Introduction
Coronary heart disease (CHD) is a prevalent
condition with a high incidence and mortality rate. The global
incidence of CHD continues to rise annually, with an increasing
trend toward a younger age of onset (1). According to the American Heart
Association (2), an estimated
254.2 million people worldwide are affected by CHD, resulting in
~9.21 million deaths each year. CHD is the leading cause of heart
failure, characterized by its long duration, challenging
management, and poor prognosis. It not only severely affects the
quality of life of patients, but also poses a significant threat to
their longevity and overall health (3). The pathogenesis of CHD is
multifactorial, with lipid deposition on the vessel walls, smooth
muscle cell proliferation, and fibrous matrix formation being the
primary contributors to atherosclerotic plaque development.
Inflammation plays a critical role throughout the stages of
atherosclerosis, involving various immune cells that contribute to
plaque instability or rupture, arterial narrowing, and obstruction.
These processes can lead to myocardial ischemia, hypoxia, necrosis,
and ultimately, acute cardiovascular events (4). Several inflammatory biomarkers, such
as C-reactive protein, interleukin-2 (IL-2), and tumor necrosis
factor-α (TNF-α), have been implicated in the progression of
atherosclerosis (5), and their
detection is valuable for early diagnosis of CHD. Despite advances
in diagnostic techniques and treatments, there remains a
significant need for simple, highly specific, and reproducible
methods to diagnose CHD effectively.
Sulfatide, an acidic sphingolipid composed of
ceramide, galactose, and sulfate, is widely distributed in the
organs and serum of both animals and humans. A previous study has
demonstrated a strong association between serum sulfatide levels
and atherosclerosis, inflammation, and thrombosis (6). The present study proposes for the
first time the measurement of fecal sulfatide levels and the
investigation of their relationship with the onset and progression
of CHD. The aim of the present study was to identify new stool
biomarkers for CHD, providing a non-invasive, convenient, and
accurate method for detecting cardiovascular disease (CVD) risk
factors. This could pave the way for a new era of fecal biomarker
detection in the prevention and treatment of CHD.
Both neutrophil and lymphocyte counts reflect the
natural physiological response of the body to stress, trauma,
surgery, and infection. The neutrophil-to-lymphocyte ratio (NLR), a
combined inflammatory marker, integrates cellular and humoral
immunity, offering a more comprehensive indication of dynamic
changes in inflammatory and immune response pathways compared to
single indicators. NLR is a simple, reliable, and effective
parameter for assessing the intensity of neuroendocrine stress and
immune-inflammatory responses (7).
In recent years, NLR has gained significant attention in CHD
research. A previous study showed a strong association between
elevated NLR levels and the severity of coronary artery disease
(8). Higher NLR levels have also
been found to predict the prognosis of patients with CHD,
recurrence of myocardial infarction, and long-term adverse events
in patients with acute coronary syndrome (ACS) following
percutaneous coronary intervention (PCI) and coronary artery bypass
grafting (9). The present study
explored the predictive value of fecal sulfatide and NLR, both
individually and in combination, for CHD, offering valuable
insights for early detection.
Materials and methods
Participants
A total of 523 patients diagnosed with CHD at the
Division of Cardiology of Hebei General Hospital (Shijiazhuang,
China) from August 2022 to September 2023 were included in the
experimental group. The mean age of the participants was 64.79±0.41
years, with 353 males and 170 females. The experimental group was
further subdivided into three categories: i) Stable angina pectoris
(194 cases), ii) unstable angina pectoris (134 cases), and iii)
acute myocardial infarction (AMI; 195 cases). The control group
consisted of 198 healthy individuals who underwent routine physical
examinations at the aforementioned hospital during the same period.
Inclusion criteria for the experimental group required patients to
meet the diagnostic criteria for CHD and provide informed consent.
Exclusion criteria included the presence of malignant tumors,
congenital heart disease, valvular disease, cardiomyopathy, thyroid
disease, immune system and blood disorders, cerebrovascular
diseases, acute and chronic infections, and mental disorders. The
present study was approved by the Ethics Committee of Hebei General
Hospital (approval no. 20220231) and was conducted in accordance
with The Declaration of Helsinki. All participants provided
informed consent before participation.
Clinical characteristics
Data on general characteristics were collected for
all participants, including age, sex, body mass index (BMI),
smoking history (defined as smoking ≥1 pack/day for at least one
year), and alcohol consumption history (defined as regular alcohol
consumption ≥100 ml/day of 50% alcohol content for at least one
year).
Biochemical analysis
Professional nurses collected 10 ml of cubital
venous blood from each participant after an 8-h overnight fast
using a sodium citrate anticoagulant tube. The blood samples were
then sent to the laboratory for analysis. Biochemical parameters
and cell flow analysis were performed using a Beckman-Coulter
automatic biochemical analyzer with the necessary reagents, all
provided by the Laboratory Department of Hebei General Hospital.
The biochemical markers assessed included albumin, alanine
aminotransferase (ALT), aspartate aminotransferase (AST),
high-density lipoprotein cholesterol (HDL-C), low-density
lipoprotein cholesterol (LDL-C), and the NLR.
Measurement of fecal sulfatide
Approximately 1 g of fresh stool was harvested from
each patient, rinsed three times with PBS, and then subjected to
ultrasonic crushing. The sample was centrifuged at 11,180 x g for
10 min at 4˚C, and the supernatant was collected for further
analysis. Fecal sulfatide levels were quantified using the ELISA
method according to the instructions provided with the kit (human
sulfatide ELISA kit; cat. no. MM-60644H2; Jiangsu Meimian
Industrial Co., Ltd.).
Statistical analysis
Statistical analyses were performed utilizing SPSS
v.26.0 (IBM Corp.) software. Normally distributed data were
summarized as the means ± standard deviation (SD). For comparisons
between two groups, an unpaired Student's t-test was used, while a
one-way analysis of variance (ANOVA) was employed for comparisons
among multiple groups and Bonferroni method was used for comparison
among groups. When significant differences were found between
groups, pairwise comparisons were conducted using LSD or
Games-Howell tests. Non-normally distributed data were presented as
median and interquartile range, with comparisons between two groups
performed using the Mann-Whitney U test, and comparisons between
multiple groups using the Kruskal-Wallis test. Frequency or
percentage data were analyzed utilizing Pearson's chi-squared test
for group comparisons. To assess the influencing factors of CHD,
multiple logistic regression was applied. Receiver operating
characteristic (ROC) curves were generated to evaluate the
diagnostic value of fecal sulfatide and NLR for CHD, and Z-tests
were conducted to compare differences in the area under the curve
(AUC). Statistical significance was set at P<0.05.
Results
The baseline characteristics of the study
participants are summarized in Table
I. Comparison between the CHD and non-CHD groups revealed no
statistically significant differences in the proportion of males,
BMI, smoking history, or alcohol consumption history (P>0.05),
suggesting that these factors may not be primary contributors to
the development of CHD in this cohort. However, the CHD group had
notably higher levels of age, ALT, AST, and LDL-C compared with the
non-CHD group (P<0.05). These findings suggest that older age,
elevated liver enzymes (ALT and AST), and increased LDL-C levels
may be associated with an elevated risk of CHD. By contrast, the
levels of albumin and HDL-C were distinctly lower in the CHD group
(P<0.05). Furthermore, it was observed that the NLR and fecal
sulfatide levels were notably higher in the CHD group compared with
the non-CHD group. These results highlight the potential role of
inflammatory markers and lipid metabolism in the development of
CHD, underscoring the need for further exploration of these
parameters.
 | Table IBaseline and clinical data of
individuals in the non-CHD and CHD groups. |
Table I
Baseline and clinical data of
individuals in the non-CHD and CHD groups.
| Characteristics | Νon-CHD group
(n=198) | CHD group
(n=523) |
t/Z/χ2 | P-value |
|---|
| Age (years) | 60 (55, 62) | 66 (58, 72) | -8.333 | <0.001 |
| Male [n (%)] | 139 (70.2) | 353 (67.5) | 0.486 | 0.486 |
| BMI (kg/m2) | 25.7±2.9 | 25.5±3.5 | 0.797 | 0.426 |
| Smoking [n (%)] | 70 (35.4) | 190 (36.3) | 0.059 | 0.808 |
| Drinking [n (%)] | 35 (17.7) | 97 (18.5) | 0.073 | 0.787 |
| Albumin (g/l) | 44.5±2.9 | 39.6±4.7 | 17.053 | <0.001 |
| ALT (U/l) | 21.2 (15.8,
30.0) | 23.6 (15.9,
41.1) | -2.713 | 0.007 |
| AST(U/l) | 23.1 (19.0,
27.6) | 25.6 (19.0,
64.6) | -4.613 | <0.001 |
| HDL-C (mmol/l) | 1.4±0.3 | 1.1±0.3 | 12.050 | <0.001 |
| LDL-C (mmol/l) | 3.2±0.8 | 4.1±0.8 | -14.567 | <0.001 |
| NLR | 1.65 (1.33,
2.41) | 2.92 (2.02,
4.92) | -12.895 | <0.001 |
| Fecal sulfatide
(µmol/l) | 1.64±0.39 | 2.40±0.48 | -21.932 | <0.001 |
Additionally, as demonstrated in Table II, when examining the sex
differences in fecal sulfatide and NLR levels across the entire
cohort, it was observed that the average sulfatide level in males
was 2.17±0.58 µmol/l, and in females, it was 2.24±0.54 µmol/l, with
no statistically significant difference between the groups
(P=0.109). Similarly, the NLR level in males was 2.52 (1.75,3.83),
while in females, it was 2.33 (1.54,3.75), with no significant
difference observed between the groups (P=0.147). When further
analyzing the differences in fecal sulfatide and NLR levels by sex
within the CHD and non-CHD groups (Table III), it was found that in male
patients, the CHD group had higher sulfatide levels (2.40±0.48
µmol/l vs. 1.60±0.36 µmol/l; P<0.001) and NLR levels [2.96
(2.09,4.85) vs. 1.69 (1.35,2.16)] compared with the non-CHD group.
Similarly, in female patients, the CHD group exhibited higher
sulfatide levels (2.41±0.47 µmol/l vs. 1.75±0.43 µmol/l;
P<0.001) and NLR levels [2.81 (1.83,5.32) vs. 1.60 (1.28,2.06)]
compared with the non-CHD group. These findings revealed that the
elevated levels of fecal sulfatide and NLR in the CHD population
are not significantly influenced by sex.
 | Table IISex differences in fecal sulfatide and
NLR levels. |
Table II
Sex differences in fecal sulfatide and
NLR levels.
| Parameter | Male (n=492) | Female (n=229) | t/Z | P-value |
|---|
| Fecal sulfatide
(µmol/l) | 2.17±0.58 | 2.24±0.54 | -1.605 | 0.109 |
| NLR | 2.52 (1.75,
3.83) | 2.33 (1.54,
3.75) | -1.450 | 0.147 |
 | Table IIISex differences in fecal sulfatide and
NLR levels within the CHD and non-CHD groups. |
Table III
Sex differences in fecal sulfatide and
NLR levels within the CHD and non-CHD groups.
| Parameter | Sex | Non-CHD group | CHD group | t/Z | P-value |
|---|
| Fecal sulfatide
(µmol/l) | Male | 1.60±0.36 | 2.40±0.48 | -20.075 | <0.001 |
| | Female | 1.75±0.43 | 2.41±0.47 | -9.444 | <0.001 |
| NLR | Male | 1.69 (1.35,
2.16) | 2.96 (2.09,
4.85) | -11.049 | <0.001 |
| | Female | 1.60 (1.28,
2.06) | 2.81 (1.83,
5.32) | -6.765 | <0.001 |
A comparison of clinical data across different
subgroups of CHD is presented in Table IV. Significant differences were
noted in age, albumin, ALT, AST, HDL-C, LDL-C, NLR and fecal
sulfatide levels among the three subgroups (P<0.05). Pairwise
comparisons revealed that patients in the stable angina pectoris
group were younger than those in the AMI group. Additionally,
albumin levels were notably higher in the stable angina and
unstable angina pectoris groups compared with the AMI group.
Furthermore, ALT, AST, LDL-C, and NLR levels were elevated in the
AMI group relative to both the stable and unstable angina groups.
The fecal sulfatide levels were also higher in the AMI group than
in the stable angina pectoris group.
 | Table IVComparison of clinical data between
different coronary heart disease subgroups. |
Table IV
Comparison of clinical data between
different coronary heart disease subgroups.
|
Characteristics | Stable angina
pectoris (n=194) | Unstable angina
pectoris (n=134) | Acute myocardial
infarction (n=195) | F/H | P-value |
|---|
| Age (years) | 64 (57, 69) | 66 (57, 73) | 68 (59,
73)a | 13.763 | 0.001 |
| Albumin (g/l) | 41.51±3.52 | 40.79±3.39 |
36.77±5.12a,b | 59.997 | <0.001 |
| ALT (U/l) | 18.7 (14.4,
29.2) | 20.2 (14.8,
29.1) | 42.4 (21.8,
105.3)a,b | 107.270 | <0.001 |
| AST (U/l) | 21.0 (17.8,
25.6) | 20.4 (17.4,
26.8) | 106.0 (50.9,
367.4)a,b | 271.031 | <0.001 |
| HDL-C (mmol/l) |
1.15±0.29b |
1.06±0.24a | 1.11±0.28 | 3.725 | 0.025 |
| LDL-C (mmol/l) | 3.87±0.71 | 3.94±0.73 |
4.57±0.64a,b | 59.160 | <0.001 |
| NLR | 2.25 (1.67,
2.93)b | 2.68 (1.99,
3.43)a | 5.55 (3.37,
11.83)a,b | 180.217 | <0.001 |
| Fecal sulfatide
(µmol/l) | 2.32±0.48 | 2.39±0.50 |
2.50±0.44a | 7.040 | 0.001 |
For the multivariate analysis, CHD was set as the
dependent variable, and age, albumin, ALT, AST, HDL-C, LDL-C, NLR,
and fecal sulfatide levels were considered as independent
variables. As demonstrated in Table
V, age, albumin, HDL-C, NLR, and fecal sulfatide were
identified as significant factors in the development of CHD. Age,
NLR, and fecal sulfatide were found to be risk factors, while
albumin and HDL-C were protective factors.
 | Table VLogistic regression analysis of the
factors influencing CHD. |
Table V
Logistic regression analysis of the
factors influencing CHD.
|
Characteristics | B | SE | Wald | OR | 95% CI | P-value |
|---|
| Age (years) | 0.007 | 0.025 | 9.344 | 1.080 | 1.028-1.135 | 0.002 |
| Albumin (g/l) | -0.383 | 0.079 | 23.418 | 0.682 | 0.584-0.796 | <0.001 |
| ALT (U/l) | 0.009 | 0.020 | 0.190 | 1.009 | 0.970-1.049 | 0.663 |
| AST (U/l) | 0.028 | 0.026 | 1.170 | 1.028 | 0.978-1.081 | 0.279 |
| HDL-C (mmol/l) | -6.041 | 0.847 | 50.879 | 0.002 | 0.000-0.013 | <0.001 |
| LDL-C (mmol/l) | -0.352 | 0.396 | 0.791 | 0.703 | 0.324-1.528 | 0.374 |
| NLR | 1.365 | 0.265 | 26.463 | 3.917 | 2.328-6.589 | <0.001 |
| Fecal sulfatide
(µmol/l | 7.243 | 0.883 | 67.311 | 1,397.627 | 247.72-7,
885.122 | <0.001 |
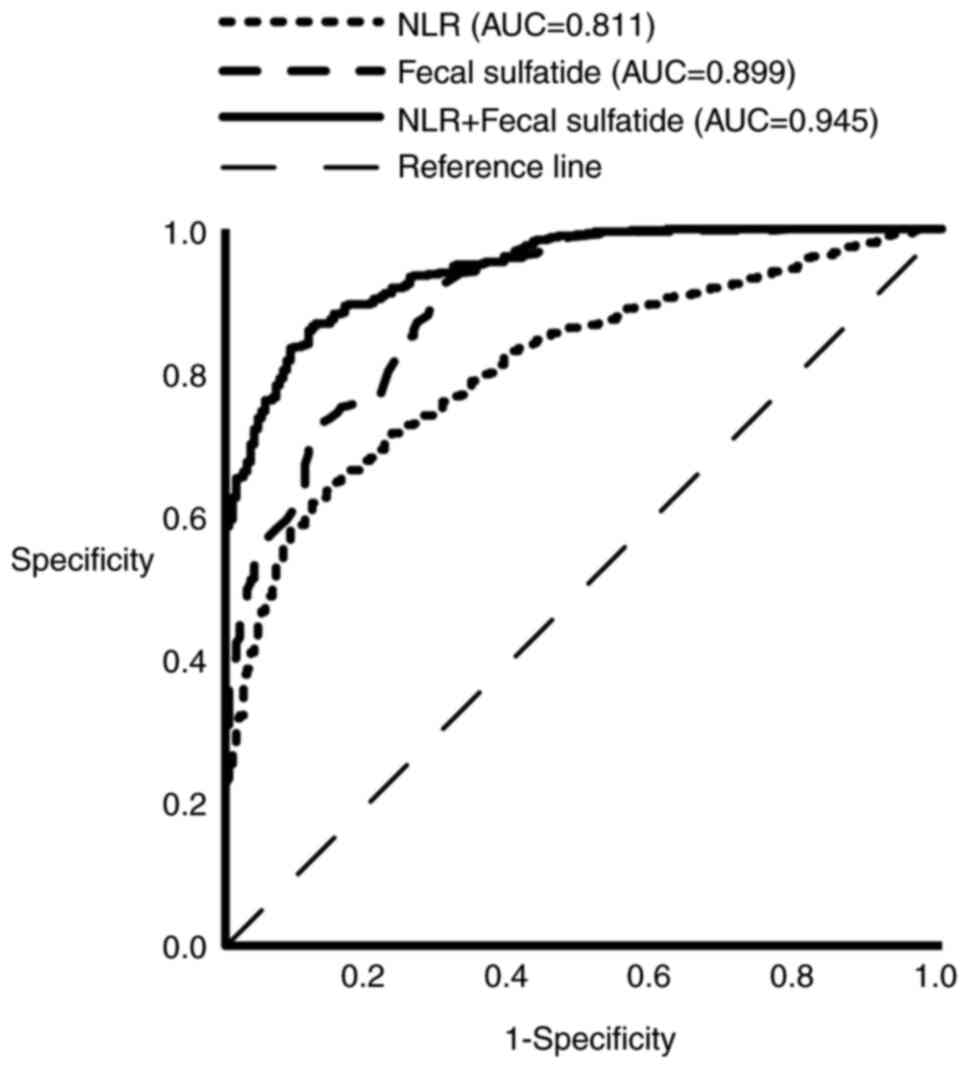
ROC analysis was implemented to evaluate the
predictive value of NLR and fecal sulfatide, both individually and
in combination, for the diagnosis of CHD. As illustrated in
Table VI the AUC for NLR was
0.811, with an NLR threshold of >2.529 providing strong
predictive power for CHD, showing a sensitivity of 0.618 and
specificity of 0.879. The AUC for fecal sulfatide was 0.899, and a
fecal sulfatide level >1.825 µmol/l was highly predictive of
CHD. The AUC for the combined use of NLR and fecal sulfatide was
0.945 (95% CI, 0.929-0.960; P<0.05). Based on Fig. 1 and Table VI, it was concluded that the
combination of NLR and fecal sulfatide demonstrates superior
predictive ability for the occurrence of CHD compared with either
marker alone.
 | Table VIComparison of the areas under ROC
curves of different indexes. |
Table VI
Comparison of the areas under ROC
curves of different indexes.
| Predictors | AUC | 95% CI | Sensitivity | Specificity | P-value |
|---|
| NLR | 0.811 | 0.779-0.843 | 0.618 | 0.879 | <0.001 |
| Fecal
sulfatide | 0.899 | 0.874-0.924 | 0.920 | 0.702 | <0.001 |
| NLR + fecal
sulfatide | 0.945 | 0.929-0.960 | 0.864 | 0.879 | <0.001 |
Discussion
CHD is a common and increasingly prevalent clinical
condition, posing a significant threat to public health worldwide
(1). The development and
progression of CHD involve the formation of coronary
atherosclerotic plaques, plaque rupture, and subsequent
intravascular thrombosis. Traditional risk factors for CHD include
age, male sex, obesity, elevated total cholesterol, high LDL-C
levels, smoking, hypertension, and a strong family history of CHD,
among others (5). Inflammation is
pivotal in the pathogenesis of atherosclerosis. During plaque
formation, endothelial cells are activated by various stimuli,
triggering the aggregation of leukocytes. Monocytes continuously
migrate and accumulate within the developing plaques, where they
differentiate into macrophages, proliferate, and form lipid-laden
foam cells that accelerate plaque progression. Persistent
pathological inflammation further exacerbates this process by
reducing collagen production in the extracellular matrix and
increasing the activity of collagenolytic enzymes. This leads to
thinning of the fibrous cap, making it more vulnerable to rupture
and thrombosis (10).
Sulfatide, a major glycolipid found in the serum
lipoproteins of various mammals and humans, is widely distributed
throughout the body and plays a significant physiological role in
the metabolism of several tissues and organs. A previous study
showed that in animal models with atherosclerotic lesions, serum
sulfatide concentrations are distinctly elevated, leading to its
accumulation in atherosclerotic plaques and thickened arterial
walls (11). This suggests that
sulfatide may play a critical role in the development of
atherosclerosis and CVDs. Previous studies from the authors have
demonstrated a strong association between sulfatide and
atherosclerosis. Specifically, serum sulfatide levels were found to
be positively associated with carotid intima-media thickness,
serving as an independent risk factor for atherosclerosis in
hypertensive patients (12).
Additionally, high serum sulfatide concentrations were revealed to
be associated with an increased risk of restenosis in patients with
CHD following PCI (6). Moreover,
serum sulfatide levels have been shown to reflect the severity of
heart injury, with significant correlations observed in patients
with ST-elevation myocardial infarction (13). Other studies have also established
a close relationship between serum sulfatide and inflammation,
thrombosis (14), and intimal
hyperplasia (15). Traditional
methods for measuring blood lipids and serum sulfatide, which
typically require fasting venous blood samples, may not provide
timely and accurate results, leading to potential inconvenience and
risks for patients. By contrast, fecal sulfatide detection is
simple, non-invasive, and more adaptable. Therefore, the aim of the
present study was to explore the association between fecal
sulfatide levels and the occurrence of CHD.
The NLR has emerged as a promising biomarker for CHD
risk stratification, reflecting the complex interactions between
systemic inflammation, immune dysregulation, and vascular
pathology. Several mechanisms help explain its prognostic value.
Neutrophils, key players in innate immunity, contribute to vascular
damage through the release of pro-inflammatory cytokines such as
IL-6 and TNF-α, as well as reactive oxygen species (16,17).
These factors impair the bioavailability of endothelial nitric
oxide and promote the oxidative modification of LDL, which
accelerates foam cell formation and the progression of
atherosclerotic plaques. Therefore, the NLR serves as a marker of
the inflammatory environment that drives atherosclerosis. Moreover,
previous studies have found that neutrophils can promote plaque
vulnerability through releasing neutrophil extracellular traps
(NETs), which expose pro-coagulant molecules (such as tissue
factor) and degrade collagen in the fibrous cap (18,19).
Elevated NLR has been associated with unstable plaque features,
such as a thin fibrous cap and a large necrotic core. Additionally,
NETs activate platelets and trigger the coagulation cascade,
linking inflammation to atherosclerotic thrombosis, particularly in
acute coronary syndrome. NLR may also reflect sympathetic-adrenal
activation and stress responses (20). Research indicates that during acute
myocardial ischemia, a surge in catecholamines stimulates the bone
marrow, leading to neutrophilia and lymphocyte apoptosis, thereby
temporarily elevating NLR. These stress-induced immune changes may
exacerbate endothelial injury and predict adverse outcomes
following myocardial infarction (21).
The use of NLR as a biomarker for cardiovascular
diseases is well-established, with numerous studies (22-26)
confirming its diagnostic, evaluative, and prognostic value across
various conditions, including cardiovascular diseases. This raises
concerns about the novelty of using NLR alone as a biomarker for
CHD. A meta-analysis has demonstrated that NLR can predict
hospitalization and long-term outcomes in patients with ST-segment
elevation myocardial infarction following PCI (22). In a study involving 364 consecutive
patients undergoing PCI, the high NLR group exhibited a notably
higher incidence of major adverse cardiovascular events compared
with the low NLR group (23).
Additionally, Xu et al (24) found that NLR could predict the
long-term prognosis of patients with myocardial infarction
involving left main and/or three-vessel lesions. In a cohort of
2,967 patients with ACS and 571 patients without ACS, NLR was
identified as a strong predictor of a high Gensini score, with an
NLR >2.04 indicating the presence of ACS (25). Another study suggested that NLR is
effective in monitoring vascular inflammation and platelet
stability, making it a timely predictor for atherosclerotic
cardiovascular and cerebrovascular diseases (26). The novel aspect of the present
study lies in the addition of fecal sulfatide as a complementary
biomarker to NLR. The reported increase in AUC from 0.811 (NLR
alone) and 0.899 (fecal sulfatide alone) to 0.945 (NLR + fecal
sulfatide) suggests a potential clinical benefit in combining these
markers for enhanced prediction of CHD.
The present study identified age as a significant
risk factor for CHD, while albumin and HDL-C were found to be
protective factors, which is consistent with previous research. It
was observed that fecal sulfatide and serum NLR levels were notably
higher in the CHD group compared with the control group.
Furthermore, NLR levels were elevated in patients with AMI compared
to those with stable and unstable angina pectoris. Similarly, fecal
sulfatide levels were higher in patients with AMI than in those
with stable angina pectoris. Multivariate logistic regression
analysis revealed that both fecal sulfatide and NLR were
independent risk factors for CHD. Notably, fecal sulfatide
(AUC=0.899) and NLR (AUC=0.811) demonstrated strong predictive
ability for CHD, and the combination of both biomarkers notably
enhanced predictive accuracy (AUC=0.945).
Despite these promising findings, the present study
has some limitations. The sample size was relatively small and
derived from a single center. Moreover, factors such as diet and
medication may have influenced the levels of the biomarkers
studied. Future research will expand the sample size and include a
multi-center approach to better assess the predictive value of
these biomarkers for CHD.
The present study is the first, to the best of our
knowledge, to measure fecal sulfatide levels in patients diagnosed
with CHD using the ELISA method. The results suggest that elevated
fecal sulfatide levels are closely associated with the onset and
progression of CHD. Additionally, the integration of fecal
sulfatide as a complementary biomarker to NLR is a novel aspect of
the present study. In order to strengthen the theoretical basis for
the early diagnosis and treatment of CHD, a larger clinical study
will be performed by the authors, to explore the mechanisms related
to the influence of NLR and fecal sulfatide on CHD.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 82103475), the Natural
Science Foundation of Hebei Province (grant no. H2023307028; and
H2024206056), the Medical Science Research Program of Health
Commission of Hebei Province (grant no. 20240370), the Hebei
Province Government-Funded Clinical Medicine Excellent Talent
Training Program (grant no. ZF2025013) and the Hebei Province
Medical Adaptation Technology Tracking Project (grant no.
GZ2024002).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KX, YL and RH jointly participated in the
preliminary design of the study, and completed the data extraction
and collation and the first draft of the paper. XH participated in
data sorting and data analysis. RG and HG participated in the
design of the experimental method. GL participated in the
preliminary design of the study, funding acquisition and reviewed
and revised the first draft of the paper. KX and YL confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hebei General Hospital (approval no. 20220231). The
methods were carried out in accordance with The Declaration of
Helsinki. Written informed consent was obtained from all
participants after they were fully informed of the study's purpose,
procedures, and potential risks.
Patient consent for publication
All the patients have been informed and signed
informed consent before the experiments.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu SS: Writing Committee of the Report on
Cardiovascular Health and Diseases in China. Epidemiology and
current management of cardiovascular disease in China. J Geriatr
Cardiol. 21:387–406. 2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Martin SS, Aday AW, Almarzooq ZI, Anderson
CAM, Arora P, Avery CL, Baker-Smith CM, Barone Gibbs B, Beaton AZ,
Boehme AK, et al: 2024 heart disease and stroke statistics: A
report of US and global data from the American heart association.
Circulation. 149:e347–e913. 2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pastena P, Frye JT, Ho C, Goldschmidt ME
and Kalogeropoulos AP: Ischemic cardiomyopathy: Epidemiology,
pathophysiology, outcomes, and therapeutic options. Heart Fail Rev.
29:287–299. 2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ
and Han M: Inflammation and atherosclerosis: Signaling pathways and
therapeutic intervention. Signal Transduct Target Ther.
7(131)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gupta L, Thomas J, Ravichandran R, Singh
M, Nag A and Panjiyar BK: Inflammation in cardiovascular disease: A
comprehensive review of biomarkers and therapeutic targets. Cureus.
15(e45483)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li G, Hu R, Wu HZ and Chen SX: High serum
concentration of sulfatide is a risk factor for restenosis in
patients with coronary heart disease after percutaneous coronary
intervention. Metabolomics (Los Angel). 5(1)2015.
|
|
7
|
Zahorec R: Neutrophil-to-lymphocyte ratio,
past, present and future perspectives. Bratisl Lek Listy.
122:474–488. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li S, Chen H, Zhou L, Cui H, Liang S and
Li H: Neutrophil-to-lymphocyte ratio predicts coronary artery
lesion severity and long-term cardiovascular mortality in patients
with unstable angina pectoris. Acta Cardiol. 77:708–715.
2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Agarwal R, Aurora RG, Siswanto BB and
Muliawan HS: The prognostic value of neutrophil-to-lymphocyte ratio
across all stages of coronary artery disease. Coron Artery Dis.
33:137–143. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Conti CR: Epidemiology, pathophysiology,
and therapeutic targets in stable ischemic heart disease.
Cardiovasc Innov App. 3:279–283. 2019.
|
|
11
|
Hara A and Taketomi T: Characterization
and changes of glycosphingolipids in the aorta of the Watanabe
hereditable hyperlipidemic rabbit. J Biochem. 109:904–908.
1991.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li G, Hu R, Gu J and Wu HZ: Relationship
between carotid artery atherosclerosis and sulfatide in
hypertensive patients. Genet Mol Res. 14:4840–4846. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li G, Hu R, Guo Y, He L, Zuo Q and Wang Y:
Circulating sulfatide, a novel biomarker for ST-segment elevation
myocardial infarction. J Atheroscler Thromb. 26:84–92.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Inoue T, Taguchi I, Abe S, Abe S, Li G, Hu
R, Nakajima T, Hara A, Aoyama T, Kannagi R, et al: Sulfatides are
associated with neointimal thickening after vascular injury.
Atherosclerosis. 211:291–296. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shimazawa M, Kondo K, Hara H, Nakashima M
and Umemura K: Sulfatides, L- and P-selectin ligands, exacerbate
the intimal hyperplasia occurring after endothelial injury. Eur J
Pharmacol. 520:118–126. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Libby P, Ridker PM and Hansson GK: Leducq
Transatlantic Network on Atherothrombosis. Inflammation in
atherosclerosis: From pathophysiology to practice. J Am Coll
Cardiol. 54:2129–2138. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Silvestre-Roig C, Braster Q, Ortega-Gomez
A and Soehnlein O: Neutrophils as regulators of cardiovascular
inflammation. Nat Rev Cardiol. 17:327–340. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hofbauer TM, Ondracek AS and Lang IM:
Neutrophil extracellular traps in atherosclerosis and thrombosis.
Handb Exp Pharmacol. 270:405–425. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Adamstein NH, MacFadyen JG, Rose LM, Glynn
RJ, Dey AK, Libby P, Tabas IA, Mehta NN and Ridker PM: The
neutrophil-lymphocyte ratio and incident atherosclerotic events:
Analyses from five contemporary randomized trials. Eur Heart J.
42:896–903. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tamhane UU, Aneja S, Montgomery D, Rogers
EK, Eagle KA and Gurm HS: Association between admission neutrophil
to lymphocyte ratio and outcomes in patients with acute coronary
syndrome. Am J Cardiol. 102:653–657. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Buonacera A, Stancanelli B, Colaci M and
Malatino L: Neutrophil to lymphocyte ratio: An emerging marker of
the relationships between the immune system and diseases. Int J Mol
Sci. 23(3636)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang S, Diao J, Qi C, Jin J, Li L, Gao X,
Gong L and Wu W: Predictive value of neutrophil to lymphocyte ratio
in patients with acute ST segment elevation myocardial infarction
after percutaneous coronary intervention: A meta-analysis. BMC
Cardiovasc Disord. 18(75)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Choi DH, Kobayashi Y, Nishi T, Kim HK, Ki
YJ, Kim SS, Park KH, Song H and Fearon WF: Combination of mean
platelet volume and neutrophil to lymphocyte ratio predicts
long-term major adverse cardiovascular events after percutaneous
coronary intervention. Angiology. 70:345–351. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xu N, Tang XF, Yao Y, Zhao X, Chen J, Gao
Z, Yang Y, Gao RL, Xu B and Yuan JQ: Predictive value of neutrophil
to lymphocyte ratio in long-term outcomes of left main and/or
three-vessel disease in patients with acute myocardial infarction.
Catheter Cardiovasc Interv. 91:551–557. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen J, Chen MH, Li S, Guo YL, Zhu CG, Xu
RX, Zhang Y, Sun J, Qing P, Liu G and Li JJ: Usefulness of the
neutrophil-to-lymphocyte ratio in predicting the severity of
coronary artery disease: A Gensini score assessment. J Atheroscler
Thromb. 21:1271–1282. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dziedzic EA, Gąsior JS, Tuzimek A,
Dąbrowski M and Jankowski P: Neutrophil-to-lymphocyte ratio is not
associated with severity of coronary artery disease and is not
correlated with vitamin D level in patients with a history of an
acute coronary syndrome. Biology (Basel). 11(1001)2022.PubMed/NCBI View Article : Google Scholar
|