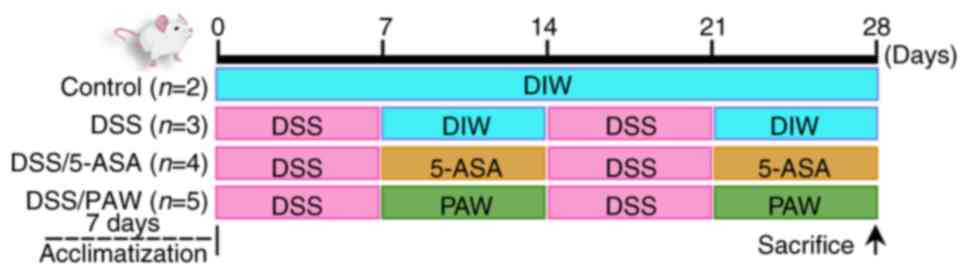
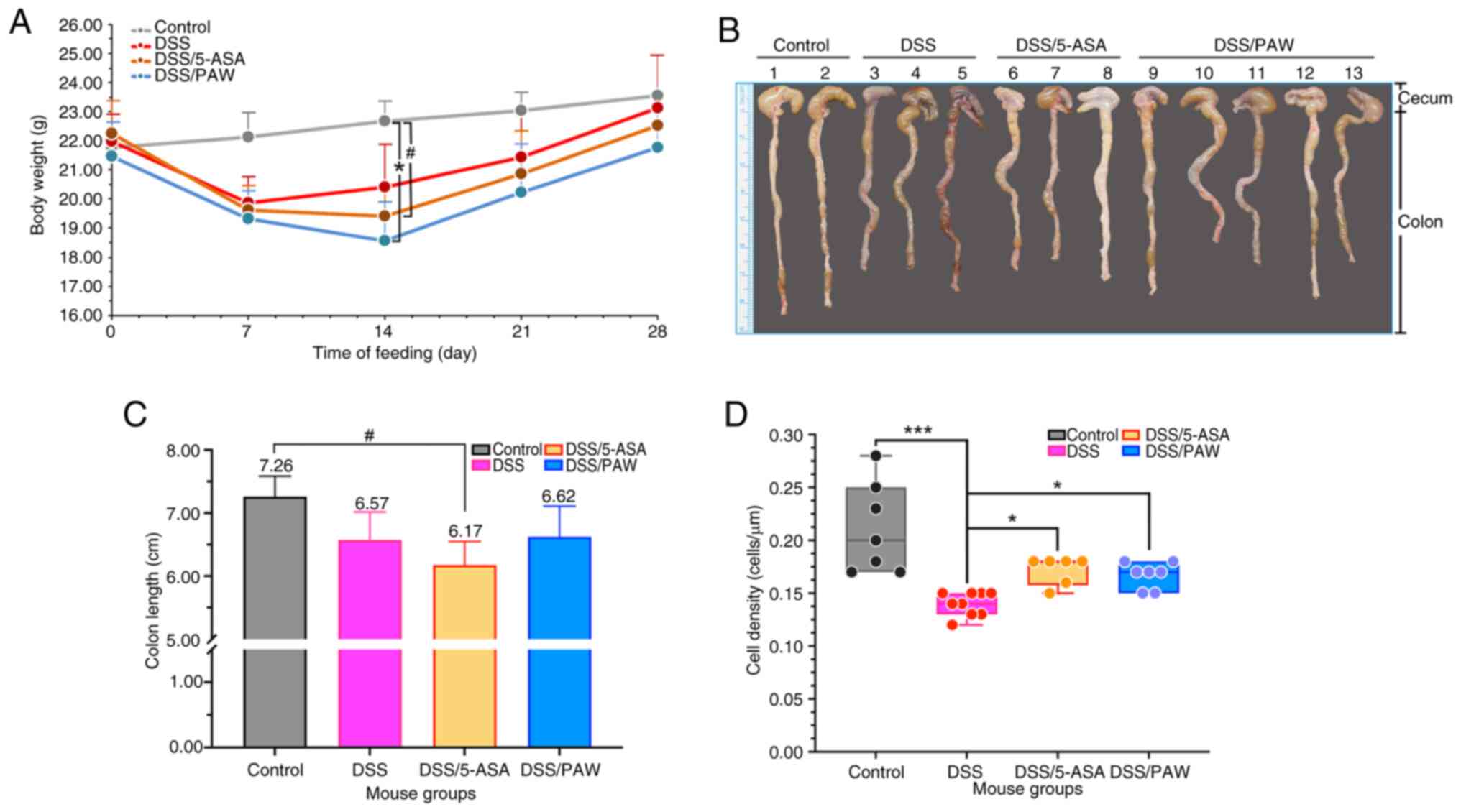
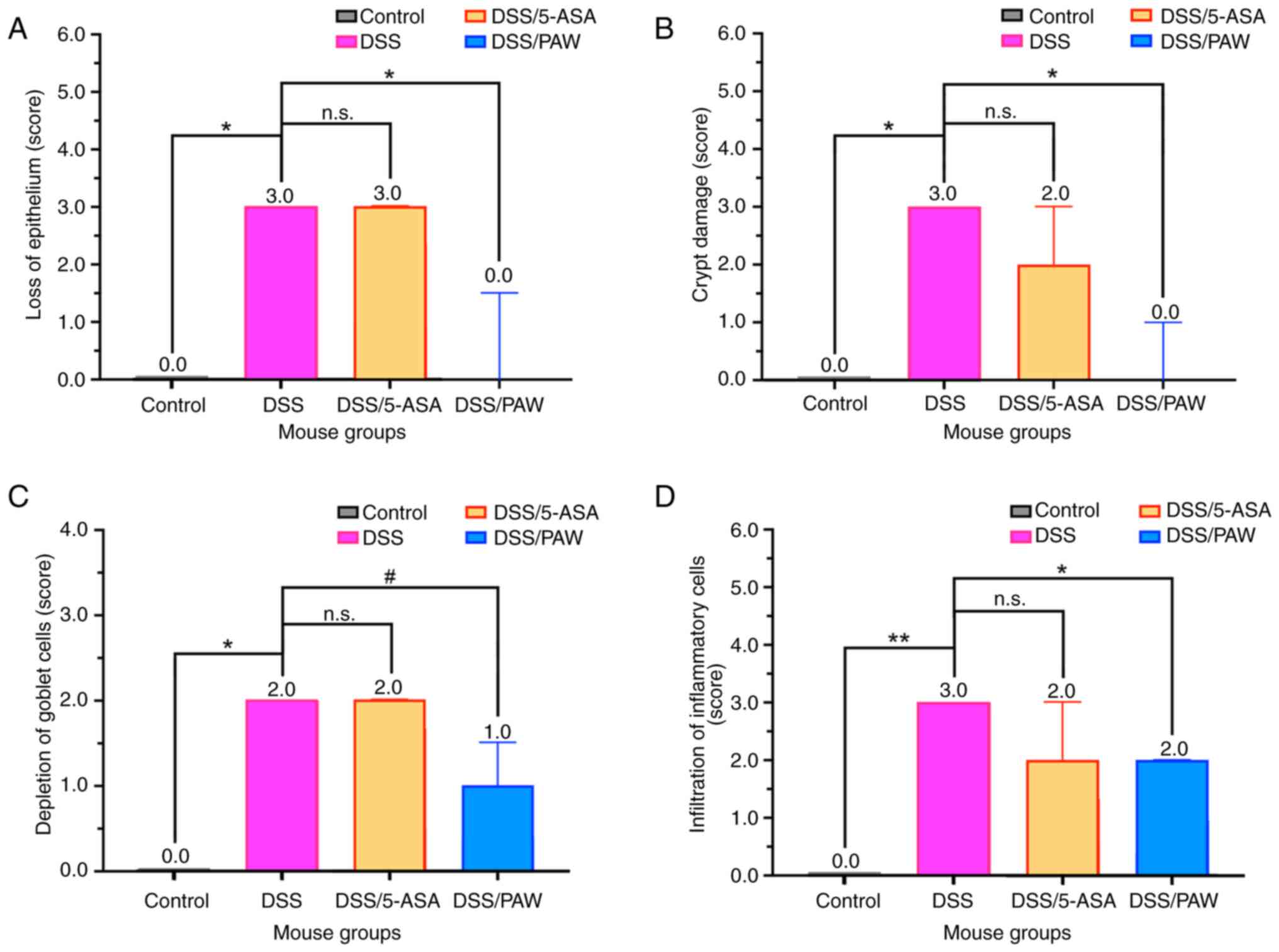
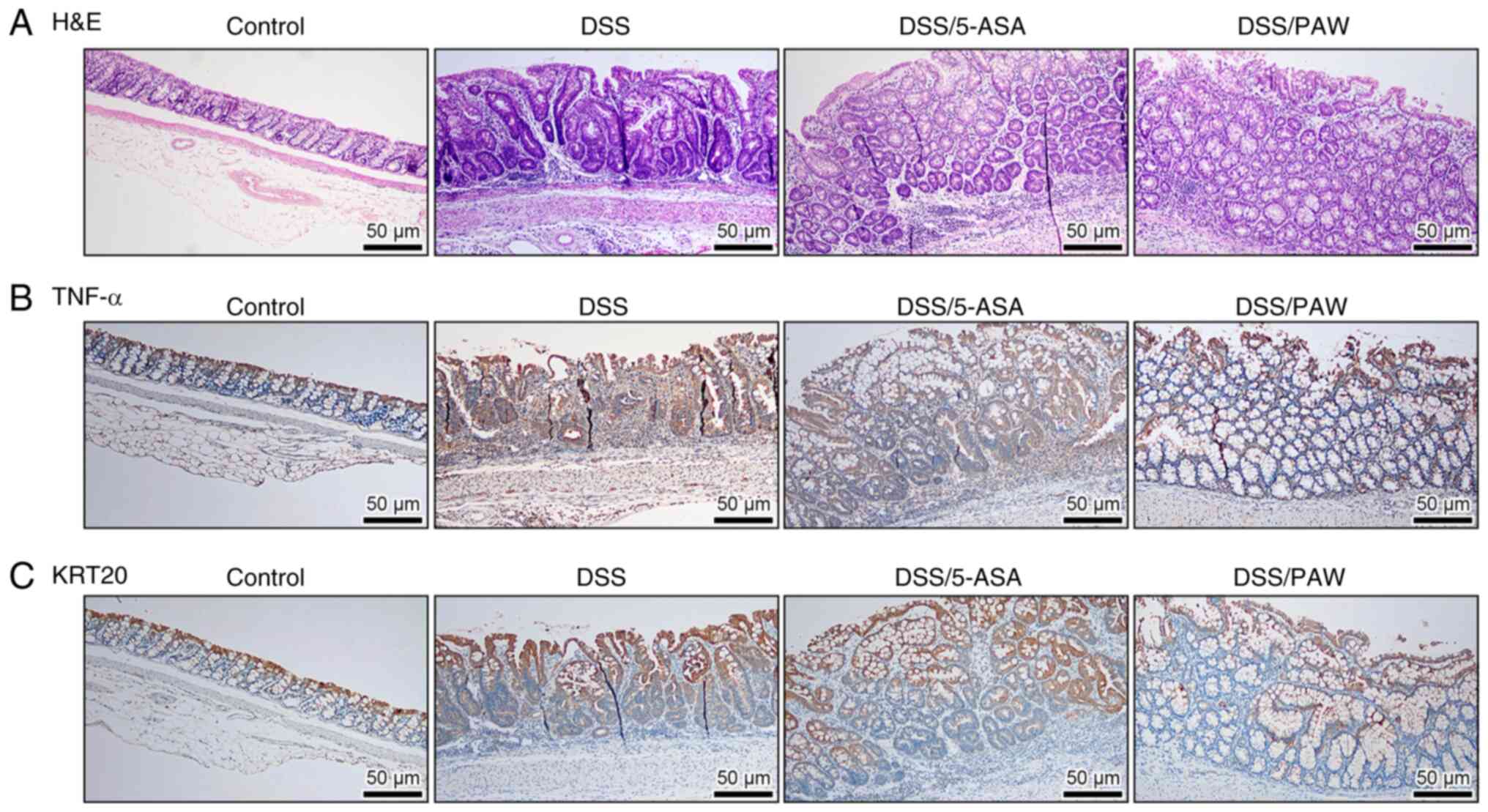
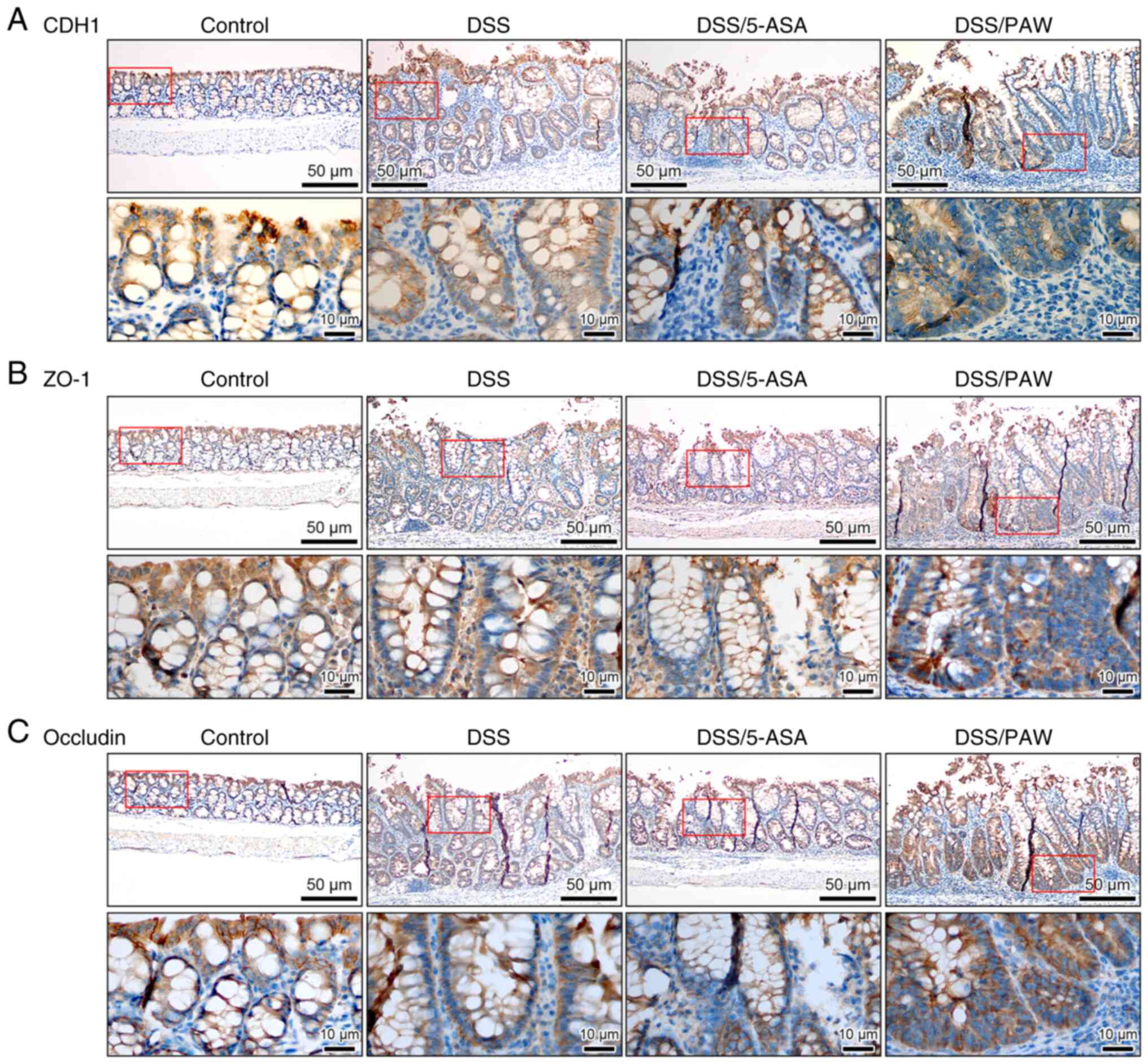
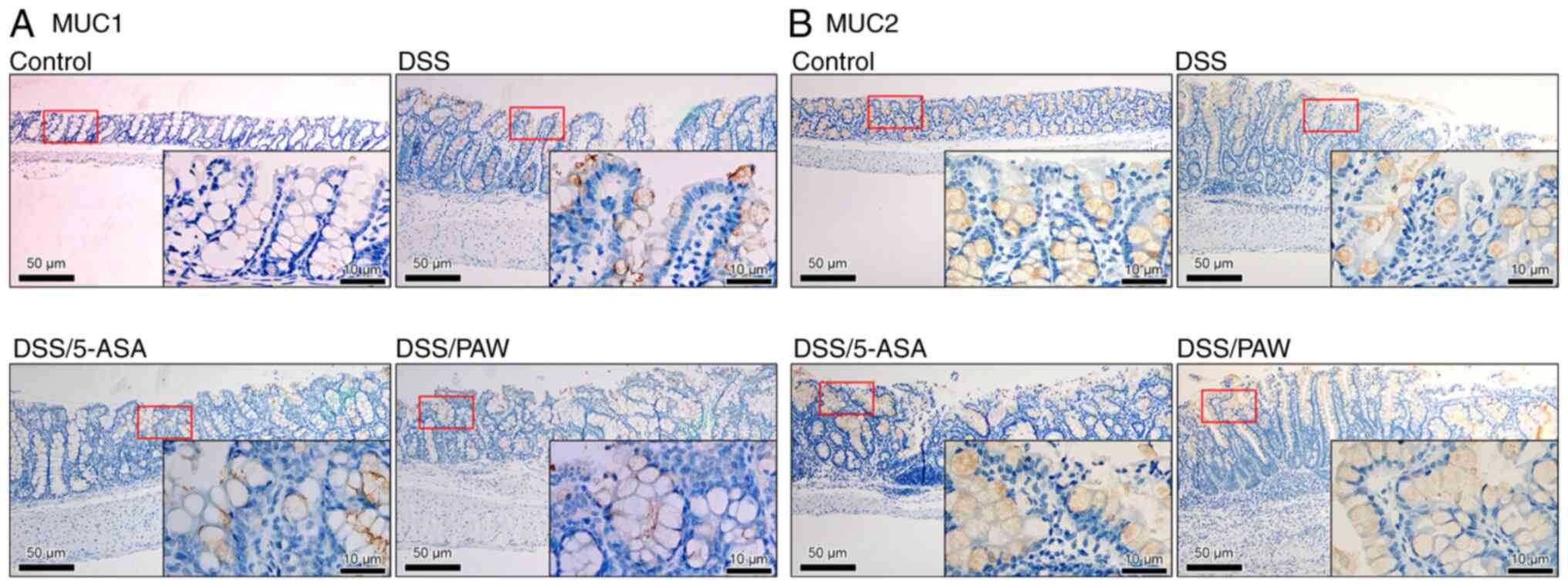
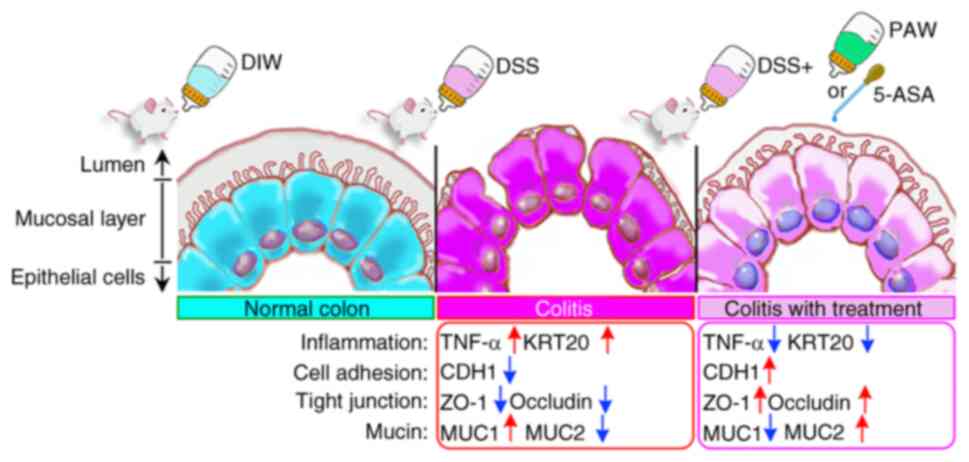
|
1
|
Le Berre C, Ananthakrishnan AN, Danese S,
Singh S and Peyrin-Biroulet L: Ulcerative colitis and Crohn's
disease have similar burden and goals for treatment. Clin
Gastroenterol Hepatol. 18:14–23. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li XX, Liu Y, Luo J, Huang ZD, Zhang C and
Fu Y: Vitamin D deficiency associated with Crohn's disease and
ulcerative colitis: A meta-analysis of 55 observational studies. J
Transl Med. 17(323)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alarfaj SJ, Mostafa SA, Negm WA, El-Masry
TA, Kamal M, Elsaeed M and El Nakib AM: Mucosal genes expression in
inflammatory bowel disease patients: New insights. Pharmaceuticals
(Basel). 16(324)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Neurath MF and Vieth M: Different levels
of healing in inflammatory bowel diseases: Mucosal, histological,
transmural, barrier and complete healing. Gut. 72:2164–2183.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sanchez de Medina F, Romero-Calvo I,
Mascaraque C and Martinez-Augustin O: Intestinal inflammation and
mucosal barrier function. Inflamm Bowel Dis. 20:2394–2404.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dong J, Liang W, Wang T, Sui J, Wang J,
Deng Z and Chen D: Saponins regulate intestinal inflammation in
colon cancer and IBD. Pharmacol Res. 144:66–72. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kotla NG, Isa ILM, Rasala S, Demir S,
Singh R, Baby BV, Swamy SK, Dockery P, Jala VR, Rochev Y, et al:
Modulation of gut barrier functions in ulcerative colitis by
hyaluronic acid system. Adv Sci (Weinh). 9(e2103189)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lopetuso LR, Scaldaferri F, Bruno G,
Petito V, Franceschi F and Gasbarrini A: The therapeutic management
of gut barrier leaking: The emerging role for mucosal barrier
protectors. Eur Rev Med Pharmacol Sci. 19:1068–1076.
2015.PubMed/NCBI
|
|
9
|
Seo K, Seo J, Yeun J, Choi H, Kim YI and
Chang SY: The role of mucosal barriers in human gut health. Arch
Pharm Res. 44:325–341. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nakase H, Hirano T, Wagatsuma K, Ichimiya
T, Yamakawa T, Yokoyama Y, Hayashi Y, Hirayama D, Kazama T, Yoshii
S, et al: Artificial intelligence-assisted endoscopy changes the
definition of mucosal healing in ulcerative colitis. Dig Endosc.
33:903–911. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang J, Zhang C, Guo C and Li X: Chitosan
ameliorates DSS-induced ulcerative colitis mice by enhancing
intestinal barrier function and improving microflora. Int J Mol
Sci. 20(5751)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Foppoli A, Maroni A, Moutaharrik S,
Melocchi A, Zema L, Palugan L, Cerea M and Gazzaniga A: In vitro
and human pharmacoscintigraphic evaluation of an oral 5-ASA
delivery system for colonic release. Int J Pharm.
572(118723)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Beiranvand M: A review of the biological
and pharmacological activities of mesalazine or 5-aminosalicylic
acid (5-ASA): An anti-ulcer and anti-oxidant drug.
Inflammopharmacology. 29:1279–1290. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Burr NE, Hall B, Hamlin PJ, Selinger CP,
Ford AC and O'Connor A: Systematic review and network meta-analysis
of medical therapies to prevent recurrence of post-operative
Crohn's disease. J Crohns Colitis. 13:693–701. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cho J, Kweon HS, Huh SO and Sadra A:
Augmented reduction in colonic inflammatory markers of dextran
sulfate sodium-induced colitis with a combination of
5-aminosalicylic acid and AD-lico from Glycyrrhiza inflata. Anim
Cells Syst (Seoul). 22:189–196. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Torres P, Canete F, Nunez L, Aguilar A,
Mesonero F, Calafat M, Fernandez C, Teniente A, Manosa M,
Lopez-Sanroman A, et al: Spacing the administration interval of
Anti-TNF agents: A valid strategy for patients with inflammatory
bowel disease? Dig Dis Sci. 65:2036–2043. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang CP and Liu YC: Therapeutics for
inflammatory-related diseases based on plasmon-activated water: A
review. Int J Mol Sci. 19(1589)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cheng CH, Liu YC, Yang YSH, Lin KJ, Wu D,
Liu YR, Chang CC, Hong CT and Hu CJ: Plasmon-activated water as a
therapeutic strategy in Alzheimer's disease by altering gut
microbiota. Aging (Albany NY). 15:3715–3737. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen HC, Hwang BJ, Mai FD, Liu YC, Lin CM,
Kuo HS, Chou DS, Lee MJ, Yang KH, Yu CC, et al: Active and stable
liquid water innovatively prepared using resonantly illuminated
gold nanoparticles. ACS Nano. 8:2704–2713. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen HC, Mai FD, Hwang BJ, Lee MJ, Chen
CH, Wang SH, Tsai HY, Yang CP and Liu YC: Creation of
Electron-doping liquid water with reduced hydrogen bonds. Sci Rep.
6(22166)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chang CC, Liu CY, Su IC, Lee YJ, Yeh HJ,
Chen WC, Yu CJ, Kao WY, Liu YC and Huang CJ: Functional
Plasmon-activated water increases Akkermansia muciniphila
abundance in gut microbiota to ameliorate inflammatory bowel
disease. Int J Mol Sci. 23(11422)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Blikslager AT, Moeser AJ, Gookin JL, Jones
SL and Odle J: Restoration of barrier function in injured
intestinal mucosa. Physiol Rev. 87:545–564. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sommer K, Wiendl M, Muller TM, Heidbreder
K, Voskens C, Neurath MF and Zundler S: Intestinal mucosal wound
healing and barrier integrity in IBD-crosstalk and trafficking of
cellular players. Front Med (Lausanne). 8(643973)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pan HH, Zhou XX, Ma YY, Pan WS, Zhao F, Yu
MS and Liu JQ: Resveratrol alleviates intestinal mucosal barrier
dysfunction in dextran sulfate sodium-induced colitis mice by
enhancing autophagy. World J Gastroenterol. 26:4945–4959.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cheng CH, Lin KJ, Hong CT, Wu D, Chang HM,
Liu CH, Hsiao IT, Yang CP, Liu YC and Hu CJ: Plasmon-activated
water reduces amyloid burden and improves memory in animals with
Alzheimer's disease. Sci Rep. 9(13252)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Iba Y, Sugimoto Y, Kamei C and Masukawa T:
Possible role of mucosal mast cells in the recovery process of
colitis induced by dextran sulfate sodium in rats. Int
Immunopharmacol. 3:485–491. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chang SC, Chiang HH, Liu CY, Li YJ, Lu CL,
Lee YP, Huang CJ and Lai CL: Intestinal mucosal barrier improvement
with prebiotics: Histological evaluation of longish glucomannan
hydrolysates-induced innate T lymphocyte activities in Mice.
Nutrients. 14(2220)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Helenius TO, Antman CA, Asghar MN, Nystrom
JH and Toivola DM: Keratins Are altered in intestinal
disease-related stress responses. Cells. 5(35)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pugliese D, Felice C, Papa A, Gasbarrini
A, Rapaccini GL, Guidi L and Armuzzi A: Anti TNF-alpha therapy for
ulcerative colitis: Current status and prospects for the future.
Expert Rev Clin Immunol. 13:223–233. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Meigs TE, Fedor-Chaiken M, Kaplan DD,
Brackenbury R and Casey PJ: Galpha12 and Galpha13 negatively
regulate the adhesive functions of cadherin. J Biol Chem.
277:24594–24600. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kuo WT, Odenwald MA, Turner JR and Zuo L:
Tight junction proteins occludin and ZO-1 as regulators of
epithelial proliferation and survival. Ann N Y Acad Sci.
1514:21–33. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bankole E, Read E, Curtis MA, Neves JF and
Garnett JA: The relationship between mucins and ulcerative colitis:
A systematic review. J Clin Med. 10(1935)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bergstrom KS, Kissoon-Singh V, Gibson DL,
Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, et
al: Muc2 protects against lethal infectious colitis by
disassociating pathogenic and commensal bacteria from the colonic
mucosa. PLoS Pathog. 6(e1000902)2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sheng YH, Hasnain SZ, Florin TH and
McGuckin MA: Mucins in inflammatory bowel diseases and colorectal
cancer. J Gastroenterol Hepatol. 27:28–38. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang CK, Chen HC, Fang SU, Ho CW, Tai CJ,
Yang CP and Liu YC: Innovatively therapeutic strategy on lung
cancer by daily drinking antioxidative plasmon-induced activated
water. Sci Rep. 8(6316)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lian YZ, Liu YC, Chang CC, Nochi T and
Chao JC: Combined Lycium barbarum polysaccharides with
plasmon-activated water affect IFN-γ/TNF-α induced inflammation in
Caco-2 cells. Pharmaceuticals (Basel). 16(1455)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen HC, Cheng CY, Chen LY, Chang CC, Yang
CP, Mai FD, Liao WC, Chang HM and Liu YC: Plasmon-activated water
effectively relieves hepatic oxidative damage resulting from
chronic sleep deprivation. RSC Adv. 8:9618–9626. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Atia O, Goren I, Fischler TS, Weisband YL,
Greenfeld S, Kariv R, Ledderman N, Matz E, Rimon RM, Dotan I, et
al: 5-aminosalicylate maintenance is not superior to no maintenance
in patients with newly diagnosed Crohn's disease-A nationwide
cohort study. Aliment Pharmacol Ther. 57:1004–1013. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chassaing B, Aitken JD, Malleshappa M and
Vijay-Kumar M: Dextran sulfate sodium (DSS)-induced colitis in
mice. Curr Protoc Immunol. 104:15.25.1–15.25.14. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Naito Y, Takagi T, Uchiyama K, Kuroda M,
Kokura S, Ichikawa H, Yanagisawa R, Inoue K, Takano H, Satoh M, et
al: Reduced intestinal inflammation induced by dextran sodium
sulfate in interleukin-6-deficient mice. Int J Mol Med. 14:191–196.
2004.PubMed/NCBI
|
|
41
|
Giuffrida P, Cococcia S, Delliponti M,
Lenti MV and Di Sabatino A: Controlling gut inflammation by
restoring anti-inflammatory pathways in inflammatory bowel disease.
Cells. 8(397)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gandhi GR, Mohana T, Athesh K, Hillary VE,
Vasconcelos ABS, Farias de Franca MN, Montalvao MM, Ceasar SA,
Jothi G, Sridharan G, et al: Anti-inflammatory natural products
modulate interleukins and their related signaling markers in
inflammatory bowel disease: A systematic review. J Pharm Anal.
13:1408–1428. 2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Magnusson K, de Wit M, Brennan DJ, Johnson
LB, McGee SF, Lundberg E, Naicker K, Klinger R, Kampf C, Asplund A,
et al: SATB2 in combination with cytokeratin 20 identifies over 95%
of all colorectal carcinomas. Am J Surg Pathol. 35:937–948.
2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen Y, Jin Y, Stanton C, Paul Ross R,
Zhao J, Zhang H, Yang B and Chen W: Alleviation effects of
Bifidobacterium breve on DSS-induced colitis depends on intestinal
tract barrier maintenance and gut microbiota modulation. Eur J
Nutr. 60:369–387. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
He Y, Ayansola H, Hou Q, Liao C, Lei J,
Lai Y, Jiang Q, Masatoshi H and Zhang B: Genistein inhibits colonic
goblet cell loss and colorectal inflammation induced by salmonella
typhimurium infection. Mol Nutr Food Res.
65(e2100209)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Huang L, Zheng J, Sun G, Yang H, Sun X,
Yao X, Lin A and Liu H: 5-Aminosalicylic acid ameliorates dextran
sulfate sodium-induced colitis in mice by modulating gut microbiota
and bile acid metabolism. Cell Mol Life Sci. 79(460)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ter Avest MM, van Hee K, Bronkhorst C and
de Jonge HJM: Mesalazine induced autoimmune hepatitis in a patient
with Crohn's disease. Clin Res Hepatol Gastroenterol.
45(101551)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ben-Horin S, Har-Noy O, Katsanos KH,
Roblin X, Chen M, Gao X, Schwartz D, Cheon JH, Cesarini M, Bojic D,
et al: Corticosteroids and mesalamine versus corticosteroids for
acute severe ulcerative colitis: A randomized controlled trial.
Clin Gastroenterol Hepatol. 20:2868–2875.e1. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Handley G and Hand J: Adverse effects of
immunosuppression: Infections. Handb Exp Pharmacol. 272:287–314.
2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Rankala R, Mustonen A, Voutilainen M and
Mattila K: Costs of medications used to treat inflammatory bowel
disease. Scand J Gastroenterol. 59:34–38. 2024.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Forgie AJ, Fouhse JM and Willing BP:
Diet-microbe-host interactions that affect gut mucosal integrity
and infection resistance. Front Immunol. 10(1802)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gustafsson JK and Johansson MEV: The role
of goblet cells and mucus in intestinal homeostasis. Nat Rev
Gastroenterol Hepatol. 19:785–803. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kanauchi O, Fukuda M, Matsumoto Y, Ishii
S, Ozawa T, Shimizu M, Mitsuyama K and Andoh A: Eubacterium limosum
ameliorates experimental colitis and metabolite of microbe
attenuates colonic inflammatory action with increase of mucosal
integrity. World J Gastroenterol. 12:1071–1077. 2006.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Luissint AC, Parkos CA and Nusrat A:
Inflammation and the intestinal barrier: Leukocyte-epithelial cell
interactions, cell junction remodeling, and mucosal repair.
Gastroenterology. 151:616–632. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Schlegel N, Boerner K and Waschke J:
Targeting desmosomal adhesion and signalling for intestinal barrier
stabilization in inflammatory bowel diseases-Lessons from
experimental models and patients. Acta Physiol (Oxf).
231(e13492)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Schneider MR, Dahlhoff M, Horst D, Hirschi
B, Trulzsch K, Muller-Hocker J, Vogelmann R, Allgauer M, Gerhard M,
Steininger S, et al: A key role for E-cadherin in intestinal
homeostasis and Paneth cell maturation. PLoS One.
5(e14325)2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Panwar S, Sharma S and Tripathi P: Role of
barrier integrity and dysfunctions in maintaining the healthy gut
and their health outcomes. Front Physiol. 12(715611)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
de Ponthaud C, Abdalla S, Belot MP, Shao
X, Penna C, Brouquet A and Bougneres P: Increased CpG methylation
at the CDH1 locus in inflamed ileal mucosa of patients with Crohn
disease. Clin Epigenetics. 16(28)2024.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Guo G, Shi F, Zhu J, Shao Y, Gong W, Zhou
G, Wu H, She J and Shi W: Piperine, a functional food alkaloid,
exhibits inhibitory potential against TNBS-induced colitis via the
inhibition of IκB-α/NF-κB and induces tight junction protein
(claudin-1, occludin, and ZO-1) signaling pathway in experimental
mice. Hum Exp Toxicol. 39:477–491. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Niv Y: Mucin gene expression in the
intestine of ulcerative colitis patients: A systematic review and
meta-analysis. Eur J Gastroenterol Hepatol. 28:1241–1245.
2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chang CC, Chao KC, Huang CJ, Hung CS and
Wang YC: Association between aberrant dynein cytoplasmic 1 light
intermediate chain 1 expression levels, mucins and chemosensitivity
in colorectal cancer. Mol Med Rep. 22:185–192. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Singh V, Johnson K, Yin J, Lee S, Lin R,
Yu H, In J, Foulke-Abel J, Zachos NC, Donowitz M, et al: Chronic
inflammation in ulcerative colitis causes long-term changes in
goblet cell function. Cell Mol Gastroenterol Hepatol. 13:219–232.
2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Grondin JA, Kwon YH, Far PM, Haq S and
Khan WI: Mucins in intestinal mucosal defense and inflammation:
Learning from clinical and experimental studies. Front Immunol.
11(2054)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Klepsch V, Gerner RR, Klepsch S, Olson WJ,
Tilg H, Moschen AR, Baier G and Hermann-Kleiter N: Nuclear orphan
receptor NR2F6 as a safeguard against experimental murine colitis.
Gut. 67:1434–1444. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kang Y, Park H, Choe BH and Kang B: The
role and function of mucins and its relationship to inflammatory
bowel disease. Front Med (Lausanne). 9(848344)2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Hamamoto Y, Kawamura M, Uchida H,
Takagahara K, Katori C, Asai H, Harada H, Shimizu S, Morii E and
Yoshida K: Aberrant MUC immunohistochemical expressions in
inflammatory bowel diseases. Appl Immunohistochem Mol Morphol.
31:107–112. 2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Liu M, Zhu D, Yan H, Dong Z, Zhang J, Kong
N, Zhang G, Xu Q, Han T, Ke P, et al: Combined administration of
anisodamine and neostigmine alleviated colitis by inducing
autophagy and inhibiting inflammation. PLoS One.
19(e0291543)2024.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wang Y, Wang W, Yang H, Shao D, Zhao X and
Zhang G: Intraperitoneal injection of 4-hydroxynonenal (4-HNE), a
lipid peroxidation product, exacerbates colonic inflammation
through activation of Toll-like receptor 4 signaling. Free Radic
Biol Med. 131:237–242. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Ryu DB, Lim JY, Lee SE, Park G and Min CK:
Induction of indoleamine 2,3-dioxygenase by Pre-treatment with
Poly(I:C) may enhance the efficacy of MSC treatment in DSS-induced
Colitis. Immune Netw. 16:358–365. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Li Y, Liu XT, Zhang PL, Li YC, Sun MR,
Wang YT, Wang SP, Yang H, Liu BL, Wang M, et al: Hydroxysafflor
Yellow A Blocks HIF-1α induction of NOX2 and protects ZO-1 protein
in cerebral microvascular endothelium. Antioxidants (Basel).
11(728)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Lapresa R, Agulla J, Bolanos JP and
Almeida A: APC/C-Cdh1-targeted substrates as potential therapies
for Alzheimer's disease. Front Pharmacol.
13(1086540)2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Yu T, Chen D, Qi H, Lin L and Tang Y:
Resolvins protect against diabetes-induced colonic oxidative
stress, barrier dysfunction, and associated diarrhea via the HO-1
pathway. Biofactors. 50:967–979. 2024.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Paco A, Leitao-Castro J and Freitas R:
Epigenetic regulation of CDH1 is altered after HOXB7-silencing in
MDA-MB-468 triple-negative breast cancer cells. Genes (Basel).
12(1575)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Li R, Liu Y, Wu J, Chen X, Lu Q, Xia K,
Liu C, Sui X, Liu Y, Wang Y, et al: Adaptive metabolic responses
facilitate blood-brain barrier repair in ischemic stroke via
BHB-mediated epigenetic modification of ZO-1 expression. Adv Sci
(Weinh). 11(e2400426)2024.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Liu D, Xu Y, Feng J, Yu J, Huang J and Li
Z: Mucins and tight junctions are severely altered in necrotizing
enterocolitis neonates. Am J Perinatol. 38:1174–1180.
2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Park YH, Kim N, Shim YK, Choi YJ, Nam RH,
Choi YJ, Ham MH, Suh JH, Lee SM, Lee CM, et al: Adequate dextran
sodium sulfate-induced colitis model in mice and effective outcome
measurement method. J Cancer Prev. 20:260–267. 2015.PubMed/NCBI View Article : Google Scholar
|