Introduction
Osteoradionecrosis (ORN) of the jaw is a major
complication of radiotherapy for head and neck cancer (HNC)
(1,2). ORN is characterized by persistent
bone exposure lasting over three months in patients with a history
of radiotherapy to the orofacial region (3-5).
The advent of intensity-modulated radiotherapy (IMRT) and
widespread use of prophylactic tooth extractions before
radiotherapy have reduced the incidence of ORN; however, its
incidence rate remains between 4 and 8% (6). The risk factors for ORN include
radiotherapy-related factors, such as radiation dose exceeding 60
Gy and the type of radiation modality, as well as patient-related
factors, including poor oral hygiene, and dental extractions within
the irradiated area, particularly involving the mandible (7-9).
It is still controversial to determine the appropriate timing of
dental extractions among patients undergoing radiotherapy; however,
the National Comprehensive Cancer Network guidelines propose that
dental extraction should be performed at least 2 weeks prior to the
initiation of radiotherapy (1). A
previous study demonstrated that the prognosis of ORN varies, with
a survival rate of 96% at 12 months and 73% at 60 months after the
diagnosis of ORN, indicating that ORN can have a significant impact
on long-term survival (10).
Hence, effective ORN management is also crucial for improving
long-term outcomes and quality of life of patients with HNC.
Conventional treatments for ORN include conservative and surgical
approaches. Conservative treatments, such as pentoxifylline and
tocopherol, clodronate potentiation and hyperbaric oxygen (HBO)
therapy, offer limited efficacy as ORN advances (1,11-14).
By contrast, surgical treatments, particularly segmental
mandibulectomy, are now regarded as favorable options for advanced
ORN (1,15-17).
Although evidence increasingly supports surgical treatment,
patients with ORN often undergo other cancer therapies, such as
surgery or chemoradiotherapy, for their primary HNC. Thus, the
patients' overall prognosis must be considered when managing
ORN.
Comprehensive genomic profiling (CGP) technologies
have provided critical insights into tumorigenesis mechanisms and
identified potent therapeutic targets, advancing precision medicine
(18,19). These technologies are also employed
in companion diagnostics, helping predict therapeutic efficacy
(19,20). The present study presents a case of
ORN coinciding with thyroid cancer metastasis to the submandibular
gland. Subsequent CGP identified a RET fusion as a therapeutic
target, and treatment with its inhibitor, selpercatinib, resulted
in a partial response.
The present case report highlighted the necessity of
extensive surgical treatment for ORN among those with advanced
cancer, as well as the clinical utility of submandibular dissection
in the surgical treatment of ORN, particularly thyroid cancer
exhibiting an aggressive phenotype. Furthermore, the study
emphasized the novelty of managing ORN in patients with advanced
cancer using CGP testing. Through this strategy, the patient of the
present study successfully underwent management of ORN with
extensive surgery, while concurrently receiving treatment for
advanced PTC based on genomic profiling. The effectiveness of this
novel management approach was demonstrated by improving the
patient's condition. This suggests that the combined approach may
offer a promising treatment option for patients with both ORN and
advanced PTC.
Case presentation
A 58-year-old Japanese male was referred to the
Department of Oral Diagnosis and Medicine in Hokkaido University
Hospital (Hokkaido, Japan) by his family dentist in July 2020 for
evaluation of bone exposure in the mandible. The patient reported
severe spontaneous pain in the right mandibular area. The patient
had a history of smoking for 18 years (ages 20 to 38 years) and of
drinking alcohol regularly for 38 years. The medical family history
included no cancer cases; however, the patient's medical history
included left-sided thyroid cancer and right-sided oropharyngeal
cancer.
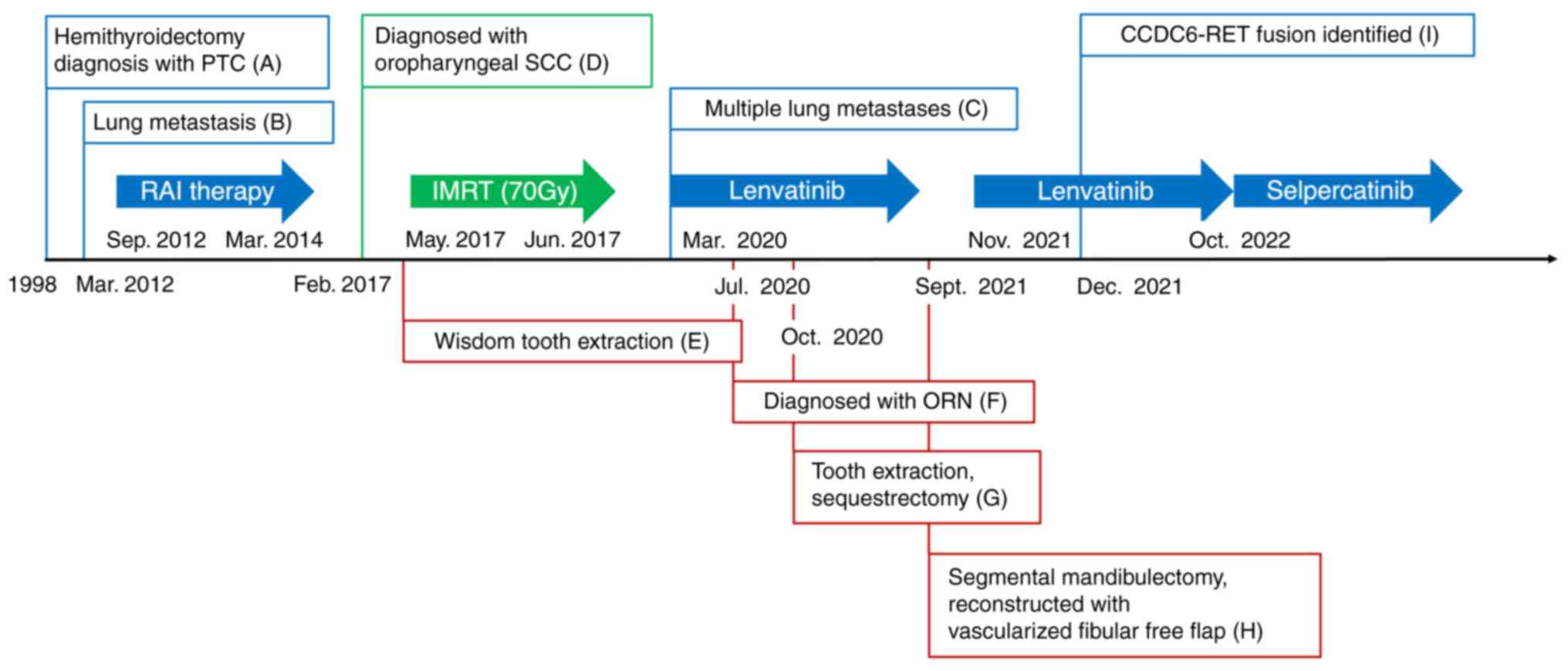
For thyroid cancer, the patient had undergone a left
hemithyroidectomy and was diagnosed with papillary thyroid
carcinoma (PTC; TNM stage unknown) in 1998 (Fig. 1A). In March 2012, routine computed
tomography (CT) identified a mass in the right lung. The patient
underwent thoracoscopic right lower lobectomy and the lesion was
diagnosed as PTC metastasis. The patient received postoperative
radioactive iodine (RI) therapy with I-131 (Fig. 1B). After three sessions of RI
therapy, a partial response was achieved and the patient was
monitored regularly. In March 2020, routine CT revealed worsening
PTC metastases in the lung, leading to initiation of lenvatinib
treatment (24 mg/day) (Fig.
1C).
Regarding oropharyngeal cancer, the patient first
experienced right neck pain and a swollen cervical lymph node in
February 2017. Fine-needle aspiration suggested squamous cell
carcinoma (SCC) (Fig. 1D). The
patient also reported discomfort in the right palatine tonsil.
Biopsy confirmed right oropharyngeal SCC with p16+/human
papillomavirus + (cT2N1M0, stage 1). The patient opted for IMRT
alone to treat the oropharyngeal cancer. In March 2017, prior to
IMRT, the right-sided wisdom tooth, which was scheduled to be in
the irradiation field (Fig. 1E),
was extracted. In June 2017, the patient completed IMRT with a dose
of 70 Gy in 35 fractions and showed no evidence of recurrence on
follow-up positron emission tomography (PET)/CT.
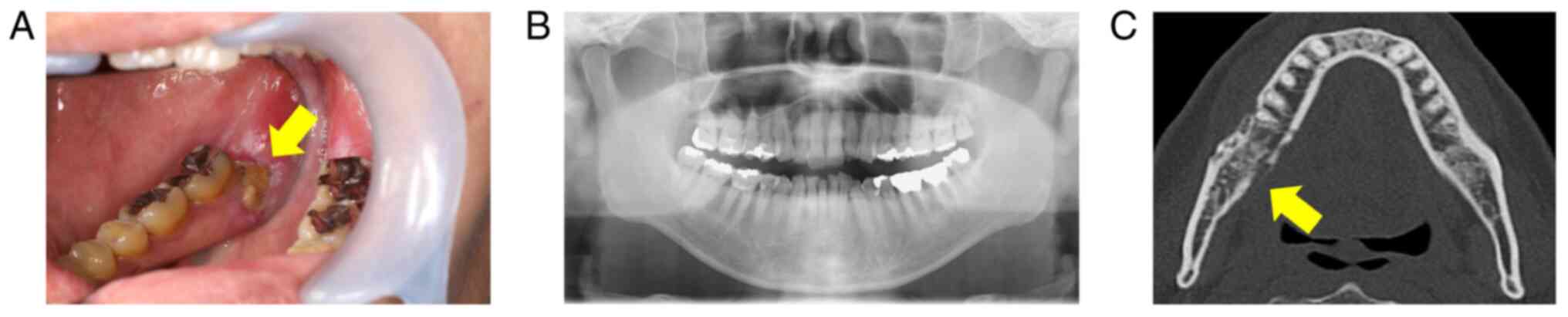
In July 2020, extraoral examination revealed
swollen, reddened skin over the right mandible. The patient also
experienced trismus, with a maximal interincisal distance of 22 mm.
Intraoral examination showed exposed bone (4 mm in diameter) in the
right mandibular gingiva (Fig.
2A). A panoramic X-ray revealed mild sclerosis around the
exposed area (Fig. 2B), and CT
also showed marked sclerosis and a partial cortical irregularity
without apparent mandibular fracture (Fig. 2C). Considering these clinical
manifestations, the patient was diagnosed with ORN in the right
mandible (Grade III according to Notani's classification) (21) (Fig.
1F). Initial antimicrobial therapy with amoxicillin (AMPC;
1,000 mg/day) and clavulanic acid/AMPC (1,000 mg/day) relieved the
swollen gingiva; however, the patient still suffered pain during
meals. In October 2020, the patient underwent extraction of the
right second mandibular molar and sequestrectomy (Fig. 1G), followed by 10 sessions of HBO
therapy. During the treatment, the patient did not feel any benefit
and therefore chose to discontinue further sessions, resulting in a
total of 10 sessions.
In April 2021, the patient reported severe pain and
pus discharge from the wound, with exposed bone observed at the
site. Given the poor response to conservative treatment, the
patient consented to right segmental mandibulectomy. At that time,
the patient had been receiving lenvatinib for 18 months, which was
discontinued one week before surgery for ORN. In September 2021,
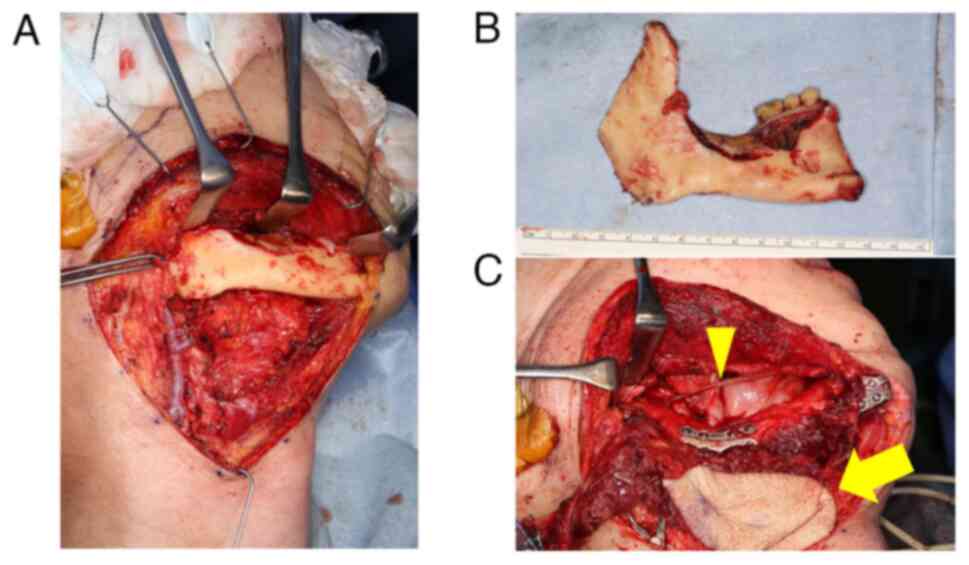
the patient underwent right segmental mandibulectomy and right
submandibular dissection (levels I and II), including right
submandibular gland resection (Figs.
1H and 3A and B). As for determining the extent of
mandibulectomy, the resected area included the bone sclerotic area
observed in the CT images, the area irradiated >45 Gy and the
area where punctuate bleeding is observed during the surgery.
Reconstruction involved a vascularized fibular free flap and
concurrent right inferior alveolar nerve repair using
Renerve® (Nipro Medical Corp.), a bioresorbable
allograft material (Fig. 3C).
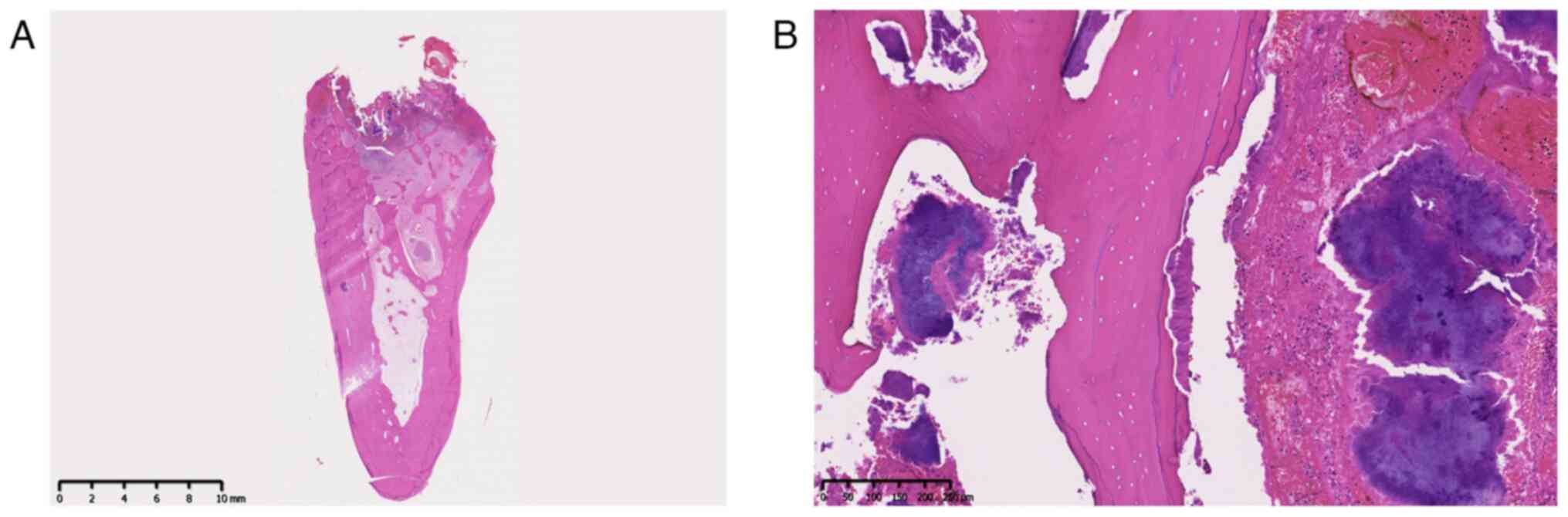
Pathological examination confirmed sequestrum with bacterial
colonies in the bone lacunae, consistent with osteonecrosis
(Fig. 4A and B). In this examination, formalin-fixed
tissue sections were processed and embedded in paraffin.
Subsequently, 4-µm sections were prepared for staining with
hematoxylin and eosin according to standard procedures.
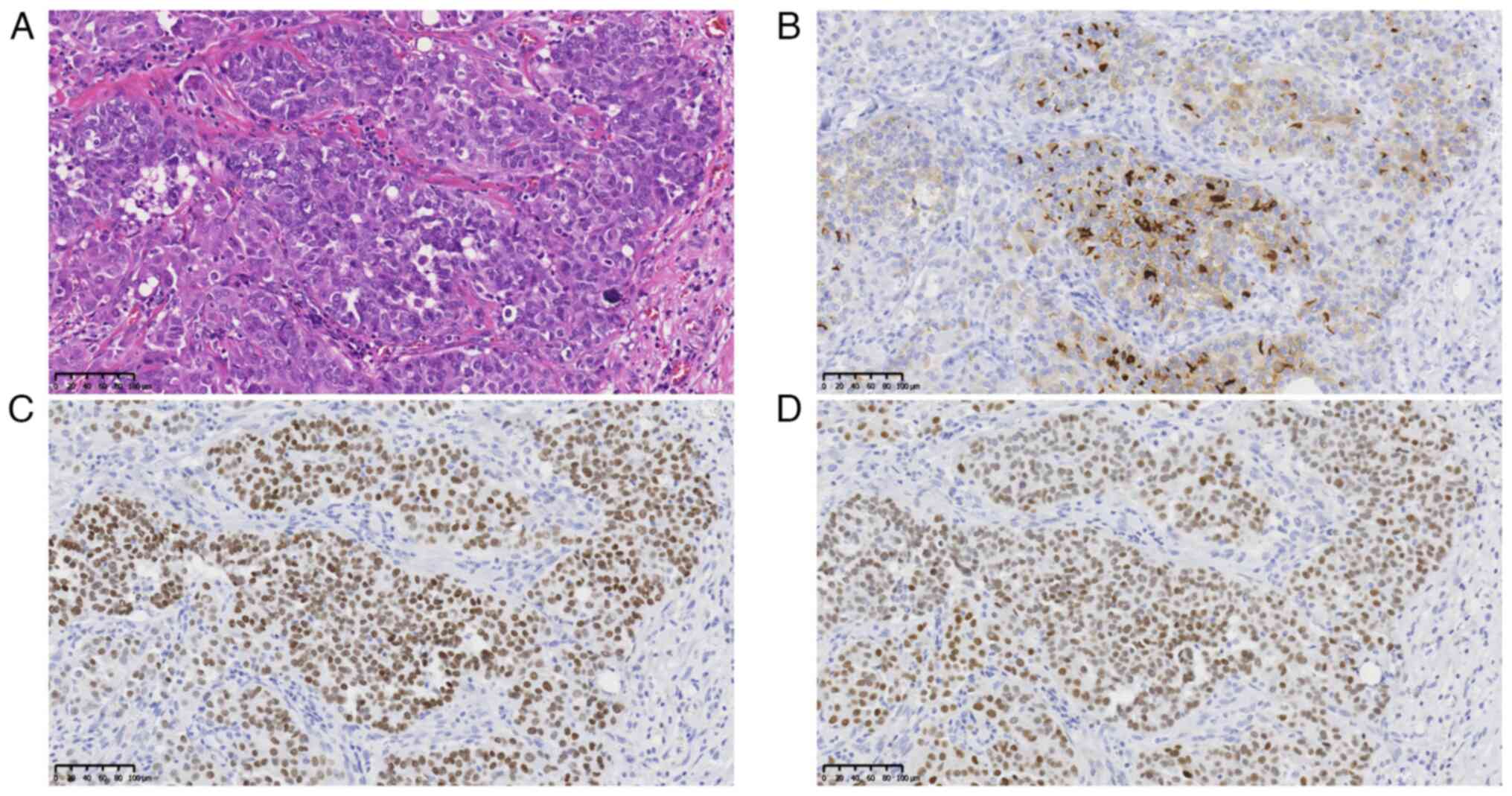
Unexpectedly, the pathological examination also revealed tumor
cells that showed optically clear nuclei, characteristic of PTC, in
the resected right submandibular gland, which had not been
identified in the previous CT images (Fig. 5A). In addition, immunohistochemical
staining was positive for thyroglobulin, thyroid transcription
factor 1 (TTF1) and paired-box 8 (PAX8), confirming PTC metastasis
(Fig. 5B-D). Regarding the
immunohistochemical staining, 4-µm paraffin sections were prepared
from blocks of tumor tissues using a microtome. The antigen was
activated by heat treatment at 97˚C for 20 min in Tris/EDTA buffer
solution (pH 9.0) after deparaffinization and dehydration. The
antibodies used were as follows: Anti-thyroglobulin (cat. no.
M078101-2; Dako; Agilent Technologies, Inc.), anti-TTF-1 (cat. no.
M3575; Dako; Agilent Technologies, Inc.) and anti-PAX8
(363M-14-RUO; Sigma-Aldrich; Merck KGaA) antibodies. Immunoreaction
was visualized with horseradish peroxidase-linked secondary
antibody (cat. no. K4001; Dako; Agilent Technologies, Inc.) and
3,3-diaminobenzidine substrate (cat. no. GV82511-2; Dako; Agilent
Technologies, Inc.). All sections were observed under a
fluorescence microscope (ECLIPSE Ci; Nikon Corp.).
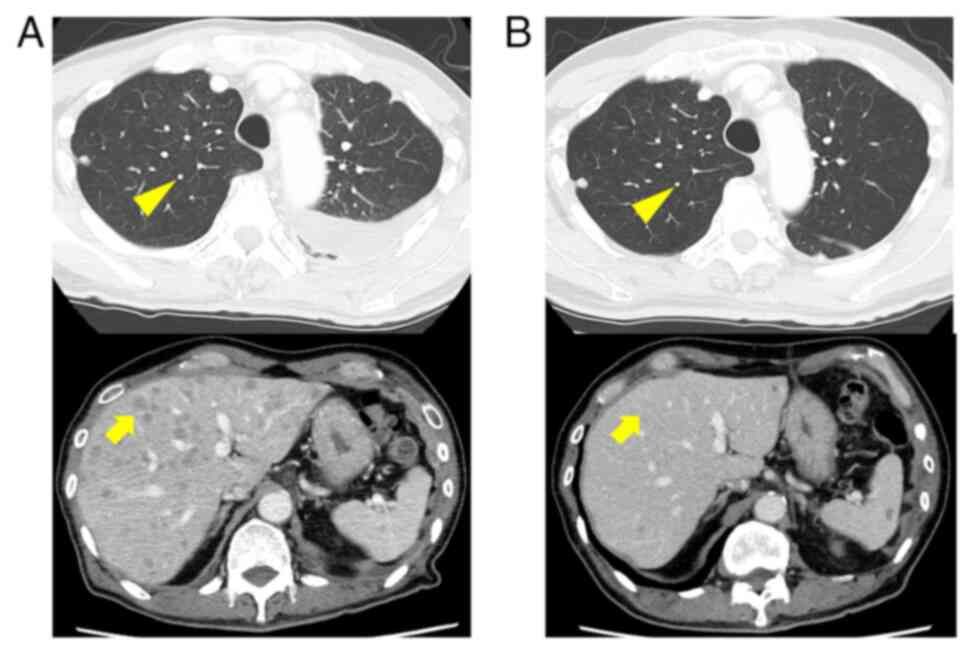
At four weeks post-surgery, lenvatinib treatment (8
mg/day) was resumed, but subsequent CT revealed further metastases
to the lungs and liver (Fig. 6A).
In December 2021, CGP using FoundationOne® CDx
(performed externally at Foundation Medicine, Inc.) identified a
CCDC6-RET fusion in tumor cells from the submandibular gland
metastasis (Fig. 1I, Table I). Consequently, the patient was
switched to selpercatinib, a selective RET inhibitor, (320 mg/day)
in October 2022, achieving a partial response with reduced
metastatic lesions in the lungs and liver (Fig. 6B).
 | Table IComprehensive genomic profiling
results. |
Table I
Comprehensive genomic profiling
results.
| Gene | Variant | Variant type | Interpretation |
|---|
| RET | CCDC6-RET | Fusion | Actionable |
| BRCA2 | V2687I
(c.8059G>A) | Missense
mutation | VUS |
| BRD4 | P1091S
(c.3271C>T) | Missense
mutation | VUS |
| SDHD | A90E
(c.269C>A) | Missense
mutation | VUS |
| TBX3 | M608T
(c.1823T>C) | Missense
mutation | VUS |
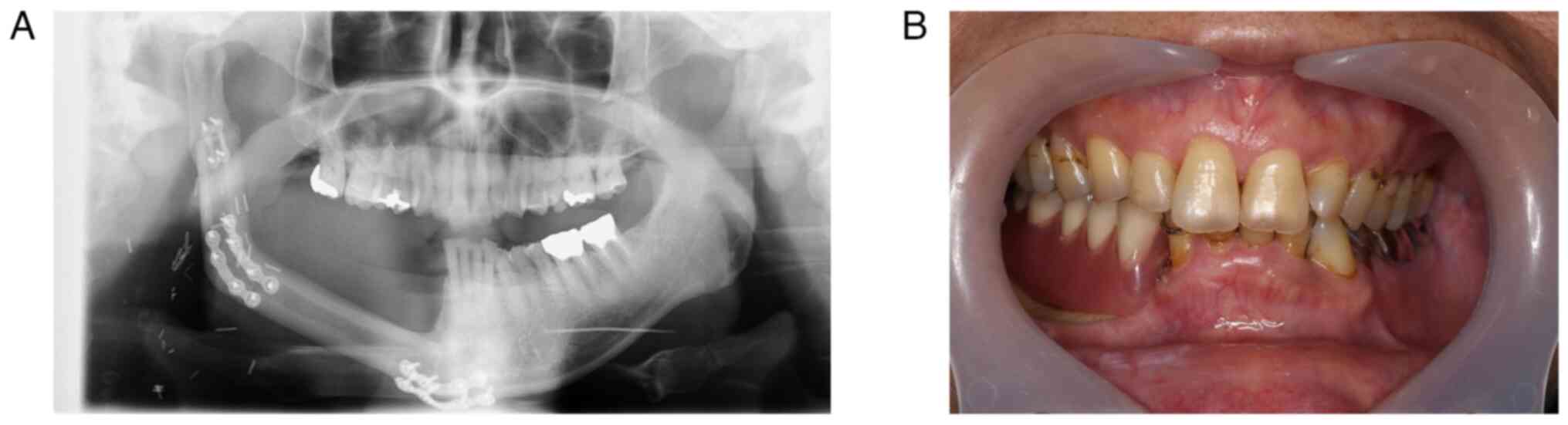
At three years after the ORN surgery, the patient
remains under regular monitoring with no signs of recurrence on
panoramic X-ray (Fig. 7A), and the
patient's occlusion has been restored with dentures (Fig. 7B). Furthermore, nerve
reconstruction has successfully preserved sensation of the skin
area around the mental region. The patient continues selpercatinib
treatment without showing any severe adverse effects, maintaining
stable disease. The patient is now being monitored regularly with
panoramic X-ray once a year for ORN and CT scans every three months
for PTC.
Discussion
This report describes a rare clinical course in a
patient with ORN of the jaw who unexpectedly presented with PTC
metastasis to the submandibular gland. The patient underwent
extensive surgical treatment for ORN, resulting in a favorable
outcome without any severe postoperative complications.
Furthermore, due to the detection of PTC metastases in the
submandibular gland, CGP was called upon, revealing RET fusion as a
therapeutic target. As a result, treatment with selpercatinib, a
selective RET inhibitor, led to the successful management of
advanced PTC with the patient showing a partial response. This case
highlights the value of extensive surgical treatment for patients
with ORN even when they have aggressive cancer phenotypes, and of
the utility of CGP in identifying therapeutic targets, particularly
in patients with cancer who generally harbor well-known actionable
targets.
Thyroid cancer is the most prevalent endocrine
malignancy (22), with PTC
accounting for ~80% of all thyroid cancers (23). The prognosis for PTC is generally
favorable, with the 10-year survival rate exceeding 90%, making it
a controllable cancer (24,25).
However, ~40% of PTC cases metastasize to the cervical lymph nodes,
lungs, liver and bones, leading to intractable disease and a
10-year survival rate <60% (26,27).
Of note, PTC metastasis to the submandibular gland is rare, with
only a few reports describing such occurrences (28-32).
In the patient of the present study, preoperative CT scans failed
to detect the metastasis. Given his multiple lung PTC metastases
despite ongoing lenvatinib treatment, it was decided to perform
submandibular dissection during the surgery for ORN and the right
submandibular gland was resected, as occult PTC metastasis was
suspected. Previously, it was reported that ultrasound (US) would
be highly effective in detecting a submandibular gland metastasis
that cannot be identified by CT or PET/CT (32). Therefore, it should be considered
to perform not only regular CT but also US for monitoring patients
with thyroid cancer to detect metastasis around the neck region in
the early period.
The CGP test is a next-generation sequencing
approach that analyzes the genomic landscape of patients with
cancer, which enables the detection of potential therapeutic
targets, leading to personalized medicine. Currently, our hospital
offers two CGP platforms, FoundationOne® CDx and
OncoGuide™ National Cancer Center (NCC) OncoPanel, to those who
complete or are expected to complete standard treatment for solid
tumors. FoundationOne® CDx examining based on
formalin-fixed, paraffin-embedded (FFPE) samples, enables the
analysis of a total of 324 genes covering various somatic mutations
in 309 genes and 36 fusion genes in addition to microsatellite
instability and tumor mutation burden (TMB), while the
OncoGuide™ NCC OncoPanel test based on both FFPE and
blood samples allows analyzing 126 genes, including various
somatic/germline mutations in 114 genes and 12 fusion genes and TMB
(33,34). As for the patient of the present
study, his desire to undergo CGP with a broader gene analysis led
to analysis with the FoundationOne® CDx platform, which
eventually identified a CCDC6-RET fusion that is covered in these
CGP platforms.
The RET proto-oncogene encodes a
transmembrane tyrosine kinase receptor protein (35). RET regulates cellular
proliferation, differentiation and apoptosis through binding with
its ligands, the glial cell line-derived neurotrophic factor family
(36,37). Genetic aberrations, including RET
alterations, fusions and amplifications, have been observed in
various cancers (38,39). These RET aberrations lead to
ligand-independent hyperactivation of downstream signaling
pathways, contributing to tumorigenesis (40). RET aberrations have become major
therapeutic targets, with several multi-kinase inhibitors,
including lenvatinib, and selective RET inhibitors, such as
selpercatinib and pralsetinib, having received Food and Drug
Administration approval (41-43).
Prior studies have shown that these selective RET inhibitors offer
substantial benefits with manageable toxicity in patients with
thyroid and lung cancers harboring RET aberrations (44,45).
Of note, patients with thyroid cancer often exhibit RET
aberrations, with ~50% of medullary thyroid carcinoma and 30% of
PTC cases exhibiting such alterations (38,46,47).
Therefore, early CGP testing is valuable for identifying actionable
targets, such as RET aberrations, in thyroid cancer cases not
well-controlled by ongoing treatments.
Management of ORN remains challenging, as
conventional treatments are often insufficient for this intractable
disease. Treatment strategies, including conservative and surgical
options, depend on disease severity (1). Several reports have described ORN
grade classification based on clinical manifestations, radiological
findings, duration of bone exposure and response to HBO therapy
(4). In our department, Notani's
classification is used, which considers the extent of bone
involvement to guide treatment decisions for patients with ORN
(Table I) (21). Accordingly, the patient of the
present study was classified as Grade III. Notani et al
(21) demonstrated that patients
with Grade III ORN show favorable outcomes with segmental
resection. Consequently, the patient of the present study underwent
segmental mandibulectomy followed by reconstruction with a
vascularized fibular free flap. Furthermore, previous studies have
emphasized early surgical intervention when conservative treatments
fail to show improvement in clinical and radiographic findings
(17,21,48).
Although the decision to perform extensive surgery in patients with
cancer is controversial, the response of the patient of the present
study indicated limited efficacy with antimicrobial treatment and
sequestrectomy. Given the patient's strong desire to alleviate the
severe pain from mandibular inflammation and the limited success of
conservative treatments, it was decided to perform surgery.
The decision to perform extensive surgery on cancer
patients should always be carefully weighed against potential risks
and benefits. This is because those who receive anti-angiogenic
inhibitors would likely show delayed wound healing as described
previously (49). Lenvatinib is a
multi-kinase inhibitor targeting vascular endothelial growth factor
(VEGF) receptors 1-3, fibroblast growth factor receptors 1-4,
platelet-derived growth factor receptor-α, RET and KIT
proto-oncogene receptor tyrosine kinases (50). From the perspective of bone
remodeling, lenvatinib can impair the normal remodeling process by
inhibiting angiogenesis. In general, bone remodeling primarily
involves two key processes: Vigorous angiogenesis and regulation of
basic multicellular units (BMUs), including osteogenic cell
activity (51). Angiogenesis plays
crucial roles in bone remodeling by recruiting osteogenic cells
toward the remodeling site and by supplying oxygen and growth
factors essential for bone regeneration (52). Although it remains elusive whether
lenvatinib directly affects BMUs, its inhibition of VEGF may
disrupt angiogenesis. Therefore, lenvatinib treatment may have
contributed to the worsening of ORN by impairing bone healing.
Furthermore, it has been reported that lenvatinib bears a risk of
developing medication osteonecrosis of the jaw (MRONJ) (53,54).
In the present study, the patient had been subjected to
radiotherapy and lenvatinib treatment, both of which can contribute
to the onset of osteonecrosis. As for the differential diagnosis,
it is noteworthy that MRONJ specifically presents as an apparent
periosteal reaction on CT imaging (55,56).
In the patient of the present study, CT revealed osteolytic lesions
accompanied by sclerotic changes around the exposed bone without
showing any apparent periosteal reaction. Thereafter, the patient
was eventually diagnosed with ORN.
Regarding segmental mandibulectomy for ORN
treatment, there are no clear guidelines on the ideal resection
area. A previous study suggested that preoperative radiographic
findings and intraoperative bleeding from native bone may help
define resection borders (1). At
our department, criteria for determining the extent of
mandibulectomy for ORN have been established (Table II). These include occlusal
factors, radiographic findings, radiation dose, intraoperative
observations and consideration of autologous bone reconstruction.
Based on these guidelines, early surgical intervention is suitable,
as previously recommended when conservative treatments failed to
yield substantial benefits (48).
In the patient of the present study, the extent of mandibulectomy
was determined by the extend of sclerotic changes observed on CT,
the area irradiated >45 Gy, intraoperative findings to observe
bleeding and availability of autologous bone that includes
patients' surgical tolerance. Nevertheless, further research is
necessary to refine these criteria for more sophisticated
decision-making regarding ORN surgery.
 | Table IIClinical/radiographic factors for
determining the extent of mandibular resection required to treat
jaw osteoradionecrosis. |
Table II
Clinical/radiographic factors for
determining the extent of mandibular resection required to treat
jaw osteoradionecrosis.
| Factors determining
the extent of mandibulectomy | Context |
|---|
| Oral
hygiene/denture assessment | Preservation of
occlusal contact |
| Imaging
assessment | CT; presence of
osteomyelitis and/or peritoneal reaction |
| | MRI; hypointensity
region in T1-weighted images |
| | PET/CT;
accumulation of FDG uptake region |
| Radiation
amount | Affected bone
irradiated >45 Gy |
| Intraoperative
findings | Punctate bleeding
at the wound edge of the affected bone |
| Reconstruction
method | Availability of
autologous bones |
Despite the success of the present strategy in
preventing postoperative complications and recurrence, there are
certain limitations to address. First, the extensive surgery for
ORN led to discontinuation of the patient's treatment for PTC
metastases, ultimately worsening the progression of PTC. The
withdrawal of tyrosine kinase inhibitors (TKIs) can trigger a rapid
disease progression known as the flare phenomenon, characterized by
an aggressive resurgence of cancer symptoms (57,58).
Specifically, patients with thyroid cancer treated with lenvatinib
have a 14.3% incidence rate of the flare phenomenon (59). Furthermore, previous studies have
shown that patients experiencing the flare phenomenon tend to have
a worse prognosis compared with those who do not exhibit this
phenomenon (59). Considering
these facts, it is highly important to manage the lenvatinib
treatment course properly in the surgical setting. Thus far,
guidelines for the perioperative discontinuation of lenvatinib and
resumption remain to be established. Several reports have
recommended the discontinuation period as seven days prior to
surgery based on the half-life of lenvatinib, 34.5 h (60-62).
As for the timing of lenvatinib resumption, a recent paper
demonstrated that the timing for resumption should be four to six
weeks after the surgery when wound healing is generally confirmed
(60). Accordingly, the patient of
the present study stopped lenvatinib treatment one week before ORN
surgery and resumed treatment four weeks post-surgery. Given the
progression of PTC metastases during this period, the timing of TKI
treatment resumption or an alternative strategy should be
reconsidered. Previously, a paper demonstrated that the
personalized treatment schedule of lenvatinib led to the successful
management of a patient with thyroid cancer who responded well to
lenvatinib; however, severe adverse effects occurred (63). Hence, when a patient exhibits an
apparent disease flare following lenvatinib discontinuation, it
should be considered to resume lenvatinib immediately with a low
dose after surgery with careful attention to wound management. As a
second limitation of the study, the CGP testing successfully
identified the actionable target; however, this technique has
several limitations. CGP requires an adequate quantity and quality
of tumor samples defined as the tumor cell content ratio, DNA
amount and tissue size, which would narrow down the patients'
accessibility to this analysis (34,64).
Furthermore, CGP testing can detect druggable gene mutations, in
other words, it cannot detect gene mutations beyond the scope of
the CGP panels (65). In addition,
the prevalence of CGP testing can have an economic impact,
increasing the financial burden on health systems (34,66).
Of note, a recent paper demonstrated that CGP testing may be
cost-effective compared with conventional single-gene testing
(65). This is proposed to be due
to the increased possibility of identifying actionable targets,
resulting in patients receiving genotype-matched therapy, which may
provide better outcomes than traditional chemotherapies (65). Furthermore, since the CGP testing
generally takes four to six weeks to get results (65), it would be preferable to perform
this analysis before the completion of standard treatment.
Therefore, based on these facts, early CGP testing should be
considered among patients with cancer harboring potentially
actionable targets. In particular, as mentioned earlier, effective
RET inhibitors have already been developed and shown therapeutic
efficacy in patients with thyroid cancer harboring RET aberrations
(67,68). Therefore, early CGP testing should
be encouraged for patients with refractory thyroid cancer to
identify actionable targets and guide personalized treatment plans.
Based on the rationale for early CGP testing, the present analysis
also identified missense forms of four genes, BRCA2, BRD4, SDHD and
TBX3, in addition to the CCDC6-RET fusion. According to the
previous papers, BRCA2 primarily functions in DNA repair pathways
(69). BRD4 is involved in the
NF-κB-mediated inflammatory gene expression system, thereby
regulating inflammation and promoting fibrosis (70). Furthermore, TBX3 facilitated
epithelial wound healing in mouse experiments (71). Although no reports describing a
relationship between SDHD function and wound healing exist, to the
best of our knowledge, it is noteworthy to consider how these
genetic alterations may influence clinical outcomes, particularly
postoperative wound healing. As a third limitation of the present
study, the patient did not receive any quantitative analyses to
assess the preservation of oral and sensory functions after the
reconstructive surgery. To support the effectiveness of the present
treatment strategy, further examinations, including repetitive
saliva swallowing test, glucose measurement by chewing gummy jelly
and Semmes Weinstein monofilament test for sensory testing, for
instance, should be performed (72-74).
In conclusion, extensive surgical treatment should
be considered for managing patients with ORN even in those with
advanced cancer. In particular, submandibular dissection should
also be included as part of the extensive surgery, particularly
when patients with thyroid cancer exhibit an aggressive phenotype,
to detect occult metastases. Lastly, the present case also
highlights the utility of CGP in managing aggressive cancers with
specific gene aberrations, underscoring its potential role in
precision medicine.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The comprehensive genomic profiling data generated
in the present study are available in the Figshare repository at
https://doi.org/10.6084/m9.figshare.28890965.v1.
Authors' contributions
TK collected the clinical data. TK and KIS wrote the
manuscript. TK, KIS, KY, TMu, TI, TMa, JK and YK acquired and
interpreted clinical data. AYM performed the pathological
examination. KIS, JS and YK revised the manuscript. KIS and JS
checked and confirmed the authenticity of the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present report was approved by Hokkaido
University Hospital Independent Clinical Research Review Committee
(Sapporo, Japan; approval no. 023-0364).
Patient consent for publication
Written informed consent for the publication of the
clinical data, including photos and images, was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peterson DE, Koyfman SA, Yarom N,
Lynggaard CD, Ismaila N, Forner LE, Fuller CD, Mowery YM, Murphy
BA, Watson E, et al: Prevention and management of
osteoradionecrosis in patients with head and neck cancer treated
with radiation therapy: ISOO-MASCC-ASCO guideline. J Clin Oncol.
42:1975–1996. 2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schwartz HC and Kagan AR:
Osteoradionecrosis of the mandible: Scientific basis for clinical
staging. Am J Clin Oncol. 25:168–171. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Peterson DE, Doerr W, Hovan A, Pinto A,
Saunders D, Elting LS, Spijkervet FK and Brennan MT:
Osteoradionecrosis in cancer patients: The evidence base for
treatment-dependent frequency, current management strategies, and
future studies. Support Care Cancer. 18:1089–1098. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chronopoulos A, Zarra T, Ehrenfeld M and
Otto S: Osteoradionecrosis of the jaws: Definition, epidemiology,
staging and clinical and radiological findings. A concise review.
Int Dent J. 68:22–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Spijkervet FKL, Brennan MT, Peterson DE,
Witjes MJH and Vissink A: Research frontiers in oral toxicities of
cancer therapies: Osteoradionecrosis of the jaws. J Natl Cancer
Inst Monogr. 2019(lgz006)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
de Almeida-Silva LA, Lupp JDS,
Sobral-Silva LA, Dos Santos LAR, Marques TO, da Silva DBR,
Caneppele TMF and Bianchi-de-Moraes M: The incidence of
osteoradionecrosis of the jaws in oral cavity cancer patients
treated with intensity-modulated radiotherapy: A systematic review
and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol.
138:66–78. 2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Aarup-Kristensen S, Hansen CR, Forner L,
Brink C, Eriksen JG and Johansen J: Osteoradionecrosis of the
mandible after radiotherapy for head and neck cancer: Risk factors
and dose-volume correlations. Acta Oncol. 58:1373–1377.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Beaumont S, Bhatia N, McDowell L, Fua T,
McCullough M, Celentano A and Yap T: Timing of dental extractions
in patients undergoing radiotherapy and the incidence of
osteoradionecrosis: A systematic review and meta-analysis. Br J
Oral Maxillofac Surg. 59:511–523. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kojima Y, Otsuru M, Hasegawa T, Ueda N,
Kirita T, Yamada SI, Kurita H, Shibuya Y, Funahara M and Umeda M:
Risk factors for osteoradionecrosis of the jaw in patients with
oral or oropharyngeal cancer: Verification of the effect of tooth
extraction before radiotherapy using propensity score matching
analysis. J Dent Sci. 17:1024–1029. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chieng CY, Davies A, Aziz A, Lowe D and
Rogers SN: Health related quality of life and patient concerns in
patients with osteoradionecrosis. Br J Oral Maxillofac Surg.
59:1061–1066. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Forner LE, Dieleman FJ, Shaw RJ, Kanatas
A, Butterworth CJ, Kjeller G, Alsner J, Overgaard J, Hillerup S,
Hyldegaard O, et al: Hyperbaric oxygen treatment of mandibular
osteoradionecrosis: Combined data from the two randomized clinical
trials DAHANCA-21 and NWHHT2009-1. Radiother Oncol. 166:137–144.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lombardi N, Varoni E, Villa G, Salis A and
Lodi G: Pentoxifylline and tocopherol for prevention of
osteoradionecrosis in patients who underwent oral surgery: A
clinical audit. Spec Care Dentist. 43:136–143. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Robard L, Louis MY, Blanchard D, Babin E
and Delanian S: Medical treatment of osteoradionecrosis of the
mandible by PENTOCLO: preliminary results. Eur Ann Otorhinolaryngol
Head Neck Dis. 131:333–338. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Annane D, Depondt J, Aubert P, Villart M,
Géhanno P, Gajdos P and Chevret S: Hyperbaric oxygen therapy for
radionecrosis of the jaw: A randomized, placebo-controlled,
double-blind trial from the ORN96 study group. J Clin Oncol.
22:4893–4900. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Alam DS, Nuara M and Christian J: Analysis
of outcomes of vascularized flap reconstruction in patients with
advanced mandibular osteoradionecrosis. Otolaryngol Head Neck Surg.
141:196–201. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Baumann DP, Yu P, Hanasono MM and Skoracki
RJ: Free flap reconstruction of osteoradionecrosis of the mandible:
A 10-year review and defect classification. Head Neck. 33:800–807.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bettoni J, Olivetto M, Duisit J, Caula A,
Testelin S, Dakpé S, Lengele B and Devauchelle B: The value of
reconstructive surgery in the management of refractory jaw
osteoradionecrosis: A single-center 10-year experience. Int J Oral
Maxillofac Surg. 48:1398–1404. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vogt A, Schmid S, Heinimann K, Frick H,
Herrmann C, Cerny T and Omlin A: Multiple primary tumours:
Challenges and approaches, a review. ESMO Open.
2(e000172)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pankiw M, Brezden-Masley C and Charames
GS: Comprehensive genomic profiling for oncological advancements by
precision medicine. Med Oncol. 41(1)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Milbury CA, Creeden J, Yip WK, Smith DL,
Pattani V, Maxwell K, Sawchyn B, Gjoerup O, Meng W, Skoletsky J, et
al: Clinical and analytical validation of
FoundationOne®CDx, a comprehensive genomic profiling
assay for solid tumors. PLoS One. 17(e0264138)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Notani Ki, Yamazaki Y, Kitada H,
Sakakibara N, Fukuda H, Omori K and Nakamura M: Management of
mandibular osteoradionecrosis corresponding to the severity of
osteoradionecrosis and the method of radiotherapy. Head Neck.
25:181–186. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Prete A, Borges de Souza P, Censi S, Muzza
M, Nucci N and Sponziello M: Update on fundamental mechanisms of
thyroid cancer. Front Endocrinol (Lausanne). 11(102)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhu J, Zhu X, Tu C, Li YY, Qian KQ, Jiang
C, Feng TB, Li C, Liu GJ and Wu L: Parity and thyroid cancer risk:
A meta-analysis of epidemiological studies. Cancer Med. 5:739–752.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Tuttle RM, Leboeuf R and Martorella AJ:
Papillary thyroid cancer: Monitoring and therapy. Endocrinol Metab
Clin North Am. 36:753–778, vii. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang J, Yu F, Shang Y, Ping Z and Liu L:
Thyroid cancer: Incidence and mortality trends in China, 2005-2015.
Endocrine. 68:163–173. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shaha A: Treatment of thyroid cancer based
on risk groups. J Surg Oncol. 94:683–691. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Toraih EA, Elshazli RM, Trinh LN, Hussein
MH, Attia AA, Ruiz EML, Zerfaoui M, Fawzy MS and Kandil E:
Diagnostic and prognostic performance of liquid biopsy-derived
exosomal microRNAs in thyroid cancer patients: A systematic review
and meta-analysis. Cancers (Basel). 13(4295)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sarda AK, Pandey D, Bhalla SA and Goyal A:
Isolated submandibular gland metastasis from an occult papillary
thyroid cancer. Indian J Cancer. 41:89–91. 2004.PubMed/NCBI
|
|
29
|
Davies RJ, Pring M, Aw J, Hughes CW and
Thomas SJ: Isolated submandibular metastasis from a contralateral
thyroid papillary microcarcinoma: An unusual case. Dentomaxillofac
Radiol. 38:546–549. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Radia S, Singh CV and Chamoli P: Right
submandibular gland metastasis from an occult papillary thyroid
cancer. Int J Otorhinolaryngol Head Neck Surg. 4:836–838. 2018.
|
|
31
|
Mittal RP, Yadav RR, Mhashal SK and Mittal
PR: Occult metastatic papillary thyroid carcinoma presenting as
submandibular mass: An unusual case. Int J Head Neck Surg.
9(131)2018.
|
|
32
|
Golant BT, Velez-Perez A, Krishnamurthy S,
Guo M, Mousavi S, Hu MI, Varghese JM, Zafereo ME and Debnam JM:
Thyroid carcinoma metastasizing to the submandibular gland:
Sonographic findings. J Clin Ultrasound. 48:227–230.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sunami K, Ichikawa H, Kubo T, Kato M,
Fujiwara Y, Shimomura A, Koyama T, Kakishima H, Kitami M,
Matsushita H, et al: Feasibility and utility of a panel testing for
114 cancer-associated genes in a clinical setting: A hospital-based
study. Cancer Sci. 110:1480–1490. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yatabe Y, Sunami K, Goto K, Nishio K,
Aragane N, Ikeda S, Inoue A, Kinoshita I, Kimura H, Sakamoto T, et
al: Multiplex gene-panel testing for lung cancer patients. Pathol
Int. 70:921–931. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Takahashi M, Ritz J and Cooper GM:
Activation of a novel human transforming gene, ret, by DNA
rearrangement. Cell. 42:581–588. 1985.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tallini G, Asa SL and Fuller GN: RET
oncogene activation in papillary thyroid carcinoma. Adv Anat
Pathol. 8:345–354. 2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wells SA Jr and Santoro M: Targeting the
RET pathway in thyroid cancer. Clin Cancer Res. 15:7119–7123.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kato S, Subbiah V, Marchlik E, Elkin SK,
Carter JL and Kurzrock R: RET aberrations in diverse cancers:
Next-generation sequencing of 4,871 patients. Clin Cancer Res.
23:1988–1997. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Takahashi M, Kawai K and Asai N: Roles of
the RET proto-oncogene in cancer and development. JMA J. 3:175–181.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Abdullah MI, Junit SM, Ng KL, Jayapalan
JJ, Karikalan B and Hashim OH: Papillary thyroid cancer: Genetic
alterations and molecular biomarker investigations. Int J Med Sci.
16:450–460. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bradford D, Larkins E, Mushti SL,
Rodriguez L, Skinner AM, Helms WS, Price LSL, Zirkelbach JF, Li Y,
Liu J, et al: FDA approval summary: Selpercatinib for the treatment
of lung and thyroid cancers with RET gene mutations or fusions.
Clin Cancer Res. 27:2130–2135. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Regua AT, Najjar M and Lo HW: RET
signaling pathway and RET inhibitors in human cancer. Front Oncol.
12(932353)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Thein KZ, Velcheti V, Mooers BHM, Wu J and
Subbiah V: Precision therapy for RET-altered cancers with RET
inhibitors. Trends Cancer. 7:1074–1088. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hadoux J, Elisei R, Brose MS, Hoff AO,
Robinson BG, Gao M, Jarzab B, Isaev P, Kopeckova K, Wadsley J, et
al: Phase 3 trial of selpercatinib in advanced RET-mutant medullary
thyroid cancer. N Engl J Med. 389:1851–1861. 2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhou C, Solomon B, Loong HH, Park K, Pérol
M, Arriola E, Novello S, Han B, Zhou J, Ardizzoni A, et al:
First-line selpercatinib or chemotherapy and pembrolizumab in RET
fusion-positive NSCLC. N Engl J Med. 389:1839–1850. 2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mulligan LM: RET revisited: Expanding the
oncogenic portfolio. Nat Rev Cancer. 14:173–186. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Vodopivec DM and Hu MI: RET kinase
inhibitors for RET-altered thyroid cancers. Therapeutic Advances in
Medical Oncology. 14(17588359221101691)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hato H, Sakata KI, Sato J, Satoh A,
Hayashi T and Kitagawa Y: Clinical study of treatment methods and
associated factors in mandibular osteoradionecrosis. J Oral Sci.
63:289–291. 2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Cabanillas ME and Habra MA: Lenvatinib:
Role in thyroid cancer and other solid tumors. Cancer Treat Rev.
42:47–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zschäbitz S and Grüllich C: Lenvantinib: A
tyrosine kinase inhibitor of VEGFR 1-3, FGFR 1-4, PDGFRα, KIT and
RET. Recent Results Cancer Res. 211:187–198. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bolamperti S, Villa I and Rubinacci A:
Bone remodeling: An operational process ensuring survival and bone
mechanical competence. Bone Res. 10(48)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Stegen S, van Gastel N and Carmeliet G:
Bringing new life to damaged bone: The importance of angiogenesis
in bone repair and regeneration. Bone. 70:19–27. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Mauceri R, Panzarella V, Morreale I and
Campisi G: Medication-related osteonecrosis of the jaw in a cancer
patient receiving lenvatinib. Int J Oral Maxillofac Surg.
48:1530–1532. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Monteiro L, Vasconcelos C, Pacheco JJ and
Salazar F: Photobiomodulation laser therapy in a lenvatinib-related
osteonecrosis of the jaw: A case report. J Clin Exp Dent.
13:e626–e629. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Akashi M, Wanifuchi S, Iwata E, Takeda D,
Kusumoto J, Furudoi S and Komori T: Differences between
osteoradionecrosis and medication-related osteonecrosis of the jaw.
Oral Maxillofac Surg. 22:59–63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kimura T, Sakata KI, Imamachi K and
Kitagawa Y: Osteoradionecrosis of the jaw suspicious of correlation
with bone-modifying agent treatment: A case report. Med Case Rep
Stud Protoc. 5(e00302)2024.
|
|
57
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Tori M and Shimo T: Long-term efficacy of
lenvatinib for recurrent papillary thyroid carcinoma after
multimodal treatment and management of complications: A case
report. BMC Cancer. 18(698)2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Yamazaki H, Sugino K, Matsuzu K, Masaki C,
Akaishi J, Hames K, Tomoda C, Suzuki A, Uruno T, Ohkuwa K, et al:
Rapid disease progression after discontinuation of lenvatinib in
thyroid cancer. Medicine (Baltimore). 99(e19408)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Toda S, Iwasaki H, Murayama D, Nakayama H,
Suganuma N and Masudo K: Invasive procedures in patients undergoing
treatment with lenvatinib for thyroid cancer. Mol Clin Oncol.
14(81)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Yamazaki H, Masudo K, Kanada S, Inayama Y,
Hayashi H, Fujii Y and Rino Y: Conversion surgery after lenvatinib
treatment for anaplastic thyroid carcinoma: A case report. Surg
Case Rep. 9(38)2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Dubbelman AC, Rosing H, Nijenhuis C,
Huitema AD, Mergui-Roelvink M, Gupta A, Verbel D, Thompson G,
Shumaker R, Schellens JH and Beijnen JH: Pharmacokinetics and
excretion of (14)C-lenvatinib in patients with advanced solid
tumors or lymphomas. Invest New Drugs. 33:233–240. 2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Resteghini C, Locati LD, Bossi P,
Bergamini C, Guzzo M and Licitra L: Do not throw the baby out with
the bathwater: SELECT a personalized, de-escalated lenvatinib
schedule allows response in locally advanced DTC while controlling
major drug-related bleeding. Ann Oncol. 28:2321–2322.
2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Tomlins SA, Hovelson DH, Suga JM, Anderson
DM, Koh HA, Dees EC, McNulty B, Burkard ME, Guarino M, Khatri J, et
al: Real-world performance of a comprehensive genomic profiling
test optimized for small tumor samples. JCO Precis Oncol 5:
PO.20.00472, 2021.
|
|
65
|
Tang W, Hanada K, Motoo Y, Sakamaki H, Oda
T, Furuta K, Abutani H, Ito S and Tsutani K: Budget impact analysis
of comprehensive genomic profiling for untreated advanced or
recurrent solid cancers in Japan. J Med Econ. 26:614–626.
2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Gamboa O, Bonilla CE, Quitian D, Torres
GF, Buitrago G and Cardona AF: Cost-effectiveness of comprehensive
genomic profiling in patients with non-small cell lung cancer for
the colombian health system. Value Health Reg Issues. 39:115–125.
2024.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Subbiah V, Hu MI, Wirth LJ, Schuler M,
Mansfield AS, Curigliano G, Brose MS, Zhu VW, Leboulleux S, Bowles
DW, et al: Pralsetinib for patients with advanced or metastatic
RET-altered thyroid cancer (ARROW): A multi-cohort, open-label,
registrational, phase 1/2 study. Lancet Diabetes Endocrinol.
9:491–501. 2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wirth LJ, Brose MS, Subbiah V, Worden F,
Solomon B, Robinson B, Hadoux J, Tomasini P, Weiler D,
Deschler-Baier B, et al: Durability of response with selpercatinib
in patients with RET-activated thyroid cancer: Long-term safety and
efficacy from LIBRETTO-001. J Clin Oncol. 42:3187–3195.
2024.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Andreassen PR, Seo J, Wiek C and Hanenberg
H: Understanding BRCA2 function as a tumor suppressor based on
domain-specific activities in DNA damage responses. Genes (Basel).
12(1034)2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Wei Q, Gan C, Sun M, Xie Y, Liu H, Xue T,
Deng C, Mo C and Ye T: BRD4: An effective target for organ
fibrosis. Biomark Res. 12(92)2024.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Ichijo R, Kobayashi H, Yoneda S, Iizuka Y,
Kubo H, Matsumura S, Kitano S, Miyachi H, Honda T and Toyoshima F:
Tbx3-dependent amplifying stem cell progeny drives interfollicular
epidermal expansion during pregnancy and regeneration. Nat Commun.
8(508)2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Kaplan J, Lee ZH, Grome L, Yao CMKL,
Mericli AF, Roubaud MS, Largo RD and Garvey PB: Sensory outcomes
for inferior alveolar nerve reconstruction with allograft following
free fibula mandible reconstruction. Plast Reconstr Surg.
152:499e–506e. 2023.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Takeuchi N, Sawada N, Ekuni D and Morita
M: Oral factors as predictors of frailty in community-dwelling
older people: A prospective cohort study. Int J Environ Res Public
Health. 19(1145)2022.PubMed/NCBI View Article : Google Scholar
|
|
74
|
van der Bilt A, Engelen L, Pereira LJ, van
der Glas HW and Abbink JH: Oral physiology and mastication. Physiol
Behav. 89:22–27. 2006.PubMed/NCBI View Article : Google Scholar
|