Introduction
Ulcerative colitis (UC) is a chronic inflammatory
bowel disease (IBD) characterized by frequent relapses and
remission phases (1,2). Disruption of the intestinal
epithelial barrier makes a crucial contribution to the development
of IBD, and the maintenance of appropriate mucosal gene expression
is essential for intestinal integrity (3). In the large intestine, preservation
of the protective mucosal barrier strengthens the innate defenses
of the host (4,5). Given the risk of colorectal cancer
(CRC) associated with UC, innovative treatments that are capable of
restoring gut-barrier function and suppressing intestinal
inflammation are necessary to manage UC and other digestive
diseases involving chronic inflammation (6,7).
Following acute or chronic injury, restoration of
the mucus layer using intestinal barrier protectants helps to
reestablish physiological homeostasis (8). This is important given the continuous
turnover of epithelial cells from crypts to villi, which is
accompanied by physiological epithelial cell death (9). Maintaining a healthy mucosa promotes
long-term clinical remission and improves outcomes in patients with
UC (4,10). Therefore, strengthening the
intestinal barrier has been indicated to be a viable strategy to
achieve long-term therapeutic success in patients with UC (11).
Mesalamine, also known as 5-aminosalicylic acid
(5-ASA), is widely used to treat gastrointestinal inflammation
(12,13). As a synthetic anti-inflammatory
drug, 5-ASA is a first-line treatment for UC (1). However, it does not significantly
affect the remission or relapse rates of IBD (14). Combining 5-ASA with additional
therapies has been shown to improve clinical outcomes and reduce
the inflammatory markers for colorectal sites (15,16).
Plasmon-activated water (PAW) is a non-toxic form of water that has
shown promise in the treatment of inflammatory diseases, including
Alzheimer's disease and chronic kidney disease (17,18).
As previously reported (19,20),
the presence of hydrogen bonds (HBs) in PAW is diminished due to
hot electron transfer from supported gold nanoparticles (AuNPs)
under resonant illumination, which disrupts the strong hydrogen
bonding network of bulk water. In addition, PAW has an
electron-doped structure helps to maintain the stability of the
water by preserving its reduced HBs for at least one week. A recent
study indicated that PAW is able to alleviate UC symptoms in animal
models of IBD (21).
Maintaining the integrity of the gut mucosa ensures
resistance to infection, promotes the resolution of inflammation,
and supports the restoration of epithelial barrier function
(22,23). Although current treatments target
gut-barrier restoration, challenges remain. To the best of our
knowledge, the present study is the first to examine the effects of
PAW on gut barriers in a mouse model of UC.
Materials and methods
Animal study design and housing
conditions
A total of 14 male mice (7 weeks old; weight, ~25 g)
were purchased from the National Laboratory Animal Center in Taiwan
and maintained in the Animal Research Center of Cathay General
Hospital (Taipei, Taiwan). The animal experiment was approved by
the Institutional Animal Care and Use Committee of Cathay General
Hospital (approval no. CGH-IACUC-110-006) and conducted in
accordance with the Guide for the Care and Use of Laboratory
Animals (8th edition). The AVMA Guidelines for the Euthanasia of
Animals: 2020 Edition were adhered to, ensuring that euthanasia was
performed with minimal distress. The mice were housed in plastic
cages (3-5 mice per cage) under controlled conditions comprising
50±10% humidity, a 12-h light/dark cycle and a temperature of
23±2˚C. The animals had free access to water and were fed a
pelleted mouse diet (cat no. D12450H; Research Diets, Inc.). The
number of mice per cage was limited to a maximum of five to provide
adequate space for movement. In addition, to provide environmental
enrichment, wooden blocks and small shelters were provided within
the cages.
To induce UC, 3% dextran sodium sulfate (DSS;
molecular weight, 36,000-50,000; MP Biomedicals, LLC) was
administered to the mice in their drinking water for 7 days,
followed by deionized water for the next 7 days. This cycle was
repeated once (24). Mice
exhibiting any symptoms, including weight changes, fecal
consistency and overt bleeding, were kept under close observation.
Technicians monitored the activity of all experimental mice daily,
replaced drinking water or PAW every 2 days, and measured body
weight weekly. The following humane endpoints were established:
Body weight loss >15%, infection, severely under-conditioned
appearance and abnormal behavior. After 28 days, all mice were
euthanized as previously described (21). In brief, the mice were exposed to
CO₂ at a flow rate of ~50% vol/min. The CO₂ flow was maintained for
≥1 min after the cessation of respiration, which was confirmed by
observing the absence of breathing and fading of eye color for each
mouse.
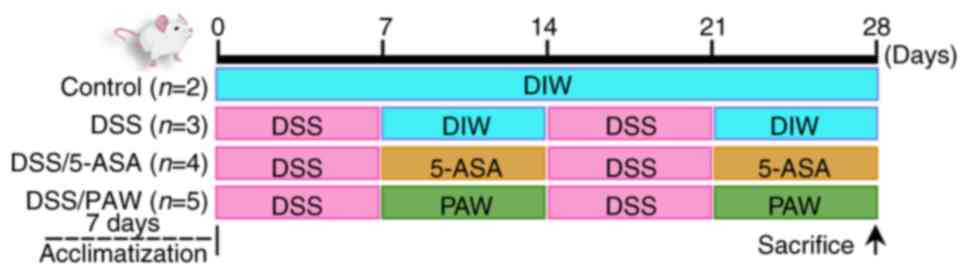
The experimental timeline for DSS induction, 5-ASA
administration and PAW consumption is illustrated in Fig. 1. Following a 7-day quarantine, the
mice were randomly divided into four groups based on body weight:
Control group (n=2) in which mice were given deionized water every
day of the 28-day experimental period; DSS group (n=3) comprising
mice with DSS-induced UC; DSS/5-ASA group (n=4) comprising mice
with DSS-induced UC receiving 5-ASA via gavage: and DSS/PAW group
(n=5) comprising mice with DSS-induced UC who were given PAW as
drinking water for the duration of the 28-day study period.
PAW preparation and 5-ASA
treatment
PAW was prepared following the procedure described
in our previous study (25).
Briefly, distilled water was passed through a glass tube containing
ceramics with AuNPs adsorbed on their surface. The water was
exposed to resonant light emitted by green light-emitting diodes
(LEDs), with a wavelength peak centered at 530 nm. PAW (pH 6.96,
23.5˚C) was collected within 2 h in glass sample bottles for
subsequent use. The 5-ASA treatment involved the administration of
200 mg/kg 5-ASA once daily by gavage in the week following DSS
induction.
Histological and immunohistochemical
analyses
Colons were isolated from the mice. After measuring
the length of each colon, the colon tissue was fixed with 4%
paraformaldehyde in phosphate-buffered saline for 10 min at room
temperature and then embedded in paraffin wax. Sections were cut to
a thickness of 5 µm and mounted on slides for hematoxylin and eosin
(H&E) staining and the immunohistochemical detection of tumor
necrosis factor α (TNF-α), keratin 20 (KRT20), E-cadherin (CDH1),
tight junction protein 1 (ZO-1), occludin, mucin 1 (MUC1) and MUC2.
H&E staining was performed at room temperature using a
Tissue-Tek DRS™ 2000 Automated Slide Stainer (Sakura Finetek USA,
Inc.) according to a standard sequential protocol. Tissue sections
were first deparaffinized with two consecutive xylene baths (5 min
each), followed by a third xylene bath for 7 min. Rehydration was
carried out through a graded ethanol series (100% ethanol for 60
and 90 sec, 95% ethanol for 60 sec and 75% ethanol for 60 sec),
followed by rinsing in running water for 3 min. Slides were then
stained with hematoxylin for 5 min and briefly differentiated by
dipping five times in 1% acid alcohol (1% HCl in 70% ethanol).
After rinsing, slides were counterstained with eosin for 3 min at
room temperature, dehydrated through graded alcohols and cleared in
xylene before mounting. Immunohistochemical staining was performed
using a BenchMark GX automated slide stainer (Roche Diagnostics)
under closed fixation conditions. The process included
deparaffinization with EZ Prep solution (cat. no. 950-102) for 8
min at 75˚C, followed by antigen retrieval with a Cell Conditioning
1 solution (CC1; cat. no. 950-124) or Cell Conditioning 2 solution
(CC2; cat. no. 950-123) (all from Roche Tissue Diagnostics; Roche
Diagnostics, Ltd.) for 48 min at 95˚C. Primary antibody
hybridization was conducted at 37˚C for various durations depending
on the antigen. CDH-1, ZO-1, occludin and TNF-α antigens were
retrieved with CC1 for 64 min, while MUC1 was retrieved with CC1
for 92 min. Each section was hybridized with specific primary
antibodies as follows: Anti-CDH-1 antibody (1:500) for 32 min,
anti-ZO-1 antibody (1:500) and anti-occludin antibody (1:200) for 1
h, and anti-TNF-α antibody (1:1,200) and anti-MUC1 antibody (1:50)
for 2 h. KRT20 and MUC2 were retrieved using CC2 for 48 min and
hybridized with anti-KRT20 antibody (1:400) and anti-MUC2 antibody
(1:400) for 2 h. Primary antibodies against TNF-α (cat. no. A0277),
CDH1 (cat. no. A20798), ZO-1 (cat. no. A0659) and MUC2 (cat. no.
A14659) were purchased from ABclonal Biotech Co., Ltd.; those
against MUC1 (cat. no. Ab109185) were purchased from Abcam
(Cambridge, UK); and those against KRT20 (cat. no. 17329-1-AP) and
occludin (cat. no. 27260-1-AP) were purchased from Proteintech
Group, Inc.
All sections were hybridized twice for 12 min at
37˚C with 100 µl Histofine® Simple Stain Mouse MAX PO
(R) (cat. no. 414341F; Nichirei Biosciences, Inc.), which comprises
anti-rabbit antibody and a universal immunoperoxidase polymer.
Visualization was performed with an OptiView DAB IHC detection kit
(cat. no. 760-700; Roche Diagnostics), followed by counterstaining
with hematoxylin II for 8 min at 25˚C (cat. no. 790-2208; Roche
Tissue Diagnostics; Roche Diagnostics, Ltd.) and Bluing Reagent for
4 min at 25˚C (cat. no. 760-2037; Roche Tissue Diagnostics; Roche
Diagnostics, Ltd.). After staining, the sections were scanned using
a Zeiss Mirax scanner (Carl Zeiss AG) and analyzed independently by
a pathologist who was blinded to the experimental groups.
Inflammation characterization of colon
tissues and measurement of epithelial cell density
Following euthanasia, the colons of all mice were
removed and dissected longitudinally. Inflammation of the colon was
assessed based on epithelial loss, crypt damage, goblet cell
depletion and inflammatory cell infiltration. The histological
evaluation of colonic tissue was performed on the H&E-stained
sections in a blinded manner, using a scoring system adapted from
that described by Iba et al (26). The four parameters were scored as
follows: i) Loss of epithelium: 0, no staining; 1, 0-5% (mild); 2,
5-10% (moderate); or 3, >10% (severe); ii) Crypt damage: 0,
none; 1, 0-10% (mild); 2, 10-20% (moderate); or 3, >20%
(severe), with crypt loss evaluated as the percentage relative to
the total mucosal area; ii) Depletion of goblet cells: 0, none; 1,
mild; 2, moderate; or 3, severe; and iv) Infiltration of
inflammatory cells: 0, none; 1, mild; 2, moderate; or 3, severe.
The integrity of the colonic epithelium was evaluated by manually
measuring the density of the colonic epithelial cells along the
edge of the basement membrane (27).
Statistical analyses
Statistical analysis was conducted using IBM SPSS
Statistics (version 27.0; IBM Corp.). One-way analysis of variance
followed by Tukey's honestly significant difference post hoc test
was employed for comparisons among multiple groups with normally
distributed data, and Kruskal-Wallis followed by Dunn's post hoc
test was used for comparisons among multiple groups with ordinal
data. P<0.05 was considered to indicate a statistically
significant difference.
Results
5-ASA and PAW increase epithelial cell
density in inflamed intestines
During the experiment, one mouse in the DSS/5-ASA
group died on day 10, while no mortality was observed in the DSS
and DSS/PAW groups. The death was likely associated with the DSS
treatment; however, the mortality rate (~8.3%) is lower than that
reported in a study by Pan et al (24), where mice with 3% DSS-induced with
UC using had a mortality rate of ~11.1%. Among the groups with 3%
DSS-induced UC, the DSS/PAW group exhibited a significant reduction
in body weight and the DSS/5-ASA showed a trend towards reduced
body weight on day 14 (Fig. 2A),
and a non-significant reduction in colon length was observed in the
DSS/5-ASA group (Fig. 2B and
C) compared with those in the
control group. Epithelial cell density was also assessed to
evaluate the integrity of the intracolonic epithelium based on
H&E staining (Fig. 2D). The
DSS group had the lowest cell density, while the DSS/5-ASA and
DSS/PAW groups exhibited significantly increased cell density
compared with that in the DSS group.
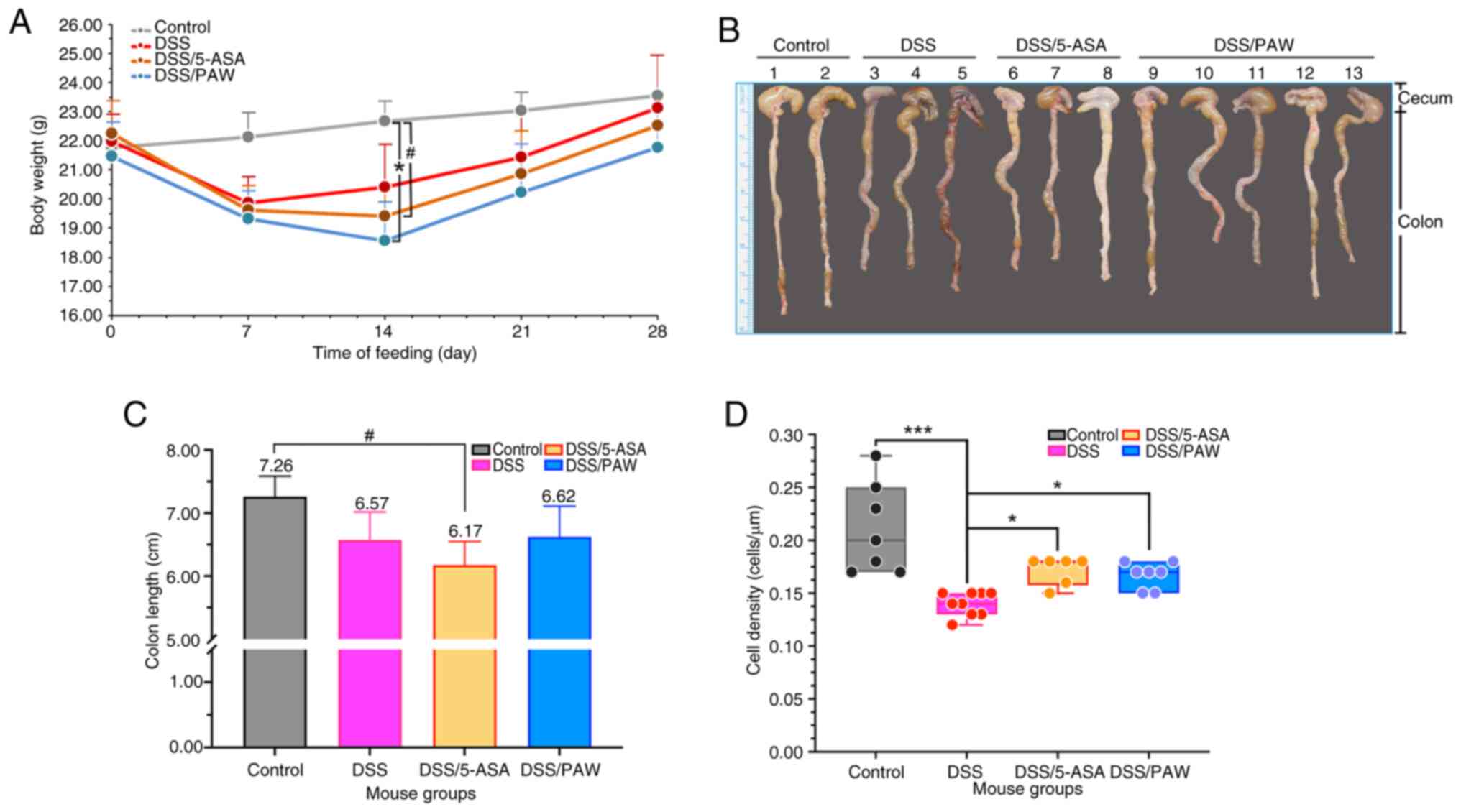 | Figure 2Analysis of body weight and
epithelial cell density in the inflamed intestines of mice with
DSS-induced ulcerative colitis. (A) Body weights, (B) photographs
of the colons, (C) colon lengths and (D) average epithelial cell
density in four groups of mice: Control group, deionized water; DSS
group, 3% DSS; DSS/5-ASA group, 3% DSS + 200 mg/kg 5-ASA group;
DSS/PAW 3% DSS + PAW. Mean values and the positive SD are shown.
#0.05<P<0.1, *P<0.05,
**P<0.01 and n.s. as indicated. DSS, dextran sodium
sulfate; 5-ASA, 5-aminosalicylic acid; PAW, plasmon-activated
water; SD, standard deviation; n.s., not significant. |
PAW modulates intestinal inflammatory
responses
Three inflammatory indicators, namely epithelial
loss, crypt damage and goblet cell depletion, were used to evaluate
the extent of colonic epithelial damage (26). The infiltration of inflammatory
cells was also assessed. As shown in Fig. 3, comparison of the results in the
DSS and DSS/5-ASA groups revealed that 5-ASA did not significantly
reduce the scores for these inflammatory indicators, indicating
that 5-ASA did not ameliorate the DSS-induced colonic epithelial
damage. Compared with the DSS group, the DSS/PAW group showed a
significant reduction in epithelial loss (Fig. 3A), crypt damage (Fig. 3B) and inflammatory cell
infiltration (Fig. 3D), along with
a non-significant decrease in goblet cell depletion (Fig. 3C) compared with that in the DSS
group.
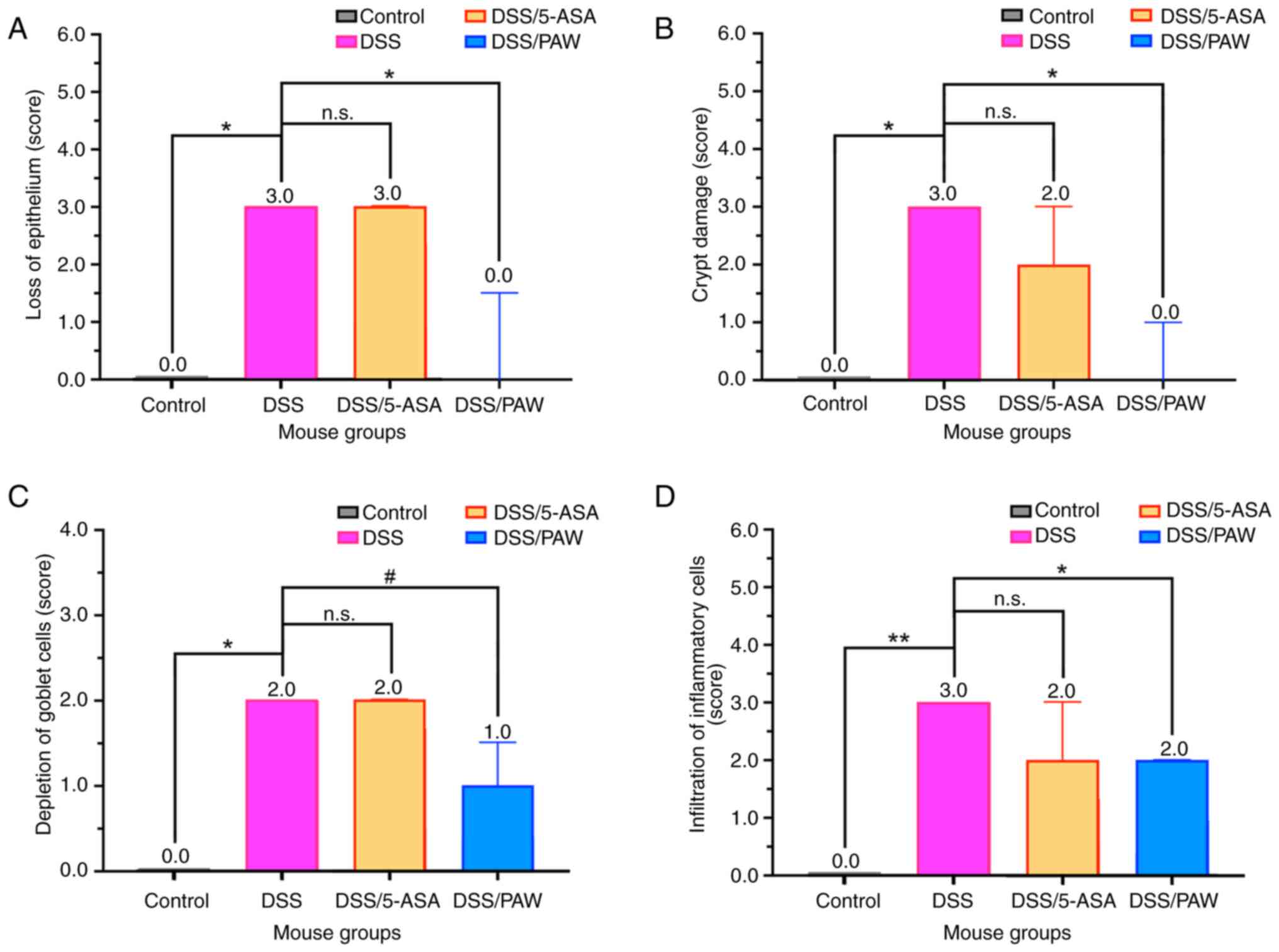 | Figure 3Histological scores for indicators of
colonic inflammation in mice with DSS-induced ulcerative colitis.
(A) Loss of epithelium, (B) crypt damage, (C) depletion of goblet
cells and (D) infiltration of inflammatory cells in the four groups
of mice: Control group, deionized water; DSS group, 3% DSS;
DSS/5-ASA group, 3% DSS + 200 mg/kg 5-ASA; DSS/PAW group, 3% DSS +
PAW. Median values with interquartile range are shown for each
group. #0.05<P<0.1, *P<0.05,
**P<0.01 and n.s. as indicated. DSS, dextran sodium
sulfate; 5-ASA, 5-aminosalicylic acid; PAW, plasmon-activated
water; n.s., not significant. |
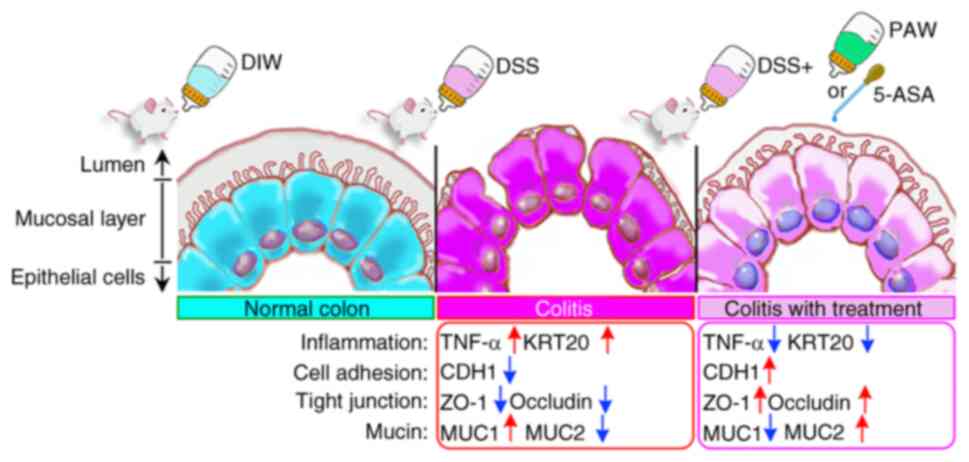
Histological examination, as shown in Fig. 4A, revealed that DSS induced
structural abnormalities compared with the control group, with
dense inflammatory cell infiltration and frequent crypt abscesses.
However, both 5-ASA and PAW mitigated these inflammatory responses,
with the DSS/PAW group showing the most marked improvement. The
DSS-induced inflammatory status was further assessed using two
colitis markers, TNF-α and KRT20 (28,29).
Immunostaining revealed low expression of TNF-α (Fig. 4B) and KRT20 (Fig. 4C) at the base of the colonic crypts
with marked elevation in the superficial epithelium of the normal
tissue. DSS treatment upregulated the expression of TNF-α and KRT20
in the mucosal layer. However, treatment with 5-ASA or PAW
attenuated the expression of these markers in the epithelial cells
and lamina propria. Notably, PAW induced a more pronounced
reduction than 5-ASA.
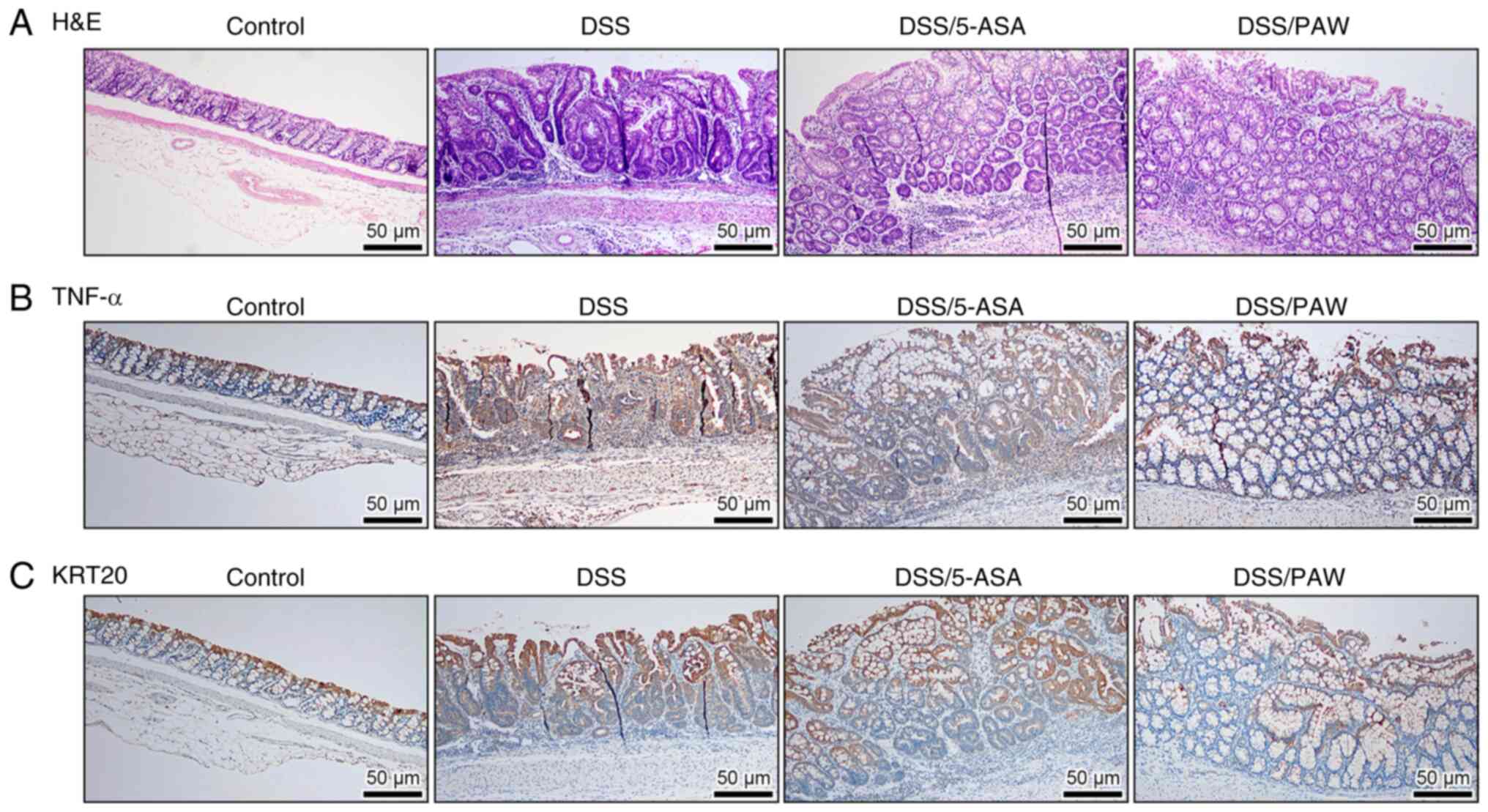 | Figure 4Histological and immunohistochemical
analysis of the colonic tissues of mice with DSS-induced ulcerative
colitis. (A) Representative H&E staining images and
immunostaining of (B) TNF-α and (C) KRT20 in the four groups:
Control group, deionized water; DSS group, 3% DSS; DSS/5-ASA group,
3% DSS + 200 mg/kg 5-ASA; and DSS/PAW group, 3% DSS + PAW. Scale
bar, 50 µm. H&E, hematoxylin and eosin; DSS, dextran sodium
sulfate; TNF-α, tumor necrosis factor-α; KRT20, keratin 20; 5-ASA,
5-aminosalicylic acid; PAW, plasmon-activated water. |
PAW enhances the adhesion and tight
junctions of epithelial cells in inflamed intestines
Immunohistochemical analysis was used to analyze the
expression of the adhesion molecule CDH1 (Fig. 5A) and the tight junction-associated
proteins ZO-1 (Fig. 5B) and
occludin (Fig. 5C). These three
proteins play key roles in cell proliferation and survival via the
regulation of epithelial adhesion and tight junction integrity
(30,31). The expression of CDH1, ZO-1 and
occludin was increased in the colonic epithelium of the DSS/PAW
group, particularly in the intestinal crypts, compared with that in
the DSS group. However, the expression of these proteins did not
appear to increase in the colons of the DSS/5-ASA group.
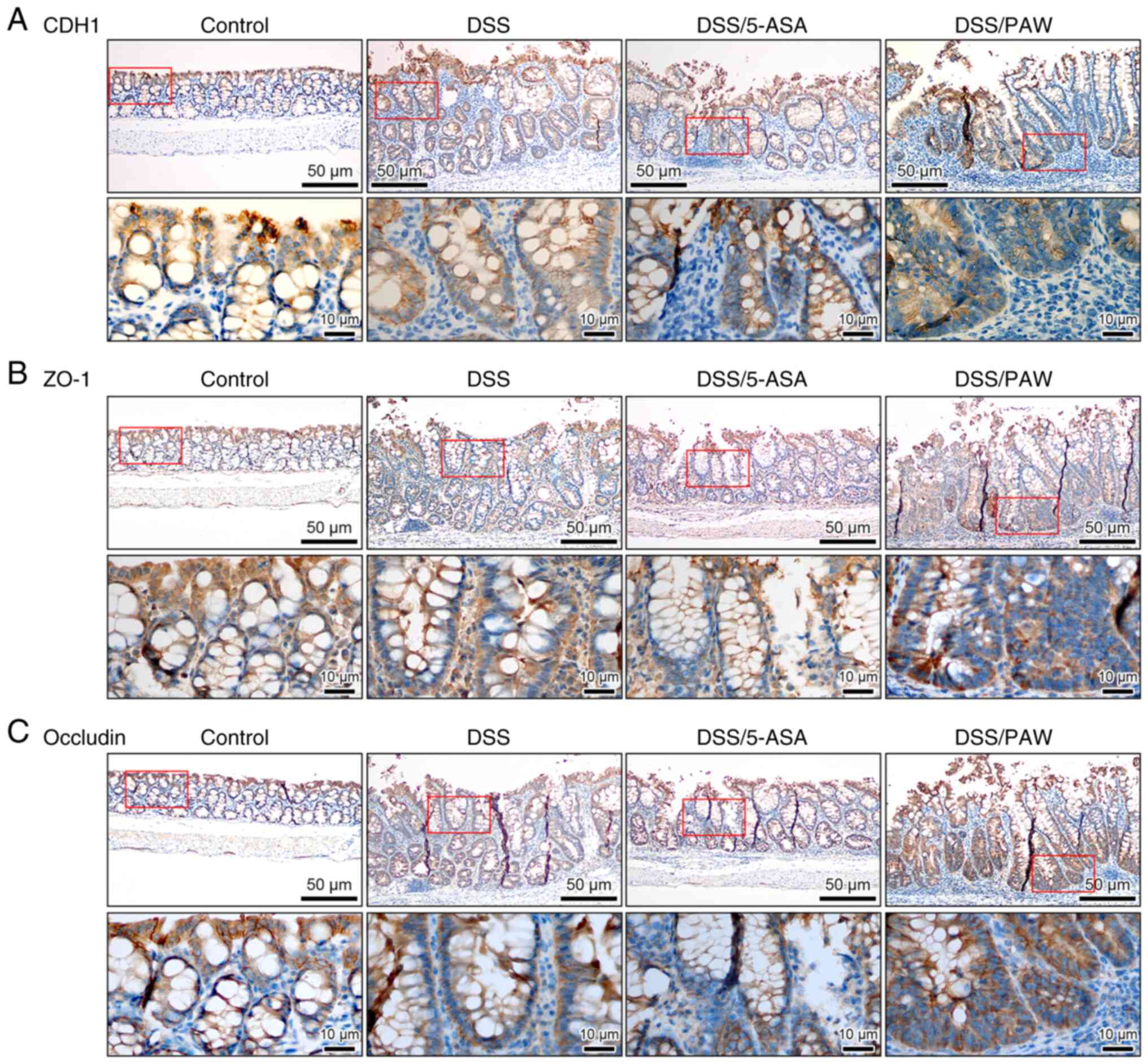 | Figure 5Immunohistochemical analysis of cell
adhesion and tight junction molecules in the colonic tissues of
mice with DSS-induced ulcerative colitis. (A) CDH1, (B) ZO-1 and
(C) occludin expression in the four groups: Control group,
deionized water; DSS group, 3% DSS; DSS/5-ASA group, 3% DSS + 200
mg/kg 5-ASA; DSS/PAW group, 3% DSS + PAW. Scale bar, 50 µm. The
lower image (scale bar, 10 µm) in each panel represents a closer
view of the area in the red box of the upper image. DSS, dextran
sodium sulfate; CDH1, E-cadherin; ZO-1, tight junction protein 1;
5-ASA, 5-aminosalicylic acid; PAW, plasmon-activated water. |
PAW influences mucin content in the
inflamed intestinal mucosa
Mucins are commonly detected in the colons of
patients with UC (32). Among
them, MUC1 and MUC2 modulate mucus composition and are implicated
in the pathogenesis of UC (33,34).
In the present study, MUC2 was evenly distributed in the mucosal
epithelial cells of the control group (Fig. 6B), whereas MUC1 expression was
minimal (Fig. 6A). In the DSS
group, representing the active phase of UC, MUC1 expression was
markedly upregulated, whereas MUC2 expression was reduced.
Treatment with either 5-ASA or PAW attenuated the change in mucin
composition in the inflamed intestinal cells by downregulating MUC1
expression and increasing MUC2 expression. Notably, numerous
MUC2-positive cells were detected in the mucosal epithelium of the
colons in the DSS/PAW group.
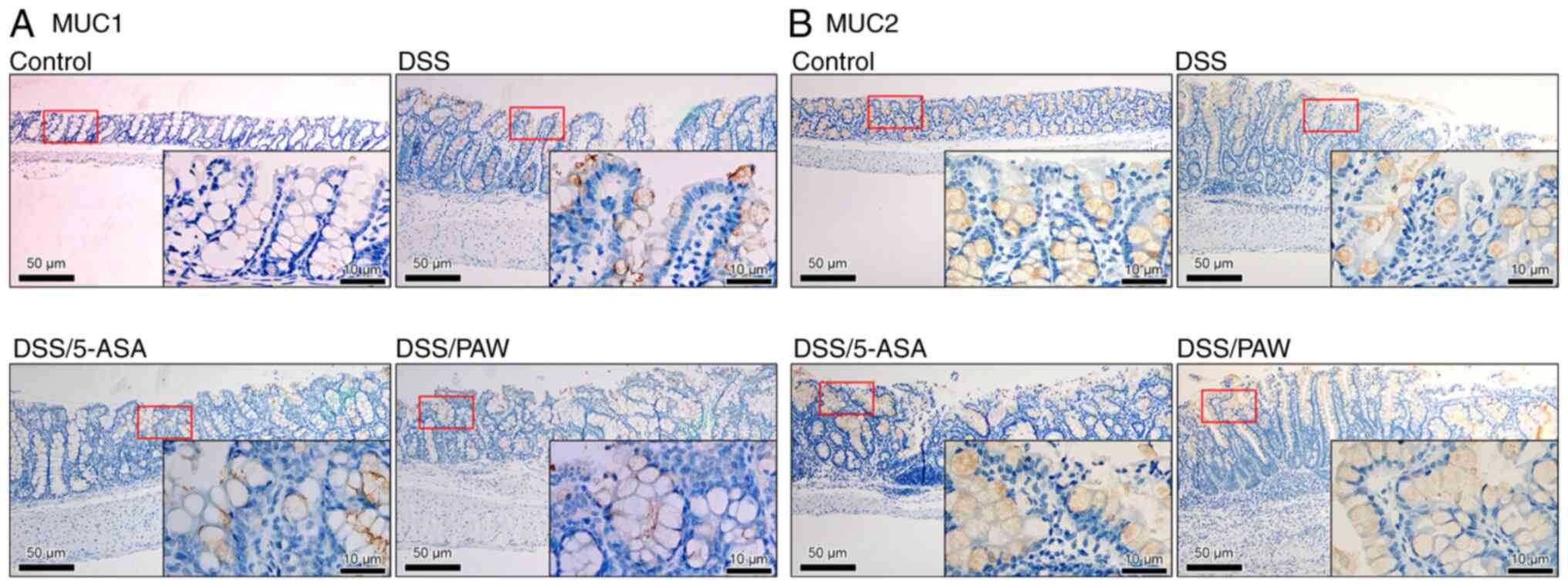 | Figure 6Immunohistochemical analysis of
mucins in the colonic tissues of mice with DSS-induced ulcerative
colitis. (A) MUC1 and (B) MUC2 expression in the four groups:
Control group, deionized water; DSS group, 3% DSS; DSS/5-ASA group,
3% DSS + 200 mg/kg 5-ASA; DSS/PAW group, 3% DSS + PAW. DSS, dextran
sodium sulfate; Scale bar, 50 µm. The inset (scale bar, 10 µm) in
each panel represents a closer view of the area in the red box.
MUC1, mucin 1; MUC2, mucin 2; 5-ASA, 5-aminosalicylic acid; PAW,
plasmon-activated water. |
Discussion
PAW is typically synthesized by irradiating
AuNP-adsorbed ceramics with resonant light emitted by green LEDs
(25,35). As shown in our previous studies
(19,20), PAW is a form of pure water
characterized by an electron-doped structure and weakened hydrogen
bonding. Since PAW is pure water, its concentration is not a
relevant parameter in its use. This unique type of water has shown
clinical potential and anti-inflammatory effects in colonic
epithelial cells (21,36). In the present study, the
anti-inflammatory effects of PAW were compared with those of 5-ASA
in mice with DSS-induced UC. Notably, cycling treatment previously
has been applied in both DSS-induced UC and PAW-treated diseases,
serving as an important reference for the design of the present
study (24,37).
First-line 5-ASA monotherapy has been reported to
have limited efficacy in the treatment of IBD (38), and the results of the animal
experiments performed in the present study are consistent with
this. As in previous studies, body weight and colon length were
measured to assess the extent of colonic inflammation (39,40).
The results indicated that 5-ASA did not increase body weight or
restore colon length in mice with inflamed intestines, although it
did reduce inflammatory responses in the colon. Previous studies
have demonstrated that TNF-α and KRT20 are upregulated in chronic
DSS-induced UC (28,41), which is consistent with the
immunostaining results for these markers in the present study,
where elevated levels of TNF-α, an inflammatory cytokine (42), and KRT20, a tumor marker associated
with intestinal malignancy (43),
were observed. In the present study, 5-ASA treatment reduced the
immunohistochemical staining of TNF-α and KRT20 in the inflamed
intestines of the UC model mice. Notably, PAW treatment resulted in
a more marked reduction in the levels of these two markers. These
results suggest that although neither 5-ASA nor PAW completely
resolved DSS-induced UC during the study period, both treatments
mitigated inflammation in the intestinal microenvironment.
Microscopically, PAW was shown to alleviate colonic epithelial
damage, further reducing inflammation.
As reported in earlier studies, epithelial injury,
crypt damage and goblet cell loss can be used to evaluate the
severity of DSS-induced UC (44,45).
In the present study, a comparison of these indicators revealed
that PAW exerted a significant anti-inflammatory effect while
5-ASA, a drug commonly used in clinical practice to treat UC, did
not (46). In addition, while
short-term treatment with PAW did not attenuate the DSS-induced
loss of body weight or shortening of colon length, it did enhance
the microenvironmental immunity of the colon and reduce
inflammation. Moreover, PAW was more effective than 5-ASA in
suppressing inflammation in mice with DSS-induced UC.
In the present study, PAW did not induce any
inflammatory response in the colonic tissues; instead it
demonstrated clear anti-inflammatory effects. Previous studies have
shown that PAW is safe for long-term consumption, with no observed
toxicity; in two extended animal experiments, mice exhibited no
abnormalities after consuming PAW for 9 months (25) or 16 months (18). Additionally, results from a human
clinical trial (study no. Y800N20A01; sponsored by Taipei Medical
University-Shuang Ho Hospital) revealed that PAW caused no adverse
effects in humans. These findings are consistent with those of our
previous study, which showed that PAW possesses potent antioxidant
and anti-inflammatory properties without any evident toxicity
(18). In the present study, it
was also observed that the anti-inflammatory effect of PAW was
stronger than that of 5-ASA and effectively mitigated IBD severity.
Consequently, it appears that PAW treatment not only ameliorates
intestinal inflammation but could also help to avoid the potential
effects of 5-ASA, such as autoimmune hepatitis (47). As an alternative to 5-ASA,
corticosteroids are commonly used to provide short-term relief;
however, they are associated with significant side effects
(48). Immunosuppressants and
biologics offer improved disease control but pose risks such as
infections, and are expensive (49,50).
In the present study, PAW outperformed 5-ASA in the restoration of
epithelial integrity and modulation of mucin expression,
highlighting its potential as a novel treatment strategy for
UC.
Maintaining the integrity of the gut mucosa,
including the formation of tight epithelial junctions, is essential
for enhancing pathogen resistance and supporting gut health
(51). The intestinal barrier,
protected by a layer of mucus, performs various site-specific
protective functions (52).
Therefore, the preservation of mucosal integrity helps to modulate
the mucosal defense system and promote anti-inflammatory responses
(53). During inflammation, the
paracellular space between gut epithelial cells is typically sealed
by apical junctional complexes composed of tight and adherens
junctions (54,55). CDH1 is an adhesion molecule, while
occludin and ZO-1 are tight junction proteins involved in
maintaining the mechanical integrity of the intestinal epithelium
(31,56,57).
Consistent with the findings of de Ponthaud et al (58), a reduction in CDH1 levels was
observed in the inflamed ileal mucosa in the present study.
However, PAW treatment partially restored CDH1 levels, contributing
to improved gut health in mice with DSS-induced UC. This recovery
may be important, as a loss of CDH1 expression is associated with
cancer progression, including the increased proliferation, invasion
and metastasis of colorectal neoplasm cells (30). The findings of the present study
suggest that PAW may help to restore gut-barrier function by
upregulating the expression of tight junction proteins. Barrier
integrity and dysfunction are key factors in the maintenance of gut
health and related outcomes (57).
Consistent with the findings of Guo et al (59), the present study indicated that an
increase in the expression levels of tight junction proteins, such
as occludin and ZO-1, may attenuate the progression of UC.
In addition to adhesion and tight junction
molecules, damage to the mucus layer and goblet cells in the
intestinal lining is frequently observed during inflammation and
plays a key role in disease pathogenesis (52). In the colon, mucus acts as the
primary barrier between intestinal microorganisms and the mucosa,
contributing to the defense system of the host (60). The efficacy of certain
chemotherapeutic agents for CRC has been linked to the differential
expression of mucins, particularly MUC1 and MUC2(61). The present study found that PAW
influences the expression of specific genes that may alter the fate
of colonic cells. For example, UC leads to long-term changes in
goblet cell function (62). Goblet
cells are responsible for producing mucins, which are essential for
intestinal lubrication, cell signaling, and protecting the
epithelium from pathogens, toxins and other irritants (63). Previous studies have shown that
mucin expression is dysregulated in intestinal epithelial cells
during active inflammation and contributes to the pathological
changes observed in IBD (64-66).
In inflamed intestinal tissues, a physiological characteristic
pattern of high MUC1 and low MUC2 expression levels is associated
with UC exacerbation (64,65). MUC2, a secreted mucin produced by
goblet cells, forms the primary component of the intestinal mucus
barrier, safeguarding epithelial cells from pathogens and
maintaining gut homeostasis. Conversely, MUC1 is a membrane-bound
mucin expressed on epithelial surfaces; its upregulation during
inflammation and infection suggests a role in modulating immune
responses and epithelial repair mechanisms. In particular, the
downregulation of MUC2 is indicated to play a crucial role in UC
pathogenesis (65). In the present
study, reduced MUC2 levels were observed within the inflammatory
cells of UC model mice; however, MUC2 expression was restored in
the DSS/PAW group. These results suggest that PAW treatment
increases MUC2 levels, thereby preserving the mucus layer and
protecting the colonic epithelium from damage and pathogen
infiltration.
The present study has certain limitations. First,
the precise mechanism by which PAW improves the gut barrier in UC
was not fully elucidated. Second, the sample size used was small;
while adhering to the refinement, reduction and replacement
principle of animal research, this may impact the generalizability
and statistical power of the findings. Third, the unequal group
sizes and absence of power calculations represent further
limitations of the study. While previous studies have used similar
experimental animal numbers (67-69),
the small and imbalanced sample sizes may have limited the ability
to detect subtle differences between experimental groups,
particularly in assays for which the observed changes did not reach
statistical significance.
Despite these limitations, the findings are
noteworthy and warrant further investigation. While PAW
demonstrated stronger anti-inflammatory effects than 5-ASA, future
studies should incorporate formal power analyses and use larger,
more balanced sample groups. The inclusion of additional
experimental groups, such as mice treated with both PAW and 5-ASA,
along with increased sample sizes, will enhance statistical
robustness and facilitate a deeper understanding of the underlying
molecular mechanisms. The molecular effects of PAW on key proteins
in the colonic epithelium, including CDH1, ZO-1 and MUC1/2,
highlights its potential influence on cell adhesion, tight junction
stabilization and mucin regulation. However, further investigation
of oxidative signaling (70-72),
epigenetic regulation (73,74)
and the modulation of tight junctions/mucins (75) may be necessary to clarify the
precise molecular mechanisms. In addition, long-term studies
involving repeated dosing and varied treatment durations are
essential to determine the sustainability and reproducibility of
the observed effects. The evaluation of multiple animal models of
UC or progression to clinical trials will help establish the
broader applicability of PAW and confirm its therapeutic potential
in human contexts.
In conclusion, the colonic mucosa serves as a
suitable site for the evaluation of epithelial damage and
inflammatory cell infiltration (76). As depicted in Fig. 7, both 5-ASA and PAW exhibit
anti-inflammatory effects and modulate the expression of mucins,
adhesion molecules and tight junction proteins. PAW significantly
ameliorated DSS-induced colitis in mice by enhancing gut-barrier
integrity and modulating inflammation-related proteins and mucins.
Notably, PAW outperformed 5-ASA in restoring epithelial cell
density, reducing crypt damage, and increasing the levels of tight
junction proteins such as CDH1, ZO-1 and occludin. In addition, PAW
restored the balance of MUC1 and MUC2 in the colonic mucosa, a
critical factor for maintaining intestinal barrier function. By
contrast, while 5-ASA effectively reduced the expression of the
inflammation markers TNF-α and KRT20, its effects on epithelial and
mucosal repair were less pronounced compared with those of PAW.
These findings highlight the therapeutic potential of PAW as a
novel intervention for UC and support further investigation into
its mechanisms and long-term efficacy.
In summary, the findings of the present study
indicate that PAW ameliorates DSS-induced colitis in mice by
modulating the expression of CDH1, occludin, ZO-1, MUC1 and MUC2.
These results highlight the therapeutic potential of PAW in the
treatment of UC.
Acknowledgements
Not applicable.
Funding
Funding: The study received funding from Taipei Medical
University Hospital (TMUH; grant no, 111TMUH-MOST-08) and the
National Science and Technology Council of Taiwan (grant nos. MOST
111-2314-B-038-121 and MOST 111-2221-E-038-001-MY3).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CCC, CJC, YCL and CJH took the lead in data
curation, contributed equally to formal analysis, investigation and
methodology, and took the lead in writing, reviewing and editing
the manuscript. CYL and HJY supported data curation, and
contributed equally to formal analysis, investigation and
methodology. YJL, WCC, CWC, and JLP were involved in formal
analysis and investigation, and provided equal supervision. CCC and
YCL led conceptualization and supervision, and were responsible for
funding acquisition and use, and project administration. CCC, YCL
and CJH confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The Institutional Animal Care and Use Committee of
Cathay General Hospital approved this study (approval no.
CGH-IACUC-110-006).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Le Berre C, Ananthakrishnan AN, Danese S,
Singh S and Peyrin-Biroulet L: Ulcerative colitis and Crohn's
disease have similar burden and goals for treatment. Clin
Gastroenterol Hepatol. 18:14–23. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li XX, Liu Y, Luo J, Huang ZD, Zhang C and
Fu Y: Vitamin D deficiency associated with Crohn's disease and
ulcerative colitis: A meta-analysis of 55 observational studies. J
Transl Med. 17(323)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alarfaj SJ, Mostafa SA, Negm WA, El-Masry
TA, Kamal M, Elsaeed M and El Nakib AM: Mucosal genes expression in
inflammatory bowel disease patients: New insights. Pharmaceuticals
(Basel). 16(324)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Neurath MF and Vieth M: Different levels
of healing in inflammatory bowel diseases: Mucosal, histological,
transmural, barrier and complete healing. Gut. 72:2164–2183.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sanchez de Medina F, Romero-Calvo I,
Mascaraque C and Martinez-Augustin O: Intestinal inflammation and
mucosal barrier function. Inflamm Bowel Dis. 20:2394–2404.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dong J, Liang W, Wang T, Sui J, Wang J,
Deng Z and Chen D: Saponins regulate intestinal inflammation in
colon cancer and IBD. Pharmacol Res. 144:66–72. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kotla NG, Isa ILM, Rasala S, Demir S,
Singh R, Baby BV, Swamy SK, Dockery P, Jala VR, Rochev Y, et al:
Modulation of gut barrier functions in ulcerative colitis by
hyaluronic acid system. Adv Sci (Weinh). 9(e2103189)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lopetuso LR, Scaldaferri F, Bruno G,
Petito V, Franceschi F and Gasbarrini A: The therapeutic management
of gut barrier leaking: The emerging role for mucosal barrier
protectors. Eur Rev Med Pharmacol Sci. 19:1068–1076.
2015.PubMed/NCBI
|
|
9
|
Seo K, Seo J, Yeun J, Choi H, Kim YI and
Chang SY: The role of mucosal barriers in human gut health. Arch
Pharm Res. 44:325–341. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nakase H, Hirano T, Wagatsuma K, Ichimiya
T, Yamakawa T, Yokoyama Y, Hayashi Y, Hirayama D, Kazama T, Yoshii
S, et al: Artificial intelligence-assisted endoscopy changes the
definition of mucosal healing in ulcerative colitis. Dig Endosc.
33:903–911. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang J, Zhang C, Guo C and Li X: Chitosan
ameliorates DSS-induced ulcerative colitis mice by enhancing
intestinal barrier function and improving microflora. Int J Mol
Sci. 20(5751)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Foppoli A, Maroni A, Moutaharrik S,
Melocchi A, Zema L, Palugan L, Cerea M and Gazzaniga A: In vitro
and human pharmacoscintigraphic evaluation of an oral 5-ASA
delivery system for colonic release. Int J Pharm.
572(118723)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Beiranvand M: A review of the biological
and pharmacological activities of mesalazine or 5-aminosalicylic
acid (5-ASA): An anti-ulcer and anti-oxidant drug.
Inflammopharmacology. 29:1279–1290. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Burr NE, Hall B, Hamlin PJ, Selinger CP,
Ford AC and O'Connor A: Systematic review and network meta-analysis
of medical therapies to prevent recurrence of post-operative
Crohn's disease. J Crohns Colitis. 13:693–701. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cho J, Kweon HS, Huh SO and Sadra A:
Augmented reduction in colonic inflammatory markers of dextran
sulfate sodium-induced colitis with a combination of
5-aminosalicylic acid and AD-lico from Glycyrrhiza inflata. Anim
Cells Syst (Seoul). 22:189–196. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Torres P, Canete F, Nunez L, Aguilar A,
Mesonero F, Calafat M, Fernandez C, Teniente A, Manosa M,
Lopez-Sanroman A, et al: Spacing the administration interval of
Anti-TNF agents: A valid strategy for patients with inflammatory
bowel disease? Dig Dis Sci. 65:2036–2043. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang CP and Liu YC: Therapeutics for
inflammatory-related diseases based on plasmon-activated water: A
review. Int J Mol Sci. 19(1589)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cheng CH, Liu YC, Yang YSH, Lin KJ, Wu D,
Liu YR, Chang CC, Hong CT and Hu CJ: Plasmon-activated water as a
therapeutic strategy in Alzheimer's disease by altering gut
microbiota. Aging (Albany NY). 15:3715–3737. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen HC, Hwang BJ, Mai FD, Liu YC, Lin CM,
Kuo HS, Chou DS, Lee MJ, Yang KH, Yu CC, et al: Active and stable
liquid water innovatively prepared using resonantly illuminated
gold nanoparticles. ACS Nano. 8:2704–2713. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen HC, Mai FD, Hwang BJ, Lee MJ, Chen
CH, Wang SH, Tsai HY, Yang CP and Liu YC: Creation of
Electron-doping liquid water with reduced hydrogen bonds. Sci Rep.
6(22166)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chang CC, Liu CY, Su IC, Lee YJ, Yeh HJ,
Chen WC, Yu CJ, Kao WY, Liu YC and Huang CJ: Functional
Plasmon-activated water increases Akkermansia muciniphila
abundance in gut microbiota to ameliorate inflammatory bowel
disease. Int J Mol Sci. 23(11422)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Blikslager AT, Moeser AJ, Gookin JL, Jones
SL and Odle J: Restoration of barrier function in injured
intestinal mucosa. Physiol Rev. 87:545–564. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sommer K, Wiendl M, Muller TM, Heidbreder
K, Voskens C, Neurath MF and Zundler S: Intestinal mucosal wound
healing and barrier integrity in IBD-crosstalk and trafficking of
cellular players. Front Med (Lausanne). 8(643973)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pan HH, Zhou XX, Ma YY, Pan WS, Zhao F, Yu
MS and Liu JQ: Resveratrol alleviates intestinal mucosal barrier
dysfunction in dextran sulfate sodium-induced colitis mice by
enhancing autophagy. World J Gastroenterol. 26:4945–4959.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cheng CH, Lin KJ, Hong CT, Wu D, Chang HM,
Liu CH, Hsiao IT, Yang CP, Liu YC and Hu CJ: Plasmon-activated
water reduces amyloid burden and improves memory in animals with
Alzheimer's disease. Sci Rep. 9(13252)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Iba Y, Sugimoto Y, Kamei C and Masukawa T:
Possible role of mucosal mast cells in the recovery process of
colitis induced by dextran sulfate sodium in rats. Int
Immunopharmacol. 3:485–491. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chang SC, Chiang HH, Liu CY, Li YJ, Lu CL,
Lee YP, Huang CJ and Lai CL: Intestinal mucosal barrier improvement
with prebiotics: Histological evaluation of longish glucomannan
hydrolysates-induced innate T lymphocyte activities in Mice.
Nutrients. 14(2220)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Helenius TO, Antman CA, Asghar MN, Nystrom
JH and Toivola DM: Keratins Are altered in intestinal
disease-related stress responses. Cells. 5(35)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pugliese D, Felice C, Papa A, Gasbarrini
A, Rapaccini GL, Guidi L and Armuzzi A: Anti TNF-alpha therapy for
ulcerative colitis: Current status and prospects for the future.
Expert Rev Clin Immunol. 13:223–233. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Meigs TE, Fedor-Chaiken M, Kaplan DD,
Brackenbury R and Casey PJ: Galpha12 and Galpha13 negatively
regulate the adhesive functions of cadherin. J Biol Chem.
277:24594–24600. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kuo WT, Odenwald MA, Turner JR and Zuo L:
Tight junction proteins occludin and ZO-1 as regulators of
epithelial proliferation and survival. Ann N Y Acad Sci.
1514:21–33. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bankole E, Read E, Curtis MA, Neves JF and
Garnett JA: The relationship between mucins and ulcerative colitis:
A systematic review. J Clin Med. 10(1935)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bergstrom KS, Kissoon-Singh V, Gibson DL,
Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, et
al: Muc2 protects against lethal infectious colitis by
disassociating pathogenic and commensal bacteria from the colonic
mucosa. PLoS Pathog. 6(e1000902)2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sheng YH, Hasnain SZ, Florin TH and
McGuckin MA: Mucins in inflammatory bowel diseases and colorectal
cancer. J Gastroenterol Hepatol. 27:28–38. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang CK, Chen HC, Fang SU, Ho CW, Tai CJ,
Yang CP and Liu YC: Innovatively therapeutic strategy on lung
cancer by daily drinking antioxidative plasmon-induced activated
water. Sci Rep. 8(6316)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lian YZ, Liu YC, Chang CC, Nochi T and
Chao JC: Combined Lycium barbarum polysaccharides with
plasmon-activated water affect IFN-γ/TNF-α induced inflammation in
Caco-2 cells. Pharmaceuticals (Basel). 16(1455)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen HC, Cheng CY, Chen LY, Chang CC, Yang
CP, Mai FD, Liao WC, Chang HM and Liu YC: Plasmon-activated water
effectively relieves hepatic oxidative damage resulting from
chronic sleep deprivation. RSC Adv. 8:9618–9626. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Atia O, Goren I, Fischler TS, Weisband YL,
Greenfeld S, Kariv R, Ledderman N, Matz E, Rimon RM, Dotan I, et
al: 5-aminosalicylate maintenance is not superior to no maintenance
in patients with newly diagnosed Crohn's disease-A nationwide
cohort study. Aliment Pharmacol Ther. 57:1004–1013. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chassaing B, Aitken JD, Malleshappa M and
Vijay-Kumar M: Dextran sulfate sodium (DSS)-induced colitis in
mice. Curr Protoc Immunol. 104:15.25.1–15.25.14. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Naito Y, Takagi T, Uchiyama K, Kuroda M,
Kokura S, Ichikawa H, Yanagisawa R, Inoue K, Takano H, Satoh M, et
al: Reduced intestinal inflammation induced by dextran sodium
sulfate in interleukin-6-deficient mice. Int J Mol Med. 14:191–196.
2004.PubMed/NCBI
|
|
41
|
Giuffrida P, Cococcia S, Delliponti M,
Lenti MV and Di Sabatino A: Controlling gut inflammation by
restoring anti-inflammatory pathways in inflammatory bowel disease.
Cells. 8(397)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gandhi GR, Mohana T, Athesh K, Hillary VE,
Vasconcelos ABS, Farias de Franca MN, Montalvao MM, Ceasar SA,
Jothi G, Sridharan G, et al: Anti-inflammatory natural products
modulate interleukins and their related signaling markers in
inflammatory bowel disease: A systematic review. J Pharm Anal.
13:1408–1428. 2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Magnusson K, de Wit M, Brennan DJ, Johnson
LB, McGee SF, Lundberg E, Naicker K, Klinger R, Kampf C, Asplund A,
et al: SATB2 in combination with cytokeratin 20 identifies over 95%
of all colorectal carcinomas. Am J Surg Pathol. 35:937–948.
2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen Y, Jin Y, Stanton C, Paul Ross R,
Zhao J, Zhang H, Yang B and Chen W: Alleviation effects of
Bifidobacterium breve on DSS-induced colitis depends on intestinal
tract barrier maintenance and gut microbiota modulation. Eur J
Nutr. 60:369–387. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
He Y, Ayansola H, Hou Q, Liao C, Lei J,
Lai Y, Jiang Q, Masatoshi H and Zhang B: Genistein inhibits colonic
goblet cell loss and colorectal inflammation induced by salmonella
typhimurium infection. Mol Nutr Food Res.
65(e2100209)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Huang L, Zheng J, Sun G, Yang H, Sun X,
Yao X, Lin A and Liu H: 5-Aminosalicylic acid ameliorates dextran
sulfate sodium-induced colitis in mice by modulating gut microbiota
and bile acid metabolism. Cell Mol Life Sci. 79(460)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ter Avest MM, van Hee K, Bronkhorst C and
de Jonge HJM: Mesalazine induced autoimmune hepatitis in a patient
with Crohn's disease. Clin Res Hepatol Gastroenterol.
45(101551)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ben-Horin S, Har-Noy O, Katsanos KH,
Roblin X, Chen M, Gao X, Schwartz D, Cheon JH, Cesarini M, Bojic D,
et al: Corticosteroids and mesalamine versus corticosteroids for
acute severe ulcerative colitis: A randomized controlled trial.
Clin Gastroenterol Hepatol. 20:2868–2875.e1. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Handley G and Hand J: Adverse effects of
immunosuppression: Infections. Handb Exp Pharmacol. 272:287–314.
2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Rankala R, Mustonen A, Voutilainen M and
Mattila K: Costs of medications used to treat inflammatory bowel
disease. Scand J Gastroenterol. 59:34–38. 2024.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Forgie AJ, Fouhse JM and Willing BP:
Diet-microbe-host interactions that affect gut mucosal integrity
and infection resistance. Front Immunol. 10(1802)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gustafsson JK and Johansson MEV: The role
of goblet cells and mucus in intestinal homeostasis. Nat Rev
Gastroenterol Hepatol. 19:785–803. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kanauchi O, Fukuda M, Matsumoto Y, Ishii
S, Ozawa T, Shimizu M, Mitsuyama K and Andoh A: Eubacterium limosum
ameliorates experimental colitis and metabolite of microbe
attenuates colonic inflammatory action with increase of mucosal
integrity. World J Gastroenterol. 12:1071–1077. 2006.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Luissint AC, Parkos CA and Nusrat A:
Inflammation and the intestinal barrier: Leukocyte-epithelial cell
interactions, cell junction remodeling, and mucosal repair.
Gastroenterology. 151:616–632. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Schlegel N, Boerner K and Waschke J:
Targeting desmosomal adhesion and signalling for intestinal barrier
stabilization in inflammatory bowel diseases-Lessons from
experimental models and patients. Acta Physiol (Oxf).
231(e13492)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Schneider MR, Dahlhoff M, Horst D, Hirschi
B, Trulzsch K, Muller-Hocker J, Vogelmann R, Allgauer M, Gerhard M,
Steininger S, et al: A key role for E-cadherin in intestinal
homeostasis and Paneth cell maturation. PLoS One.
5(e14325)2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Panwar S, Sharma S and Tripathi P: Role of
barrier integrity and dysfunctions in maintaining the healthy gut
and their health outcomes. Front Physiol. 12(715611)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
de Ponthaud C, Abdalla S, Belot MP, Shao
X, Penna C, Brouquet A and Bougneres P: Increased CpG methylation
at the CDH1 locus in inflamed ileal mucosa of patients with Crohn
disease. Clin Epigenetics. 16(28)2024.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Guo G, Shi F, Zhu J, Shao Y, Gong W, Zhou
G, Wu H, She J and Shi W: Piperine, a functional food alkaloid,
exhibits inhibitory potential against TNBS-induced colitis via the
inhibition of IκB-α/NF-κB and induces tight junction protein
(claudin-1, occludin, and ZO-1) signaling pathway in experimental
mice. Hum Exp Toxicol. 39:477–491. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Niv Y: Mucin gene expression in the
intestine of ulcerative colitis patients: A systematic review and
meta-analysis. Eur J Gastroenterol Hepatol. 28:1241–1245.
2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chang CC, Chao KC, Huang CJ, Hung CS and
Wang YC: Association between aberrant dynein cytoplasmic 1 light
intermediate chain 1 expression levels, mucins and chemosensitivity
in colorectal cancer. Mol Med Rep. 22:185–192. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Singh V, Johnson K, Yin J, Lee S, Lin R,
Yu H, In J, Foulke-Abel J, Zachos NC, Donowitz M, et al: Chronic
inflammation in ulcerative colitis causes long-term changes in
goblet cell function. Cell Mol Gastroenterol Hepatol. 13:219–232.
2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Grondin JA, Kwon YH, Far PM, Haq S and
Khan WI: Mucins in intestinal mucosal defense and inflammation:
Learning from clinical and experimental studies. Front Immunol.
11(2054)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Klepsch V, Gerner RR, Klepsch S, Olson WJ,
Tilg H, Moschen AR, Baier G and Hermann-Kleiter N: Nuclear orphan
receptor NR2F6 as a safeguard against experimental murine colitis.
Gut. 67:1434–1444. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kang Y, Park H, Choe BH and Kang B: The
role and function of mucins and its relationship to inflammatory
bowel disease. Front Med (Lausanne). 9(848344)2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Hamamoto Y, Kawamura M, Uchida H,
Takagahara K, Katori C, Asai H, Harada H, Shimizu S, Morii E and
Yoshida K: Aberrant MUC immunohistochemical expressions in
inflammatory bowel diseases. Appl Immunohistochem Mol Morphol.
31:107–112. 2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Liu M, Zhu D, Yan H, Dong Z, Zhang J, Kong
N, Zhang G, Xu Q, Han T, Ke P, et al: Combined administration of
anisodamine and neostigmine alleviated colitis by inducing
autophagy and inhibiting inflammation. PLoS One.
19(e0291543)2024.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wang Y, Wang W, Yang H, Shao D, Zhao X and
Zhang G: Intraperitoneal injection of 4-hydroxynonenal (4-HNE), a
lipid peroxidation product, exacerbates colonic inflammation
through activation of Toll-like receptor 4 signaling. Free Radic
Biol Med. 131:237–242. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Ryu DB, Lim JY, Lee SE, Park G and Min CK:
Induction of indoleamine 2,3-dioxygenase by Pre-treatment with
Poly(I:C) may enhance the efficacy of MSC treatment in DSS-induced
Colitis. Immune Netw. 16:358–365. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Li Y, Liu XT, Zhang PL, Li YC, Sun MR,
Wang YT, Wang SP, Yang H, Liu BL, Wang M, et al: Hydroxysafflor
Yellow A Blocks HIF-1α induction of NOX2 and protects ZO-1 protein
in cerebral microvascular endothelium. Antioxidants (Basel).
11(728)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Lapresa R, Agulla J, Bolanos JP and
Almeida A: APC/C-Cdh1-targeted substrates as potential therapies
for Alzheimer's disease. Front Pharmacol.
13(1086540)2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Yu T, Chen D, Qi H, Lin L and Tang Y:
Resolvins protect against diabetes-induced colonic oxidative
stress, barrier dysfunction, and associated diarrhea via the HO-1
pathway. Biofactors. 50:967–979. 2024.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Paco A, Leitao-Castro J and Freitas R:
Epigenetic regulation of CDH1 is altered after HOXB7-silencing in
MDA-MB-468 triple-negative breast cancer cells. Genes (Basel).
12(1575)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Li R, Liu Y, Wu J, Chen X, Lu Q, Xia K,
Liu C, Sui X, Liu Y, Wang Y, et al: Adaptive metabolic responses
facilitate blood-brain barrier repair in ischemic stroke via
BHB-mediated epigenetic modification of ZO-1 expression. Adv Sci
(Weinh). 11(e2400426)2024.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Liu D, Xu Y, Feng J, Yu J, Huang J and Li
Z: Mucins and tight junctions are severely altered in necrotizing
enterocolitis neonates. Am J Perinatol. 38:1174–1180.
2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Park YH, Kim N, Shim YK, Choi YJ, Nam RH,
Choi YJ, Ham MH, Suh JH, Lee SM, Lee CM, et al: Adequate dextran
sodium sulfate-induced colitis model in mice and effective outcome
measurement method. J Cancer Prev. 20:260–267. 2015.PubMed/NCBI View Article : Google Scholar
|