Introduction
Hyalinizing trabecular tumors (HTTs) of the thyroid,
which were first described by Carney et al (1) in 1987, represent a rare subtype of
thyroid neoplasm that continues to present both clinical and
pathological diagnostic challenges. These tumors are defined by
their characteristic hyalinized stroma and trabecular growth
pattern (2). Clinically, HTTs that
usually present as asymptomatic, well circumscribed and solitary
masses exhibit a benign course, with no evidence of capsular or
vascular invasion (3). Even in
rare cases where malignant features, such as focal invasion, are
observed, the prognosis remains favorable, with no reports of
recurrence or metastasis following surgical resection (1,4).
Therefore, HTTs have traditionally been classified and managed as
benign entities. Despite their benign nature, HTTs are frequently
misdiagnosed as papillary thyroid carcinomas (PTCs) due to
overlapping nuclear features, such as nuclear grooves, and
intranuclear pseudoinclusions observed under light microscopy
(5,6). These shared features can lead to
confusion in the preoperative setting, particularly because PTC is
a much more common malignant tumor of the thyroid with distinct
clinical and histological implications (5,6).
Furthermore, the amyloid-like appearance of the hyalinized stroma
in HTTs may mimic the amyloid deposits characteristic of medullary
thyroid carcinoma (MTC), leading to diagnostic confusion (5). Preoperative differentiation of HTTs
from these malignancies is particularly challenging using fine
needle aspiration biopsy (FNAB), often resulting in unnecessary
overtreatment such as total thyroidectomy or lymph node dissection
that is inappropriate for benign tumors. The potential harm of
overtreatment of these cases emphasizes the need for a more nuanced
understanding of HTTs and highlights the importance of refining
diagnostic tools to differentiate them from other thyroid tumors.
The present study aimed to address these diagnostic challenges by
analyzing the clinical characteristics of HTTs over an 11-year
period at a tertiary referral center. The findings of the present
study may aid in the establishment of more accurate differential
diagnostic criteria and guide the development of appropriate
management strategies for similar cases.
Materials and methods
Patients
A retrospective analysis was conducted on the
medical record data for 11 patients with pathologically confirmed
HTTs based on their final histological examinations following
thyroid surgeries at Chonnam National University Hwasun Hospital
(CNUHH; Hwasun, South Korea), which functions as a tertiary
referral hospital and regional national cancer institution. The
patients underwent thyroid surgery between March 2011 and December
2021. Patients diagnosed with HTT were included, while patients
without appropriate follow-up were excluded. The patients'
characteristics, including the age and sex, are described in the
results section and Table I.
Clinical data were collected and analyzed retrospectively. The
clinical information obtained included the patients' sex, age, past
medical history, type of surgery (lobectomy or total
thyroidectomy), tumor size, FNAB results, ultrasonography (US)
features, final histopathological findings and follow-up duration.
The present study was approved by the Institutional Review Board of
CNUHH (approval no. CNUHH-2024-175).
 | Table IClinical characteristics of patients
with hyalinizing trabecular tumors. |
Table I
Clinical characteristics of patients
with hyalinizing trabecular tumors.
| Characteristic | Value |
|---|
| Sex | |
|
Male | 1 |
|
Female | 10 |
| Age, years | 54.9±11.2 |
| Underlying
disease | |
|
Hypertension | 4 |
|
Diabetes | 1 |
|
Asthma | 1 |
|
Other
malignancy or tumor | 3 |
|
None | 5 |
| Tumor long diameter,
cm | 1.7±1.1 |
| Fine needle
aspiration biopsy result | |
|
Hurtle cell
neoplasm | 1 |
|
Suspicious
for papillary thyroid carcinoma | 5 |
|
Suspicious
for medullary thyroid carcinoma | 1 |
|
Benign
nodule (adenomatous goiter, follicular nodule) | 3 |
|
Atypia of
undetermined significance | 2 |
|
Suspicious
for carcinoma, unspecified | 1 |
|
Hyalinizing
trabecular adenoma | 1 |
| Feature of
ultrasonography | |
|
Well-defined | 8 |
|
Ill-defined | 3 |
|
Hypoechoic | 10 |
|
Isoechoic | 1 |
|
Heterogenous | 10 |
|
Homogenous | 1 |
FNAB process
All FNAB procedures were performed under US guidance
using a 25-gauge needle. The aspirated cellular material was
immediately smeared onto glass slides, fixed with 95% ethanol for
15 min, and subsequently stained in hematoxylin for 1-3 min and
then stained in Papanicolaou (PAP) for 1-3 min, at room temperature
(7). Cytological interpretations
(8,9) were carried out by experienced
pathologists. The Korean Thyroid Association recommends FNAB for
thyroid nodules >10 mm (10);
however, FNAB is often performed on smaller nodules based on
patient requests or clinical judgment. For this reason, FNAB was
performed on some HTT cases with 3- or 5-mm nodules.
Immunohistochemical (IHC)
analysis
Resected tumor samples were fixed at room
temperature in 10% formalin for 3 days, embedded in paraffin and
cut in 3-µm tissue sections. An automated immunostainer (BOND-MAX
DC2002; Leica Microsystems, Inc.) was used for staining. The
sections were stained with hematoxylin for 5-15 min and eosin for
1-3 min, at room temperature, and anti-human Ki-67 (MIB-1 clone;
cat. no. 790-4286; Roche Diagnostics), calcitonin (cat. no. A0576;
Dako; Agilent Technologies, Inc.), high molecular weight
cytokeratin (HMCK; cat. no. M0630; Dako; Agilent Technologies,
Inc.), CD56 (cat. no. M7304; Dako; Agilent Technologies, Inc.),
paired box 8 (PAX8; cat. no 363M-15; Cell Marque; MilliporeSigma),
thyroid transcription factor 1 (TTF-1, cat. no. M3575; Dako;
Agilent Technologies, Inc.), BRAF V600E (cat. no. 760-5095; Roche
Diagnostics) or CD31 (cat. no. M0823; Dako; Agilent Technologies,
Inc.) antibodies. The specimens were reviewed by pathologists to
confirm the diagnosis of HTT under a light microscope (11,12).
Results
Patients characteristics
The demographic and clinical characteristics of the
11 patients diagnosed with HTTs are summarized in Table I. Between March 2011 and December
2021, a total of 9,169 patients underwent thyroid surgery at CNUHH.
Among these, 11 patients (0.12%) were histologically confirmed to
have HTTs. Individual patient characteristics are detailed in
Table II. Of the 11 patients, 1
(9.1%) was male and 10 (90.9%) were female, with a mean age of
54.9±11.2 years (range, 39-69 years). A total of 10 patients
presented with an incidentally detected thyroid mass identified by
US during routine medical checkups, while 1 patient presented with
a lateral neck mass as the primary symptom.
 | Table IIIndividual characteristics of
patients with hyalinizing trabecular tumors. |
Table II
Individual characteristics of
patients with hyalinizing trabecular tumors.
| Sex | Age, years | Underlying
disease | Operation type | Size, cm | FNA biopsy
result | Feature of
ultrasonography | Biopsy result | IHC staining |
|---|
| F | 69 | None | Rt. lobectomy | 3.1 | Hurtle cell
neoplasm | Well-defined,
heterogenous and hypoechoic | HTT and
oncocytoma | MIB-1(-) |
| F | 41 | HTN | TT with CLND | 0.5 | Some irregular
clusters of follicular cells and numerous histocytes, suggestive of
cystic change of adenomatous goiter | Well-defined,
heterogeneous and isoechoic | HTT accompanied by
PTC in the contralateral lobe | MIB-1(+) |
| F | 62 | HTN | TT | 0.6 | No aspiration for
HTT (FNA for other nodules: Suspicious for PTC) | Well-defined,
heterogenous and hypoechoic | HTC accompanied by
PTC in the bilateral lobe | MIB-1(+) |
| F | 64 | HTN | TT | 2.2 | Suspicious for PTC
and MTC | Well-defined,
heterogenous and hypoechoic | Hyalinizing
trabecular adenoma, nodular hyperplasia with papillary epithelial
growth and Hashimoto's thyroiditis | MIB-1(+);
calcitonin(-) |
| F | 55 | None | TT with CLND | 2.4 | Suspicious for
PTC | Well-defined,
heterogenous and hypoechoic | HTT | MIB-1(+); HMCK
(focal+); CD56(+) |
| F | 50 | None | TT with CLND | 1.4 | Benign, suggestive
for adenomatous goiter | Well-defined,
heterogeneous and hypoechoic | HTT accompanied by
PTC in ipsilateral lobe | MIB-1(+);
HMCK(-) |
| M | 52 | Thymoma | Rt. lobectomy | 3.9 | Atypia of
undetermined significance | Ill-defined,
heterogeneous and hypoechoic | HTT, nodular
hyperplasia with papillary epithelial growth and Hashimoto's
thyroiditis | None performed |
| F | 39 | HTN, DM and breast
cancer | Rt. lobectomy | 1.0 | Suspicious for PTC
and hyalinizing trabecular adenoma | Well-defined,
homogeneous and hypoechoic | Hyalinizing
trabecular adenoma | MIB-1(+) |
| F | 69 | None | Lt. lobectomy with
CLND | 1.2 | Atypia of
undetermined significance (first aspiration); suspicious for PTC
(second aspiration) | Ill-defined,
heterogeneous and hypoechoic | HTT | MIB-1(+) |
| F | 41 | None | Rt. lobectomy with
CLND; Rt. MRND type III | 2.2 | Suspicious for
carcinoma or metastatic carcinoma | Well-defined,
heterogeneous and hypoechoic | HTT | MIB-1(+); PAX8(+);
TTF-1(+); BRAF(-); calcitonin(-); CD31(-) |
| F | 62 | Asthma and
leukemia | Lt. lobectomy | 0.3 | Benign follicular
nodule (first aspiration); suspicious for PTC (second
aspiration) | Ill-defined,
heterogeneous and hypoechoic | HTT accompanied by
PTC in ipsilateral lobe | MIB-1(+) |
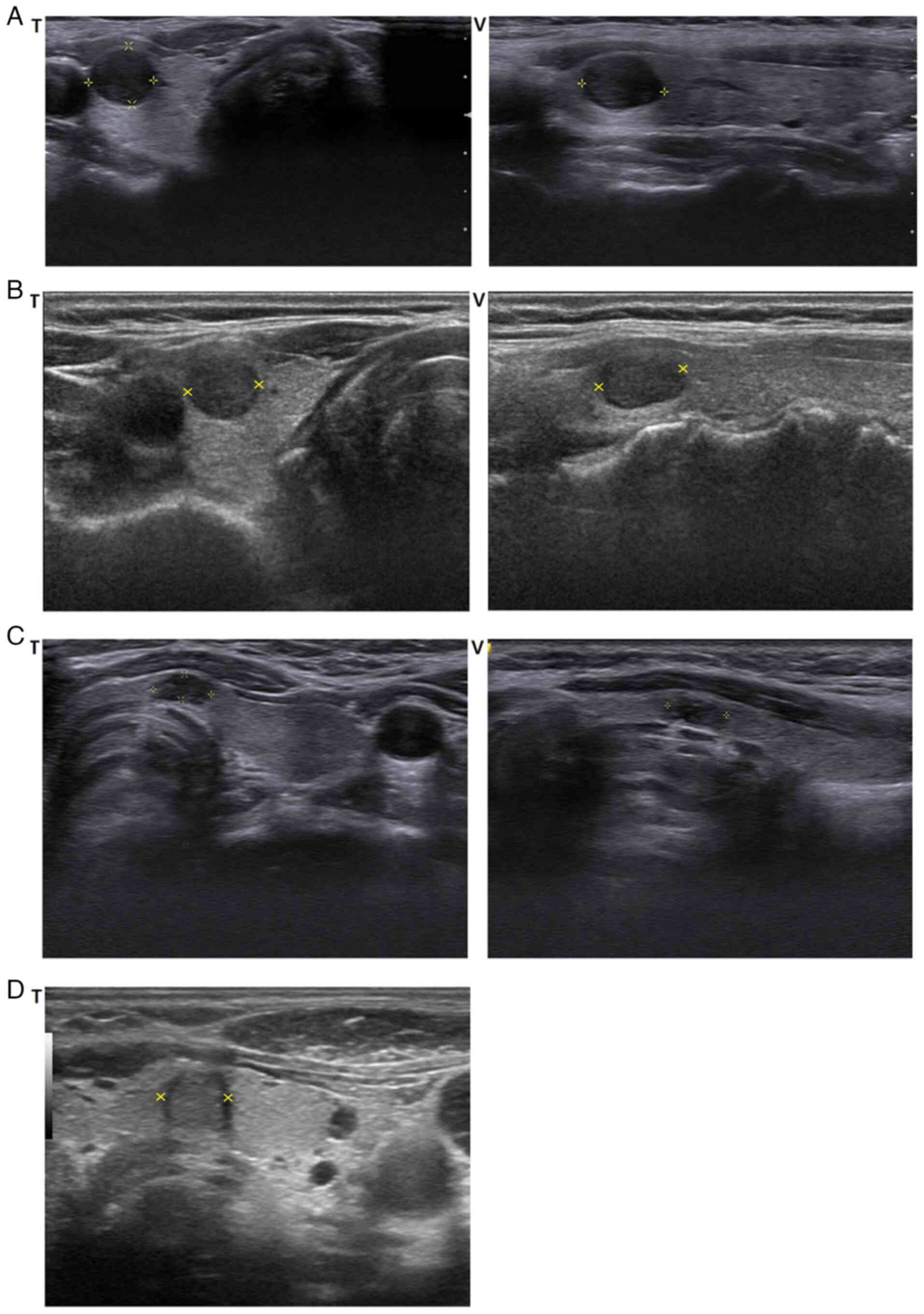
US findings
The mean tumor size, as measured by the longest
dimension on US, was 1.7±1.1 cm (range, 0.3-3.9 cm). Although no
consistent US features were observed, most tumors (7 cases)
appeared as hypoechoic lesions with well-defined margins (6 cases,
heterogenous, Fig. 1A; 1 case,
homogenous, Fig. 1B). Three tumors
appeared as ill-defined, hypoechoic heterogenous lesions (Fig. 1C), and one tumor appeared as a
well-defined, heterogenous isoechoic lesion (Fig. 1D).
FNAB findings
FNAB was performed on all 11 patients as part of the
preoperative evaluation. A total of 10 out of the 11 patients
underwent FNAB for a mass identified as HTT in the permanent biopsy
after surgery. Out of 10 patients, 8 patients underwent FNAB once,
while 2 patients underwent FNAB twice. However, in 1 patient, FNAB
targeted another nodule that suggested PTC, rather than HTT. Of the
10 FNAB-targeted HTT masses, cytological findings suggested the
inclusion of HTT in the differential diagnosis in only 1 patient.
Nevertheless, even in this instance, the findings could not
reliably differentiate between PTC and HTT. Additionally, in total
6 patients including 1 patient in whom HTT and PTC could not be
differentiated, the FNAB findings indicated suspicious malignancy,
including PTC or unspecified carcinoma. Detailed FNAB findings for
the 11 cases are presented in Table
II.
Surgery, histopathological findings
and prognosis
Surgical interventions included thyroid lobectomies
in 6 patients and total thyroidectomies in 5 patients. Among the 5
patients who underwent total thyroidectomies, 3 patients exhibited
both HTTs and PTCs in the same, contralateral or bilateral lobes
upon final histological examination. Similarly, 1 patient who
underwent a lobectomy was diagnosed with both a PTC and an HTT
within the same lobe. One patient underwent total thyroidectomy due
to FNAB findings that were inconclusive between MTC and PTC.
Another patient underwent total thyroidectomy upon personal
request. Notably, intraoperative frozen-section biopsies were not
performed in any of the 11 cases based on the surgeon's judgement
during the surgery. In all cases, the final histopathological
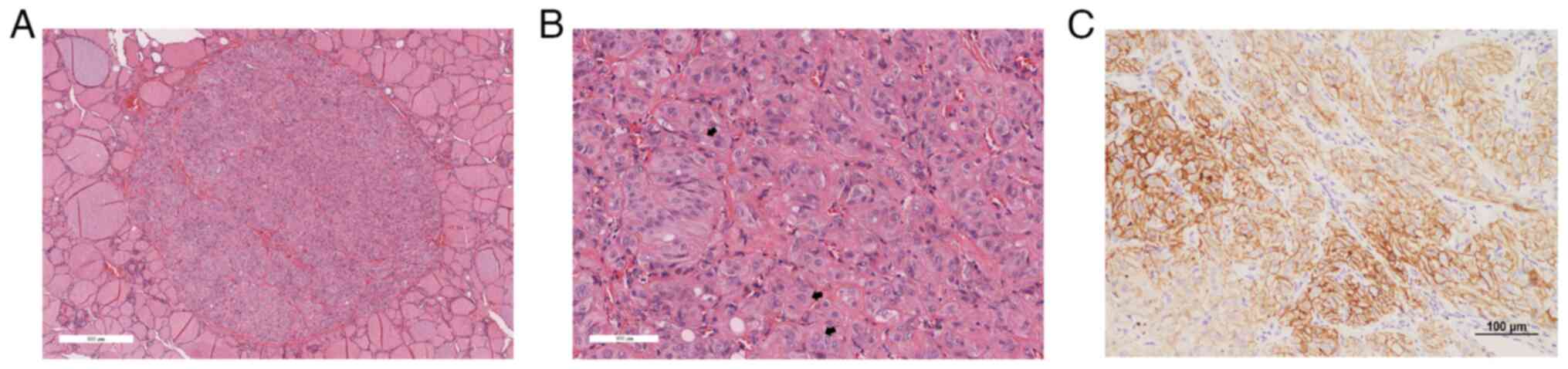
evaluation revealed tumors that were clearly demarcated from the
surrounding non-neoplastic thyroid tissue (Fig. 2A). Under high magnification, tumor
cells exhibited trabecular and organoid arrangements, abundant
eosinophilic cytoplasm with hyaline material, nuclear grooves and
intranuclear inclusions (Fig. 2B),
which supported the definitive diagnosis of HTT. IHC analysis
revealed membranous positivity for Ki-67/MIB-1 clone in 9 cases
(Fig. 2C). This membranous
staining of MIB-1 is the characteristic pathological finding of
HTT. The IHC staining results used for differential diagnosis are
summarized in Table II. To
exclude PTC from the differential diagnosis of HTT, the
immunohistochemical markers HMCK, CD56 and BRAF were utilized
(11-13).
Calcitonin staining was employed to distinguish HTT from MTC
(11-13),
while CD31 was used to assess vascular invasion (14). Thyroid follicular origin was
confirmed using PAX8 and TTF-1 (11,12).
The present study involved patients over an 11-year-long period,
and as a result the IHC slides have faded, making them unsuitable
for publication. Therefore, a MIB-1 IHC image from a representative
patient was presented. Postoperative follow-up was conducted for a
mean duration of 38 months (range, 3-132 months). All patients
remained free of recurrence or metastasis, and no major
postoperative complications were reported.
Discussion
HTTs are described in the literature as rare
neoplasms originating from follicular cells, occurring in
middle-aged women between 40 and 70 years and typically exhibiting
benign behavior (15-18).
The etiology of HTTs remains unclear; however, associations with
chronic lymphocytic thyroiditis, Hashimoto's thyroiditis,
multinodular goiter, a history of neck radiation exposure and PTC
have been documented (17-19).
Notably, instances of HTTs coexisting with micropapillary thyroid
carcinoma have also been reported (20). In the present study, 4 out of 11
cases (36.4%) were accompanied by PTC in either the contralateral
or ipsilateral lobe, and 2 patients presented with concurrent
Hashimoto's thyroiditis. These findings are consistent with the
aforementioned previous reports highlighting conditions associated
with HTTs.
HTTs were first described by Carney et al
(1) in 1987, who characterized
their clinical and pathological features as either solitary lesions
or components of multinodular presentations. These tumors are
generally small, measuring ≤2 cm, with gross pathology revealing
well-defined margins and capsular formation (1,2).
Microscopically, HTTs are distinguished by the trabecular
arrangement of polygonal, oval or spindle-shaped tumor cells set
within a hyalinizing stroma (21-23).
Certain trabeculae may appear curved, forming ribbon- or
festoon-like patterns (24).
Although cases of HTTs with capsular invasion,
vascular invasion, lymph node metastases and even lung metastases
have been reported (25,26), Carney et al (2) noted that of 19 patients with HTTs, 18
exhibited benign clinical courses without malignancies, even in
tumors with features suggestive of malignancy, and no recurrences
or metastases were observed during long-term follow-up. Similarly,
in the present study, none of the 11 patients demonstrated evidence
of recurrence or metastasis during the follow-up period.
On preoperative FNAB, HTTs may present nuclear
grooves, intranuclear inclusions and occasionally psammoma bodies,
which can result in a misdiagnosis of PTC (19,21,22).
Additionally, the hyalinizing stroma can mimic amyloid deposits,
leading to potential diagnostic confusion with MTC (27). In certain cases, the tumor cells
form glandular patterns, posing challenges in distinguishing HTTs
from paragangliomas (23).
Misdiagnoses due to cytological similarities with PTC or MTC have
been documented, underscoring the necessity for meticulous
histopathological evaluation and further research to refine
diagnostic approaches for HTTs (20,22,24,27).
The clues for an accurate diagnosis of HTTs include the absence of
papillary structures or fibrovascular stalks and the presence of
elongated nuclei in association with hyaline stroma (11,12).
Although certain studies have explored the use of preoperative US,
FNAB and frozen-section histopathology to identify HTTs, no
definitive clinical diagnostic criteria for preoperative
differentiation have been established (28,29).
In the present study, only 1 patient was suspected to represent HTT
based on preoperative FNAB, while diagnostic challenges were
evident in 6 patients suspected as having PTC or unspecific
carcinoma.
The final diagnosis of HTTs can only be confirmed
through pathological examination. In cases where distinguishing
tumor types based on histological features is challenging, IHC
analysis plays a pivotal role. Unlike PTCs, which typically
demonstrate minimal reactivity to the MIB-1 antibody, HTTs exhibit
strong positive reactivity to MIB-1, localized along the tumor cell
membranes. In the present study, 9 cases displayed MIB-1
positivity. Additionally, while PTCs exhibit strong positive IHC
staining for galectin-3, HMCK and cytokeratin 19, HTTs generally
show negative or weakly positive staining for these markers
(30-32).
IHC staining also distinguishes HTTs from MTCs, as HTTs are
positive for thyroglobulin and negative for neuroendocrine markers,
such as calcitonin, synaptophysin and chromogranin, whereas MTCs
exhibit the opposite staining pattern (27).
Molecular analyses have further identified
RET/PTC rearrangements in both PTCs and HTTs; however,
BRAF and N-Ras mutations commonly observed in PTCs
are absent in HTTs (13,33,34).
Notably, Nikiforova et al (35) reported a high prevalence of
GLIS rearrangements, particularly PAX8-GLIS3, in HTTs
but not in PTCs. Among indeterminate FNAB results,
PAX8-GLIS3 was detected in 0.1% of cases, and all 5
surgically confirmed cases were diagnosed as HTT, underscoring the
utility of molecular testing using preoperative FNAB material
(35). The incidence of HTT in the
present study (~0.12%) aligns with the aforementioned reported
rate, further highlighting the rarity of this entity. The lack of
genetic testing is the main limitation of the present study. The
current study was conducted over an 11-year-long period, which
includes a notable time before the introduction of molecular
testing for HTT. However, further research on the clinical impact
of molecular testing, including GLIS rearrangements, on the
diagnosis and treatment of HTT will be an important topic for
future studies.
Despite these diagnostic advances, the utility of US
and FNAB in reliably diagnosing HTTs preoperatively remains limited
(28,29). Nevertheless, when preoperative US
findings suggest the occurrence of benign tumors, but FNAB results
raise suspicion for malignancy, particularly PTC, the differential
diagnosis should include HTT. Particularly for FNAB results
suspicious for PTC, the absence of BRAF mutations may
heighten the suspicion for HTT. However, as demonstrated by the low
incidence of HTT in the present study (only 11 cases among 9,169
thyroidectomy patients), its rarity poses an important diagnostic
challenge for clinicians and pathologists (11). Limited exposure of clinicians to
HTTs and their cytological features may further contribute to the
difficulty in achieving a preoperative diagnosis. It is imperative
for clinicians and pathologists to recognize the key clinical,
cytological and molecular characteristics of HTT, including the use
of additional tests, such as GLIS rearrangements, to improve
diagnostic accuracy (36).
However, in cases of thyroid nodules, >30% of
patients fall into the non-diagnostic, indeterminate and follicular
neoplasm categories under the Bethesda System (8,9),
where treatment decisions are unclear. However, cytological IHC and
molecular testing are not performed for all cytologically difficult
cases. IHC and molecular testing on preoperative cytological
material could be helpful in the differential diagnosis, but this
is only possible when clinicians or pathologists suspect HTT
through conventional clinical aspects, including basic FNAB and US
findings. As is often the case with rare entities, the main
obstacle to diagnostic accuracy seems to be simply considering the
diagnosis of HTT. Therefore, the present study is likely to help
clinicians in suspecting HTT and considering further diagnostic
tests.
Early clinical suspicion for HTT is crucial, as it
may prevent unnecessary surgical procedures, such as total
thyroidectomy. Compared with total thyroidectomy, thyroid lobectomy
offers several advantages, including preservation of thyroid
function, reduced dependence on lifelong thyroid hormone
replacement therapy and avoidance of complications, such as
recurrent laryngeal nerve injury and hypocalcemia (37). The type of thyroid surgery
significantly impacts patient quality of life; therefore, when HTT
is suspected preoperatively, a diagnostic lobectomy is preferable
to total thyroidectomy to optimize patient outcomes through reduced
surgical intervention (38).
In conclusion, while the diagnostic utility of US
and FNAB for HTTs remains limited, their findings, in conjunction
with molecular and IHC analyses, can aid in the identification of
HTT preoperatively. In cases where preoperative US findings appear
benign but discrepancies with FNAB results raise suspicion for PTC,
HTT should be considered in the differential diagnosis. When HTT is
clinically suspected, diagnostic lobectomy is recommended over
total thyroidectomy to minimize surgical burden and preserve
quality of life.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Chonnam National
University (grant nos. 2022-2747 and 2023-0898).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HRL, HBJ and TMY analyzed the data and drafted the
manuscript. JSL and KHL analyzed pathological data. TMY
participated in the design of the study. HRL, HBJ and TMY
contributed to the interpretation of the data. All authors read and
approved the final version of the manuscript. HBJ and TMY confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Chonnam National University Hwasun Hospital
(Hwasun, South Korea; approval no. CNUHH-2024-175). Patients
provided written informed consent for the use of resected tissue
specimens in research.
Patient consent for publication
Patients provided written informed consent for the
publication of research results on their resected tissue
specimens.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Carney JA, Ryan J and Goellner JR:
Hyalinizing trabecular adenoma of the thyroid gland. Am J Surg
Pathol. 11:583–591. 1987.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Carney JA, Hirokawa M, Lloyd RV, Papotti M
and Sebo TJ: Hyalinizing trabecular tumors of the thyroid gland are
almost all benign. Am J Surg Pathol. 32:1877–1889. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gupta S, Modi S, Gupta V and Marwah N:
Hyalinizing trabecular tumor of the thyroid gland. J Cytol.
27:63–65. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Katoh R, Jasani B and Williams ED:
Hyalinizing trabecular adenoma of the thyroid. A report of three
cases with immunohistochemical and ultrastructural studies.
Histopathology. 15:211–224. 1989.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Evenson A, Mowschenson P, Wang H, Connolly
J, Mendrinos S, Parangi S and Hasselgren PO: Hyalinizing trabecular
adenoma-an uncommon thyroid tumor frequently misdiagnosed as
papillary or medullary thyroid carcinoma. Am J Surg. 193:707–712.
2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
McCluggage WG and Sloan JM: Hyalinizing
trabecular carcinoma of thyroid gland. Histopathology. 28:357–362.
1996.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bongiovanni M, Cibas ES and Faquin WC: The
role of thyroid fine needle aspiration cytology and the Bethesda
system for reporting thyroid cytopathology. Diagn Histopathol.
17:95–105. 2011.
|
|
8
|
Baloch ZW, LiVolsi VA, Asa SL, Rosai J,
Merino MJ, Randolph G, Vielh P, DeMay RM, Sidawy MK and Frable WJ:
Diagnostic terminology and morphologic criteria for cytologic
diagnosis of thyroid lesions: A synopsis of the national cancer
institute thyroid fine-needle aspiration state of the science
conference. Diagn Cytopathol. 36:425–437. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Crippa S, Mazzucchelli L, Cibas ES and Ali
SZ: The Bethesda system for reporting thyroid fine-needle
aspiration specimens. Am J Clin Pathol. 134:343–345.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yi KH, Lee EK, Kang HC, Kim SW, Kim IJ,
Park SY, Nam KH, Park JW, Bae SK, Baek SK, et al: 2016 Reivised
Korean thyroid association management guidelines for patients with
thyroid nodules and thyroid cancer. Int J Thyroidol. 9:59–126.
2016.
|
|
11
|
Bishop JA and Ali SZ: Hyalinizing
trabecular adenoma of the thyroid gland. Diagn Cytopathol.
39:306–310. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Rossi ED, Papotti M, Faquin W, Larocca LM
and Pantanowiz L: The diagnosis of hyalinizing trabecular tumor: A
difficult and controversial thyroid entity. Head Neck Pathol.
14:778–784. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Podany P and Gilani SM: Hyalinizing
trabecular tumor: Cytologic, histologic and molecular features and
diagnostic considerations. Ann Diagn Pathol.
54(151803)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lin X, Zhu B, Liu Y and Silverman JF:
Follicular thyroid carcinoma invades venous rather than lymphatic
vessels. Diagn Pathol. 5(8)2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
DeLellis RA, Lloyd RV, Heitz PU and Eng C:
Pathology and genetics of tumours of endocrine organs: WHO
Classification of Tumours. Vol 8. 3rd edition.
PathologyOutlines.com, Inc., Michigan, p300, 2004.
|
|
16
|
Ergün S, Akıncı O, Öztürk T and Karataş A:
Hyalinizing trabecular tumor of the thyroid gland. Turk J Surg.
34:149–151. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nosé V, Volante M and Papotti M:
Hyalinizing trabecular tumor of the thyroid: An update. Endocr
Pathol. 19:1–8. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Thompson LDR: Hyalinizing trabecular
adenoma of the thyroid gland. Ear Nose Throat J. 90:416–417.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Seo JH, Kim JP and Woo SH: A case of
hyalinizing trabecular adenoma of the thyroid gland. Int J
Thyroidol. 10:46–49. 2017.
|
|
20
|
Jong HS, Kim EJ and Kim SW: A case of
thyroid haylinizing trabecular tumor mistaken for papillary
carcinoma in aspiration cytology. Korean J Head Neck Oncol.
34:33–36. 2018.
|
|
21
|
Howard BE, Gnagi SH, Ocal IT and Hinni M:
Hyalinizing trabecular tumor masquerading as papillary thyroid
carcinoma on fine-needle aspiration. ORL J Otorhinolaryngol Relat
Spec. 75:309–313. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee HK, Kim HS, Hur MH, Kang SS, Lee JH
and Lee SK: Hyalinizing trabecular adenoma of thyroid gland. J
Korean Surg Soc. 62:87–90. 2002.
|
|
23
|
Park KS, Kim SW, Min HS, Han WS, Noh DY,
Park SH, Youn YK, Oh SK and Choe KJ: Hyalinizing trabecular adenoma
of thyroid. J Korean Surg Soc. 65:572–575. 2003.
|
|
24
|
Yim H, Shim C and Soh EY: Hyalinizing
trabecular adenoma of the thyroid: A case report. Korean J Pathol.
32:226–230. 1998.
|
|
25
|
Sambade C, Franssila K, Cameselle-Teijeiro
J, Nesland J and Sobrinho-Simões M: Hyalinizing trabecular adenoma:
A misnomer for a peculiar tumor of the thyroid gland. Endocr
Pathol. 2:83–91. 1991.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gowrishankar S, Pai SA and Carney JA:
Hyalinizing trabecular carcinoma of the thyroid gland.
Histopathology. 52:529–531. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Han JJ, Lee YJ, Choi MC, Kwon M, Chon S
and Lee J: A case of hyalinizing trabecular tumor of the thyroid
gland misdiagnosed as medullary carinoma at cytologic examination.
J Korean Endocr Soc. 23:327–331. 2008.
|
|
28
|
Kuma S, Hirokawa M, Miyauchi A, Kakudo K
and Katayama S: Cytologic features of hyalinizing trabecular
adenoma of the thyroid. Acta Cytol. 47:399–404. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sung SY, Shen HY, Hsieh CB, Duh QY, Su TF,
Chan DC and Shih ML: Hyalinizing trabecular tumor of thyroid: Does
frozen section prevent unnecessarily aggressive operation? Six new
cases and a literature review. J Chin Med Assoc. 77:573–577.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hirokawa M, Carney JA and Ohtsuki Y:
Hyalinizing trabecular adenoma and papillary carcinoma of the
thyroid gland express different cytokeratin patterns. Am J Surg
Pathol. 24:877–881. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hirokawa M and Carney JA: Cell membrane
and cytoplasmic staining for MIB-1 in hyalinizing trabecular
adenoma of the thyroid gland. Am J Surg Pathol. 24:575–578.
2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gaffney RL, Carney JA, Sebo TJ, Erickson
LA, Volante M, Papotti M and Lloyd RV: Galectin-3 expression in
hyalinizing trabecular tumors of the thyroid gland. Am J Surg
Pathol. 27:494–498. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Salvatore G, Chiappetta G, Nikiforov YE,
Decaussin-Petrucci M, Fusco A, Carney JA and Santoro M: Molecular
profile of hyalinizing trabecular tumours of the thyroid: High
prevalence of RET/PTC rearrangements and absence of B-raf and N-ras
point mutations. Eur J Cancer. 41:816–821. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dell'Aquila M, Gravina C, Cocomazzi A,
Capodimonti S, Musarra T, Sfregola S, Fiorentino V, Revelli L,
Martini M, Fadda G, et al: A large series of hyalinizing trabecular
tumors: Cytomorphology and ancillary techniques on fine needle
aspiration. Cancer Cytopathol. 127:390–398. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nikiforova MN, Nikitski AV, Panebianco F,
Kaya C, Yip L, Williams M, Chiosea SI, Seethala RR, Roy S, Condello
V, et al: GLIS rearrangement is a genomic hallmark of hyalinizing
trabecula tumor of the thyroid gland. Thyroid. 29:161–173.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nikiforova MN, Nikiforov YE and Ohori NP:
GLIS rearrangements in thyroid nodules: A key to preoperative
diagnosis of hyalinizing trabecular tumor. Cancer Cytopathol.
127:560–566. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Farkas EA, King TA, Bolton JS and Fuhrman
GM: A comparison of total thyroidectomy and lobectomy in the
treatment of dominant thyroid nodules. Am Surg. 68:678–683.
2002.PubMed/NCBI
|
|
38
|
Kim BC, Pak SJ, Cho JW, Kim WW, Lee YM,
Sung TY, Baek JH and Chung KW: Clinical characteristics of the
hyalinizing trabecular tumor. J Endocr Surg. 22:116–122. 2022.
|