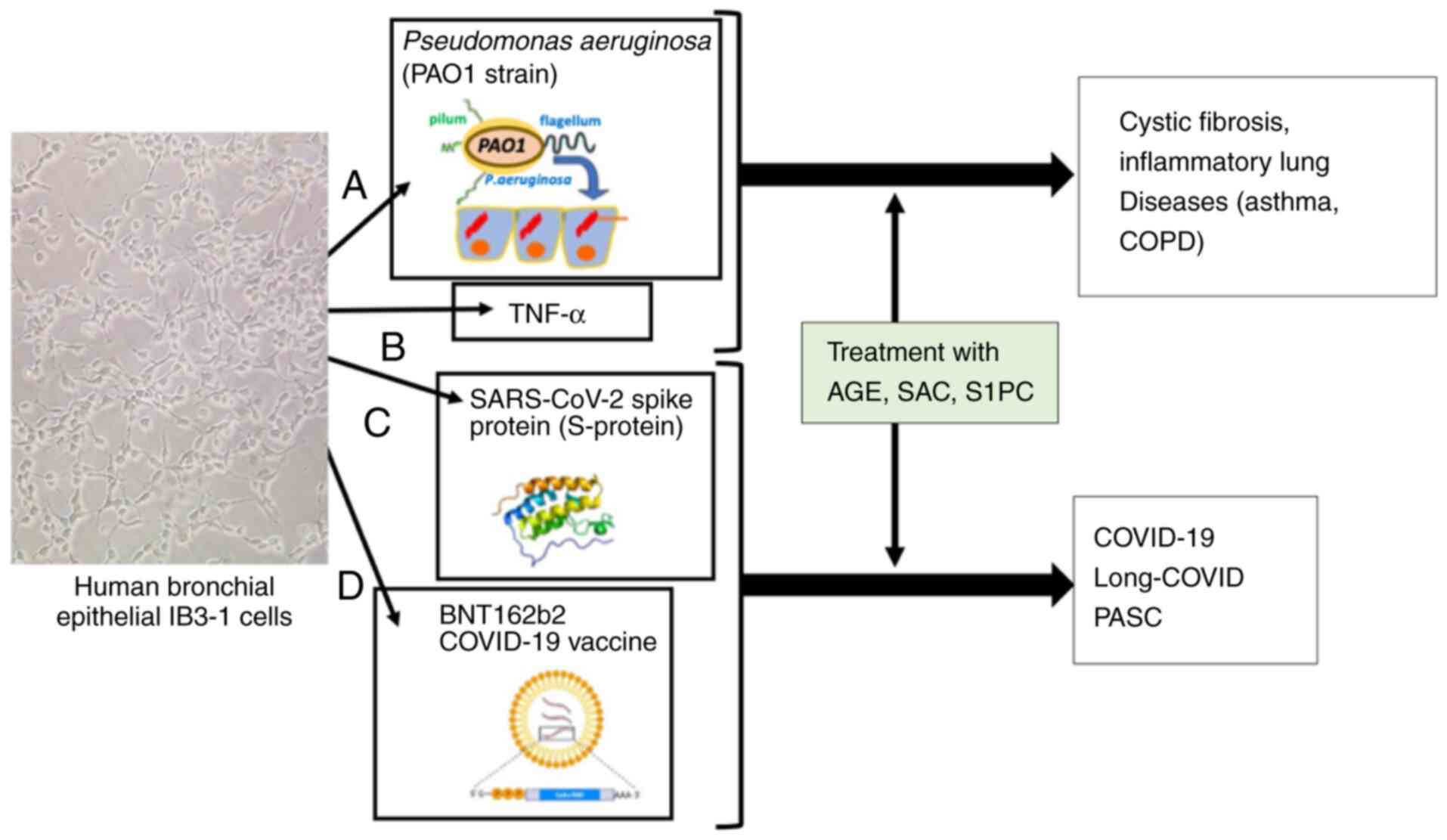
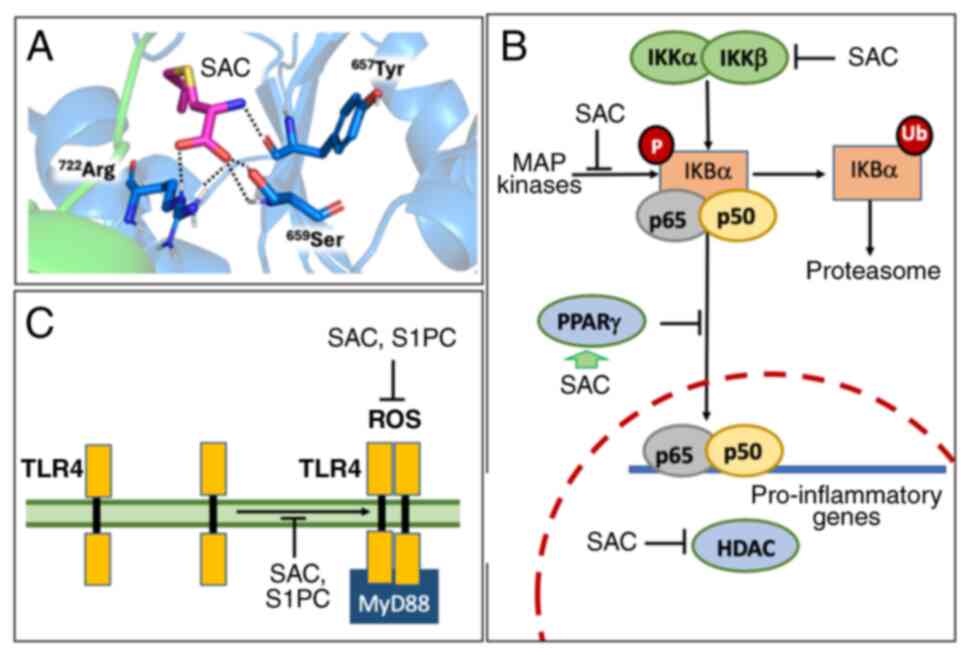
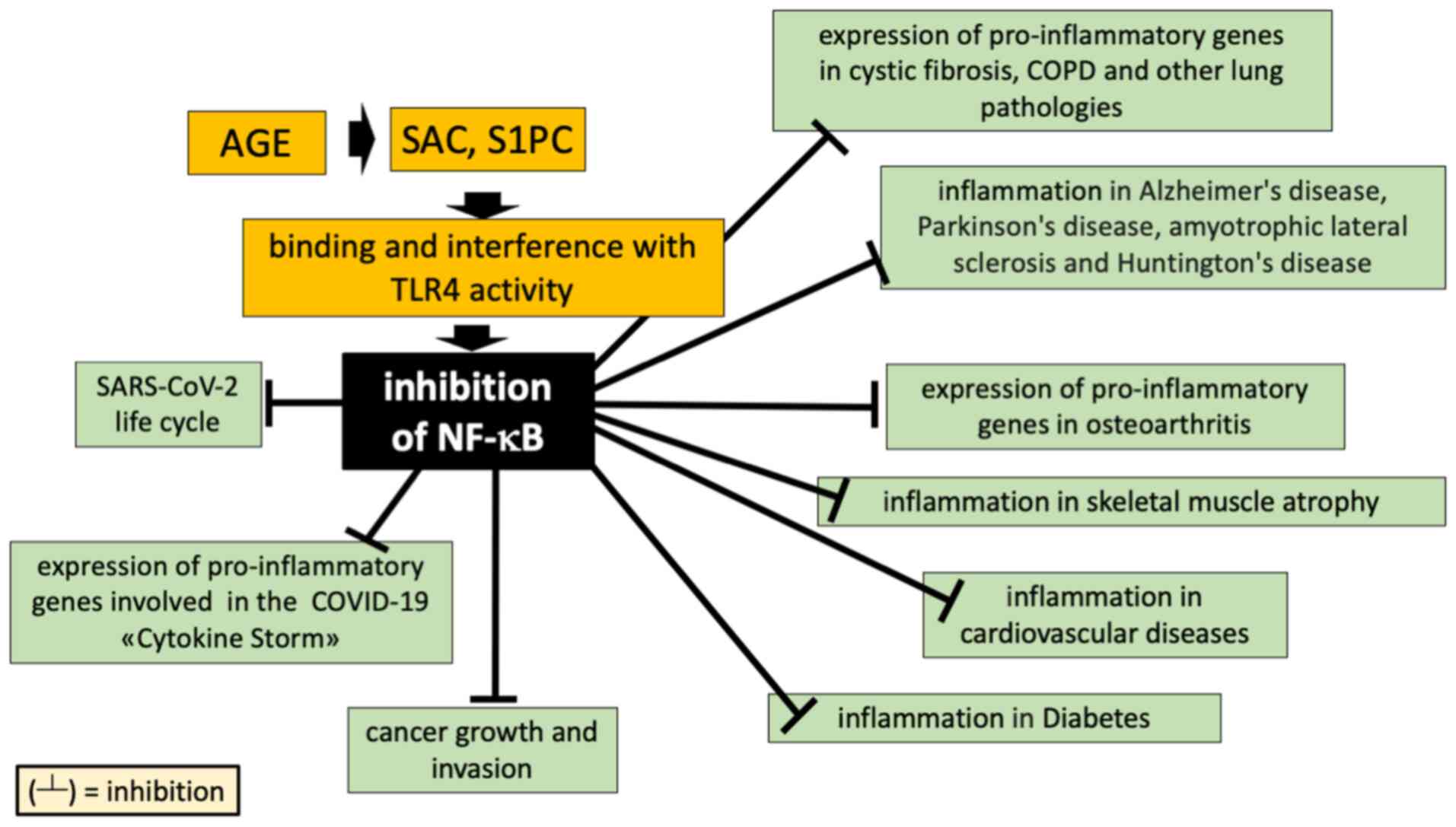
|
1
|
Ohkubo S, Dalla Via L, Grancara S, Kanamori Y, García-Argáez AN, Canettieri G, Arcari P, Toninello A and Agostinelli E: The anti-oxidant, aged garlic extract, exerts cytotoxic effects on wild-type and multidrug-resistant human cancer cells by altering mitochondrial permeability. Int J Oncol. 53:1257–1268. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H and Itakura Y: Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Medica. 60:417–420. 1994.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Serrano JCE, Castro-Boqué E, García-Carrasco A, Morán-Valero MI, González-Hedström D, Bermúdez-López M, Valdivielso JM, Espinel AE and Portero-Otín M: Antihypertensive effects of an optimized aged garlic extract in subjects with grade I hyper-tension and antihypertensive drug therapy: A Randomized, triple-blind controlled trial. Nutrients. 15(3691)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang Q, Li F, Jia G and Liu R: Aged black garlic extract inhibits the growth of estrogen receptor-positive breast cancer cells by downregulating MCL-1 expression through the ROS-JNK pathway. PLoS One. 18(e0286454)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Das D, M K, Mitra A, Zaky MY, Pathak S and Banerjee A: A review on the efficacy of plant-derived bio-active compounds cur-cumin and aged garlic extract in modulating cancer and age-related diseases. Curr Rev Clin Exp Pharmacol. 19:146–162. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Borek C: Antioxidant health effects of aged garlic extract. J Nutr. 131(3s)(1010S-5S)2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xu C, Mathews AE, Rodrigues C, Eudy BJ, Rowe CA, O'Donoughue A and Percival SS: Aged garlic extract supplementation modifies inflammation and immunity of adults with obesity: A randomized, double-blind, placebo-controlled clinical trial. Clin Nutr ESPEN. 24:148–155. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Libero ML, Lucarini E, Recinella L, Ciampi C, Veschi S, Piro A, Chiavaroli A, Acquaviva A, Nilofar N, Orlando G, et al: Anti-inflammatory and anti-hyperalgesic effects induced by an aqueous aged black garlic extract in rodent models of ulcerative colitis and colitis-associated visceral pain. Phytother Res. 38:4177–4188. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wlosinska M, Nilsson AC, Hlebowicz J, Fakhro M, Malmsjö M and Lindstedt S: Aged garlic extract reduces IL-6: A double-blind placebo-controlled trial in females with a low risk of cardiovascular disease. Evid Based Complement Alternat Med. 2021(6636875)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Albrakati A: Aged garlic extract rescues ethephon-induced kidney damage by modulating oxidative stress, apoptosis, inflammation, and histopathological changes in rats. Environ Sci Pollut Res Int. 28:6818–6829. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McElvaney OJ and McElvaney NG: Targeting IL-8 in cystic fibrosis: Enough but Not too much. Am J Respir Cell Mol Biol. 59:401–402. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Escotte S, Tabary O, Dusser D, Majer-Teboul C, Puchelle E and Jacquot J: Fluticasone reduces IL-6 and IL-8 production of cystic fibrosis bronchial epithelial cells via IKK-beta kinase pathway. Eur Respir J. 21:574–581. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rennard SI, Dale DC, Donohue JF, Kanniess F, Magnussen H, Sutherland ER, Watz H, Lu S, Stryszak P, Rosenberg E and Staudinger H: CXCR2 antagonist MK-7123. A phase 2 proof-of-concept trial for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 191:1001–1011. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Planagumà A, Domènech T, Pont M, Calama E, García-González V, López R, Aulí M, López M, Fonquerna S, Ramos I, et al: Combined anti CXC receptors 1 and 2 therapy is a promising anti-inflammatory treatment for respiratory diseases by reducing neutrophil migration and activation. Pulm Pharmacol Ther. 34:37–45. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang J, Wang X, Zhang Y, He R, Fu Z, Wang R, Ma Y, Fu D, Meng S, Cai W, et al: Intra-articular injection of interleukin-8 neutralizing monoclonal antibody effectively attenuates osteoarthritis progression in rabbits. Cartilage: Mar 25, 2024 (Epub ahead of print).
|
|
16
|
Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, Skiest D, Aziz MS, Cooper N, Douglas IS, et al: Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 384:1503–1516. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pinzon RT, Wijaya VO and Buana RB: Interleukin-6 (IL-6) inhibitors as therapeutic agents for coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J Infect Public Health. 14:1001–1009. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Simonetti A, Restaino A, Bernardi E, Ferrara OM, Margoni S, D'Onofrio AM, Ranieri F, Janiri D, Galluzzo V, Tosato M, et al: Effect of anti-interleukin-6 agents on psychopathology in a sample of patients with post-COVID-19 syndrome: an observational study. Brain Sci. 14(47)2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Holms R: Long COVID (PASC) is maintained by a self-sustaining pro-inflammatory TLR4/RAGE-Loop of S100A8/A9 > TLR4/RAGE signalling, inducing chronic expression of IL-1b, IL-6 and TNFa: Anti-inflammatory ezrin peptides as potential therapy. Immuno: Sep 8, 2022 (Epub ahead of print).
|
|
20
|
Dinarello CA, Simon A and van der Meer JW: Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 11:633–652. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Asaba CN, Ekabe CJ, Ayuk HS, Gwanyama BN, Bitazar R and Bukong TN: Interplay of TLR4 and SARS-CoV-2: Unveiling the complex mechanisms of inflammation and severity in COVID-19 infections. J Inflamm Res. 17:5077–5091. 2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bartels YL, van Lent PLEM, van der Kraan PM, Blom AB, Bonger KM and van den Bosch MHJ: Inhibition of TLR4 signalling to dampen joint inflammation in osteoarthritis. Rheumatology (Oxford). 63:608–618. 2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Greene CM, Branagan P and McElvaney NG: Toll-like receptors as therapeutic targets in cystic fibrosis. Expert Opin Ther Targets. 12:1481–1495. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kodera Y, Suzuki A, Imada O, Kasuga S, Sumioka I, Kanezawa A, Taru N, Fujikawa M, Nagae S, Masamoto K, et al: Physical, chemical, and biological properties of s-allylcysteine, an amino acid derived from garlic. J Agric Food Chem. 50:622–632. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kanamori Y, Via LD, Macone A, Canettieri G, Greco A, Toninello A and Agostinelli E: Aged garlic extract and its constituent, S-allyl-L-cysteine, induce the apoptosis of neuroblastoma cancer cells due to mitochondrial membrane depolarization. Exp Ther Med. 19:1511–1521. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kodera Y, Ushijima M, Amano H, Suzuki JI and Matsutomo T: Chemical and biological properties of S-1-Propenyl-l-cysteine in aged garlic extract. Molecules. 22(570)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
DiMango E, Ratner AJ, Bryan R, Tabibi S and Prince A: Activation of NF-kappaB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J Clin Invest. 101:2598–2605. 1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bezzerri V, Borgatti M, Finotti A, Tamanini A, Gambari R and Cabrini G: Mapping the transcriptional machinery of the IL-8 gene in human bronchial epithelial cells. J Immunol. 187:6069–6081. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Finotti A, Borgatti M, Bezzerri V, Nicolis E, Lampronti I, Dechecchi M, Mancini I, Cabrini G, Saviano M, Avitabile C, et al: Effects of decoy molecules targeting NF-kappaB transcription factors in Cystic fibrosis IB3-1 cells: Recruitment of NF-kappaB to the IL-8 gene promoter and transcription of the IL-8 gene. Artif DNA PNA XNA. 3:97–296. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fabbri E, Borgatti M, Montagner G, Bianchi N, Finotti A, Lampronti I, Bezzerri V, Dechecchi MC, Cabrini G and Gambari R: Expression of microRNA-93 and Interleukin-8 during Pseudomonas aeruginosa-mediated induction of proinflammatory responses. Am J Respir Cell Mol Biol. 50:1144–1155. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lampronti I, Dechecchi MC, Rimessi A, Bezzerri V, Nicolis E, Guerrini A, Tacchini M, Tamanini A, Munari S, D'Aversa E, et al: β-sitosterol reduces the expression of chemotactic cytokine genes in cystic fibrosis bronchial epithelial cells. Front Pharmacol. 8(236)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tupini C, Chilin A, Rossi A, De Fino I, Bragonzi A, D'Aversa E, Cosenza LC, Vaccarin C, Sacchetti G, Borgatti M, et al: New TMA (4,6,4'-Trimethyl angelicin) analogues as anti-inflammatory agents in the treatment of cystic fibrosis lung disease. Int J Mol Sci. 23(14483)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Montagner G, Bezzerri V, Cabrini G, Fabbri E, Borgatti M, Lampronti I, Finotti A, Nielsen PE and Gambari R: An antisense peptide nucleic acid against Pseudomonas aeruginosa inhibiting bacterial-induced inflammatory responses in the cystic fibrosis IB3-1 cellular model system. Int J Biol Macromol. 99:492–498. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hawdon NA, Aval PS, Barnes RJ, Gravelle SK, Rosengren J, Khan S, Ciofu O, Johansen HK, Høiby N and Ulanova M: Cellular responses of A549 alveolar epithelial cells to serially collected Pseudomonas aeruginosa from cystic fibrosis patients at different stages of pulmonary infection. FEMS Immunol Med Microbiol. 59:207–220. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cerqueira AM, Khaper N, Lees SJ and Ulanova M: The antioxidant resveratrol down-regulates inflammation in an in-vitro model of Pseudomonas aeruginosa infection of lung epithelial cells. Can J Physiol Pharmacol. 91:248–255. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Aval PS, Werner J, Cerqueira A, Balfour-Boehm J and Ulanova M: Piceatannol modulates lung epithelial cellular responses to Pseudomonas aeruginosa. Inflamm Allergy Drug Targets. 12:297–307. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
De Stefano D, Ungaro F, Giovino C, Polimeno A, Quaglia F and Carnuccio R: Sustained inhibition of IL-6 and IL-8 expression by decoy ODN to NF-ĸB delivered through respirable large porous particles in LPS-stimulated cystic fibrosis bronchial cells. J Gene Med. 13:200–208. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Muselet-Charlier C, Roque T, Boncoeur E, Chadelat K, Clement A, Jacquot J and Tabary O: Enhanced IL-1beta-induced IL-8 production in cystic fibrosis lung epithelial cells is dependent of both mitogen-activated protein kinases and NF-kappaB signaling. Biochem Biophys Res Commun. 357:402–407. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dechecchi MC, Nicolis E, Norez C, Bezzerri V, Borgatti M, Mancini I, Rizzotti P, Ribeiro CM, Gambari R, Becq F and Cabrini G: Anti-inflammatory effect of miglustat in bronchial epithelial cells. J Cyst Fibros. 7:555–565. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gambari R, Borgatti M, Lampronti I, Fabbri E, Brognara E, Bianchi N, Piccagli L, Yuen MC, Kan CW, Hau DK, et al: Corilagin is a potent inhibitor of NF-kappaB activity and downregulates TNF-alpha induced expression of IL-8 gene in cystic fibrosis IB3-1 cells. Int Immunopharmacol. 13:308–315. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Borgatti M, Chilin A, Piccagli L, Lampronti I, Bianchi N, Mancini I, Marzaro G, dall'Acqua F, Guiotto A and Gambari R: Development of a novel furocoumarin derivative inhibiting NF-κB dependent biological functions: Design, synthesis and biological effects. Eur J Med Chem. 46:4870–4877. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gasparello J, D'Aversa E, Papi C, Gambari L, Grigolo B, Borgatti M, Finotti A and Gambari R: Sulforaphane inhibits the expression of interleukin-6 and interleukin-8 induced in bronchial epithelial IB3-1 cells by exposure to the SARS-CoV-2 Spike protein. Phytomedicine. 87(153583)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gasparello J, d'Aversa E, Breveglieri G, Borgatti M, Finotti A and Gambari R: In vitro induction of interleukin-8 by SARS-CoV-2 Spike protein is inhibited in bronchial epithelial IB3-1 cells by a miR-93-5p agomiR. Int Immunopharmacol. 101(108201)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cosenza LC, Marzaro G, Zurlo M, Gasparello J, Zuccato C, Finotti A and Gambari R: Inhibitory effects of SARS-CoV-2 spike protein and BNT162b2 vaccine on erythropoietin-induced globin gene expression in erythroid precursor cells from patients with β-thalassemia. Exp Hematol. 129(104128)2024.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gasparello J, Papi C, Marzaro G, Macone A, Zurlo M, Finotti A, Agostinelli E and Gambari R: Aged Garlic Extract (AGE) and its constituents S-allyl-cysteine (SAC) inhibit the expression of pro-inflammatory genes induced in bronchial epithelial IB3-1 cells by exposure to the SARS-CoV-2 Spike protein and the BNT162b2 vaccine. Molecules. 29(5938)2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Papi C, Gasparello J, Marzaro G, Macone A, Zurlo M, Di Padua F, Fino P, Agostinelli E, Gambari R and Finotti A: S-1-propenyl-l-cysteine (S1PC), a major constituent of Aged Garlic Extract (AGE), exhibits inhibitory effects on pro-inflammatory gene expression in bronchial epithelial IB3-1 cells exposed to the BNT162b2 vaccine. Exp Ther Med (In press).
|
|
47
|
Puck A, Künig S, Modak M, May L, Fritz P, Battin C, Radakovics K, Steinberger P, Reipert BM, Crowe BA and Stöckl J: The soluble cytoplasmic tail of CD45 regulates T-cell activation via TLR4 signaling. Eur J Immunol. 51:3176–3185. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Geng Z, Rong Y and Lau BH: S-allyl cysteine inhibits activation of nuclear factor kappa B in human T cells. Free Radic Biol Med. 23:345–350. 1997.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hoffmann N, Rasmussen TB, Jensen PØ, Stub C, Hentzer M, Molin S, Ciofu O, Givskov M, Johansen HK and Høiby N: Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect Immun. 73:2504–2514. 2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bjarnsholt T, Jensen PØ, Rasmussen TB, Christophersen L, Calum H, Hentzer M, Hougen HP, Rygaard J, Moser C, Eberl L, et al: Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology (Reading). 151:3873–3880. 2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Smyth AR, Cifelli PM, Ortori CA, Righetti K, Lewis S, Erskine P, Holland ED, Givskov M, Williams P, Cámara M, et al: Garlic as an inhibitor of Pseudomonas aeruginosa quorum sensing in cystic fibrosis-a pilot randomized controlled trial. Pediatr Pulmonol. 45:356–362. 2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Libero ML, Montero-Hidalgo AJ, Recinella L, Luque RM, Generali D, Acquaviva A, Orlando G, Ferrante C, Menghini L, Di Simone SC, et al: The protective effects of an aged black garlic water extract on the prostate. Nutrients. 16(3025)2024.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Liu J: Aged garlic therapeutic intervention targeting inflammatory pathways in pathogenesis of bowel disorders. Heliyon. 10(e33986)2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kurita M, Matsutomo T and Kodera Y: 3-Allyltrisulfanyl-alanine formation during the preparation of aged garlic extract. J Agric Food Chem. 68:14577–14583. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Padiya R and Banerjee SK: Garlic as an anti-diabetic agent: Recent progress and patent reviews. Recent Pat Food Nutr Agric. 5:105–127. 2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kodera Y, Kurita M, Nakamoto M and Matsutomo T: Chemistry of aged garlic: Diversity of constituents in aged garlic extract and their production mechanisms via the combination of chemical and enzymatic reactions. Exp Ther Med. 19:1574–1584. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Park JM, Han YM, Kangwan N, Lee SY, Jung MK, Kim EH and Hahm KB: S-allyl cysteine alleviates nonsteroidal anti-inflammatory drug-induced gastric mucosal damages by increasing cyclooxygenase-2 inhibition, heme oxygenase-1 induction, and histone deacetylation inhibition. J Gastroenterol Hepatol 29 Suppl. 4:S80–S92. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Colín-González AL, Ali SF, Túnez I and Santamaría A: On the antioxidant, neuroprotective and anti-inflammatory properties of S-allyl cysteine: An update. Neurochem Int. 89:83–91. 2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Shao Z, Pan Z, Lin J, Zhao Q, Wang Y, Ni L, Feng S, Tian N, Wu Y, Sun L, et al: S-allyl cysteine reduces osteoarthritis pathology in the tert-butyl hydroperoxide-treated chondrocytes and the destabilization of the medial meniscus model mice via the Nrf2 signaling pathway. Aging (Albany NY). 12:19254–19272. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhang G and Ghosh S: Toll-like receptor-mediated NF-kappaB activation: A phylogenetically conserved paradigm in innate immunity. J Clin Invest. 107:13–19. 2001.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB signaling in inflammation. Sig Transduct Target Ther. 2(17023)2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Li C, Wang J, Zhao M, Zhang S and Zhang Y: Toll-like receptor 4 antagonist FP7 alleviates lipopolysaccharide-induced septic shock via NF-kB signaling pathway. Chem Biol Drug Des. 97:1151–1157. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Doyle SL and O'Neill LA: Toll-like receptors: From the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 72:1102–1113. 2006.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Vallabhapurapu S and Karin M: Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 27:693–733. 2009.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Israel A: The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol. 2(a000158)2010.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kawai T and Akira S: Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 13:460–469. 2007.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Orozco-Morales M, Hernández-Pedro NY, Barrios-Bernal P, Arrieta O, Ruiz-Godoy LM, Aschner M, Santamaría A and Colín-González AL: S-allylcysteine induces cytotoxic effects in two human lung cancer cell lines via induction of oxidative damage, downregulation of Nrf2 and NF-ĸB, and apoptosis. Anticancer Drugs. 32:117–126. 2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Basu C, Chatterjee A, Bhattacharya S, Dutta N and Sur R: S-allyl cysteine inhibits TNF-α-induced inflammation in HaCaT keratinocytes by inhibition of NF-κB-dependent gene expression via sustained ERK activation. Exp Dermatol. 28:1328–1335. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Qin L, Li G, Qian X, Liu Y, Wu X, Liu B, Hong JS and Block ML: Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia. 52:78–84. 2005.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Pi Y, Zhang LL, Li BH, Guo L, Cao XJ, Gao CY and Li JC: Inhibition of reactive oxygen species generation attenuates TLR4-mediated proinflammatory and proliferative phenotype of vascular smooth muscle cells. Lab Invest. 93:880–887. 2013.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Karin M and Ben-Neriah Y: Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev Immunol. 18:621–663. 2000.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Tanaka K, Kawakami T, Tateishi K, Yashiroda H and Chiba T: Control of IkappaBalpha proteolysis by the ubiquitin-proteasome pathway. Biochimie. 83:351–356. 2001.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kanarek N and Ben-Neriah Y: Regulation of NF-κB by ubiquitination and degradation of the IκBs. Immunol Rev. 246:77–94. 2012.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Ortuño Sahagún D, Márquez-Aguirre AL, Quintero-Fabián S, López-Roa RI and Rojas-Mayorquín AE: Modulation of PPAR-γ by nutraceutics as complementary treatment for obesity-related disorders and inflammatory diseases. PPAR Res. 2012(318613)2012.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Remels AH, Langen RC, Gosker HR, Russell AP, Spaapen F, Voncken JW, Schrauwen P and Schols AM: PPARgamma inhibits NF-kappaB-dependent transcriptional activation in skeletal muscle. Am J Physiol Endocrinol Metab. 297:E174–E183. 2009.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Nian H, Delage B, Ho E and Dashwood RH: Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: Studies with sulforaphane and garlic organosulfur compounds. Environ Mol Mutagen. 50:213–221. 2009.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Aboudounya MM and Heads RJ: COVID-19 and toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Mediators Inflamm. 2021(8874339)2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Sahanic S, Hilbe R, Dünser C, Tymoszuk P, Löffler-Ragg J, Rieder D, Trajanoski Z, Krogsdam A, Demetz E, Yurchenko M, et al: SARS-CoV-2 activates the TLR4/MyD88 pathway in human macrophages: A possible correlation with strong pro-inflammatory responses in severe COVID-19. Heliyon. 9(e21893)2023.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Alamin MH, Rahaman MM, Ferdousi F, Sarker A, Ali MA, Hossen MB, Sarker B, Kumar N and Mollah MNH: In-silico discovery of common molecular signatures for which SARS-CoV-2 infections and lung diseases stimulate each other, and drug repurposing. PLoS One. 19(e0304425)2024.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Hasan MT, Abdulrazak LF, Alam MK, Islam MR, Sathi YH, Al-Zahrani FA, Ahmed K, Bui FM and Moni MA: Discovering common pathophysiological processes between COVID-19 and cystic fibrosis by differential gene expression pattern analysis. Biomed Res Int. 2022(8078259)2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Rais N, Ved A, Ahmad R, Kumar M, Barbhai MD, Chandran D, Dey A, Dhumal S, Senapathy M, Deshmukh VP, et al: S-Allyl-L-Cysteine-A garlic Bioactive: Physicochemical nature, mechanism, pharmacokinetics, and health promoting activities. J Funct Foods. 107(105657)2023.
|
|
82
|
Nie Y, Yu K, Li B, Hu Y, Zhang H, Xin R, Xiong Y, Zhao P and Chai G: S-allyl-l-cysteine attenuates bleomycin-induced pulmonary fibrosis and inflammation via AKT/NF-κB signaling pathway in mice. J Pharmacol Sci. 139:377–384. 2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Ray B, Chauhan NB and Lahiri DK: The ‘aged garlic extract:’ (AGE) and one of its active ingredients S-allyl-L-cysteine (SAC) as potential preventive and therapeutic agents for Alzheimer's disease (AD). Curr Med Chem. 18:3306–3313. 2011.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Kosuge Y: Neuroprotective mechanisms of S-allyl-L-cysteine in neurological disease. Exp Ther Med. 19:1565–1569. 2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Elmazoglu Z, Aydın Bek Z, Sarıbaş SG, Özoğul C, Goker B, Bitik B, Aktekin CN and Karasu Ç: S-allylcysteine inhibits chondrocyte inflammation to reduce human osteoarthritis via targeting RAGE, TLR4, JNK, and Nrf2 signaling: comparison with colchicine. Biochem Cell Biol. 99:645–654. 2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Gupta P, Dutt V, Kaur N, Kalra P, Gupta S, Dua A, Dabur R, Saini V and Mittal A: S-allyl cysteine: A potential compound against skeletal muscle atrophy. Biochim Biophys Acta Gen Subj. 1864(129676)2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Dutt V, Saini V, Gupta P, Kaur N, Bala M, Gujar R, Grewal A, Gupta S, Dua A and Mittal A: S-allyl cysteine inhibits TNFα-induced skeletal muscle wasting through suppressing proteolysis and expression of inflammatory molecules. Biochim Biophys Acta Gen Subj. 1862:895–906. 2018.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Chuah SC, Moore PK and Zhu YZ: S-allylcysteine mediates cardioprotection in an acute myocardial infarction rat model via a hydrogen sulfide-mediated pathway. Am J Physiol Heart Circ Physiol. 293:H2693–H2701. 2007.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Yue LJ, Zhu XY, Li RS, Chang HJ, Gong B, Tian CC, Liu C, Xue YX, Zhou Q, Xu TS and Wang DJ: S-allyl-cysteine sulfoxide (alliin) alleviates myocardial infarction by modulating cardiomyocyte necroptosis and autophagy. Int J Mol Med. 44:1943–1951. 2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Sheela CG and Augusti KT: Antidiabetic effects of S-allyl cysteine sulphoxide isolated from garlic Allium sativum Linn. Indian J Exp Biol. 30:523–526. 1992.PubMed/NCBI
|
|
91
|
Zhai B, Zhang C, Sheng Y, Zhao C, He X, Xu W, Huang K and Luo Y: Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci Rep. 8(3527)2018.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Pandey P, Khan F, Alshammari N, Saeed A, Aqil F and Saeed M: Updates on the anticancer potential of garlic organosulfur compounds and their nanoformulations: Plant therapeutics in cancer management. Front Pharmacol. 14(1154034)2023.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Miraghajani M, Rafie N, Hajianfar H, Larijani B and Azadbakht L: Aged garlic and cancer: A systematic review. Int J Prev Med. 9(84)2018.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Reyes-Soto CY, Ramírez-Carreto RJ, Ortíz-Alegría LB, Silva-Palacios A, Zazueta C, Galván-Arzate S, Karasu Ç, Túnez I, Tinkov AA, Aschner M, et al: S-allyl-cysteine triggers cytotoxic events in rat glioblastoma RG2 and C6 cells and improves the effect of temozolomide through the regulation of oxidative responses. Discov Oncol. 15(272)2024.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Ohnishi ST and Ohnishi T: In vitro effects of aged garlic extract and other nutritional supplements on sickle erythrocytes. J Nutr. 131(3s):1085S–1092S. 2001.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Takasu J, Uykimpang R, Sunga MA, Amagase H and Niihara Y: Aged garlic extract is a potential therapy for sickle-cell anemia. J Nutr. 136 (Suppl 3):803S–805S. 2006.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Takasu J, Uykimpang R, Sunga M, Amagase H and Niihara Y: Aged garlic extract therapy for sickle cell anemia patients. BMC Blood Disord. 2(3)2002.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Gambari R and Finotti A: Interplay of TLR4 and SARS-CoV-2: Possible involvement of microRNAs [Letter]. J Inflamm Res. 17:7963–7964. 2024.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Gambari R, Papi C, Gasparello J, Agostinelli E and Finotti A: Preliminary results and a theoretical perspective of co-treatment using a miR-93-5p mimic and aged garlic extract to inhibit the expression of the pro-inflammatory interleukin-8 gene. Exp Ther Med. 29(85)2025.PubMed/NCBI View Article : Google Scholar
|