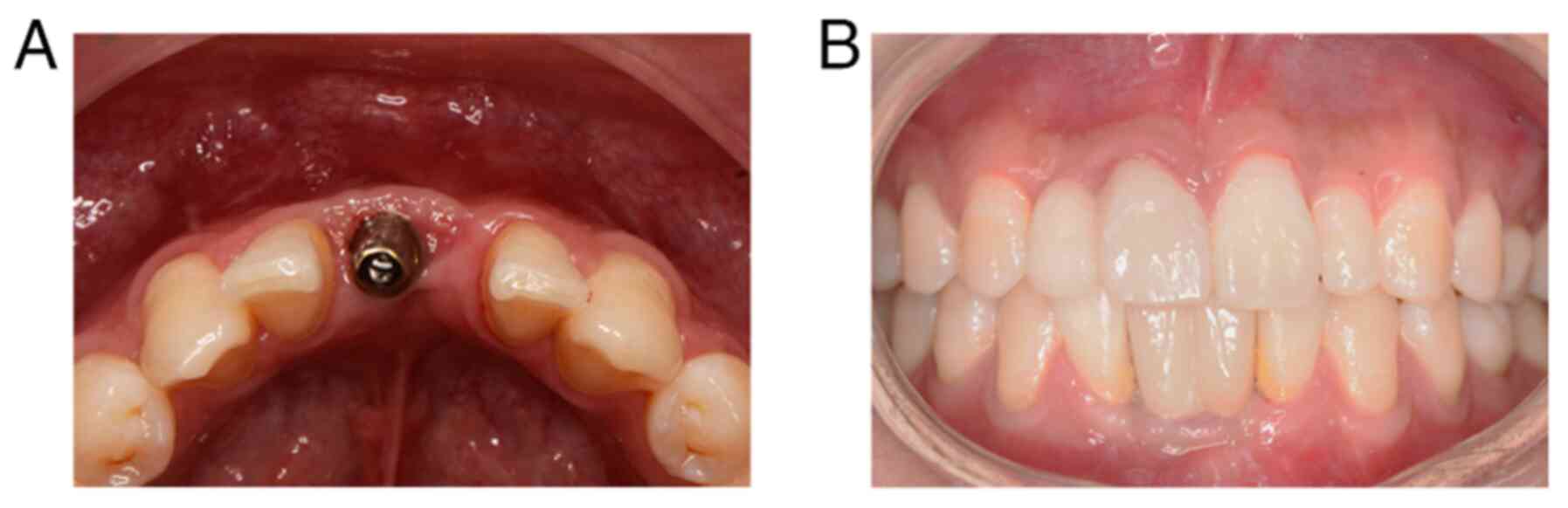
(Fig. 6). The implant stability
quotient (ISQ) value was tested using an Osstell ISQ device
(Osstell AB).
The wound healed well with no signs of tissue
dehiscence or infection during follow-up. When assessed 10 months
after CHA block grafting, the bone width had increased to 4-8 mm,
and the implant was placed with an insertion torque of 35 Ncm.
However, some residual CHA granules were observed, and partial
detachment of the CHA block was observed labially after osteotomy
preparation. The ISQ value was 70 at the time of definitive
restoration. The bone volume results are presented in Figs. 7 and 8. The last follow-up date was June 2023
and the patient was satisfied with the clinical outcome of
implant-supported restoration.
Discussion
The identification of alternatives to autogenous
bone for grafting is essential as, although autogenous bone
possesses osteogenesis, osteoconductive and osteoinductive
properties, it remains limited by the morbidity associated with
harvesting (14). Treatment
options considered for the present patient included autogenous bone
grafting, titanium mesh with guided bone regeneration, and
allogeneic or xenogenic bone block grafting. While a combination of
bone powder and a resorbable collagen membrane provides inadequate
support and poor stability, the application of a titanium mesh
makes the maintenance of space using bone powder feasible (15). Although titanium mesh has certain
advantages, it is also associated with a high rate of
complications, including early or late exposures, consequent
infections, and interference with bone healing due to the
soft-tissue layer beneath the titanium mesh (16-18).
Allogeneic grafts are typically derived from cadavers, and while
other studies have used customized allogeneic bone blocks for
augmentation prior to implantation (19-21),
biological safety remains a concern due to the potential for
disease transmission, immune reactions and ethical considerations
(22,23).
In the present case, a customized CHA block was
fabricated using natural coral. CHA has a three-dimensional
structure with interconnected channels, a porosity of 50-70%, and
pore diameter of 200-500 µm; and the porous structure is conducive
to bone tissue ingrowth, nutrient transport and waste elimination;
furthermore, its osteoconductive potential contributes to CHA being
a desirable bone substitute (12).
Manual contouring prior to fixation was not necessary since the
block was pre-shaped to fit the defect.
The main component of natural coral is calcium
carbonate (CaCO3), which can be converted into
hydroxyapatite (HA) through a hydrothermal reaction. HA undergoes
bioresorption more slowly than CaCO3, which
significantly impacts bone remodeling (24). The resorption rate of HA varies
between 0 and 5% per year (25).
Therefore, regulation of the hydrothermal conversion process
enables the degradation rate of the CHA block to be adjusted
(26). Therefore, to ensure
optimal coordination between CHA degradation and new bone formation
rates, the surface layer of coral was transformed into HA, while
CaCO3 was retained in the core. However, a healing
period of 10 months in the present case indicated relatively slow
osteogenesis. Therefore, it is suggested that adjusting the
proportion of HA in future applications could help to accelerate
bone remodeling. The alveolar ridge width increased from 2-4.7 mm
prior to bone augmentation to 4-8 mm after 10 months. This increase
in bone width, along with the lack of any signs of tissue
dehiscence or infection, demonstrates the safety and feasibility of
this technique.
The customized bone block fabricated using CAD/CAM
achieved optimal fit with the recipient site, simplified the
surgical procedure, eliminated the need for intraoperative bone
harvesting and shaping, and reduced surgical time. However, some
deviation in fit was inevitable due to factors such as data
acquisition, design and manufacturing errors. To compensate for
these deviations and ensure a uniform contour, CHA powder was used
to fill gaps and margins. Successful bone grafting was achieved
through the stable and rigid fixation of the graft to the recipient
bed (27), which may be attributed
to the customized shape of the block and the use of a tenting
screw.
In a previous study, Yao et al (11) found that CHA blocks and autogenous
bone grafts both effectively restored mandibular height. They
successfully placed implants 6 months following a bone augmentation
procedure; however, preparation of a box-shaped socket was required
for fixation. In a study on the osteoconductivity of CHA blocks in
rabbits (12), the blocks
dissolved, bony trabeculae thickened and fused with each other, and
a small amount of new bone appeared in the center at 8 weeks. By 12
weeks, there was a further increase in the peripheral new bone,
characterized by the formation of lamellar bone and bone marrow, as
well as increased blood vessel formation and thickened trabeculae
in the center. In another study using rabbits (28), histological observations at 8-15
months showed that the CHA block degraded into linear remnants, and
the areas of degradation were filled with regenerated bone and some
bone trabecula.
Sufficient blood supply is a key factor in
osteogenesis, particularly given the lack of osteogenic potential
of CHA. Therefore, proper case selection is crucial. The Lekholm
and Zarb classification system (type I-IV) is a widely method in
dental implantation to assess bone quality: Completely homogeneous
cortical bone is classified as type I; dense trabecular bone with
thick cortical bone layer is classified as type II; dense
trabecular bone with thin cortical bone layer is classified as type
III; and porous trabecular bone with thin cortical bone layer is
classified as type IV (29).
Homogeneous cortical bone has the worst blood supply. In the
present patient, the bone was classified as type III, which had
dense trabecular bone and met blood supply requirements.
At the 10-month assessment after bone augmentation,
the CHA block had not completely degraded or transformed into bone
tissue, and some granules remained. Furthermore, a part of the
labial CHA block detached after osteotomy preparation. Therefore,
secondary bone grafting using CHA powder was performed at the time
of implant placement. The definitive restoration was completed
after 6 months of healing. The ISQ value can be used to assess
implant stability and osseointegration. It ranges from 0 to 100,
with 100 representing the highest stability (30,31).
The ISQ value was 70 in the present patient at the second-stage
surgery, indicating good osseointegration.
Poor mechanical strength and high brittleness are
limitations of CHA blocks. A previous study on the physical and
mechanical properties of three natural corals reported compressive
strengths of 2.62-12.06 MPa; by contrast, the compressive strength
of the human femur is 131-283 MPa (32). Therefore, to reduce the possibility
of block fragmentation during fixation, a perforation was designed
for the fixation screw in the present case. In addition, a screw
with a large head was used to increase the retention force of the
CHA block.
In the present case, the customized CHA block used
for augmentation of a severe bone defect reduced surgical time and
postoperative reactions, although secondary bone grafting was
necessary. Nonetheless, implant restoration was successful. The
customized CHA block may serve as a potential alternative to the
autogenous bone graft. However, while CHA is osteoconductive and
osteoinductive, it lacks osteogenic potential. Also, it has a slow
bone turnover rate, poor mechanical strength and high brittleness.
Therefore, further research and modifications are necessary to
enhance the mechanical and osteogenic properties of CHA blocks and
improve their osteogenic ability. Studies including a larger number
of patients and longer follow-ups are required to explore and
evaluate the long-term effects of customized CHA blocks.
Additionally, it would be interesting to test the present technique
in combination with other recently introduced adjunctive treatments
that have shown promising results, such as ozone treatment
(33), photobiomodulation
(34), paraprobiotics (35), platelet-rich plasma or growth
factors (25), in order to
evaluate their mutual effect on tissue healing.
Acknowledgements
The authors thank Beijing YHJ Science and Trade Co.,
Ltd. for technical support.
Funding
Funding: Support was provided by the Shandong Municipal Health
Commission (Project 202208050566), the Qingdao Natural Science
Foundation (projects 23-2-1-133-zyyd-jch and 23-2-1-144-zyyd-jch)
and the Qingdao Municipal Health Commission (Project
2021-WJZD183).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SJ and WXW interpreted and analyzed the data and
drafted the manuscript. CZ and XJL acquired the data and revised
the manuscript. XL and BDZ designed the study, conducted the
treatment and revised the manuscript. XL and BDZ confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Affiliated Hospital of Qingdao University (QYFYWZLL27281).
Patient consent for publication
Written informed consent for publication was
obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benic GI and Hämmerle CH: Horizontal bone
augmentation by means of guided bone regeneration. Periodontol
2000. 66:13–40. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marković A, Mišić T, Mančić D, Jovanović
I, Šćepanović M and Jezdić Zl: Real-time thermographic analysis of
low-density bone during implant placement: A randomized
parallel-group clinical study comparing lateral condensation with
bone drilling surgical technique. Clin Oral Implants Res.
25:910–918. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Issa DR, Elamrousy W and Gamal AY:
Alveolar ridge splitting and simvastatin loaded xenograft for
guided bone regeneration and simultaneous implant placement:
Randomized controlled clinical trial. Clin Oral Investig.
28(71)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schwartz-Arad D, Ofec R, Eliyahu G, Ruban
A and Sterer N: Long term follow-up of dental implants placed in
autologous onlay bone graft. Clin Implant Dent Relat Res.
18:449–461. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Esposito M, Grusovin MG, Felice P,
Karatzopoulos G, Worthington HV and Coulthard P: The efficacy of
horizontal and vertical bone augmentation procedures for dental
implants-a cochrane systematic review. Eur J Oral Implantol.
2:167–184. 2009.PubMed/NCBI
|
|
6
|
Singh S: Management of infrabony defects
in mandibular molars in a patient with generalized aggressive
periodontitis using autogenous bone graft from maxillary
tuberosity. J Indian Soc Periodontol. 14:53–56. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Koshino T, Murase T, Takagi T and Saito T:
New bone formation around porous hydroxyapatite wedge implanted in
opening wedge high tibial osteotomy in patients with
osteoarthritis. Biomaterials. 22:1579–1582. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang S, Xu SL, Xiao XJ, Yao ZX and Wang C:
Comparison of graft height between coral hydroxyapatite and
dimenerialized bovine bone after maxillary sinus floor elevation. J
Pract Stomatol. 29:334–338. 2013.
|
|
9
|
Zhou M, Li SY, Terheyden H, Cao SS, Che YJ
and Geng YM: Particulate coral hydroxyapatite sheltered by titanium
mesh for localized alveolar rehabilitation after onlay graft
failure: A case report. J Oral Implantol. 44:147–152.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jiang S, Jiang YP, Li XJ, Wang WX, Teng
MH, Li X, Zhao BD and Mei DM: Short-term therapeutic evaluation on
bone augmentation technology without applying membrane in slight
posterior buccal bone substitute implantation. Chin J Oral
Maxillofac Surg. 17:251–256. 2019.
|
|
11
|
Yao ZX, Xu SL, Wang C and Yang S: Clinical
effect of coral hydroxyapatite blocks in reconstructing alveolar
bone height defects. Guangdong Med J. 35:1229–1232. 2014.
|
|
12
|
Yao ZX, Xu SL and Shao J: A preliminary
animal study on osteoconduction capacity of coralline
hydroxyapatite cylinders. Chin J Oral Implantol. 23:57–60.
2018.
|
|
13
|
Liu F, Pelekos G and Jin LJ: The gingival
biotype in a cohort of Chinese subjects with and without history of
periodontal disease. J Periodontal Res. 52:1004–1010.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Heimes D, Pabst A, Becker P, Hartmann A,
Kloss F, Tunkel J, Smeets R and Kämmerer PW: Comparison of
morbidity-related parameters between autologous and allogeneic bone
grafts for alveolar ridge augmentation from patients' perspective-A
questionnaire-based cohort study. Clin Implant Dent Relat Res.
26:170–182. 2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li L, Wang C, Li X, Fu G, Chen D and Huang
Y: Research on the dimensional accuracy of customized bone
augmentation combined with 3D-printing individualized titanium
mesh: A retrospective case series study. Clin Implant Dent Relat
Res. 23:5–18. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pellegrino G, Lizio G, Corinaldesi G and
Marchetti C: Titanium mesh technique in rehabilitation of totally
edentulous atrophic maxillae: A retrospective case series. J
Periodontol. 87:519–528. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Atef M, Tarek A, Shaheen M, Alarawi RM and
Askar N: Horizontal ridge augmentation using native collagen
membrane vs titanium mesh in atrophic maxillary ridges: Randomized
clinical trial. Clin Implant Dent Relat Res. 22:156–166.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Briguglio F, Falcomatà D, Marconcini S,
Fiorillo L, Briguglio R and Farronato D: The use of titanium mesh
in guided bone regeneration: A systematic review. Int J Dent.
2019(9065423)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kloss FR, Offermanns V, Donkiewicz P and
Kloss-Brandstätter A: Customized allogeneic bone grafts for
maxillary horizontal augmentation: A 5-year follow-up radiographic
and histologic evaluation. Clin Case Rep. 8:886–893.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Stopa Z, Siewert-Gutowska M, Abed K,
Szubińska-Lelonkiewicz D, Kamiński A and Fiedor P: Evaluation of
the safety and clinical efficacy of allogeneic bone grafts in the
reconstruction of the maxilla and mandible. Transplant Proc.
50:2199–2201. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Blume O, Back M, Born T, Smeets R, Jung O
and Barbeck M: Treatment of a bilaterally severely resorbed
posterior mandible due to early tooth loss by guided bone
regeneration using customized allogeneic bone blocks: A case report
with 24 months follow-up data. J Esthet Restor Dent. 30:474–479.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sanz M, Dahlin C, Apatzidou D, Artzi Z,
Bozic D, Calciolari E, De Bruyn H, Dommisch H, Donos N, Eickholz P,
et al: Biomaterials and regenerative technologies used in bone
regeneration in the craniomaxillofacial region: Consensus report of
group 2 of the 15th European workshop on periodontology on bone
regeneration. J Clin Periodontol. 46 (Suppl 21):S82–S91.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fillingham Y and Jacobs J: Bone grafts and
their substitutes. Bone Joint J. 98-B (1 Suppl A):S6–S9.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang N, Zhong Q, Zhou Y, Kundu SC, Yao J
and Cai Y: Controlled degradation pattern of hydroxyapatite/calcium
carbonate composite microspheres. Microsc Res Tech. 79:518–524.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pountos I and Giannoudis PV: Is there a
role of coral bone substitutes in bone repair? Injury.
47:2606–2613. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang T, Zheng J, Hu T, Zhang H, Fu K, Yin
R and Zhang W: Three-dimensional printing of calcium
carbonate/hydroxyapatite scaffolds at low temperature for bone
tissue engineering. 3D Print Addit Manuf. 8:1–13. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Proussaefs P, Lozada J, Kleinman A and
Rohrer MD: The use of ramus autogenous block grafts for vertical
alveolar ridge augmentation and implant placement: A pilot study.
Int J Oral Maxillofac Implants. 17:238–248. 2002.PubMed/NCBI
|
|
28
|
Ning Y, Wei T, Defu C, Yonggang X, Da H,
Dafu C, Lei S and Zhizhong G: The research of degradability of a
novel biodegradable coralline hydroxyapatite after implanted into
rabbit. J Biomed Mater Res A. 88:741–746. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lekholm U, Zarb GA and Albrektsson T:
Patient selection and preparation. Tissue Integrated Prostheses.
Brånemark PI, Zarb GA and Albrektsson T (eds). Quintessence
Publishing Co., Inc., Chicago IL, pp199-209, 1985.
|
|
30
|
El-Hady AIA, Eid HI, Mohamed SL and Fadl
SM: Influence of titanium and titanium-zirconium alloy as implant
materials on implant stability of maxillary implant retained
overdenture: A randomized clinical trial. BMC Oral Health.
24(902)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Parmar V, Elhammali NA, Altaher Mohammed
OB, Chauhan M, Gupta P, Manas A, Raj A and Chetani H: Dependability
of Osstell ISQ's for measuring implant stability. Bioinformation.
20:921–925. 2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu YC, Lee TM, Chiu KH, Shaw SY and Yang
CY: A comparative study of the physical and mechanical properties
of three natural corals based on the criteria for bone-tissue
engineering scaffolds. J Mater Sci Mater Med. 20:1273–1280.
2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Scribante A, Gallo S, Pascadopoli M, Frani
M and Butera A: Ozonized gels vs chlorhexidine in non-surgical
periodontal treatment: A randomized clinical trial. Oral Dis.
30:3993–4000. 2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Elbay M, Elbay ÜŞ, Kaya E and Kalkan ÖP:
Effects of photobiomodulation with different application parameters
on injection pain in children: A randomized clinical trial. J Clin
Pediatr Dent. 47:54–62. 2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Butera A, Pascadopoli M, Nardi MG, Ogliari
C, Chiesa A, Preda C, Perego G and Scribante A: Clinical use of
paraprobiotics for pregnant women with periodontitis: Randomized
clinical trial. Dent J (Basel). 12(116)2024.PubMed/NCBI View Article : Google Scholar
|