Introduction
Pituitary apoplexy (PA) is a rare clinical syndrome,
occurring in ~2-12% of pituitary adenomas (1,2), a
tumor with an overall incidence of 3.9-7.4 per 100,000 person-years
in the general population (3). It
predominantly affects individuals aged 50-69 years and exhibits a
significant male predilection, with a male-to-female ratio ranging
from 1.1:1 to 2.3:1 (2,4). Several factors may trigger PA,
including surgery, pregnancy, thrombocytopenia, head trauma,
anticoagulation, infections and other iatrogenic causes (4-7).
Common symptoms include sudden, severe headache, vision loss,
blindness, cranial nerve palsy, altered consciousness, hypotension,
hypoglycemia and other manifestations of pituitary dysfunction.
Given its acute and life-threatening nature, PA is considered a
neurosurgical emergency. However, the optimal treatment remains
undefined, with management primarily relying on the expertise of a
multidisciplinary team. A systematic review of 708 patients with PA
across 13 clinical series (1993-2024) reported that 30.6% of
patients received conservative management (serial neurologic
assessments, visual function monitoring, renal/metabolic
surveillance with electrolyte panels, pharmacologic therapy with
glucocorticoids or dopamine agonists for prolactinoma-associated
cases), while 69.4% underwent surgical intervention as the primary
approach (4). The current study
presents a rare case of PA in a patient who had been taking aspirin
for ~4 months following coronary stent implantation and discusses
its clinical features, management and outcome.
Case report
In November 2022, a 45-year-old male was admitted to
Chongqing General Hospital (Chongqing, China) with a 2-month
history of intermittent headaches and progressive right-sided
vision impairment, which had acutely worsened over the past 6 h. At
4 months before admission, the patient had been diagnosed with
acute myocardial infarction due to triple-vessel coronary artery
disease and subsequently underwent coronary stent implantation.
Following the procedure, the patient was placed on enteric-coated
aspirin tablets for antiplatelet therapy. In addition, the patient
had a history of left eye trauma at the age of 23 years, resulting
in severe visual impairment, with only light perception
remaining.
On initial physical examination, the left eye had no
light perception, while visual acuity in the right eye was limited
to finger counting at a distance of 30 cm. On the second day of
hospitalization, endocrine evaluation revealed decreased levels of
prolactin (1.74 ng/ml; normal range, 2.64-13.13 ng/ml) and
testosterone (1.23 ng/ml; normal range, 1.75-7.81 ng/ml), while all
other hormonal parameters were within normal limits. The
coagulation profile obtained on hospital day 1 included normal
values for all measured parameters: Prothrombin time, 10.40 sec
(normal range, 9-14 sec); activated partial thromboplastin time,
31.40 sec (normal range, 20-40 sec); thrombin time, 12.50 sec
(normal range, 10-18 sec); fibrinogen, 4.54 g/l (normal range, 2-4
g/l); and international normalized ratio, 0.93 (normal range,
0.80-1.20), collectively indicating normal coagulation function.
The complete blood count on the first day of hospitalization
demonstrated increased levels of white blood cells
(11.78x109/l; normal range, 3.50-9.50x109/l)
with otherwise normal parameters, including erythrocytes
(5.12x10¹²/l; normal range, 4.30-5.80x10¹²/l), hemoglobin (149 g/l;
normal range, 130-175 g/l), platelets (191x109/l; normal
range, 100-300x109/l), and platelet indices: Mean volume
(11.60 fl; normal range, 9-13 fl), distribution width (16.80 fl;
normal range, 9-17 fl), plateletcrit (0.22%; normal range,
0.11-0.28%) and large cell ratio (38.40%; normal range, 11-45%).
The increased white blood cells indicated that the patient was in a
stress state following disease onset.
Preoperative visual field assessment was not
performed. Computed tomography (CT) of the head performed on
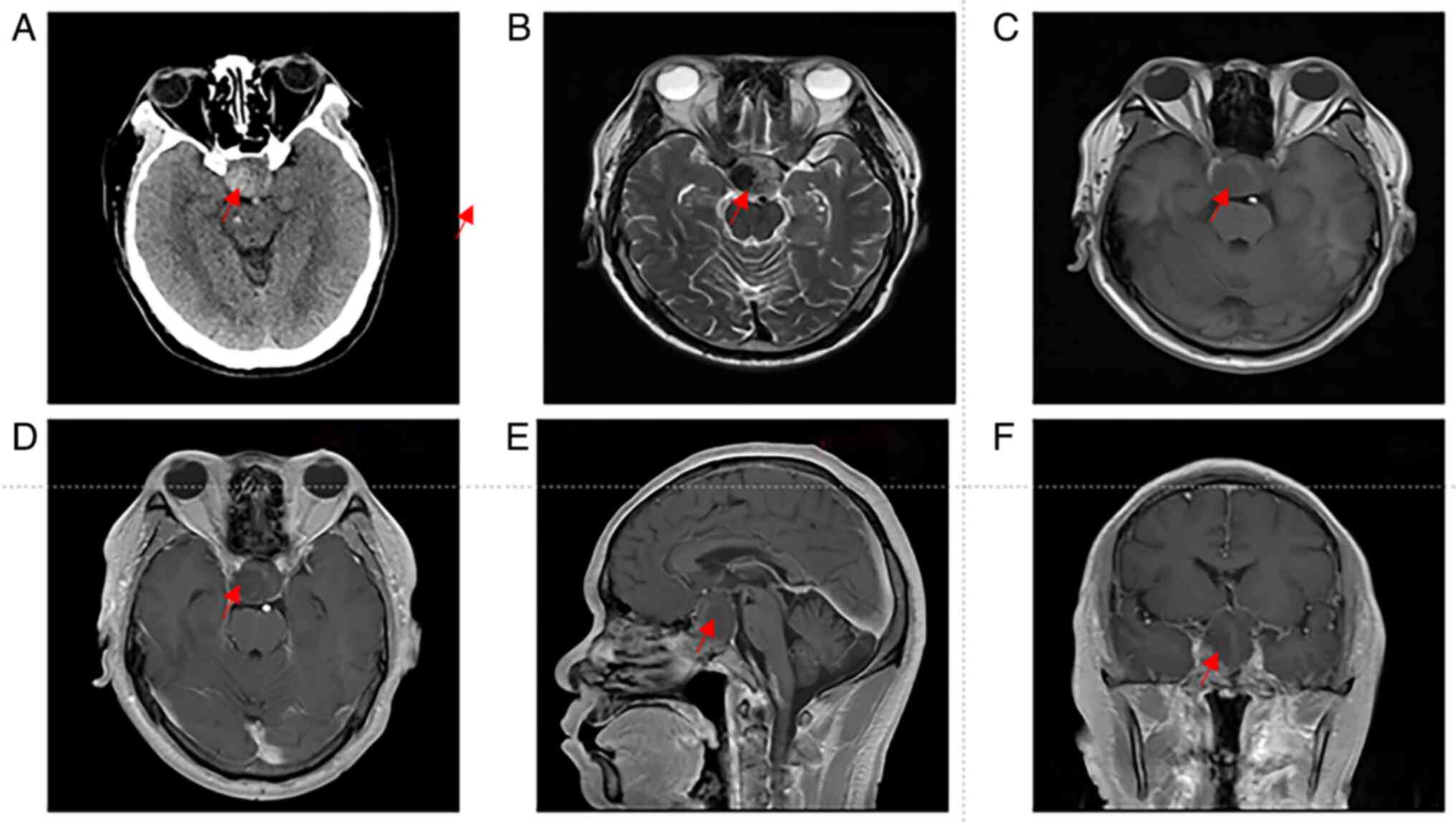
hospital day 1, revealed a sellar mass measuring 2.7x2.9x4.1 cm,
exhibiting iso- to slightly hyperdense characteristics (Fig. 1A). On the second day of
hospitalization, magnetic resonance imaging (MRI) showed an
irregular mass in the sellar region, measuring ~2.6x2.9x4.2 cm
(Fig. 1B-F). The lesion appeared
iso- to slightly hypointense on T1-weighted imaging (Fig. 1C) and iso- to slightly hyperintense
on T2-weighted imaging (Fig. 1B),
while post-contrast imaging revealed localized nodular enhancement
(Fig. 1D-F). After comprehensive
preoperative evaluation, the patient underwent transsphenoidal
endoscopic total resection of the lesion on the second day of
hospitalization.
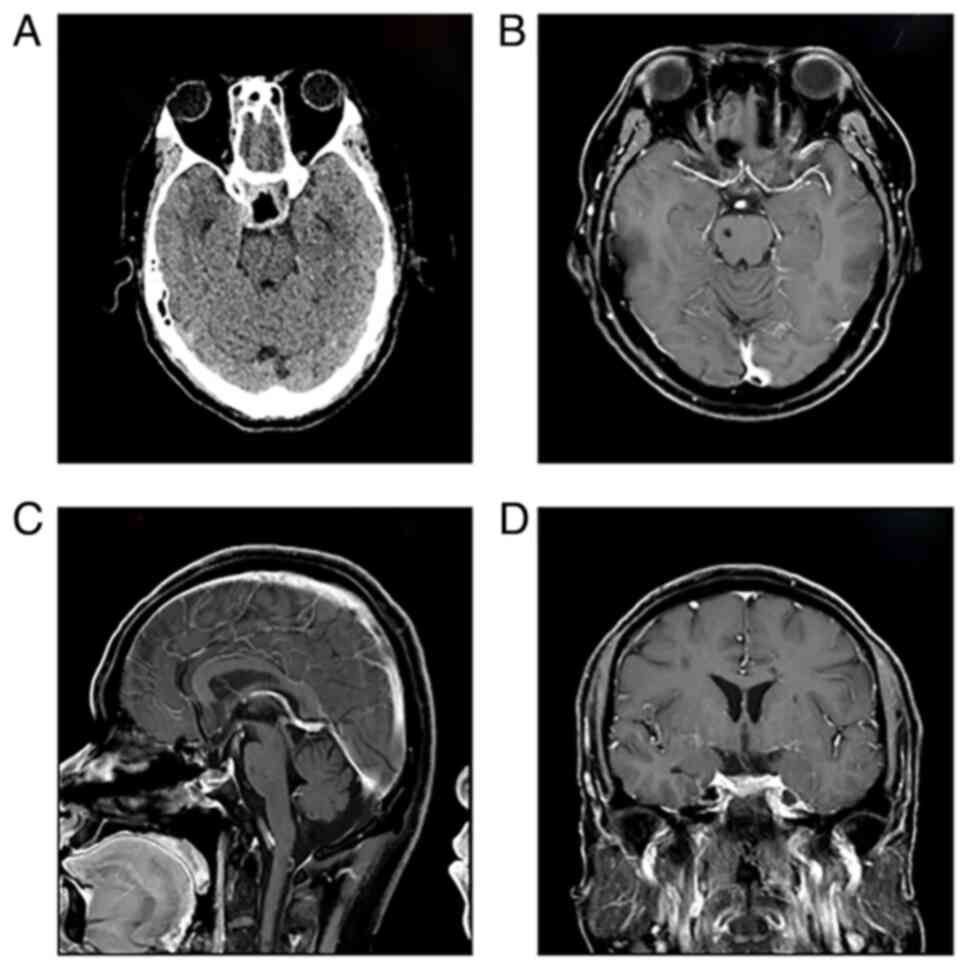
On postoperative day 3, CT scans showed no evidence
of bleeding in the sellar region (Fig.
2A). To prevent coronary stent thrombosis, the patient was
started on low-molecular-weight heparin. On postoperative day 3,
endocrine evaluation revealed decreased levels of cortisol (2.16
µg/dl; normal range, 4.26-24.85 µg/dl), adrenocorticotropic hormone
(2.68 pg/ml; normal range, 7.2-63.4 pg/ml) and human
thyroid-stimulating hormone (0.322 µIU/ml; normal range,
0.560-5.910 µIU/ml), while all other hormonal parameters remained
within normal limits. To manage adrenal insufficiency, the patient
was promptly initiated on intravenous hydrocortisone (100 mg) on
postoperative day 3. Additionally, a daily oral prednisone regimen
was prescribed, with 5 mg administered in the morning and 2.5 mg in
the afternoon. By postoperative day 5, the patient reported
significant improvement in right-sided vision. Visual field testing
showed a right-eye visual acuity of 0.5, with 3/4 visual field
defects, while light perception in the left eye was restored. On
postoperative day 9, endocrine evaluation revealed slightly
decreased free triiodothyronine (3.31 pmol/l; normal range,
3.53-7.37 pmol/l) and free thyroxine (6.52 pmol/l; normal range,
7.98-16.02 pmol/l), while other hormonal levels remained normal.
Consequently, prednisone was discontinued.
Surgically resected tumor tissues underwent routine
histopathological examination with hematoxylin and eosin staining.
Subsequent to specimen collection, tumor tissues were fixed in 4%
formaldehyde at room temperature for 24 h, followed by paraffin
embedding. The embedded blocks were sectioned into 4-µm slices and
deparaffinized in xylene at 60˚C for 2 h. For staining, sections
were sequentially treated with 0.5% hematoxylin (3 min) and 0.5%
eosin (3 min) at room temperature. Histopathological evaluation was
subsequently performed using light microscopy, with representative
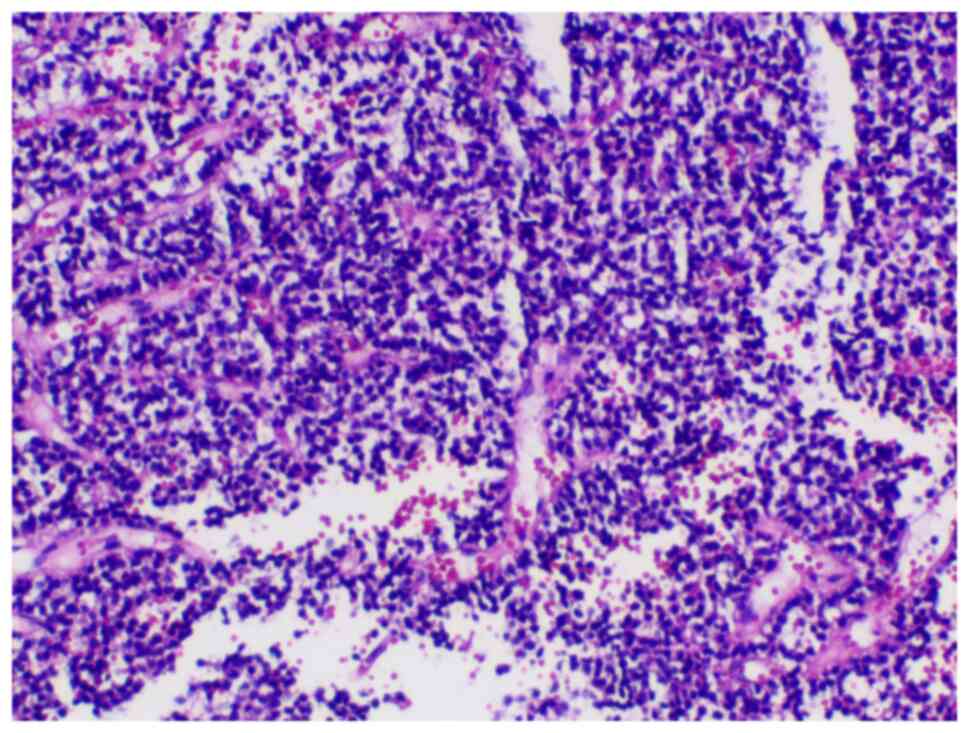
microphotographs captured for documentation. As indicated in
Fig. 3, a pituitary neuroendocrine
tumor was confirmed, which was likely a null cell adenoma.
At 6 months post-operatively, follow-up MRI showed
no evidence of tumor recurrence (Fig.
2B-D), and endocrine assessment confirmed the recovery of
pituitary function. However, the patient declined further vision
and visual field examinations, stating that he had fully recovered.
At 1 year post-operatively, a telephone follow-up indicated that
the patient remained asymptomatic and continued to decline
additional evaluations, including MRI, vision and visual field
examinations, and endocrine evaluation.
Discussion
Apoplexy is a rare but serious complication of
pituitary adenomas. Several case reports have documented
occurrences of PA following cardiac surgery, involving
cardiopulmonary bypass, mitral and aortic valve replacement and
stent placement (7-12).
Table I shows the major
characteristics of previous cases. All patients were male with a
minimum age of 53 years. With the exception of one case managed
conservatively, all patients underwent surgical intervention for
PA, with operative timing ranging from 2 h to 2 weeks
post-diagnosis. Postoperative assessment revealed functional
recovery in all surgical cases, although the extent of improvement
varied significantly among individuals. The key distinguishing
feature of the present case compared to previous reports is the
timing of PA onset and different triggering factors driving PA
development. While prior cases typically occurred within the
immediate postoperative period, the present study reported a rare
case of PA in a patient who had been taking aspirin for ~4 months
following coronary stent implantation. Regarding previously
reported perioperative PA occurrences during cardiac surgery, it
was hypothesized that cardiac surgery may increase the
susceptibility of abnormal pituitary tissue to hypoperfusion or
ischemia, while also elevating the risk of hemorrhage from the
fragile vasculature of adenomas, particularly due to
heparin-induced anticoagulation (2,10).
In the present case, the patient's aspirin use may represent one of
the contributing factors to PA development. Aspirin
(acetylsalicylic acid) is a well-established inhibitor of platelet
aggregation (13,14). Its primary mechanism involves the
irreversible inhibition of cyclooxygenase (COX) activity within
platelets, specifically targeting the COX-1 isoform. COX-1
catalyzes the conversion of arachidonic acid to thromboxane A2
(TXA2). TXA2 serves as a crucial mediator of platelet activation
and vasoconstriction. Deficiency in TXA2 production significantly
inhibits both platelet aggregation and activation processes.
Aspirin exerts its effect by acetylating a specific serine residue
in the COX-1 active site, thereby permanently blocking TXA2
synthesis. This irreversible inhibition persists throughout the
platelet's lifespan.
 | Table IMajor characteristics of previous
studies. |
Table I
Major characteristics of previous
studies.
| Author/s, year | Age, years | Sex | Cardiac surgery | Time after
surgery | Treatment for PA | Surgery time after
cardiac surgery | Outcome of PA | (Refs.) |
|---|
| Semenov et al,
2000 | 82 | Male | Coronary artery
bypass surgery | Immediate
post-operative | Transsphenoidal
surgery | 13 days | Right visual field
defect and need for hormone replacement therapy at 15-month
follow-up | (7) |
| Cooper et al,
1986 | 63 | Male | Coronary artery
bypass surgery | 12 h | Transsphenoidal
surgery | 2 weeks | Ophthalmoplegia
resolved at 2-month follow-up | (8) |
| | 62 | Male | Mitral and aortic
valve replacement | 12 h | Transsphenoidal
surgery | 21 h | Sixth cranial nerve
paresis and slight right ptosis at three weeks
post-operatively | |
| | 55 | Male | Coronary artery
bypass surgery | Immediate
post-operative | Transsphenoidal
surgery | 18 h | Slight ptosis at
2-month follow-up | |
| Fuchs et al,
1998 | 53 | Male | Coronary stent
implantation | NA | Conservative
management | NA | Ophthalmoplegia
improved markedly at 3-month follow-up | (9) |
| | 65 | Male | Coronary stent
implantation | On postoperative day
1 | Transsphenoidal
surgery | Emergency
operation | Right eye blindness
and left visual field defect at 3-month follow-up | |
| Loubani et al,
2001 | 60 | Male | Coronary artery
bypass surgery | On postoperative day
1 | Surgery | On postoperative day
5 | Minor visual defect
on the right. MRI shows complete resolution of the pituary adenoma
at 1-year follow-up | (10) |
| Mattke et al,
2002 | 64 | Male | Coronary artery
bypass surgery | Immediate
post-operative | Transsphenoidal
surgery | 9 days | Visual acuity and
visual field defects were both markedly improved after surgery | (11) |
| Chen et al,
2004 | 62 | Male | Coronary artery
bypass surgery | 3 h | Transsphenoidal
surgery | 4 days | Almost recovery and
no residual pituitary tumor at four-month follow-up | (12) |
The diagnosis of PA can be readily confirmed through
clinical symptoms, endocrine evaluations and imaging studies.
However, the optimal management approach remains a subject of
ongoing debate. Earlier guidelines from the UK recommended surgical
decompression for patients presenting with significant
neuro-ophthalmic symptoms or reduced consciousness (15). By contrast, conservative management
with close monitoring is generally preferred for patients without
neuro-ophthalmological symptoms, hemodynamic stability or altered
consciousness (4). A meta-analysis
comparing 259 surgical and 198 conservative cases, along with a
multicenter international prospective registry study from 2024
involving 67 surgical and 30 medical cases, demonstrated that both
surgical and non-surgical approaches can lead to the restoration of
visual and endocrine functions, yielding comparable clinical
outcomes in PA (16,17). The impact of surgical timing on
neurological recovery remains an area of active investigation,
though findings are inconsistent. Certain studies suggest that
early surgical intervention significantly improves visual acuity,
visual fields and pituitary function recovery (18-20),
while others report no significant effect of surgical timing on
visual outcomes in patients with PA (21,22).
These discrepancies may be attributed to factors such as variations
in sample size, differing definitions of early vs. late surgery
(based on the interval between symptom onset and surgery) and
potential biases inherent in observational studies. Given the
rarity of PA, conducting randomized controlled trials is
particularly challenging. However, despite being derived from
observational research, these findings provide valuable insights
into PA management.
In the present case, emergency surgical intervention
was performed at 14 h after symptom onset. The decision to proceed
with surgery was based on four critical factors: i) The patient had
severe bilateral visual impairment, with the right eye affected by
PA and the left eye already compromised due to prior trauma.
Emergency transsphenoidal decompression was prioritized to maximize
the chances of visual recovery in the PA-affected right eye; ii)
although spontaneous tumor regression following PA has been
reported in certain studies (2,16),
there are no reliable predictors to identify patients who would
benefit from conservative management; iii) the procedure was
performed by a highly experienced neurosurgical team specializing
in pituitary surgery, significantly reducing the risk of
complications such as cerebrospinal fluid leakage or iatrogenic
damage to functional pituitary tissue; and iv) no surgical
contraindications were identified during the comprehensive
preoperative evaluation. As anticipated, the patient experienced
significant visual improvement without any major postoperative
complications.
In conclusion, PA is a rare complication,
particularly in this case, where it occurred after nearly four
months of continuous aspirin use following coronary stent
implantation. However, complete recovery was achieved through a
multidisciplinary approach, including transsphenoidal endoscopic
surgery, endocrine management and cardiovascular therapy. This case
also broadens the understanding of the pathophysiological
mechanisms and temporal occurrence of PA. Traditionally associated
with the perioperative period due to intraoperative heparinization
and hemodynamic fluctuations, PA may also develop after prolonged
exposure to antiplatelet therapy, suggesting a potential risk
factor in its pathogenesis. While routine screening and
prophylactic measures may not be cost-effective given the low
incidence of pituitary adenomas and PA, it is crucial for
clinicians to educate patients undergoing long-term antiplatelet
therapy after coronary stent implantation. If symptoms suggestive
of PA-such as headaches or visual disturbances-arise, prompt
referral to a high-volume pituitary disease center with specialized
expertise is essential.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CS, DZ, JW, JL and NW designed the study. CS, DZ, JW
and NW collected and analyzed the clinical data. CS and DZ reviewed
previous cases. CS, DZ, JW, JL and NW wrote and revised the paper.
CS and NW confirmed the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were performed in in accordance with
the 1964 Helsinki declaration and its later amendments or
comparable ethical standards.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this manuscript and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abbara A, Clarke S, Eng PC, Milburn J,
Joshi D, Comninos AN, Ramli R, Mehta A, Jones B, Wernig F, et al:
Clinical and biochemical characteristics of patients presenting
with pituitary apoplexy. Endocr Connect. 7:1058–1066.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Briet C, Salenave S, Bonneville JF, Laws
ER and Chanson P: Pituitary Apoplexy. Endocr Rev. 36:622–645.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Daly AF and Beckers A: The epidemiology of
pituitary adenomas. Endocrinol Metab Clin North Am. 49:347–355.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Iglesias P: Pituitary apoplexy: An updated
review. J Clin Med. 13(2508)2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gheorghe AM, Trandafir AI, Ionovici N,
Carsote M, Nistor C, Popa FL and Stanciu M: Pituitary apoplexy in
patients with pituitary neuroendocrine tumors (PitNET).
Biomedicines. 11(680)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Muthukumar N: Pituitary apoplexy: A
comprehensive review. Neurol India. 68 (Supplement):S72–S78.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Semenov A, Denoix E, Thiebaut M, Michon A
and Pouchot J: Pituitary apoplexy following coronary bypass
surgery: A case report and literature review. Rev Med Interne.
41:852–857. 2020.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
8
|
Cooper DM, Bazaral MG, Furlan AJ, Sevilla
E, Ghattas MA, Sheeler LR, Little JR, Hahn JF, Sheldon WC and Loop
FD: Pituitary apoplexy: A complication of cardiac surgery. Ann
Thorac Surg. 41:547–550. 1986.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fuchs S, Beeri R, Hasin Y, Weiss AT,
Gotsman MS and Zahger D: Pituitary apoplexy as a first
manifestation of pituitary adenomas following intensive
thrombolytic and antithrombotic therapy. Am J Cardiol. 81:110–111.
1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Loubani M and Galiñanes M: Pituitary gland
macroadenoma: A cause of transient blindness after cardiac surgery.
Ann Thorac Surg. 72:929–931. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mattke AF, Vender JR and Anstadt MR:
Pituitary apoplexy presenting as addisonian crisis after coronary
artery bypass grafting. Tex Heart Inst J. 29:193–199.
2002.PubMed/NCBI
|
|
12
|
Chen Z, Murray AW and Quinlan JJ:
Pituitary apoplexy presenting as unilateral third cranial nerve
palsy after coronary artery bypass surgery. Anesth Analg. 98:46–48.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kulikov A, Konovalov A, Pugnaloni PP and
Bilotta F: Aspirin interruption before neurosurgical interventions:
A controversial problem. World J Cardiol. 16:191–198.
2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Crescente M, Menke L, Chan MV, Armstrong
PC and Warner TD: Eicosanoids in platelets and the effect of their
modulation by aspirin in the cardiovascular system (and beyond). Br
J Pharmacol. 176:988–999. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rajasekaran S, Vanderpump M, Baldeweg S,
Drake W, Reddy N, Lanyon M, Markey A, Plant G, Powell M, Sinha S,
et al: UK guidelines for the management of pituitary apoplexy. Clin
Endocrinol (Oxf). 74:9–20. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Goshtasbi K, Abiri A, Sahyouni R, Mahboubi
H, Raefsky S, Kuan EC, Hsu FPK and Cadena G: Visual and endocrine
recovery following conservative and surgical treatment of pituitary
apoplexy: A meta-analysis. World Neurosurg. 132:33–40.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mamelak AN, Little AS, Gardner PA, Almeida
JP, Recinos P, Soni P, Kshettry VR, Jane JA Jr, Barkhoudarian G,
Kelly DF, et al: A prospective, multicenter, observational study of
surgical vs nonsurgical management for pituitary apoplexy. J Clin
Endocrinol Metab. 109:e711–e725. 2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Randeva HS, Schoebel J, Byrne J, Esiri M,
Adams CB and Wass JA: Classical pituitary apoplexy: Clinical
features, management and outcome. Clin Endocrinol (Oxf).
51:181–188. 1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Singh TD, Valizadeh N, Meyer FB, Atkinson
JL, Erickson D and Rabinstein AA: Management and outcomes of
pituitary apoplexy. J Neurosurg. 122:1450–1457. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cabuk B, Kaya NS, Polat C, Geyik AM, Icli
D, Anik I and Ceylan S: Outcome in pituitary apoplexy patients,
stratified by delay between symptom appearance and surgery: A
single center retrospective analysis. Clin Neurol Neurosurg.
210(106991)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Paredes I, Rodríguez-Berrocal V,
Pérez-López C, García-Feijoo P, Alvarez-Escola C, Acitores Cancela
A, Araujo-Castro M, Calatayud M, Librizzi MS and Lagares A:
Influence of surgical timing on the visual prognosis of patients
suffering from a pituitary apoplexy with visual impairment.
Neurosurg Rev. 47(852)2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kelly PD, Fernando SJ, Malenke JA, Chandra
RK, Turner JH and Chambless LB: The effect of timing of surgery in
pituitary apoplexy on continuously valued visual acuity. J Neurol
Surg B Skull Base. 82 (Suppl 3):e70–e78. 2021.PubMed/NCBI View Article : Google Scholar
|