Introduction
Diurnal variation, which is defined as the
fluctuation of physiological parameters over a 24-h cycle, is a
fundamental aspect of human physiology and has important
implications in various medical fields, particularly in respiratory
medicine. In the context of pulmonary function testing, diurnal
variation is prominently observed in key metrics, such as forced
vital capacity (FVC), forced expiratory volume in 1 sec
(FEV1) and peak expiratory flow (PEF) (1,2).
These parameters are essential for assessing lung function,
diagnosing respiratory conditions, and monitoring disease
progression. Understanding the diurnal patterns of these metrics in
both healthy individuals and patients with chronic obstructive
pulmonary disease (COPD) is critical for optimizing clinical
assessments and therapeutic strategies such as optimizing the dose
and timing of bronchodilator therapy (for example, administering
long-acting bronchodilators in the morning for better symptom
control), and tailoring spirometry interpretation based on
time-of-day fluctuations (3-8).
In healthy individuals, the diurnal variation of
FVC, FEV1, and PEF typically follows a predictable and
consistent pattern (9,10). Studies have shown that these
parameters are often at their highest in the morning, staying
rather stable throughout the day to reach lower values in the early
evening before declining further overnight. This fluctuation is
primarily influenced by circadian rhythms, which govern various
physiological processes, including hormonal release, airway
dynamics and lung volume changes due to postural effects and
physical activity (10,11). For instance, increased airway
resistance is commonly observed at night, likely due to elevated
vagal tone and increased bronchial reactivity during sleep. As the
day progresses and physical activity increases, improvements in
lung volumes and reduced airway resistance contribute to enhanced
pulmonary function (8).
Conversely, the diurnal variation of pulmonary
function parameters in patients with COPD is often more complex and
less predictable. COPD, which is characterized by chronic
inflammation, airway remodeling and mucus hypersecretion, can
notably alter the normal diurnal patterns compared with those
observed in healthy individuals (12,13).
While some patients with COPD may exhibit a diurnal variation
similar to that of healthy individuals, a number of patients
experience more pronounced fluctuations in lung function. Factors
such as increased airway resistance, compromised lung compliance
and the presence of comorbidities, including cardiovascular
disease, obstructive sleep apnea and metabolic syndrome, can
exacerbate these diurnal variations. For instance, patients with
COPD may experience heightened symptoms, such as dyspnea and
wheezing, during periods of increased bronchial reactivity, which
are often associated with environmental triggers, such as
allergens, pollution or changes in weather (11-14).
Several studies have documented the diurnal
variation of FVC, FEV1 and PEF in both healthy
individuals and patients with COPD, revealing important insights
into how these populations respond differently to time-of-day
effects (7,8-16).
In healthy individuals, the predictable rise in lung function
throughout the day aligns with physiological expectations related
to increased activity and diminished airway resistance. However, in
patients with COPD, the diurnal pattern may be disrupted,
reflecting the underlying pathophysiology of the disease (12,13).
This disruption can lead to marked declines in lung function during
specific times of the day, particularly in the morning, which may
exacerbate the management challenges faced by healthcare providers
(14).
The diurnal variation of FVC, FEV1 and
PEF represents a critical aspect of pulmonary physiology that
warrants thorough investigation (10). The differences observed between
healthy individuals and patients with COPD highlight the complex
interplay of biological rhythms, disease mechanisms and
environmental influences on lung function (11). The present meta-analysis aims to
provide a detailed examination of these diurnal variations,
ultimately contributing to enhanced understanding and management of
respiratory health in diverse populations. It is anticipated that
the insights gained will not only enrich the comprehension of lung
function dynamics but also pave the way for improved clinical
practices that address the unique needs of patients with COPD and
other respiratory disorders.
Materials and methods
Search strategy
A systematic search was conducted in three main
databases [Pubmed (https://pubmed.ncbi.nlm.nih.gov/), Cochrane Library
(https://www.cochranelibrary.com/) and
Google Scholar (https://scholar.google.com)], with a time limit
between 1980 and 2024. The search was limited to studies published
from 1980 onwards to focus on spirometric measurements using
standardized modern techniques and guidelines, which were not
consistently applied in earlier literature. Gray literature was
also included in the search. The references of the included studies
were manually and individually searched to identify additional
relevant studies.
Regarding the search strategy, both free-text terms
and Medical Subject Headings terms were used. Boolean operators and
special filters were applied to maximize search efficiency. Special
filters included restriction to English-language studies, human
subjects and studies involving adult populations. The complete
search string used was as follows: (‘Diurnal’ OR ‘during the day’
OR ‘all day’ OR ‘24 h’ OR ‘daily’ OR ‘every day’) AND
(‘measurement’ OR ‘assessment’ OR ‘score’ OR ‘evaluation’ OR
‘quantification’ OR ‘estimation’ OR ‘gauge’ OR ‘calculation’) AND
(‘FVC’ OR ‘forced vital capacity’) AND (‘FEV1’ OR
‘forced expiratory volume in 1 sec’) AND ‘spirometry’ AND (‘healthy
adults’ OR ‘health’ OR ‘normal’) AND (‘COPD’ OR ‘chronic’ OR
‘bronchitis’ OR ‘emphysema’ OR ‘airflow limitation disorder’ OR
‘chronic lung disease’ OR ‘pulmonary obstructive disease’).
Inclusion and exclusion criteria
The present review and meta-analysis included
studies with populations consisting of either healthy individuals
or patients diagnosed with COPD, irrespective of age, disease
severity, therapeutic regimen or sex. The diagnosis of COPD was
established based on the Global Initiative for Chronic Obstructive
Lung Disease (GOLD) guidelines (https://goldcopd.org/). The primary outcome of
interest was the diurnal fluctuation of various spirometric
parameters, including FVC and FEV1. Spirometric
measurements were conducted both in the morning and at nighttime,
and the results were compared.
The present study encompassed observational studies,
randomized controlled trials (RCTs), quasi-randomized trials,
pre-post studies and historical control studies. Letters to the
editor, editorials, comments, case reports and case series were
excluded. Additionally, studies involving healthy individuals
exposed to occupational hazards, patients with overlapping
syndromes such as obstructive sleep apnea, morbidly obese
individuals, patients with neuromuscular diseases and smokers were
excluded. Non-human studies were also not considered.
Preferred Reporting Items for
Systematic Reviews and Meta-Analyses (PRISMA) process
The present study was conducted according to the
PRISMA guidelines (17). The
current systematic review has been registered in the International
Prospective Register of Systematic Reviews (PROSPERO ID no.
CRD420250654168).
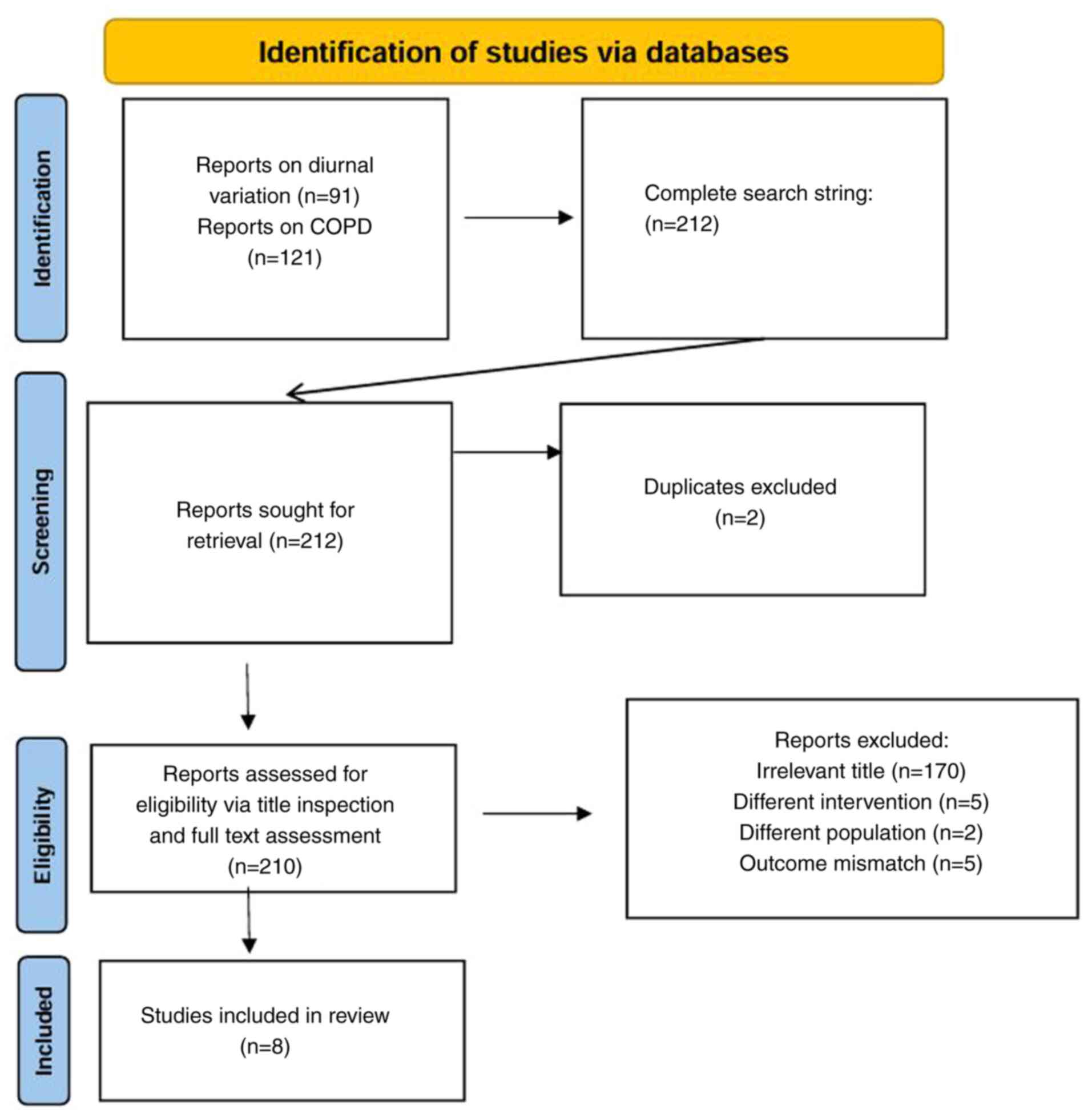
The database search for spirometric diurnal
variation in healthy subjects yielded 91 studies, while the search
for COPD-related studies resulted in 121 studies. Combining both
searches yielded a total of 212 studies. Following duplicate
removal (n=2), 210 studies were screened based on their titles,
with 170 studies excluded due to irrelevance. The remaining 40
studies underwent full-text evaluation, leading to the exclusion of
32 studies due to differences in intervention (such as timing or
protocol of spirometric measurements that did not compare morning
and nighttime values) (n=5), population (n=22) or outcome mismatch
(n=5). Ultimately, eight studies met the inclusion criteria and
were incorporated into the meta-analysis. The flow diagram
illustrating the search strategy is presented in Fig. 1.
Data extraction
Data extraction was independently conducted by two
authors (KD and VΕG) using a data extraction sheet that contained
all the following information about each included study: Study
characteristics, author, year, location, sample size, population
demographics, intervention details, outcome measures, results
effect sizes and confidence intervals (CIs). Any possible
discrepancies were resolved through constructive dialogue. The
information included in the extraction sheets was computed in a
local database in Microsoft Excel format allowing easy retrieval
and analysis during the synthesis phase of the present study.
Quality assessment and risk of
bias
To evaluate the quality of the included studies, the
Newcastle-Ottawa Scale for non-randomized observational studies
(18) was used, which assesses
study quality based on three domains: Selection of study
participants, comparability of groups and outcome assessment. This
tool provides a structured approach to evaluating potential sources
of bias and methodological rigor.
For RCTs, the Critical Appraisal Skills Program
(CASP) checklist was applied (19), which systematically assesses key
methodological aspects. The checklist examines whether the
randomization process was adequately generated and concealed to
prevent selection bias. It evaluates the extent to which blinding
was implemented for participants, investigators and outcome
assessors. The comparability of groups at baseline is assessed to
ensure balanced characteristics between intervention and control
groups. Follow-up duration and completeness are reviewed to
determine whether attrition was accounted for appropriately. The
checklist also considers whether outcomes were clearly defined,
measured using valid methods and analyzed appropriately. Finally,
the checklist assesses the applicability of results to the target
population and the feasibility of the intervention in clinical
practice.
The overall quality score of each study, based on
the aforementioned assessment tools, is presented in Tables I and II. Based on the Newcastle-Ottawa Scale,
the observational studies were rated as moderate-to-high quality
(scores ranging from 6 to 8). The RCTs were evaluated using the
CASP checklist and were rated as high quality.
 | Table IBasic characteristics of the included
studies with a population or sub-population of healthy
individuals. |
Table I
Basic characteristics of the included
studies with a population or sub-population of healthy
individuals.
| First author,
year | Population, n | Morning mean value
(FVC/FEV1), l | Morning SD
value | Night mean value
(FVC/FEV1), l | Night SD value | Quality
assessment | (Refs.) |
|---|
| Teramoto et
al, 1999 | 120 | 4.50/4.00 | 0.12/0.01 | 4.40/3.85 | 0.12/0.01 | NOS 6 | (8) |
| Zhang et al,
2024 | 35 | 3.85/3.15 | 0.02/0.05 | 3.75/3.10 | 0.02/0.05 | NOS 7 | (9) |
| Zhang et al,
2022 | 36 | 3.83/3.14 | 0.79/0.70 | 3.77/3.10 | 0.90/0.80 | NOS 7 | (10) |
| Borsboom et
al, 1999 | 404 | 4.30/3.29 | 0.10/0.01 | 4.10/3.20 | 0.10/0.01 | NOS 6 | (11) |
| Goyal et al,
2019 | 130 | -/3.20 | -/1.00 | -/3.00 | -/0.90 | NOS 8 | (14) |
 | Table IIBasic characteristics of the included
studies with a population or sub-population of patients with
chronic obstructive pulmonary disease. |
Table II
Basic characteristics of the included
studies with a population or sub-population of patients with
chronic obstructive pulmonary disease.
| First author,
year | Population, n | Morning mean value
(FVC/FEV1), l | Morning SD
value | Night mean value
(FVC/FEV1), l | Night SD value | Quality
assessment | (Refs.) |
|---|
| Fregonezi et
al, 2012 | 7 | 2.50/1.24 | 1.00/0.62 | 1.99/0.96 | 1.00/0.50 | NOS 8 | (1) |
| Teramoto et
al, 1999 | 30 | 1.90/1.34 | 0.60/0.30 | 1.60/1.10 | 0.30/0.05 | CASP RCT high
quality | (8) |
| Martin et
al, 1992 | 14 | 2.66/1.18 | 0.21/0.13 | 2.31/1.21 | 0.19/0.14 | CASP RCT high
quality | (12) |
| Calverley et
al, 2003 | 121 | 2.12/1.11 | 0.04/0.03 | 2.07/1.06 | 0.04/0.03 | CASP RCT high
quality | (13) |
Population, Intervention, Comparison
and Outcome (PICO) framework
The present study followed the PICO framework
(20) to structure the research
question. The population includes healthy individuals and patients
diagnosed with COPD according to the GOLD criteria. The
intervention involves measuring FVC and FEV1 at two
different time points, in the morning and at nighttime. The
comparators include both healthy individuals and patients with
COPD, allowing for intra-group and inter-group comparisons. The
outcome focuses on identifying potential differences in the
measured spirometric parameters between the different time points
and populations.
Statistical analysis
Meta-analysis was conducted using the Meta-Mar
platform (version 2.1; https://www.meta-mar.com). Mean diurnal differences in
FVC and FEV1 levels were measured in both groups
(healthy individuals and patients with COPD) using the
random-effects model. A heterogeneity assessment was also conducted
using the I2 statistics to quantify any possible total
variation due to heterogeneity. I² values were interpreted as
follows: 0-25% (low heterogeneity), 26-50% (moderate
heterogeneity), 51-75% (substantial heterogeneity) and >75%
(considerable heterogeneity). Lastly, the interpretation of the
results included not only the mean differences of the
aforementioned spirometric values in the two groups but also the
possible clinical significance of the observed differences.
Publication bias was assessed using Egger's test for funnel plot
asymmetry. P<0.05 was considered to indicate a statistically
significant difference.
Results
Overview of the included studies
The present meta-analysis synthesized data from
eight different studies (1,8-14)
that measured the diurnal variation of FVC and/or FEV1
using healthy individuals or patients with COPD as the target
population. Subgroup analyses comparing the FVC and FEV1
spirometric measurements between healthy adults and patients with
COPD were conducted. Healthy adults were defined as subgroup 1 and
patients with COPD were defined as subgroup 2. In total, 595 for
FVC (or 725 for FEV1) healthy adults and 172 patients
with COPD were analyzed.
Description of the included
studies
The basic characteristics of the included studies
with a population or sub-population of healthy individuals are
presented in Table I, while those
of patients with COPD are presented in Table II.
Among the studies examining healthy individuals,
Teramoto et al (8)
evaluated diurnal variation in both healthy elderly subjects and
patients with respiratory conditions including COPD, performing
spirometric measurements at multiple time points across the day.
Their findings demonstrated that diurnal fluctuations in FVC and
FEV1 were more pronounced in healthy subjects. Zhang
et al (10) and Zhang et
al (9) conducted studies using
electronic portable spirometers for home-based, multi-day
monitoring in healthy non-smoking adults. Both studies reported
consistent circadian patterns, with peak spirometric values
observed in the morning and progressive declines during the night.
Borsboom et al (11)
investigated diurnal lung function in two Dutch cohorts,
emphasizing the impact of the time of measurement on longitudinal
lung function analysis and supporting the concept of marked
morning-to-evening variability in healthy individuals. Goyal et
al (14) investigated the
circadian variability of airway caliber in healthy young male
adults by assessing spirometric indices, including FEV1
and mid-expiratory flow rates, across seven time points from early
morning (5:00 a.m.) to late evening (23:00 p.m.). Using Cosinor
rhythm analysis, they demonstrated marked temporal variability in
spirometric values, with peak values generally occurring during
daytime and troughs at night. Although only 31% of subjects
exhibited statistically significant circadian rhythms in
FEV1, variability patterns suggested marked
inter-individual differences (chronophenotypes).
The COPD-related studies included that by Fregonezi
et al (1), who measured
both pulmonary function and respiratory muscle strength across time
intervals in patients with stable COPD. The study found that
diurnal variation existed in both FVC and respiratory muscle
strength parameters (such as maximal inspiratory and expiratory
pressures), although the magnitude of change was smaller compared
with that in healthy individuals. Teramoto et al (8) also examined patients with COPD in the
same dataset as aforementioned and confirmed the reduced amplitude
of spirometric fluctuations in these patients. Martin and Pak
(12) observed slight improvements
in nighttime FEV1 and symptom scores in patients
receiving overnight theophylline, possibly reflecting a
pharmacological effect compared with baseline morning values.
Lastly, Calverley et al (13) found that tiotropium bromide
improved overall airflow in patients with COPD; however, it did not
significantly alter the diurnal variation in FVC or
FEV1, suggesting that bronchodilator therapy alone may
not restore normal circadian lung function patterns.
Forest plots
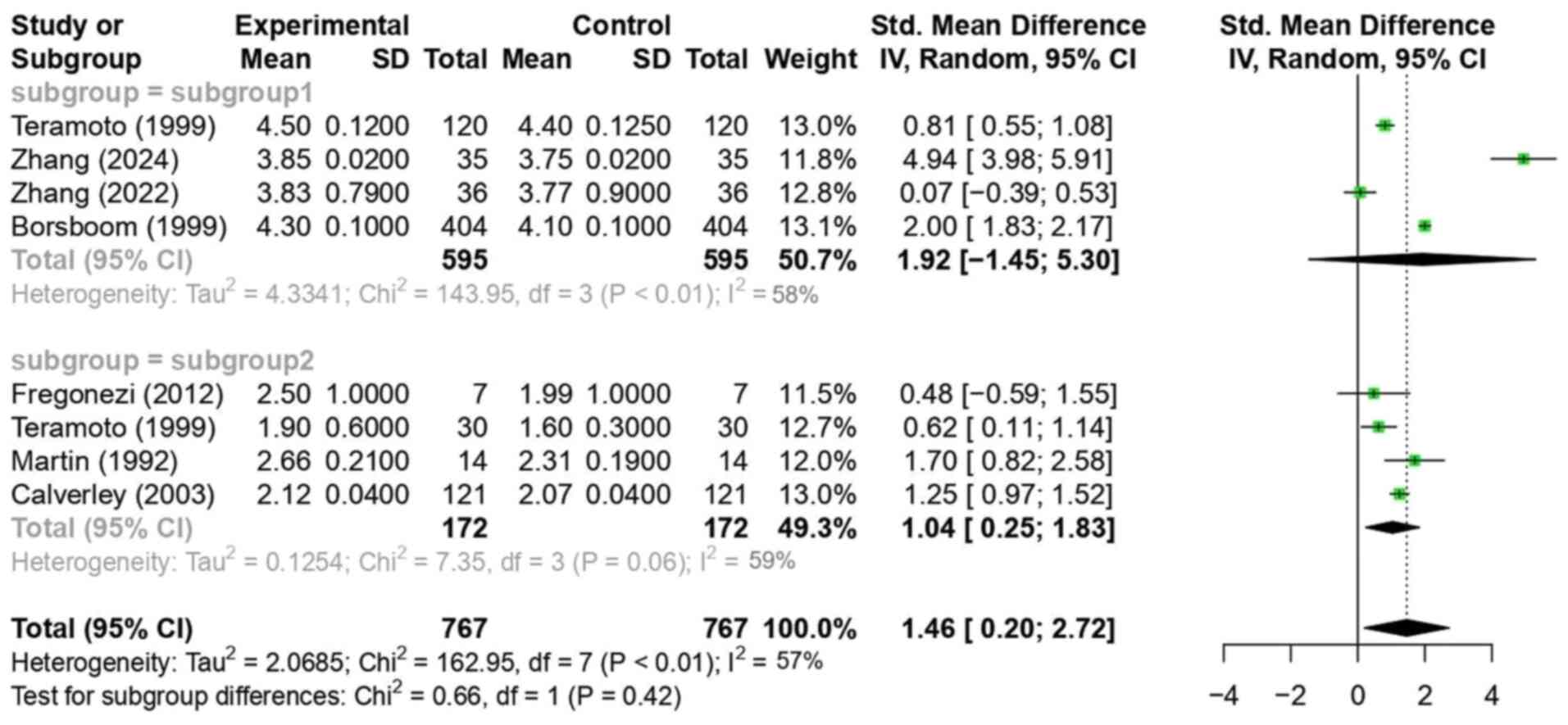
In Fig. 2, the mean
differences in diurnal FVC between healthy individuals and patients
with COPD were synthesized and analyzed using a random-effects
model. The goal was to compare the observed mean differences
between the two subgroups.
Several studies, including those by Teramoto et
al (8), Zhang et al
(9), Zhang et al (10) and Borsboom et al (11), reported higher FVC values in
healthy adults during the morning compared with those at nighttime.
In subgroup 1 (healthy individuals), a statistically significant
mean difference in diurnal FVC (morning FVC-nighttime FVC) of 1.92
(CI, -1.45, 5.30; P<0.01) was observed. However, the CI was
relatively large, including zero. Although the P-value indicated
statistical significance (P<0.01), the wide confidence interval
includes zero, suggesting uncertainty about the direction or
magnitude of the effect. Therefore, the result should be
interpreted with caution. The heterogeneity, assessed using the I²
statistic, was 58%, indicating substantial variability among the
studies. Sources of heterogeneity included differences in study
location (for example, Japan, Netherlands and Brazil), participant
characteristics (for example, the mean age ranged from 19 to 70
years and there were male-only vs. mixed-gender cohorts), timing of
spirometric measurements (certain studies used two while others
used seven time points) and types of devices (such as portable
electronic spirometers vs. standard clinical spirometers).
In subgroup 2 (patients with COPD), Fregonezi et
al (1), Teramoto et al
(8) and Martin and Pak (12) reported higher morning FVC values
compared with those at nighttime. By contrast, Calverley et
al (13) observed the opposite
effect, with lower FVC values in the morning than at night.
Although the study by Calverley et al (13) reported nighttime FVC values
slightly higher than morning values, the overall effect size
direction in the forest plot aligns due to standardized mean
differences, which adjust for variation across studies. The overall
mean difference in subgroup 2 was 1.04 (CI, 0.25, 1.83; P=0.06)
with a large CI value excluding zero, which was not statistically
significant but approached the threshold of 0.05. In subgroup 2
(patients with COPD), heterogeneity was 59%, indicating substantial
variability. This could reflect differences in disease severity,
bronchodilator use and measurement timing protocols among the
studies.
Comparing the mean differences between the two
subgroups, a difference of 1.46 (CI, 0.20, 2.72; P<0.01) was
observed, indicating a statistically significant difference in
diurnal FVC variation between healthy individuals and patients with
COPD. This suggests that the diurnal variation in FVC among healthy
individuals was 1.46 times greater than that observed in the COPD
group. The overall heterogeneity was 57%, again indicating
substantial variability.
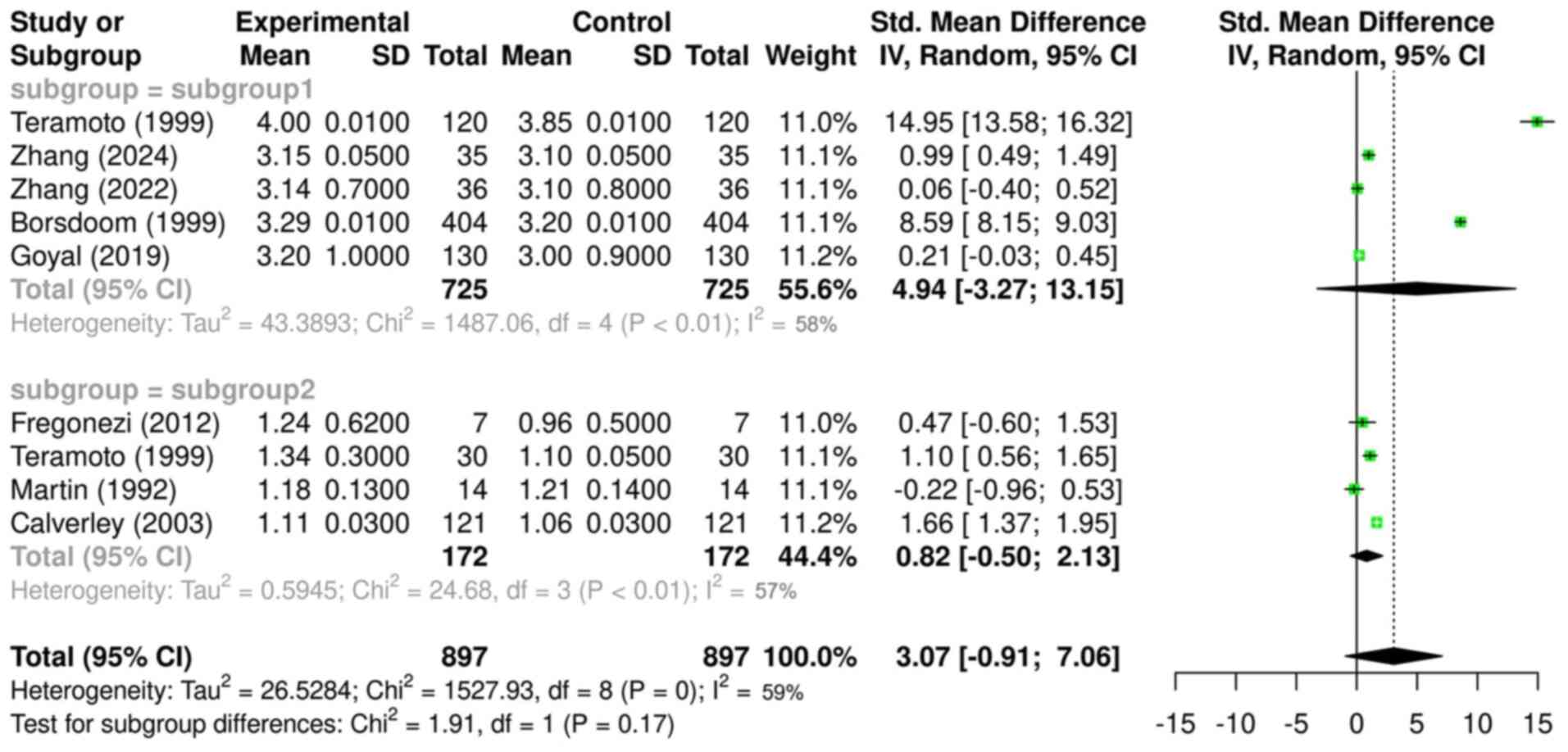
In Fig. 3, the mean
differences in diurnal FEV1 in healthy individuals and
patients with COPD were synthesized and analyzed, and the observed
mean differences between the two subgroups were compared using the
random-effects model.
While the analysis of diurnal FVC differences in
Fig. 2 included a total of 595
healthy individuals (from four studies), the analysis of diurnal
FEV1 differences in Fig.
3 included 725 healthy individuals due to the inclusion of an
additional eligible study (14),
bringing the total to five studies in subgroup 1.
In subgroup 1, all included studies reported higher
FEV1 values in the morning than at night, in line with
the aforementioned FVC measurements. The overall mean difference in
this subgroup was 4.94 (CI, -3.27, 13.15; P<0.01), with a large
CI that included zero, but demonstrated statistical significance.
Despite reaching statistical significance, the wide confidence
intervals that included zero suggest variability in the data and
reduce confidence in the precision of the effect estimate. This
result suggests that the mean difference in diurnal FEV1
values in subgroup 1 was 4.94 times higher in the morning compared
with that at nighttime. The heterogeneity was 58%, indicating a
relatively high variability in the included studies, which can be
attributed to the aforementioned factors.
Regarding subgroup 2, all included studies reported
higher morning FEV1 values except for the study by
Martin and Pak (12).
Nevertheless, the overall mean difference was 0.82 (CI, -0.5, 2.13;
P<0.01), with a large CI that included zero, and demonstrated
statistical significance. Based on this, it can be inferred that
the mean FEV1 in the morning in patients with COPD was
0.82 times higher than that at night.
The comparison of the mean differences between the
two subgroups yielded a value of 3.07 (CI, -0.91, 7.06; P=0.17),
indicating that the mean diurnal FEV1 difference in
subgroup 1 was 3.07 times greater than that in subgroup 2, although
this result was not statistically significant. The overall
heterogeneity was 59%, which can be attributed to the
aforementioned factors.
Finally, Egger's test yielded a regression
coefficient of -1.7 (P=0.17), indicating no statistically
significant evidence of publication bias. A slight asymmetry in the
funnel plot was observed (data not shown), possibly indicating
underrepresentation of smaller studies (for example, those with
sample sizes <30) that reported null or non-significant results.
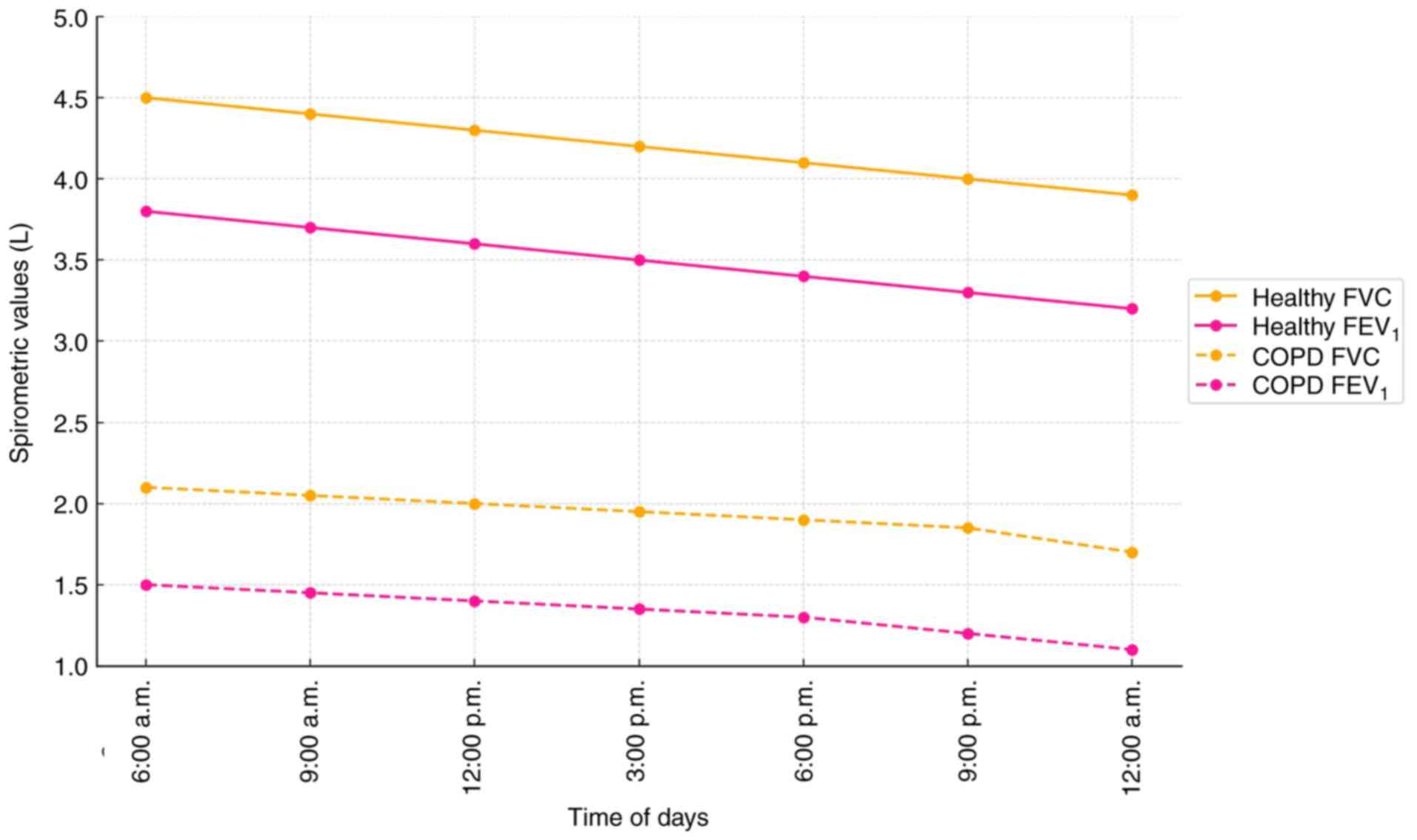
Fig. 4 graphically illustrates the
pooled diurnal trends in FVC and FEV1 values across time
points from 6:00 a.m. to 12:00 a.m. Healthy individuals show a
steeper decline in values over the day, while patients with COPD
exhibit flatter curves, reflecting blunted diurnal variation. The
steeper slope in the healthy group suggests greater diurnal
variation, whereas the relatively flat trend in the COPD group
indicates blunted respiratory fluctuations, likely due to airway
inflammation, autonomic dysregulation and reduced lung
compliance.
Discussion
The present study aimed to synthesize and critically
assess the existing literature on diurnal variations in FVC and
FEV1 measurements in healthy adults and patients with
COPD, with the goal of drawing conclusions that could be useful in
daily clinical practice and guide therapeutic decisions and
strategies.
The present findings indicated a statistically
significant difference in mean diurnal FVC measurements in healthy
adults, with morning values being 1.92 times higher than nighttime
values. A similar trend was observed in patients with COPD, where
morning FVC was 1.04 times higher than that at night; however, this
result was not statistically significant. Additionally, the mean
difference in diurnal FVC variations between healthy individuals
and patients with COPD was 1.46 times higher in healthy adults,
demonstrating statistical significance.
Similar results were observed for FEV1.
The mean FEV1 values in healthy adults were 4.94 times
higher in the morning than those at night. A similar pattern was
noted in patients with COPD, although the difference was smaller
(0.82 times). However, there was no statistically significant
difference in the mean diurnal FEV1 variations between
healthy individuals and patients with COPD.
The results of the present meta-analysis indicate
that mean diurnal differences in FVC and FEV1 are
significantly greater in healthy adults compared with individuals
diagnosed with COPD. This finding aligns with established knowledge
on COPD pathophysiology, emphasizing the impaired lung function
that characterizes the disease.
In healthy individuals, diurnal variations in FVC
and FEV1 are typically influenced by circadian rhythms,
respiratory muscle activity and fluctuations in airway resistance
(1,8,9,14,16).
These fluctuations are generally mild and reflect the ability of
the lungs to adapt to daily activities and environmental changes.
By contrast, patients with COPD experience diminished pulmonary
function due to structural airway changes and alveolar damage,
which limit their ability to maintain optimal airflow and lung
volumes throughout the day. In healthy adults, total airway
resistance (Raw) typically ranges from 1.5-2.5
cmH2O/l/sec, whereas in patients with moderate-to-severe
COPD, Raw can exceed 5.0 cmH2O/l/sec (1,14,16).
The reduced diurnal variability observed in patients
with COPD may be attributed to several underlying mechanisms
(2,3,6).
Chronic airway inflammation and remodeling, along with the
destruction of alveolar structures, contribute to fixed airflow
limitations, notably affecting respiratory mechanics. As a result,
diurnal fluctuations in FVC and FEV1 are markedly
diminished, reflecting a more static and compromised pulmonary
function (21).
Patients with COPD often experience autonomic
dysfunction, marked by increased sympathetic activity and reduced
parasympathetic tone. Factors such as chronic hypoxemia,
hypercapnia, systemic inflammation and the use of certain
medications, such as long-acting β2-agonists (for example,
salmeterol) and anticholinergics (for example, tiotropium)
contribute to this imbalance. This dysregulation affects the airway
smooth muscle tone and heart rate variability, leading to a blunted
diurnal variation in respiratory function (22). Structural changes in the lungs,
including emphysematous destruction and airway remodeling, lead to
decreased lung compliance in patients with COPD. This rigidity also
impairs the ability of the lungs to adapt to physiological changes
throughout the day (23).
Cortisol, a glucocorticoid hormone regulated by the
hypothalamic-pituitary-adrenal axis, exhibits a natural diurnal
rhythm, peaking in the early morning and declining throughout the
day. In patients with COPD, altered cortisol levels and adrenal
gland sizes have been observed, which are closely associated with
disease severity. Compared with healthy individuals, patients with
COPD exhibit lower morning serum cortisol levels and smaller
adrenal gland volumes, particularly in those with frequent
exacerbations, reflecting impaired hypothalamic-pituitary-adrenal
axis activity. These hormonal imbalances may contribute to the
reduced diurnal variation in lung function observed in these
patients (24,25).
The current findings have clinical relevance. The
reduced diurnal variation in FVC and FEV1 among patients
with COPD may affect their ability to perform daily activities and
could serve as an indicator of the disease exacerbation risk.
Recognizing these patterns can help healthcare providers to develop
personalized management strategies that account for lung function
variability, potentially leading to improved patient outcomes
through timely interventions.
Monitoring diurnal patterns in lung function may be
particularly important for patients with COPD, who may benefit from
personalized treatment regimens (7,16).
For instance, pharmacotherapeutic strategies, such as
bronchodilator administration, may need to be adjusted based on the
time of day to optimize efficacy and symptom control. Additionally,
lifestyle modifications, including smoking cessation and pulmonary
rehabilitation, play a crucial role in enhancing lung function and
increasing diurnal variability (2-4,15).
Emerging evidence has highlighted the potential of
longitudinal home spirometry as a tool for daily monitoring in
chronic lung diseases, including COPD and fibrotic interstitial
lung disease. Notably, the feasibility and reliability of portable
electronic spirometers, which allow patients to perform
self-assessments in their home environments over extended periods,
has been demonstrated. These devices have been used successfully to
detect early declines in lung function, guide therapeutic
adjustments, and assess treatment efficacy in real time (2). For example, Moor et al
(2) used home spirometry to detect
progressive FVC decline in patients with fibrotic interstitial lung
disease, which prompted timely therapeutic adjustments, including
corticosteroid tapering or initiation of antifibrotic therapy. Lung
function decline was assessed via consistent downward trends in
daily FVC values, confirmed by clinical reassessment. Similar
approaches could be applied to COPD populations to identify early
exacerbation risk. However, broader implementation requires
addressing challenges such as patient adherence, data integration
with electronic health records and standardization of measurement
protocols. Incorporating such digital tools into clinical practice
may revolutionize disease monitoring by providing continuous,
patient-specific data, ultimately facilitating personalized and
timely interventions.
The clinical importance of recognizing diurnal
variations in spirometry extends beyond academic interest, as it
has direct implications for COPD management. Awareness of daily
lung function fluctuations could influence the optimal timing of
diagnostic spirometry to avoid underestimating disease severity
during nighttime or early morning periods when pulmonary function
is at its lowest level. Moreover, time-specific tailoring of
bronchodilator therapy could improve symptom control by
synchronizing drug administration with periods of greatest airflow
limitation. Personalized scheduling of rehabilitation exercises and
daily activities may also reduce dyspnea episodes and improve the
quality of life. Importantly, longitudinal home spirometry
monitoring could detect early signs of exacerbations by identifying
deviations from the typical diurnal pattern of the patient,
allowing pre-emptive interventions. Integrating diurnal variation
assessment into routine COPD care protocols could thus enhance
disease control, reduce healthcare use, and promote precision
medicine approaches in respiratory care.
The present study exhibited moderate-to-high
heterogeneity (I²=57-59%) due to differences in study design,
population characteristics and measurement techniques, which may
limit generalizability. The included studies exhibited substantial
heterogeneity in design, population characteristics and spirometry
measurement methods, leading to moderate-to-high variability
(I²=57-59%). These differences included variation in participant
age (ranging from young adults to elderly populations), measurement
timing (two vs. seven daily time points) and spirometry equipment
(portable vs. clinical devices), which may have influenced the
observed heterogeneity. The sample size was relatively small [595
for FVC (or 725 for FEV1) healthy individuals and 172
patients with COPD], potentially limiting the generalizability of
the findings. Additionally, the marked disparity in sample sizes
between healthy individuals and patients with COPD (595/725 vs.
172) may have introduced bias into the pooled estimates and reduced
the comparative statistical power between groups. Moreover, the
exclusion of individuals with overlapping syndromes, smokers and
those exposed to occupational hazards restricts the applicability
of the results to broader populations with COPD. Furthermore, the
fact that the included patients with COPD were not stratified
according to their disease severity could affect the validity of
the observed results. Stratification is vital for external
validity, as clinicians often rely on disease staging to guide
treatment (21). By not accounting
for disease severity, the present meta-analysis limits its
applicability to real-world patient populations. Future research
should prioritize stratified analyses based on standardized
classifications, such as the GOLD criteria, to enhance the
precision, interpretability and clinical utility of the findings in
COPD research. Most included studies did not provide FVC and
FEV1 values stratified by COPD severity (GOLD stage
I-IV), preventing subgroup meta-analysis based on disease stage.
Environmental and lifestyle factors such as air pollution,
temperature, medication adherence and physical activity were not
consistently accounted for across studies, potentially affecting
lung function variability. Lastly, although not statistically
significant, the potential for publication bias suggests that
studies with small sample sizes and non-significant findings may be
underrepresented in the literature.
In conclusion, the present meta-analysis revealed
that diurnal variations in FVC and FEV1 were greater in
healthy individuals than those in patients with COPD. This may be
attributed to the airway inflammation, autonomic dysregulation and
reduced lung compliance observed in patients with COPD. Altered
cortisol rhythms may further contribute to blunted fluctuations.
These findings underscore the need for optimized spirometry timing
and tailored treatment strategies to improve COPD management.
Future research should focus on personalized therapy adjustments
and home-based lung function monitoring to enhance patient
outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KD and VEG conceptualized the study. KD, DK, TVK,
AM, NT, AC, VEG and DAS made a substantial contribution to data
interpretation and analysis and wrote and prepared the draft of the
manuscript. KD and VEG analyzed the data and performed critical
revisions. KD and VEG confirm the authenticity of all the raw data.
All authors contributed to manuscript revision and read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tool Chat GPT was used to improve the readability and
language of the manuscript, and subsequently, the authors revised
and edited the content produced by the artificial intelligence tool
as necessary, taking full responsibility for the ultimate content
of the present manuscript.
References
|
1
|
Fregonezi G, Resqueti VR, Cury JL, Paulin
E and Brunetto AF: Diurnal variations in the parameters of
pulmonary function and respiratory muscle strength in patients with
COPD. J Bras Pneumol. 38:257–263. 2012.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
2
|
Moor CC, van den Berg CAL, Visser LS,
Aerts JGJV, Cottin V and Wijsenbeek MS: Diurnal variation in forced
vital capacity in patients with fibrotic interstitial lung disease
using home spirometry. ERJ Open Res. 6:00054–2020. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kunos L, Lazar Z, Martinovszky F, Tarnoki
AD, Tarnoki DL, Kovacs D, Forgo B, Horvath P, Losonczy G and Bikov
A: Overnight changes in lung function of obese patients with
obstructive sleep apnoea. Lung. 195:127–133. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mathur T, Annepu YR, Chaitanya PDK, Ranjan
R, Verma DK, Verma N, Pandey S and Singh R: Correlation between
salivary cortisol levels and diurnal variation in spirometric
parameters in apparently healthy adults. Cureus.
16(e71493)2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mahajan KK, Mahajan SK and Mishra N:
Diurnal variations in lung transfer factor and its components.
Indian J Physiol Pharmacol. 34:209–211. 1990.PubMed/NCBI
|
|
6
|
Chan-Thim E, Dumont M, Moullec G, Rizk AK,
Wardini R, Trutschnigg B, Paquet J, de Lorimier M, Parenteau S and
Pepin V: Clinical impact of time of day on acute exercise response
in COPD. COPD. 11:204–211. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Panda A and McHardy G: Diurnal variation
in pulmonary diffusing capacity and expiratory volumes. Indian J
Physiol Pharmacol. 24:112–118. 1980.PubMed/NCBI
|
|
8
|
Teramoto S, Suzuki M, Matsui H, Ishii T,
Matsuse T and Ouchi Y: Influence of age on diurnal variability in
measurements of spirometric indices and respiratory pressures. J
Asthma. 36:487–492. 1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang Y, Wu Y, Zhang X, Lv C, Lin J, Zhao
L, Lin Y, Zhang M and Bao W: Circadian rhythm and variability of
large and small airway spirometric variables in healthy
individuals. Digit Health. 10(20552076241254698)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang X, Zhang Y, Zhou Y, Yin D, Lv C, Lin
J, Bao W and Zhang M: Age-related circadian rhythm and variability
of large- and small-airway function in healthy non-smoking adults:
Data from 7-day diurnal and nocturnal home monitoring using an
electronic portable spirometer. Front Public Health.
10(946988)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Borsboom GJ, van Pelt W, van Houwelingen
HC, van Vianen BG, Schouten JP and Quanjer PH: Diurnal variation in
lung function in subgroups from two Dutch populations: Consequences
for longitudinal analysis. Am J Respir Crit Care Med. 159 (4 Pt
1):1163–1171. 1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Martin RJ and Pak J: Overnight
theophylline concentrations and effects on sleep and lung function
in chronic obstructive pulmonary disease. Am Rev Respir Dis.
145:540–544. 1992.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Calverley PM, Lee A, Towse L, van Noord J,
Witek TJ and Kelsen S: Effect of tiotropium bromide on circadian
variation in airflow limitation in chronic obstructive pulmonary
disease. Thorax. 58:855–860. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Goyal M, Goel A, Bhattacharya S, Verma N
and Tiwari S: Circadian variability in airways characteristics: A
spirometric study. Chronobiol Int. 36:1550–1557. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim JS, Azarbarzin A, Podolanczuk AJ,
Anderson MR, Cade BE, Kawut SM, Wysoczanski A, Laine AF, Hoffman
EA, Gottlieb DJ, et al: Obstructive Sleep Apnea and Longitudinal
Changes in Interstitial Lung Imaging and Lung Function: The MESA
Study. Ann Am Thorac Soc. 20:728–737. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
McCarley C, Hanneman SK, Padhye N and
Smolensky MH: A pilot home study of temporal variations of symptoms
in chronic obstructive lung disease. Biol Res Nurs. 9:8–20.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for Assessing the Quality of Nonrandomized Studies in
Meta-Analysis. Ottawa Hospital Research Institute, Ottawa, Ontario,
2021.
|
|
19
|
Long HA, French DP and Brooks JM:
Optimising the value of the critical appraisal skills programme
(CASP) tool for quality appraisal in qualitative evidence
synthesis. Res Meth Med Health Sci. 1:31–42. 2020.
|
|
20
|
Richardson WS, Wilson MC, Nishikawa J and
Hayward RS: The well-built clinical question: A key to
evidence-based decisions. ACP J Club. 123:A12–A13. 1995.PubMed/NCBI
|
|
21
|
Matera MG, Rinaldi B, Ambrosio C and
Cazzola M: Is it preferable to administer a bronchodilator once- or
twice-daily when treating COPD? Respir Med.
219(107439)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
James AL and Wenzel S: Clinical relevance
of airway remodelling in airway diseases. Eur Respir J. 30:134–155.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
van Gestel AJ and Steier J: Autonomic
dysfunction in patients with chronic obstructive pulmonary disease
(COPD). J Thorac Dis. 2:215–222. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
O'Donnell DE and Parker CM: COPD
exacerbations. 3: Pathophysiology. Thorax. 61:354–361.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wei P, Li Y, Wu L, Wu J, Wu W, Chen S, Qin
S and Feng J: Serum cortisol levels and adrenal gland size in
patients with chronic obstructive pulmonary disease. Am J Transl
Res. 13:8150–8157. 2021.PubMed/NCBI
|