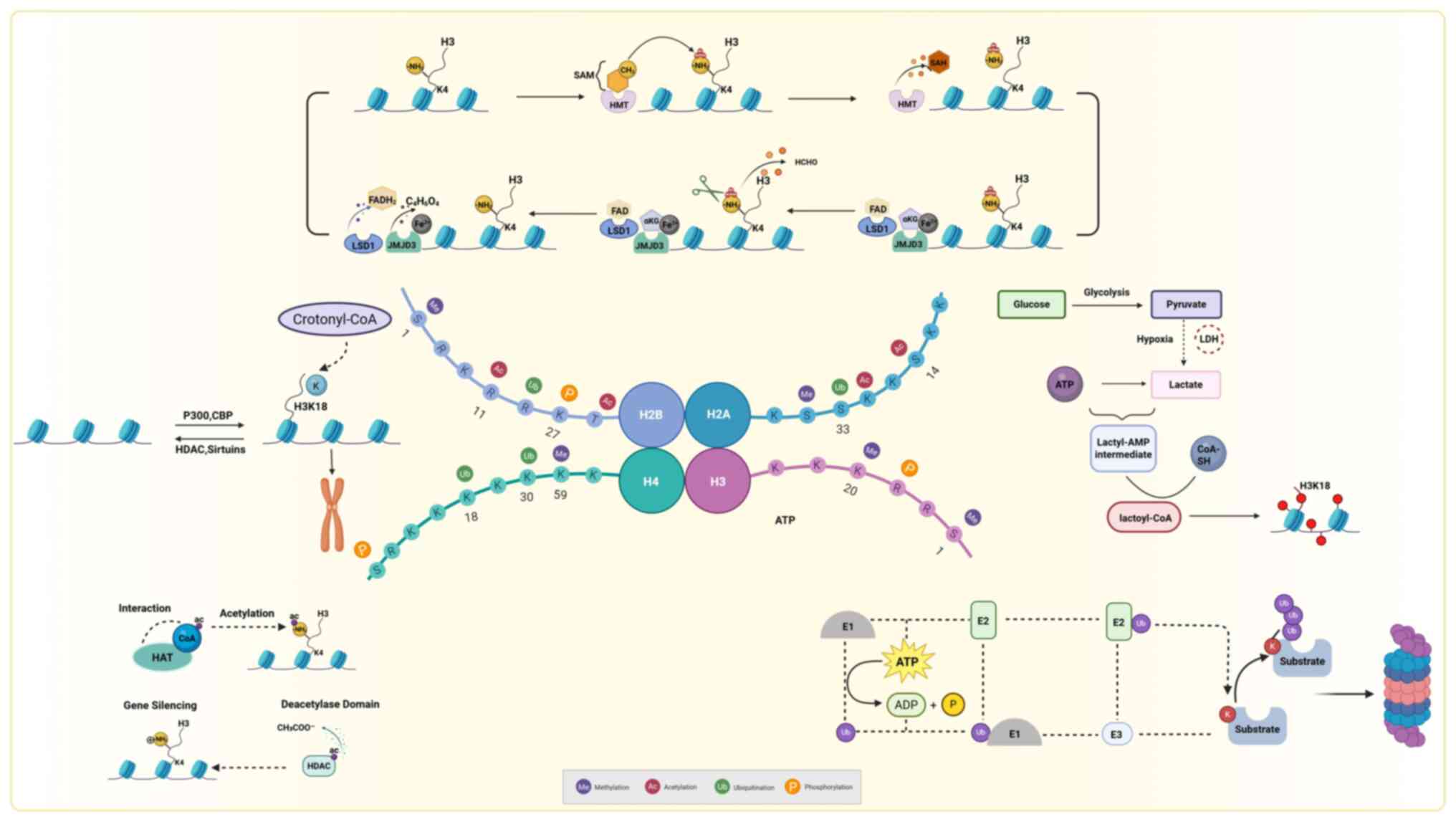
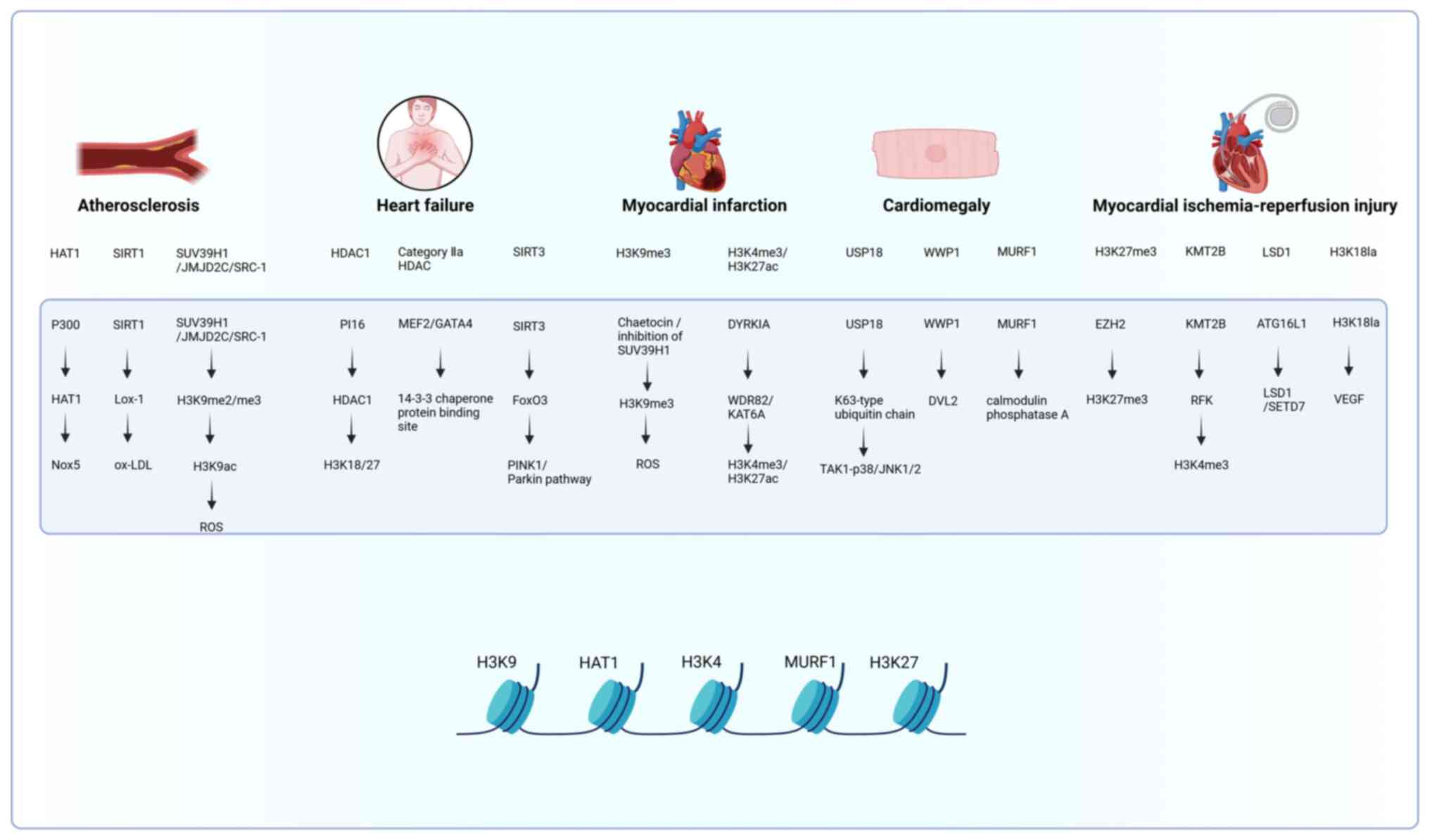
|
1
|
Feinberg AP: The key role of epigenetics
in human disease prevention and mitigation. N Engl J Med.
378:1323–1334. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhou BR and Bai Y: Chromatin structures
condensed by linker histones. Essays Biochem. 63:75–87.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ke L: Single molecule study of DNA and
nucleosome complexes. Journal 2022.
|
|
4
|
Hyun K, Jeon J, Park K and Kim J: Writing,
erasing and reading histone lysine methylations. Exp Mol Med.
49(e324)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang K, Li Y, Qiang T, Chen J and Wang X:
Role of epigenetic regulation in myocardial ischemia/reperfusion
injury. Pharmacol Res. 170(105743)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gupta I, Varshney NK and Khan S: Emergence
of members of TRAF and DUB of ubiquitin proteasome system in the
regulation of hypertrophic cardiomyopathy. Front Genet.
9(336)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
He B, Zhao YC, Gao LC, Ying XY, Xu LW, Su
YY, Ji QQ, Lin N and Pu J: Ubiquitin-specific protease 4 is an
endogenous negative regulator of pathological cardiac hypertrophy.
Hypertension. 67:1237–1248. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yan K, Ponnusamy M, Xin Y, Wang Q, Li P
and Wang K: The role of K63-linked polyubiquitination in cardiac
hypertrophy. J Cell Mol Med. 22:4558–4567. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim U and Lee DS: Epigenetic regulations
in mammalian cells: Roles and profiling techniques. Mol Cells.
46:86–98. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bannister AJ and Kouzarides T: Regulation
of chromatin by histone modifications. Cell Res. 21:381–395.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cheng X and Wang K, Zhao Y and Wang K:
Research progress on post-translational modification of proteins
and cardiovascular diseases. Cell Death Discov.
9(275)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tan M, Luo H, Lee S, Jin F, Yang JS,
Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N, et al:
Identification of 67 histone marks and histone lysine crotonylation
as a new type of histone modification. Cell. 146:1016–1028.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Soler-Botija C, Gálvez-Montón C and
Bayés-Genís A: Epigenetic biomarkers in cardiovascular diseases.
Front Genet. 10(950)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shu F, Xiao H, Li QN, Ren XS, Liu ZG, Hu
BW, Wang HS, Wang H and Jiang GM: Epigenetic and post-translational
modifications in autophagy: biological functions and therapeutic
targets. Signal Transduct Target Ther. 8(32)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vlad ML, Manea SA, Iazar AG, Raicu M,
Muresian H, Simionescu M and Manea A: Histone
acetyltransferase-dependent pathways mediate upregulation of nadph
oxidase 5 in human macrophages under inflammatory conditions: A
potential mechanism of reactive oxygen species overproduction in
atherosclerosis. Oxid Med Cell Longev. 2019(3201062)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chan SH, Hung CH, Shih JY, Chu PM, Cheng
YH, Lin HC, Hsieh PL and Tsai KL: Exercise intervention attenuates
hyperhomocysteinemia-induced aortic endothelial oxidative injury by
regulating SIRT1 through mitigating NADPH oxidase/LOX-1 signaling.
Redox Biol. 14:116–125. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lijuan L: Discovery of microbially derived
KLF2 small molecule up-regulation Study on the effect of
anti-atherosclerosis. Journal 2023.
|
|
19
|
Marks PA and Breslow R: Dimethyl sulfoxide
to vorinostat: Development of this histone deacetylase inhibitor as
an anticancer drug. Nat Biotechnol. 25:84–90. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu Y, Xu S, Liu P, Koroleva M, Zhang S, Si
S and Jin ZG: Suberanilohydroxamic acid as a pharmacological
kruppel-like factor 2 activator that represses vascular
inflammation and atherosclerosis. J Am Heart Assoc.
6(e007134)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
SenBanerjee S, Lin Z, Atkins GB, Greif DM,
Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, et
al: KLF2 is a novel transcriptional regulator of endothelial
proinflammatory activation. J Exp Med. 199:1305–1315.
2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu M, Kluger MS, D'Alessio A,
García-Cardeña G and Pober JS: Regulation of arterial-venous
differences in tumor necrosis factor responsiveness of endothelial
cells by anatomic context. Am J Pathol. 172:1088–1099.
2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bu DX, Griffin G and Lichtman AH:
Mechanisms for the anti-inflammatory effects of statins. Curr Opin
Lipidol. 22:165–170. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ridker PM and Lüscher TF:
Anti-inflammatory therapies for cardiovascular disease. Eur Heart
J. 35:1782–1791. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sen-Banerjee S, Mir S, Lin Z, Hamik A,
Atkins GB, Das H, Banerjee P, Kumar A and Jain MK: Kruppel-like
factor 2 as a novel mediator of statin effects in endothelial
cells. Circulation. 112:720–726. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang J, Xu X, Li P, Zhang B and Zhang J:
HDAC3 protects against atherosclerosis through inhibition of
inflammation via the microRNA-19b/PPARγ/NF-κB axis.
Atherosclerosis. 323:1–12. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jackson AO, Zhang J, Jiang Z and Yin K:
Endothelial-to-mesenchymal transition: A novel therapeutic target
for cardiovascular diseases. Trends Cardiovasc Med. 27:383–393.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen L, Shang C, Wang B, Wang G, Jin Z,
Yao F, Yue Z, Bai L, Wang R, Zhao S, et al: HDAC3 inhibitor
suppresses endothelial-to-mesenchymal transition via modulating
inflammatory response in atherosclerosis. Biochem Pharmacol.
192(114716)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cai L, Ma X, Huang Y, Zou Y and Chen X:
Aberrant histone methylation and the effect of SUV39H1 siRNA on
gastric carcinoma. Oncol Rep. 31:2593–2600. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cherrier T, Suzanne S, Redel L, Calao M,
Marban C, Samah B, Mukerjee R, Schwartz C, Gras G, Sawaya BE, et
al: p21(WAF1) gene promoter is epigenetically silenced by CTIP2 and
SUV39H1. Oncogene. 28:3380–3389. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang J, Chen J, Yang J, Xu C, Hu Q, Wu H,
Cai W, Guo Q, Gao W, He C, et al: SUV39H1 downregulation inhibits
neointimal hyperplasia after vascular injury. Atherosclerosis.
288:76–84. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Masi S, Ambrosini S, Mohammed SA,
Sciarretta S, Luescher TF, Paneni F and Costantino S: Epigenetic
remodeling in obesity-related vascular disease. Antioxid Redox
Signal. 34:1165–1199. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Costantino S, Paneni F and Cosentino F:
Ageing, metabolism and cardiovascular disease. J Physiol.
594:2061–2073. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Costantino S, Paneni F, Virdis A, Hussain
S, Mohammed SA, Capretti G, Akhmedov A, Dalgaard K, Chiandotto S,
Pospisilik JA, et al: Interplay among H3K9-editing enzymes SUV39H1,
JMJD2C and SRC-1 drives p66Shc transcription and vascular oxidative
stress in obesity. Eur Heart J. 40:383–391. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Karnewar S, Neeli PK, Panuganti D,
Kotagiri S, Mallappa S, Jain N, Jerald MK and Kotamraju S:
Metformin regulates mitochondrial biogenesis and senescence through
AMPK mediated H3K79 methylation: Relevance in age-associated
vascular dysfunction. Biochim Biophys Acta. 1864:1115–1128.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Greissel A, Culmes M, Napieralski R,
Wagner E, Gebhard H, Schmitt M, Zimmermann A, Eckstein HH, Zernecke
A and Pelisek J: Alternation of histone and DNA methylation in
human atherosclerotic carotid plaques. Thromb Haemost. 114:390–402.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Greissel A, Culmes M, Burgkart R,
Zimmermann A, Eckstein HH, Zernecke A and Pelisek J: Histone
acetylation and methylation significantly change with severity of
atherosclerosis in human carotid plaques. Cardiovasc Pathol.
25:79–86. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Huang Z, Song S, Zhang X, Zeng L, Sun A
and Ge J: Metabolic substrates, histone modifications, and heart
failure. Biochim Biophys Acta. 1866(194898)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jing Y, Li X, Liu Z and Li XD: Roles of
negatively charged histone lysine acylations in regulating
nucleosome structure and dynamics. Front Mol Biosci.
9(899013)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Papait R and Condorelli G: Epigenetics in
heart failure. Ann N Y Acad Sci. 1188:159–164. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chelladurai P, Boucherat O, Stenmark K,
Kracht M, Seeger W, Bauer UM, Bonnet S and Pullamsetti SS:
Targeting histone acetylation in pulmonary hypertension and right
ventricular hypertrophy. Br J Pharmacol. 178:54–71. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yan M, Chen C, Gong W, Yin Z, Zhou L,
Chaugai S and Wang DW: miR-21-3p regulates cardiac hypertrophic
response by targeting histone deacetylase-8. Cardiovasc Res.
105:340–352. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xiao-mei L, Chang P, Shu-qi W, Huan-ting Z
and Xiao-chun T: Role of histone deacetylase 2-mediated histone
acetylation imbalance in myocardial remodeling induced by pressure
overload. Chinese Journal of Pathophysiology. 38:584–591. 2022.
|
|
44
|
Yuhang C, Rui H, Yujun S and Li S:
Myocardial-specific Hdac3 deletion induces by ventricular
remodeling in mice. J Army Med Univ. 40:1205–1212. 2018.(In
Chinese).
|
|
45
|
Wang B, Zhang LD, Zhao QF, Zhu MJ and Wang
XL: Research progress of histone acetylation in prevention and
treatment of heart failure and new ideas based on traditional
Chinese medicine. China J Chinese Materia Medica. 48:2010–2019.
2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mengqing D: Mechanism of peptidase
inhibitory protein PI16 inhibiting angiotensin-ⅱinduced cardiac
hypertrophy and cardiac fibrosis by down-regulating HDAC1. Journal
2019.
|
|
47
|
Jia-pei X and Yu-hua L: Role and mechanism
of histone deacetylase 3 in cardiac fibrosis in mice. Hainan
Medical Journal. 32:2998–3002. 2021.
|
|
48
|
Min Z, Hui T and Zewen C: The role of HDAC
8 in isoprenaline-induced myocardial fibrosis of rat. Acta Univ Med
Anhui. 50:950–953. 2015.(In Chinese).
|
|
49
|
Han Y, Nie J, Wang DW and Ni L: Mechanism
of histone deacetylases in cardiac hypertrophy and its therapeutic
inhibitors. Front Cardiovasc Med. 9(931475)2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhang HN, Dai Y, Zhang CH, Omondi AM,
Ghosh A, Khanra I, Chakraborty M, Yu XB and Liang J: Sirtuins
family as a target in endothelial cell dysfunction: Implications
for vascular ageing. Biogerontology. 21:495–516. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wu B, You S, Qian H, Wu S, Lu S, Zhang Y,
Sun Y and Zhang N: The role of SIRT2 in vascular-related and
heart-related diseases: A review. J Cell Mol Med. 25:6470–6478.
2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gorski PA, Jang SP, Jeong D, Lee A, Lee P,
Oh JG, Chepurko V, Yang DK, Kwak TH, Eom SH, et al: Role of SIRT1
in modulating acetylation of the sarco-endoplasmic reticulum
Ca(2+)-ATPase in heart failure. Circ Res. 124:e63–e80.
2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li J, Chen T, Xiao M, Li N, Wang S, Su H,
Guo X, Liu H, Yan F, Yang Y, et al: Mouse Sirt3 promotes autophagy
in AngII-induced myocardial hypertrophy through the deacetylation
of FoxO1. Oncotarget. 7:86648–86659. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mehra MR, Park MH, Landzberg MJ, Lala A
and Waxman AB: Right heart failure: Toward a common language. J
Heart Lung Transplant. 33:123–126. 2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Tomson T, Battino D and Perucca E: The
remarkable story of valproic acid. Lancet Neurol.
15(141)2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Benza RL, Adamson PB, Bhatt DL, Frick F,
Olsson G, Bergh N and Dahlöf B: CS1, a controlled-release
formulation of valproic acid, for the treatment of patients with
pulmonary arterial hypertension: Rationale and design of a Phase 2
clinical trial. Pulm Circ. 14(e12323)2024.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Pang M, Li Y, Gu W, Sun Z, Wang Z and Li
L: Recent advances in epigenetics of macrovascular complications in
diabetes mellitus. Heart Lung Circ. 30:186–196. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zang R, Tan Q, Zeng F, Wang D, Yu S and
Wang Q: JMJD1A represses the development of cardiomyocyte
hypertrophy by regulating the expression of catalase. Biomed Res
Int. 2020(5081323)2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Liu X, Chen J, Zhang B, Liu G, Zhao H and
Hu Q: KDM3A inhibition modulates macrophage polarization to
aggravate post-MI injuries and accelerates adverse ventricular
remodeling via an IRF4 signaling pathway. Cell Signal.
64(109415)2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yang G, Weng X, Zhao Y, Zhang X, Hu Y, Dai
X, Liang P, Wang P, Ma L, Sun X, et al: The histone H3K9
methyltransferase SUV39H links SIRT1 repression to myocardial
infarction. Nat Commun. 8(14941)2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Jiang H, Li Y, Xiang X, Tang Z, Liu K, Su
Q, Zhang X and Li L: Chaetocin: A review of its anticancer
potentials and mechanisms. Eur J Pharmacol.
910(174459)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Schweizer S, Harms C, Lerch H, Flynn J,
Hecht J, Yildirim F, Meisel A and Märschenz S: Inhibition of
histone methyltransferases SUV39H1 and G9a leads to neuroprotection
in an in vitro model of cerebral ischemia. J Cereb Blood Flow
Metab. 35:1640–1647. 2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wang P, Alvarez-Perez JC, Felsenfeld DP,
Liu H, Sivendran S, Bender A, Kumar A, Sanchez R, Scott DK,
Garcia-Ocaña A and Stewart AF: A high-throughput chemical screen
reveals that harmine-mediated inhibition of DYRK1A increases human
pancreatic beta cell replication. Nat Med. 21:383–388.
2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Hille S, Dierck F, Kuehl C, Sosna J,
Adam-Klages S, Adam D, Luellmann-Rauch R, Frey N and Kuhn C: Dyrk1a
regulates the cardiomyocyte cell cycle via D-cyclin-dependent
Rb/E2f-signalling. Cardiovasc Res. 110:381–394. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lan C, Chen C, Qu S, Cao N, Luo H, Yu C,
Wang N, Xue Y, Xia X, Fan C, et al: Inhibition of DYRK1A, via
histone modification, promotes cardiomyocyte cell cycle activation
and cardiac repair after myocardial infarction. EBioMedicine.
82(104139)2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Young A, Bradley LA, Farrar E, Bilcheck
HO, Tkachenko S, Saucerman JJ, Bekiranov S and Wolf MJ: Inhibition
of DYRK1a enhances cardiomyocyte cycling after myocardial
infarction. Circ Res. 130:1345–1361. 2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wang N, Wang W, Wang X, Mang G, Chen J,
Yan X, Tong Z, Yang Q, Wang M, Chen L, et al: Histone lactylation
boosts reparative gene activation post-myocardial infarction. Circ
Res. 131:893–908. 2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Dal-Pra S, Hodgkinson CP, Mirotsou M,
Kirste I and Dzau VJ: Demethylation of H3K27 is essential for the
induction of direct cardiac reprogramming by miR combo. Circ Res.
120:1403–1413. 2017.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Lee S, Lee JW and Lee SK: UTX, a histone
H3-lysine 27 demethylase, acts as a critical switch to activate the
cardiac developmental program. Dev Cell. 22:25–37. 2012.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Tan J, Yang X, Zhuang L, Jiang X, Chen W,
Lee PL, Karuturi RK, Tan PB, Liu ET, Yu Q, et al: Pharmacologic
disruption of Polycomb-repressive complex 2-mediated gene
repression selectively induces apoptosis in cancer cells. Genes
Dev. 21:1050–1063. 2007.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Hou P, Li Y, Zhang X, Liu C, Guan J, Li H,
Zhao T, Ye J, Yang W, Liu K, et al: Pluripotent stem cells induced
from mouse somatic cells by small-molecule compounds. Science.
341:651–654. 2013.PubMed/NCBI View Article : Google Scholar
|
|
72
|
LncRNA H19 ameliorates myocardial
infarction-induced myocardial injury and maladaptive cardiac
remodelling by regulating KDM3A. J Cell Mol Med. 27:1757–1760.
2023.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Repetti GG, Toepfer CN, Seidman JG and
Seidman CE: Novel therapies for prevention and early treatment of
cardiomyopathies now and in the future. Circ Res. 124:1536–1550.
2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Xu J, Liang S, Wang Q, Zheng Q, Wang M,
Qian J, Yu T, Lou S, Luo W, Zhou H and Liang G: JOSD2 mediates
isoprenaline-induced heart failure by deubiquitinating CaMKIIδ in
cardiomyocytes. Cell Mol Life Sci. 81(18)2024.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Ying X, Zhao Y, Yao T, Yuan A, Xu L, Gao
L, Ding S, Ding H, Pu J and He B: Novel protective role for
ubiquitin-specific protease 18 in pathological cardiac remodeling.
Hypertension. 68:1160–1170. 2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Liu N, Chai R, Liu B, Zhang Z, Zhang S,
Zhang J, Liao Y, Cai J, Xia X, Li A, et al: Ubiquitin-specific
protease 14 regulates cardiac hypertrophy progression by increasing
GSK-3β phosphorylation. Biochem Biophys Res Commun. 478:1236–1241.
2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Zhao D, Zhong G, Li J, Pan J, Zhao Y, Song
H, Sun W, Jin X, Li Y, Du R, et al: Targeting E3 ubiquitin ligase
WWP1 prevents cardiac hypertrophy through destabilizing DVL2 via
inhibition of K27-linked ubiquitination. Circulation. 144:694–711.
2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Maejima Y, Usui S, Zhai P, Takamura M,
Kaneko S, Zablocki D, Yokota M, Isobe M and Sadoshima J:
Muscle-specific RING finger 1 negatively regulates pathological
cardiac hypertrophy through downregulation of calcineurin A. Circ
Heart Fail. 7:479–490. 2014.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Gupta MK, McLendon PM, Gulick J, James J,
Khalili K and Robbins J: UBC9-mediated sumoylation favorably
impacts cardiac function in compromised hearts. Circ Res.
118:1894–1905. 2016.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Huang CY, Kuo CH, Pai PY, Ho TJ, Lin YM,
Chen RJ, Tsai FJ, Padma VV, Kuo WW and Huang CY: Data supporting
the angiotensin II activates MEL18 to deSUMOylate HSF2 for
hypertension-related heart failure. Data Brief. 16:521–526.
2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Pai P, Shibu MA, Chang RL, Yang JJ, Su CC,
Lai CH, Liao HE, Viswanadha VP, Kuo WW and Huang CY: ERβ targets
ZAK and attenuates cellular hypertrophy via SUMO-1 modification in
H9c2 cells. J Cell Biochem. 119:7855–7864. 2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Carreras D, Martinez-Moreno R,
Pinsach-Abuin ML, Santafe MM, Gomà P, Brugada R, Scornik FS, Pérez
GJ and Pagans S: Epigenetic changes governing scn5a expression in
denervated skeletal muscle. Int J Mol Sci. 22(2755)2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Peterkin T, Gibson A and Patient R:
Redundancy and evolution of GATA factor requirements in development
of the myocardium. Dev Biol. 311:623–635. 2007.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Kuo CT, Morrisey EE, Anandappa R, Sigrist
K, Lu MM, Parmacek MS, Soudais C and Leiden JM: GATA4 transcription
factor is required for ventral morphogenesis and heart tube
formation. Genes Dev. 11:1048–1060. 1997.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Rajagopal SK, Ma Q, Obler D, Shen J,
Manichaikul A, Tomita-Mitchell A, Boardman K, Briggs C, Garg V,
Srivastava D, et al: Spectrum of heart disease associated with
murine and human GATA4 mutation. J Mol Cell Cardiol. 43:677–685.
2007.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Munshi NV, McAnally J, Bezprozvannaya S,
Berry JM, Richardson JA, Hill JA and Olson EN: Cx30.2 enhancer
analysis identifies Gata4 as a novel regulator of atrioventricular
delay. Development. 136:2665–2674. 2009.PubMed/NCBI View Article : Google Scholar
|
|
87
|
He A, Gu F, Hu Y, Ma Q, Ye LY, Akiyama JA,
Visel A, Pennacchio LA and Pu WT: Dynamic GATA4 enhancers shape the
chromatin landscape central to heart development and disease. Nat
Commun. 5(4907)2014.PubMed/NCBI View Article : Google Scholar
|
|
88
|
He A, Kong SW, Ma Q and Pu WT:
Co-occupancy by multiple cardiac transcription factors identifies
transcriptional enhancers active in heart. Proc Natl Acad Sci USA.
108:5632–5637. 2011.PubMed/NCBI View Article : Google Scholar
|
|
89
|
van den Boogaard M, Wong LY, Tessadori F,
Bakker ML, Dreizehnter LK, Wakker V, Bezzina CR, Hoen PA, Bakkers
J, Barnett P and Christoffels VM: Genetic variation in T-box
binding element functionally affects SCN5A/SCN10A enhancer. J Clin
Invest. 122:2519–2530. 2012.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Maron BJ: Clinical course and management
of hypertrophic cardiomyopathy. N Engl J Med. 379:655–668.
2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Spudich JA: Three perspectives on the
molecular basis of hypercontractility caused by hypertrophic
cardiomyopathy mutations. Pflugers Arch. 471:701–717.
2019.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Tang X, Chen XF, Sun X, Xu P, Zhao X, Tong
Y, Wang XM, Yang K, Zhu YT, Hao DL, et al: Short-Chain Enoyl-CoA
hydratase mediates histone crotonylation and contributes to cardiac
homeostasis. Circulation. 143:1066–1069. 2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Liu S, Yu H, Liu Y, Liu X, Zhang Y, Bu C,
Yuan S, Chen Z, Xie G, Li W, et al: Chromodomain protein CDYL acts
as a crotonyl-CoA hydratase to regulate histone crotonylation and
spermatogenesis. Mol Cell. 67:853–866.e855. 2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Nussbaum SS, Henry S, Yong CM, Daugherty
SL, Mehran R and Poppas A: Sex-specific considerations in the
presentation, diagnosis, and management of ischemic heart disease:
JACC focus seminar 2/7. J Am Coll Cardiol. 79:1398–1406.
2022.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Li Y, Chen B, Yang X, Zhang C, Jiao Y, Li
P, Liu Y, Li Z, Qiao B, Lau WB, et al: S100a8/a9 signaling causes
mitochondrial dysfunction and cardiomyocyte death in response to
ischemic/reperfusion injury. Circulation. 140:751–764.
2019.PubMed/NCBI View Article : Google Scholar
|
|
96
|
He J, Liu D, Zhao L, Zhou D, Rong J, Zhang
L and Xia Z: Myocardial ischemia/reperfusion injury: Mechanisms of
injury and implications for management (Review). Exp Ther Med.
23(430)2022.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Ni L, Lin B, Zhang Y, Hu L, Lin J, Fu F,
Shen M, Li C, Chen L, Yang J, et al: Histone modification landscape
and the key significance of H3K27me3 in myocardial
ischaemia/reperfusion injury. Sci China Life Sci. 66:1264–1279.
2023.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wang G, Zou X, Chen Q, Nong W, Miao W, Luo
H and Qu S: The relationship and clinical significance of
lactylation modification in digestive system tumors. Cancer Cell
Int. 24(246)2024.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Xu Y and Fang F: Histone methylation and
transcriptional regulation in cardiovascular disease. Cardiovasc
Hematol Disord Drug Targets. 14:89–97. 2014.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Ibarrola J, Xiang RR, Sun Z, Lu Q, Hill MA
and Jaffe IZ: Inhibition of the histone methyltransferase EZH2
induces vascular stiffness. Clin Sci (Lond). 138:251–268.
2024.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Klonou A, Chlamydas S and Piperi C:
Structure, activity and function of the MLL2 (KMT2B) protein lysine
methyltransferase. Life (Basel). 11(823)2021.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Zhao WK, Zhou YT and Wu Q: Ferroptosis:
Opportunities and challenges in myocardial ischemia-reperfusion
injury. Oxid Med Cell Longev. 2021(9929687)2021.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Cao Y, Luo F, Peng J, Fang Z, Liu Q and
Zhou S: KMT2B-dependent RFK transcription activates the TNF-α/NOX2
pathway and enhances ferroptosis caused by myocardial
ischemia-reperfusion. J Mol Cell Cardiol. 173:75–91.
2022.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Gao C, Liu Y, Yu Q, Yang Q, Li B, Sun L,
Yan W, Cai X, Gao E, Xiong L, et al: TNF-α antagonism ameliorates
myocardial ischemia-reperfusion injury in mice by upregulating
adiponectin. Am J Physiol Heart Circ Physiol. 308:H1583–H1591.
2015.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Pei H, Song X, Peng C, Tan Y, Li Y, Li X,
Ma S, Wang Q, Huang R, Yang D, et al: TNF-α inhibitor protects
against myocardial ischemia/reperfusion injury via Notch1-mediated
suppression of oxidative/nitrative stress. Free Radic Biol Med.
82:114–121. 2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Moe KT, Yin NO, Naylynn TM, Khairunnisa K,
Wutyi MA, Gu Y, Atan MS, Wong MC, Koh TH and Wong P: Nox2 and Nox4
mediate tumour necrosis factor-α-induced ventricular remodelling in
mice. J Cell Mol Med. 15:2601–2613. 2011.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Bravo-Sánchez E, Peña-Montes D,
Sánchez-Duarte S, Saavedra-Molina A, Sánchez-Duarte E and
Montoya-Pérez R: Effects of apocynin on heart muscle oxidative
stress of rats with experimental diabetes: Implications for
mitochondria. Antioxidants (Basel). 10(335)2021.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Du ZD, Yu S, Qi Y, Qu TF, He L, Wei W, Liu
K and Gong SS: NADPH oxidase inhibitor apocynin decreases
mitochondrial dysfunction and apoptosis in the ventral cochlear
nucleus of D-galactose-induced aging model in rats. Neurochem Int.
124:31–40. 2019.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Wang C, Zhu L, Yuan W, Sun L, Xia Z, Zhang
Z and Yao W: Diabetes aggravates myocardial ischaemia reperfusion
injury via activating Nox2-related programmed cell death in an
AMPK-dependent manner. J Cell Mol Med. 24:6670–6679.
2020.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Szekeres FLM, Walum E, Wikström P and
Arner A: A small molecule inhibitor of Nox2 and Nox4 improves
contractile function after ischemia-reperfusion in the mouse heart.
Sci Rep. 11(11970)2021.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Yu B, Meng F, Yang Y, Liu D and Shi K:
NOX2 antisense attenuates hypoxia-induced oxidative stress and
apoptosis in cardiomyocyte. Int J Med Sci. 13:646–652.
2016.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Song H, Feng X, Zhang M, Jin X, Xu X, Wang
L, Ding X, Luo Y, Lin F, Wu Q, et al: Crosstalk between lysine
methylation and phosphorylation of ATG16L1 dictates the apoptosis
of hypoxia/reoxygenation-induced cardiomyocytes. Autophagy.
14:825–844. 2018.PubMed/NCBI View Article : Google Scholar
|
|
113
|
He L, Wang Y and Luo J: Epigenetic
modification mechanism of histone demethylase KDM1A in regulating
cardiomyocyte apoptosis after myocardial ischemia-reperfusion
injury. PeerJ. 10(e13823)2022.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Thinnes CC, England KS, Kawamura A,
Chowdhury R, Schofield CJ and Hopkinson RJ: Targeting histone
lysine demethylases-progress, challenges, and the future. Biochim
Biophys Acta. 1839:1416–1432. 2014.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Li Y, Quan X, Li X, Pan Y, Zhang T, Liang
Z and Wang Y: Kdm6A protects against hypoxia-induced cardiomyocyte
apoptosis via H3K27me3 demethylation of Ncx gene. J Cardiovasc
Transl Res. 12:488–495. 2019.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Lin CF, Hsu KC, HuangFu WC, Lin TE, Huang
HL and Pan SL: Investigating the potential effects of selective
histone deacetylase 6 inhibitor ACY1215 on infarct size in rats
with cardiac ischemia-reperfusion injury. BMC Pharmacol Toxicol.
21(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Chaturvedi P, Kalani A, Givvimani S, Kamat
PK, Familtseva A and Tyagi SC: Differential regulation of DNA
methylation versus histone acetylation in cardiomyocytes during
HHcy in vitro and in vivo: An epigenetic mechanism. Physiol
Genomics. 46:245–255. 2014.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Xiao Y, Huang W, Zhang J, Peng C, Xia M
and Ling W: Increased plasma S-adenosylhomocysteine-accelerated
atherosclerosis is associated with epigenetic regulation of
endoplasmic reticulum stress in apoE-/- mice. Arterioscler Thromb
Vasc Biol. 35:60–70. 2015.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Wang J, Lin B, Zhang Y, Ni L, Hu L, Yang
J, Xu L, Shi D and Chen YH: The regulatory role of histone
modification on gene expression in the early stage of myocardial
infarction. Front Cardiovasc Med. 7(594325)2020.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Pei J, Schuldt M, Nagyova E, Gu Z, El
Bouhaddani S, Yiangou L, Jansen M, Calis JJA, Dorsch LM, Blok CS,
et al: Multi-omics integration identifies key upstream regulators
of pathomechanisms in hypertrophic cardiomyopathy due to truncating
MYBPC3 mutations. Clin Epigenetics. 13(61)2021.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Shi Y, Zhang H, Huang S, Yin L, Wang F,
Luo P and Huang H: Epigenetic regulation in cardiovascular disease:
Mechanisms and advances in clinical trials. Signal Transduct Target
Ther. 7(200)2022.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Fuster JJ, MacLauchlan S, Zuriaga MA,
Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S,
Muralidharan S, Rius C, et al: Clonal hematopoiesis associated with
TET2 deficiency accelerates atherosclerosis development in mice.
Science. 355:842–847. 2017.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Jaiswal S, Natarajan P, Silver AJ, Gibson
CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino
D, et al: Clonal hematopoiesis and risk of atherosclerotic
cardiovascular disease. N Engl J Med. 377:111–121. 2017.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Souidi A, Nakamori M, Zmojdzian M, Jagla
T, Renaud Y and Jagla K: Deregulations of miR-1 and its target
Multiplexin promote dilated cardiomyopathy associated with myotonic
dystrophy type 1. EMBO Rep. 24(e56616)2023.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Kura B, Kalocayova B, Devaux Y and
Bartekova M: Potential clinical implications of miR-1 and miR-21 in
heart disease and cardioprotection. Int J Mol Sci.
21(700)2020.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Lazar IM, Hoeschele I, de Morais J and
Tenga MJ: Cell cycle model system for advancing cancer biomarker
research. Sci Rep. 7(17989)2017.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Liu X, Xiang M, Tong Z, Luo F, Chen W, Liu
F, Wang F, Yu RQ and Jiang JH: Activatable fluorescence probe via
self-immolative intramolecular cyclization for histone deacetylase
imaging in live cells and tissues. Anal Chem. 90:5534–5539.
2018.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Hussain S, Tulsyan S, Dar SA, Sisodiya S,
Abiha U, Kumar R, Mishra BN and Haque S: Role of epigenetics in
carcinogenesis: Recent advancements in anticancer therapy. Semin
Cancer Biol. 83:441–451. 2022.PubMed/NCBI View Article : Google Scholar
|