Introduction
Pituitary abscesses are extremely rare in clinical
practice, accounting for ~1% of all pituitary lesions (1). However, as a central nervous system
infection, they pose a risk of disability and mortality (2). Pituitary abscesses are classified as
primary or secondary. Primary pituitary abscesses are more common,
accounting for ~67% of cases, and typically result from
hematogenous bacterial spread to an otherwise normal pituitary
gland. By contrast, secondary pituitary abscesses, which arise from
pre-existing sellar lesions such as pituitary adenomas, Rathke's
cleft cysts or craniopharyngiomas, represent 33% of cases (3). The pathogenetic mechanisms leading to
secondary pituitary abscesses include anatomical distortion,
impaired circulation and altered immune status, which predispose
patients to infection (4). The
clinical presentation of pituitary abscesses is variable, with
symptoms including hormonal imbalances, diabetes insipidus and
visual disturbances, often due to mass effects. When the pituitary
gland is infected, hypopituitarism may occur, resulting in
deficiencies in one or more pituitary hormones. This dysfunction
can cause severe endocrine disorders, such as diabetes insipidus
(due to antidiuretic hormone deficiency), hypothyroidism (due to
thyroid-stimulating hormone deficiency) and adrenal insufficiency
(due to adrenocorticotropic hormone deficiency) (5). These hormonal imbalances can lead to
systemic complications, including electrolyte disturbances,
metabolic abnormalities and cardiovascular instability, emphasizing
the potentially dangerous nature of pituitary abscesses (6). MRI findings typically reveal a cystic
lesion with ring enhancement, which is hyperintense on T2-weighted
images and hypointense on T1-weighted images. However, despite
advancements in imaging technology, the preoperative diagnosis of
pituitary abscesses remains challenging, particularly in cases
associated with pre-existing sellar lesions such as
craniopharyngiomas. Early surgical drainage combined with
antibiotic therapy remains the cornerstone of treatment, but
outcomes depend heavily on a timely diagnosis and the extent of the
underlying lesion. Delayed treatment may result in irreversible
hormonal deficiencies and increased morbidity (7).
The difficulty in diagnosing secondary pituitary
abscesses, particularly those associated with craniopharyngiomas,
necessitates a broader understanding of their pathophysiology and a
more systematic approach to diagnosis and management. Early
recognition and intervention are crucial for reducing the high
prevalence of pituitary dysfunction in patients treated for these
abscesses and for improving overall prognosis. The present case
report highlights the clinical features and management of a
secondary pituitary abscess arising from a craniopharyngioma and
provides a literature review of similar cases.
Case report
A 59-year-old man presented to Beijing Tiantan
Hospital, Capital Medical University (Beijing, China) in August
2023 with complaints of progressive visual deterioration in both
eyes, along with visual field constriction, dizziness, headaches,
nausea, vomiting and an intermittent fever with a peak temperature
of 39.2˚C. The visual impairment had worsened over several months,
with difficulty recognizing faces and reading, particularly in dim
lighting. The patient reported a gradual narrowing of the
peripheral vision, which was later confirmed as bitemporal
hemianopsia, a hallmark of sellar region pathology. The patient
also described intermittent diplopia and a sensation of 'pressure'
behind the eyes. In addition, a single episode of transient loss of
consciousness accompanied by convulsions lasting about 1 min was
experienced. Neurologically, the patient exhibited mild confusion,
generalized fatigue and occasional dizziness upon standing, raising
suspicion of an underlying endocrine disturbance. The patient did
not have a family history of cancer.
An MRI scan performed at a local hospital revealed a
cystic lesion with rim enhancement in the sellar and suprasellar
regions, measuring ~18x22x20 mm, with mass effect on the optic
chiasm. The lesion exhibited heterogeneous hyperintensity on
T2-weighted images and hypointensity on T1-weighted images,
suggesting a pituitary abscess (data not shown). The patient was
treated with intravenous cephalosporin antibiotics; however, the
symptoms persisted, and the patient was subsequently transferred to
the Department of Neuroinfections and Immunology at Beijing Tiantan
Hospital (Beijing, China).
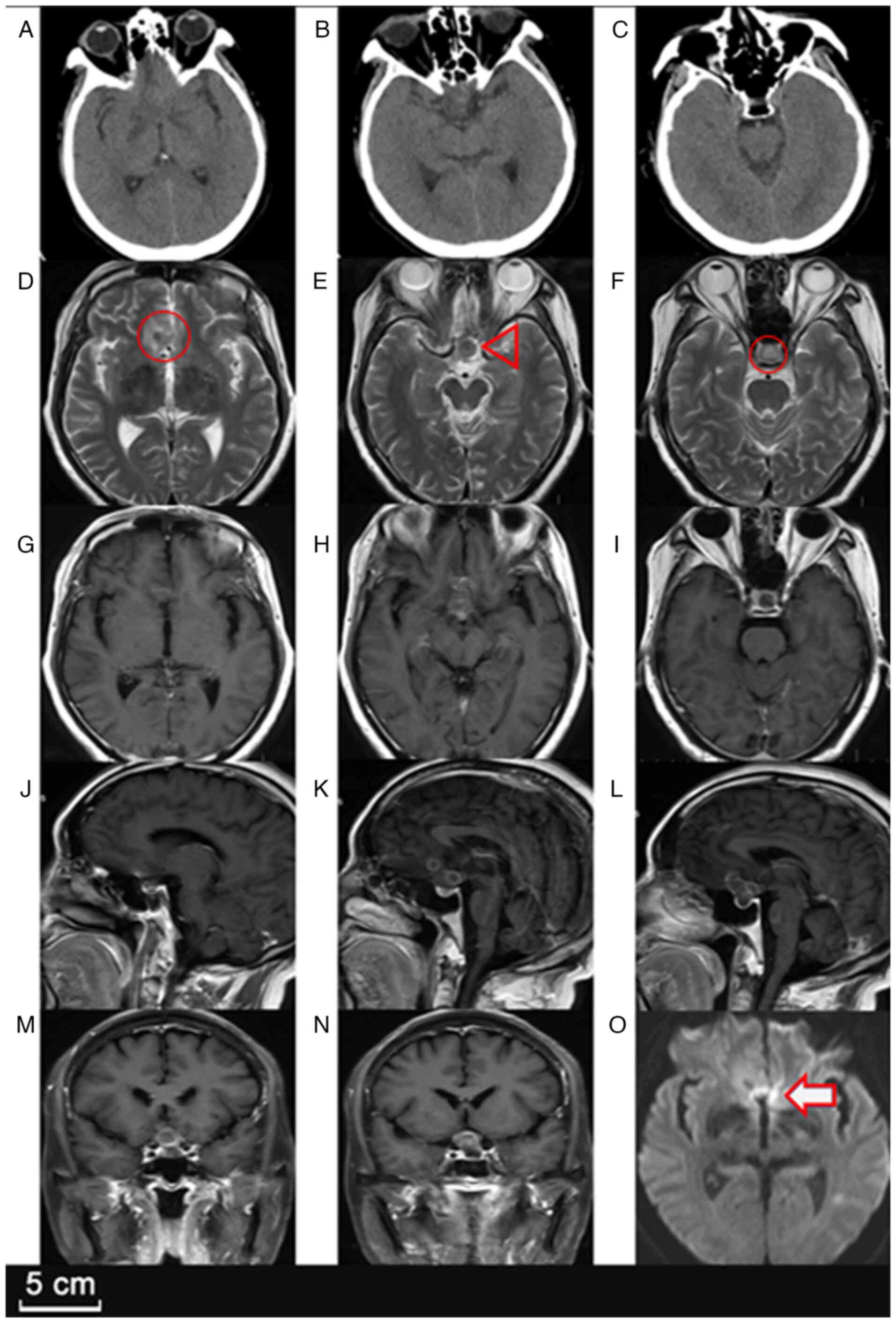
Neurological examination upon admission, 5 days
after the patient initially presented to the local hospital,
revealed bilateral visual decline with confirmed bitemporal
hemianopsia. The visual acuity was significantly reduced, measured
at 0.2 (right eye) and 0.1 (left eye) on the Snellen chart. Further
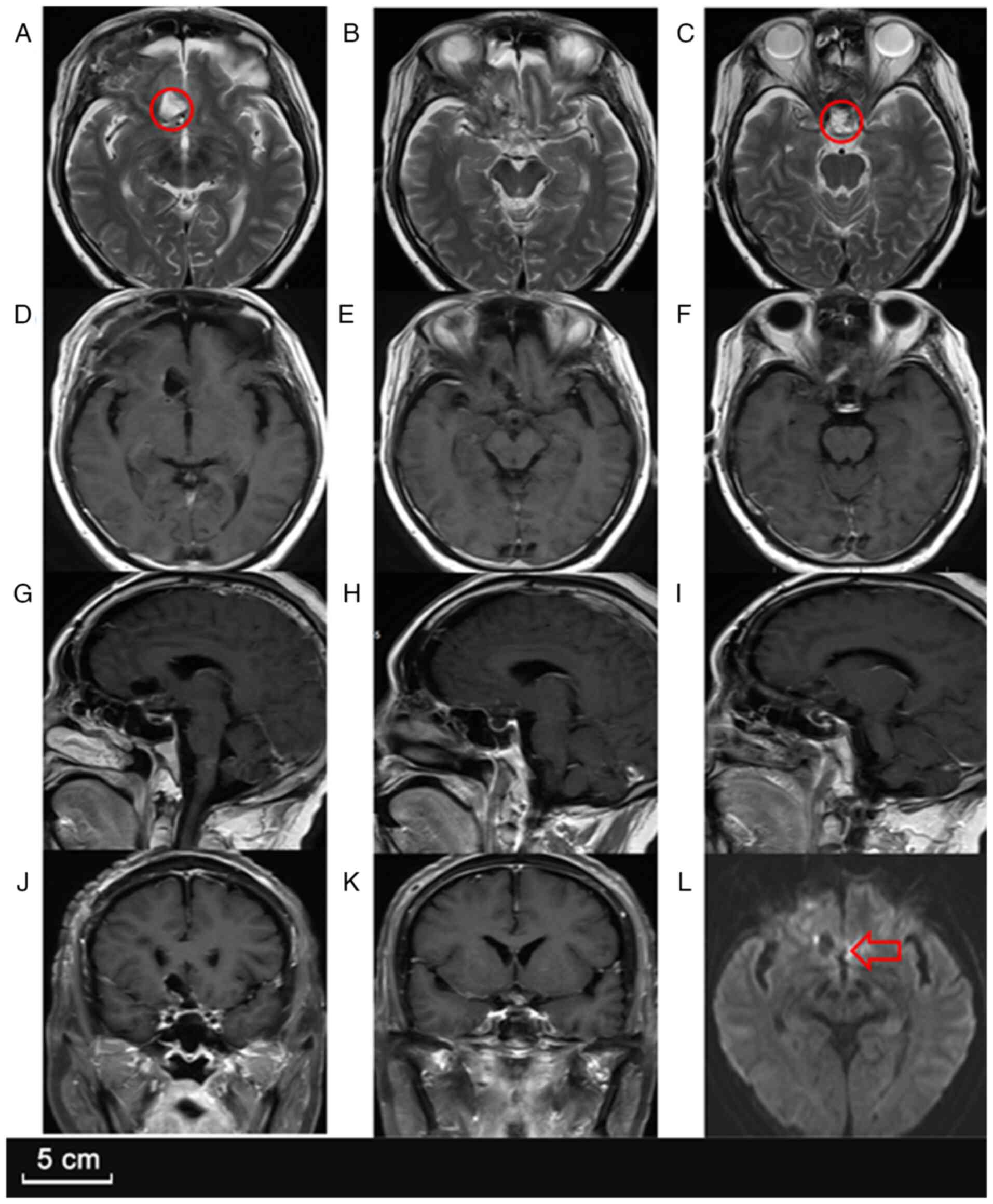
imaging studies, including a head CT scan and contrast-enhanced MRI
(Fig. 1), indicated a
well-defined, multilobulated, ring-enhancing lesion in the sellar
region, extending into the suprasellar cistern. Additionally,
another small ring-enhancing lesion was noted in the right frontal
lobe, suggesting the possibility of multifocal abscess formation.
The sellar lesion showed mild perifocal edema, with compression of
the optic chiasm and partial displacement of the third ventricle,
raising concern for obstructive hydrocephalus.
A comprehensive hormonal evaluation of the pituitary
gland (Table I) revealed
significant suppression of the thyroid and growth hormone axes,
with a free thyroxine level of 7 pmol/l (reference range,
7.64-16.03 pmol/l) and an insulin-like growth factor 1 level of
33.7 ng/ml (reference range, 45-210 ng/ml). Additionally, the
patient exhibited symptoms of adrenal insufficiency, including
postural dizziness and chronic fatigue, with a morning cortisol
level of 3.1 µg/dl (reference range, 5-25 µg/dl), necessitating
corticosteroid replacement. Oral prednisone acetate tablets were
administered at a daily dose of 10 mg from the time of diagnosis of
adrenocortical insufficiency until postoperative hormone
replacement therapy. The dosage was gradually tapered and
eventually discontinued after follow-up revealed progressive
recovery of pituitary function. Prolactin was elevated to 54.4
ng/ml (reference range, 2.50-17.00 ng/ml) due to the pituitary
stalk effect. Urine tests revealed low urine specific gravity
(1.002) and a marked increase in 24-h urinary sodium excretion
(609.96 mmol/day), supporting a diagnosis of central diabetes
insipidus. The patient underwent a lumbar puncture, and the
cerebrospinal fluid (CSF) analysis revealed a markedly elevated
white blood cell count of 1,301/µl (reference range, 0-8/µl), with
70.4% polymorphonuclear cells, consistent with a bacterial
infection. CSF protein was elevated at 251.25 mg/dl (reference
range, 15.00-45.00 mg/dl) and lactate level was increased to 3.8
mmol/l (reference range, 1.1-2.4 mmol/l), suggesting an ongoing
central nervous system (CNS) infection.
 | Table IPatient's laboratory results and
normal ranges. |
Table I
Patient's laboratory results and
normal ranges.
| Test | Pre-surgery
result | Post-surgery result
(1 week after surgery) | Normal range |
|---|
| ACTH, pg/ml | 5.34 | 5.07 | 0-46 |
| IGF-1, ng/ml | 33.7 | 72.4 | 45-210 |
| Free thyroxine,
pmol/l | 7 | 12.1 | 7.64-16.03 |
| TSH, µIU/ml | 1.62 | <0.005 | 0.49-4.91 |
| FSH, mIU/ml | 1.89 | <0.1 | 0.7-11.1 |
| LH, mIU/ml | <0.1 | <0.1 | 0.8-7.6 |
| Progesterone,
ng/ml | <0.2 | <0.2 | 0.27-0.90 |
| Testosterone,
ng/ml | <0.2 | <0.2 | 2.1-7.5 (male
>50 years) |
| Estradiol,
pg/ml | <11.8 | <11.8 | 0-39.8 (male) |
| Prolactin,
ng/ml | 54.4 | <0.5 | 2.5-17 |
| Urine specific
gravity | 1.002 | 1.002 | 1.003-1.030 |
| 24-h urine sodium
content, mmol/24 h | 609.96 | 458.65 | 40-220 |
Initial treatment and disease
progression
Empirical broad-spectrum antibiotic therapy with
meropenem (1,000 mg, bid, iv, one month) and vancomycin (500 mg,
every day, intravenously for 1 month) was initiated to control the
CNS infection, along with oral levetiracetam (500 mg, twice per
day, orally; administered long-term and continued postoperatively
for prophylactic purposes) for seizure control and desmopressin
(0.1 mg, three times a day, orally; administered long-term until
the symptoms of diabetes insipidus gradually resolved after
surgery) for central diabetes insipidus. The patient's fever and
systemic inflammatory response improved, and the patient's body
temperature returned to normal. However, despite a prolonged course
of intravenous antibiotics, the visual deficits and endocrine
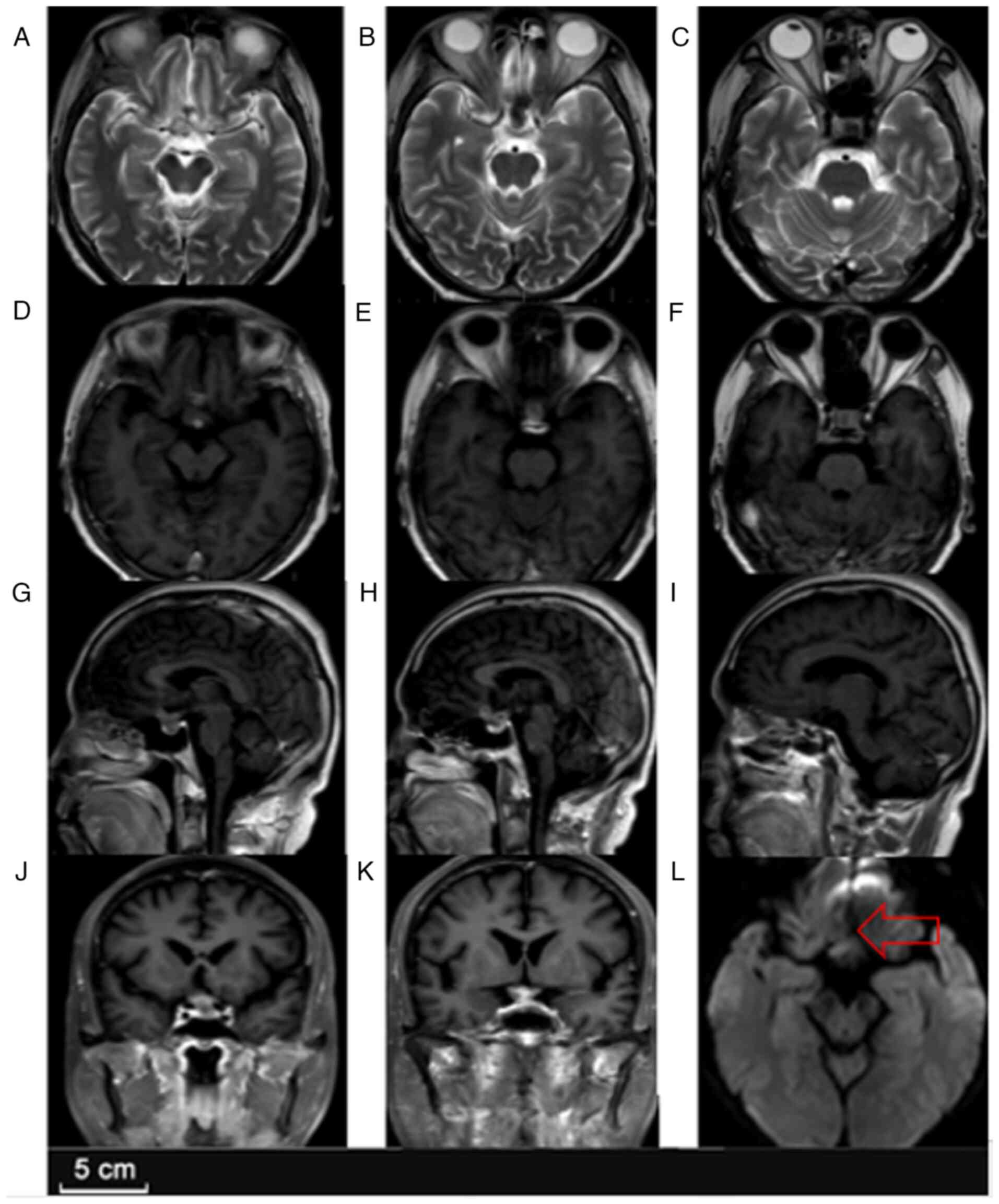
dysfunction persisted. Follow-up MRI (Fig. 2) at discharge in January 2024
showed a slight reduction in lesion size but persistent enhancement
and perifocal edema, indicating that infection had not been
completely eradicated.
After discharge, the patient continued antibiotic
treatment at a community hospital. However, in March 2024, the
fever, dizziness and lethargy recurred, and the patient again had a
seizure episode characterized by jaw clenching, loss of
consciousness and generalized tonic-clonic movements lasting ~1
min.
Surgical intervention
The patient was readmitted in April 2024 to
Neurosurgical Oncology Unit 7 at Beijing Tiantan Hospital. A
physical examination upon admission revealed that the patient was
conscious but lethargic, with further decreased visual acuity (0.1
in both eyes), constricted visual fields and persistent endocrine
symptoms. Neck stiffness was present, raising suspicion of
worsening intracranial infection.
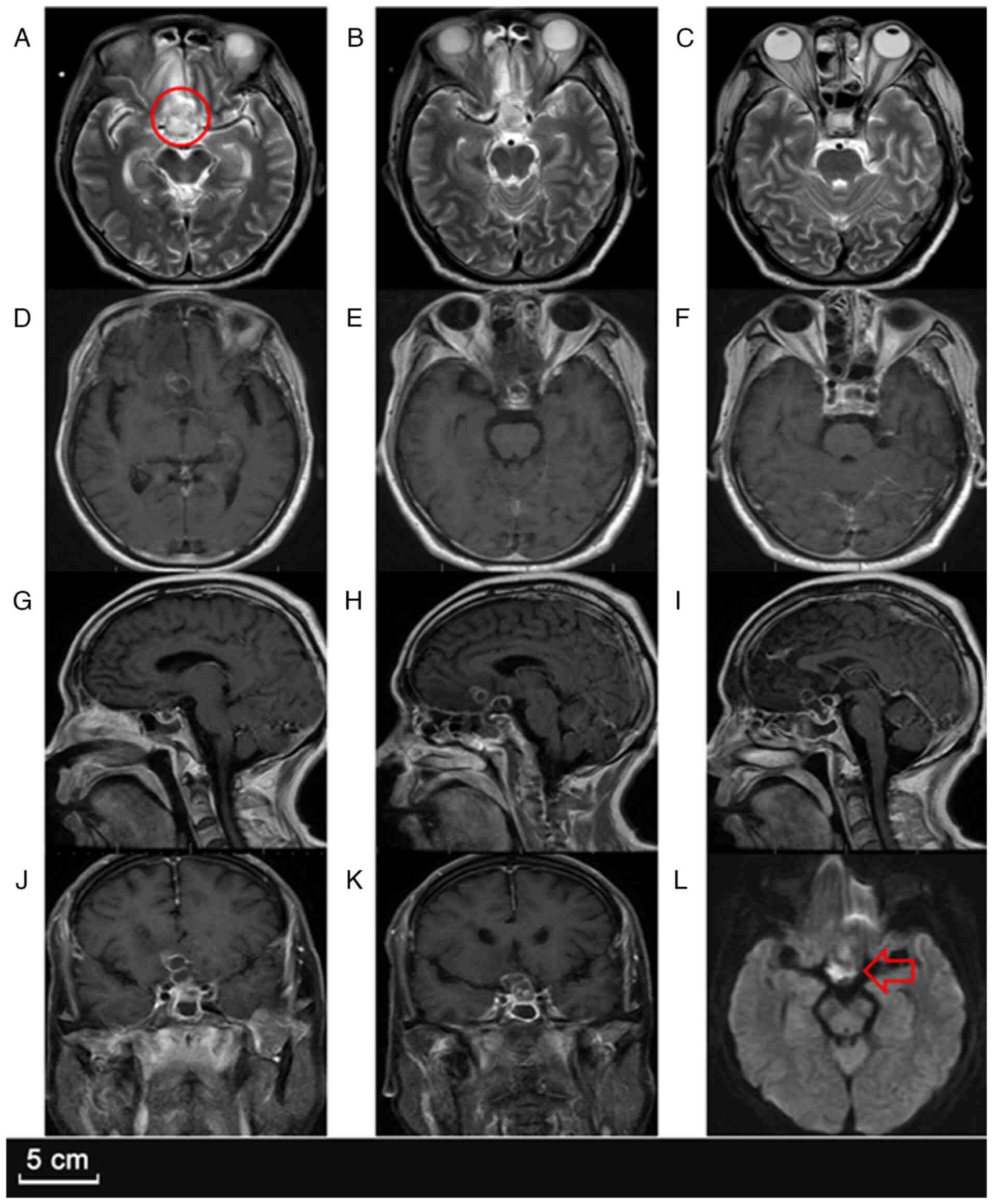
A follow-up MRI (Fig.
3) demonstrated marked enlargement of the sellar lesion, which
had increased to 26x32x28 mm, with intensified ring enhancement,
worsening perifocal edema and an increased mass effect on the optic
chiasm. The right frontal lesion had also slightly enlarged,
suggesting ongoing infection despite antibiotic therapy. Given the
failure of conservative management, the neuro-oncology team decided
to proceed with a craniotomy for lesion resection and thorough
abscess drainage.
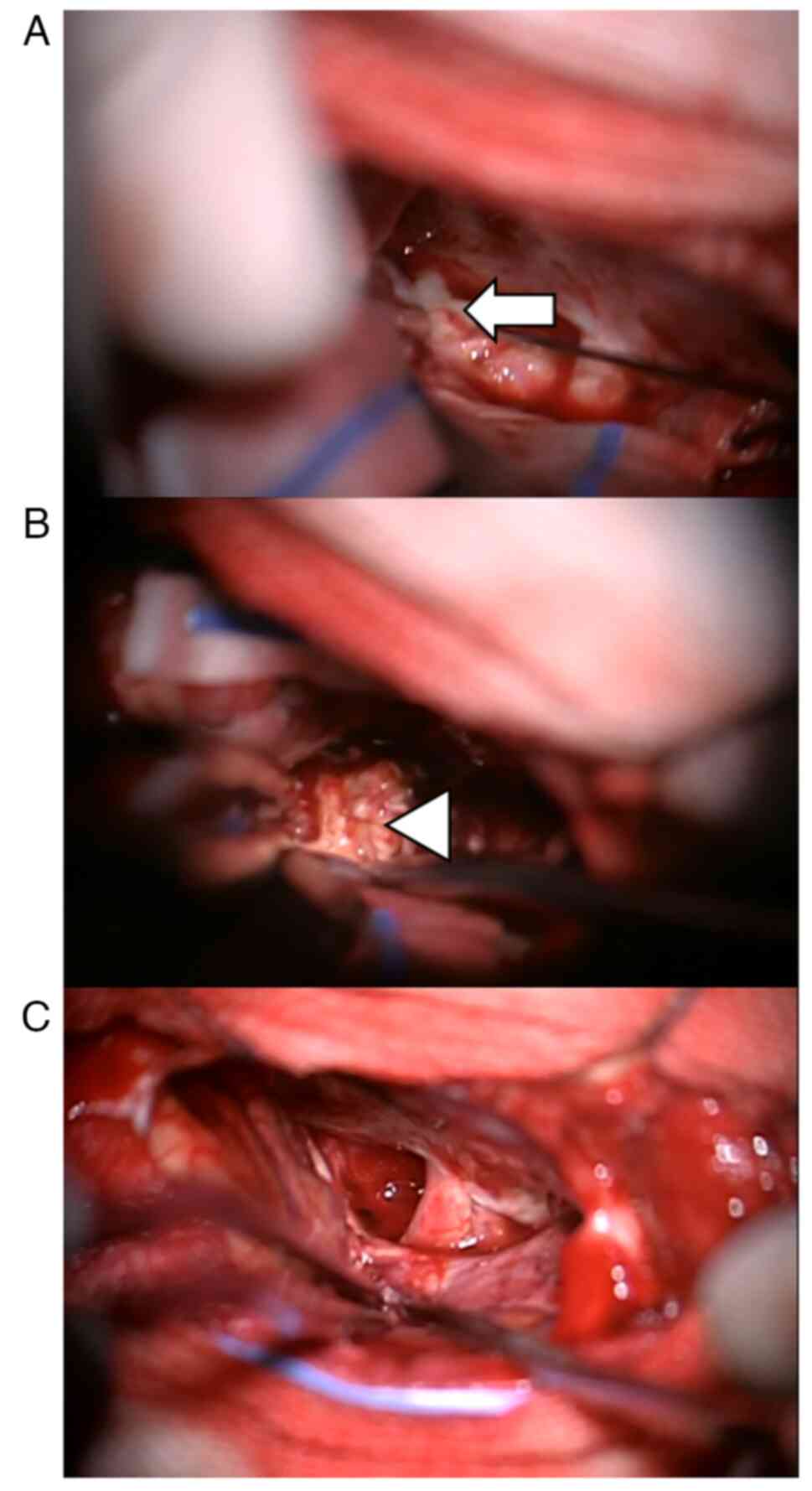
The surgery was performed via a right frontotemporal
approach, revealing a grayish-white cystic-solid lesion extending
from the right frontal base to the sellar region. Intraoperatively,
thick, white, purulent fluid was aspirated from the cystic
component, confirming an abscess (Fig.
4). The cystic cavity was irrigated with antibiotic solution
and the surrounding solid lesion was meticulously excised. The
excised specimen measured ~20x28x22 mm.
Postoperative findings and
follow-up
Tissue samples from the wall of the sellar abscess
were fixed in 10% neutral-buffered formalin at room temperature
(~22˚C) for 24 h. The fixed tissues were then processed and
embedded in paraffin. Sections were cut at a thickness of 4 µm
using a microtome. Routine hematoxylin and eosin staining was
performed at room temperature, with hematoxylin staining for 5 min
and eosin for 2 min. The stained slides were examined using a light
microscope (Olympus BX53; Olympus Corporation). Images were
captured at x200 magnification. A targeted next-generation
sequencing approach (BGI Genomics Co., Ltd.) was used to detect a
BRAFV600E mutation in the tumor sample. Genomic DNA was extracted
from formalin-fixed, paraffin-embedded (FFPE) tumor tissue using
the QIAamp DNA FFPE Tissue Kit (cat. no. 56404; Qiagen, Inc.)
according to the manufacturer's protocol. DNA quantity and quality
were assessed using a Qubit 4.0 Fluorometer (Thermo Fisher
Scientific, Inc.) and an Agilent 2100 Bioanalyzer (Agilent
Technologies, Inc.). Library preparation was conducted using the
MGIEasy Universal DNA Library Prep Set (cat. no. 1000006985; BGI
Genomics Co., Ltd.) following the manufacturer's instructions.
Sequencing was performed on the MGISEQ-2000 platform with
paired-end 150 base pair reads (PE150). The sequencing kit was the
MGISEQ-2000RS High-throughput Sequencing Set (FCL PE150; cat. no.
1000005267; BGI Genomics Co., Ltd.). The final library was loaded
at a concentration of 10 pM, quantified using a Qubit Fluorometer
and calculated based on molar concentration. Raw sequencing data
were processed with SOAPnuke (v1.5.6, https://github.com/BGI-flexlab/SOAPnuke) for quality
control, and downstream analysis, including alignment, variant
calling and annotation, was performed using Sentieon DNAseq
(v202112.05; https://www.sentieon.com/), following the standard
bioinformatics pipeline for BGI Genomics Co., Ltd. The histological
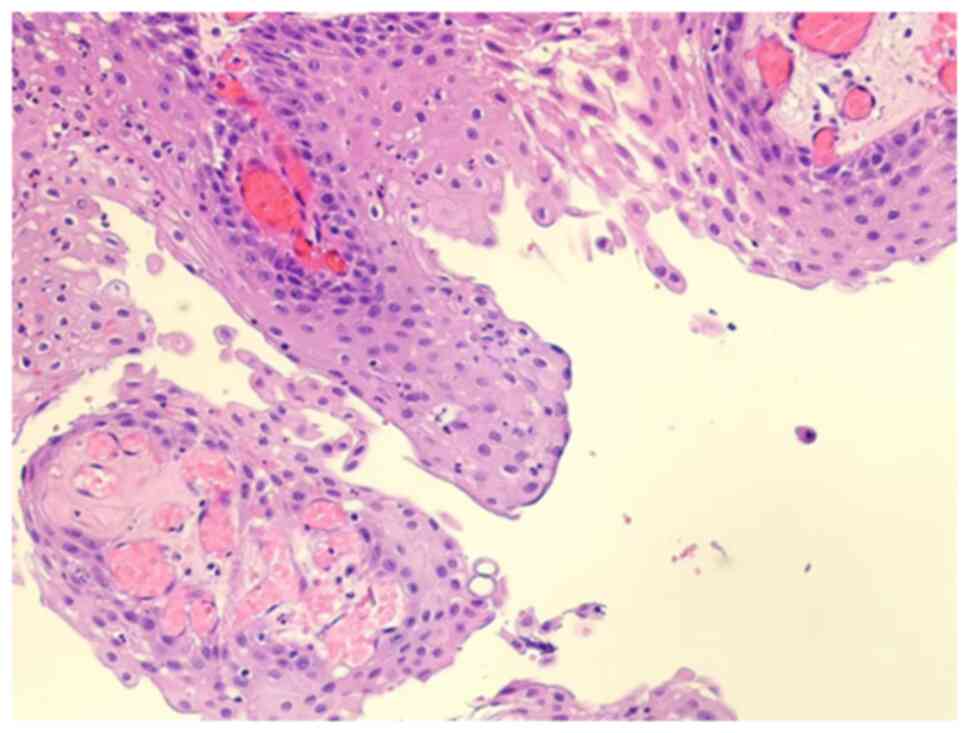
and genetic analysis confirmed the presence of a papillary
craniopharyngioma with a BRAFV600E mutation, accompanied by a
marked inflammatory infiltrate (Fig.
5). Microbial cultures from intraoperative specimens were
negative; however, next-generation sequencing (NGS) identified a
Gram-positive Staphylococcus species, confirming a secondary
pituitary abscess. Immediate postoperative MRI (Fig. 6) revealed complete resection of the
lesion and a marked reduction in the mass effect. A follow-up
hormone level assessment (Table I)
was performed, confirming persistent hypopituitarism. The patient
continued on antibiotic therapy (1,000 mg ceftriaxone, every day,
intravenously for 3 weeks), levetiracetam (500 mg, twice per day,
orally; continued postoperatively for prophylactic purposes) for
seizure control, and hormone replacement therapy (50 µg
levothyroxine, every day, orally; 10 mg prednisone, every day,
orally; long-term postoperative replacement therapy was maintained
until pituitary function recovered). The drainage tube was removed
on the fifth postoperative day.
At the 3-month follow-up, the patient's vision had
markedly improved, with better peripheral vision and reduced visual
field defects. However, long-term hormone replacement therapy,
including hydrocortisone, levothyroxine and desmopressin, was still
required to manage the pituitary insufficiency and diabetes
insipidus. The patient remains under regular follow-up (initially
once a month, and then once every 3 months.) to monitor endocrine
function and assess for potential recurrence.
Discussion
Pituitary abscesses are rare in clinical practice
and poorly understood. Pituitary abscesses are classified as either
primary or secondary, depending on the presence or absence of
underlying pituitary lesions. Secondary pituitary abscesses can
occur in association with pre-existing conditions such as
craniopharyngiomas, Rathke's cleft cysts and pituitary adenomas
(8).
In a systematic review of 488 cases of pituitary
abscesses, Stringer et al (6) found that 68% were primary and 32%
were secondary. Secondary infections can be further divided into
iatrogenic infections following surgery and spontaneous infections.
Iatrogenic infections may be caused by a disruption of the normal
blood supply to the pituitary, leading to decreased immune function
in the sellar region (9), or by
retrograde infection from sphenoid sinusitis (10,11).
In addition to iatrogenic infections, there are a number of reports
(2,12-14)
of pituitary abscesses secondary to pre-existing sellar lesions,
accounting for ~37% of secondary pituitary abscesses (15). This situation is the primary issue
that the present study aims to discuss in relation to the present
patient.
The English research literature was searched for
isolated case reports of pituitary abscesses secondary to sellar
lesions. A systematic literature search was performed using the
PubMed database. The following search terms were used: ['pituitary
abscess'(All Fields) OR 'sellar abscess'(All Fields)] AND ['case
report'(Title/Abstract) OR 'case series'(Title/Abstract)] AND
['secondary'(Title/Abstract) OR 'sellar lesion'(Title/Abstract) OR
'sellar mass'(Title/Abstract) OR 'sellar tumor'(Title/Abstract)].
These scattered cases suggested that the pathophysiological
mechanisms of such abscess formation are poorly understood
(Table II) (2,9,13-47).
 | Table IIReported cases of secondary sellar
infections following sellar lesions (1952-2024). |
Table II
Reported cases of secondary sellar
infections following sellar lesions (1952-2024).
| First author,
year | Cases, n | Pathological
diagnosis | Culture | Treatment | Outcome | (Refs.) |
|---|
| Whalley, 1952 | 1 | Pituitary
adenoma | Escherichia
coli | None | Died before
treatment | (16) |
| De Villiers
Hammann, 1956 | 1 | Pituitary
adenoma | Staphylococcus
aureus | Transcranial
surgery | Recovered | (17) |
| Obenchain and
Becker, 1972 | 1 | Rathke's cleft
cyst | Staphylococcus
aureus | Transcranial
surgery | Recovered | (18) |
| Obrador and
Blazquez, 1972 | 1 |
Craniopharyngioma | Negative | Transcranial
surgery | Recovered | (19) |
| Domingue and
Wilson, 1977 | 2 | Pituitary
adenoma | 1 negative; 1
Diplococcus pneumoniae | 1 Transcranial
surgery; 1 transnasal surgery | 1 died after
recurrence; 1 died after sepsis | (20) |
| Zorub et al,
1979 | 1 | Pituitary
adenoma | Streptococcus
pneumoniae | Transnasal
surgery | Died after acute
hydrocephalus | (21) |
| Gomez Perun et
al, 1981 | 1 | Rathke's cleft
cyst | NS | Transcranial
surgery | NS | (22) |
| Holck and Laursen,
1983 | 1 | Pituitary
adenoma | Negative | Transnasal
surgery | NS | (23) |
| Nelson et
al, 1983 | 3 | Pituitary
adenoma |
Bacteroides | 1 transcranial
surgery; 2 transnasal surgery | 3 recovered | (24) |
| Bossard et
al, 1992 | 1 | Pituitary
adenoma | NS | Transnasal
surgery | NS | (25) |
| Bognàr et
al, 1992 | 2 | Rathke's cleft
cyst | Staphylococcus
aureus and Streptococcus pyogenes | 1 transcranial and
transnasal surgery; 1 transnasal surgery | 1 died after
secondary surgery; 1 recovered | (26) |
| Shanley and Holmes,
1994 | 1 |
Craniopharyngioma | Salmonella
typhi | Transnasal
surgery | Recovered | (27) |
| Jain et al,
1997 | 2 | 1 Rathke's cleft
cyst, 1 pituitary adenoma | 1 negative; 1
Aspergillus | 1 transcranial
surgery, 1 transnasal surgery | 2 recovered | (28) |
| Thomas et
al, 1998 | 1 | Rathke's cleft
cyst | Anaerobic
streptococci | Transnasal
surgery | NS | (29) |
| Jadhav et
al, 1998 | 1 | Pituitary
adenoma | Staphylococcus
epidermidis | Transcranial
surgery | Recovered | (30) |
| Sharma et
al, 2000 | 1 | Pituitary
adenoma | Tuberculosis | NS | Recovered | (31) |
| Kroppenstedt et
al, 2001 | 1 | Pituitary
adenoma | Negative | Transnasal
surgery | Recovered | (32) |
| Vates et al,
2001 | 5 | 3 pituitary
adenoma, 1 craniopharyngioma; 1 rathke's cleft cyst | 2 negative; 1
Streptococcus pneumoniae; 1 Staphylococcus aureus; 1
alpha Streptococcus | NS | NS | (9) |
| Zhang et al,
2002 | 1 | Pituitary
adenoma | Toxoplasma
gondii | NS | NS | (33) |
| Jaiswal et
al, 2004 | 1 | Pituitary
adenoma | Escherichia
coli | Transnasal
surgery | Recovered | (34) |
| Hatiboglu et
al, 2006 | 1 | Pituitary
adenoma | Gram-positive
cocci | Transnasal
surgery | Recovered | (35) |
| Dutta et al,
2006 | 1 | Pituitary
adenoma | MRSA | Transnasal
surgery | Recovered | (36) |
| Celikoglu et
al, 2006 | 2 | Rathke's cleft
cyst | NS | Transnasal
surgery | 2 recovered | (37) |
| Takayasu et
al, 2006 | 1 | Rathke's cleft
cyst | Pseudomonas
aeruginosa | Transnasal
surgery | Recovered | (38) |
| Salinas-Lara et
al, 2008 | 1 | Pituitary
adenoma | Mucormycosis | Transnasal
surgery | Died during
surgery | (39) |
| Ciappetta et
al, 2008 | 1 | Pituitary
adenoma | Negative | Transnasal
surgery | Recovered | (15) |
| Qi et al,
2009 | 1 |
Craniopharyngioma | Staphylococcus
aureus | Transcranial
surgery | Recovered | (40) |
| Bakker and Hoving,
2010 | 1 | Pituitary
adenoma | MRSA | Transcranial
surgery | Recovered | (41) |
| Kuge et al,
2011 | 1 | Pituitary
adenoma | Gram-positive
cocci | Transnasal
surgery | Recovered | (42) |
| Kotani et
al, 2012 | 1 | Pituitary
adenoma | Gram-negative
cocci | None | Died | (43) |
| Awad et al,
2014 | 4 | Pituitary
adenoma | 1 group A
Streptococcus; 1 staphylococcus epidermidis; 1 MRSA;
1 Streptococcus pneumoniae | Transnasal
surgery | 3 recovered; 1
died | (14) |
| Safaee et
al, 2016 | 1 | Pituitary
adenoma | Staphylococcus
aureus | Transnasal
surgery | Recovered | (2) |
| Muscas et
al, 2017 | 1 | Pituitary
adenoma | Negative | Transcranial
surgery | Died after
surgery | (44) |
| Bhaisora et
al, 2018 | 1 |
Craniopharyngioma | Staphylococcus
aureus | Transnasal
surgery | Recovered | (45) |
| Coulden et
al, 2022 | 1 | Rathke's cleft
cyst | Staphylococcus
aureus | Transnasal
surgery | Recovered | (46) |
| López Goméz et
al, 2022 | 1 |
Caniopharyngioma | Negative | Transnasal
surgery | Recovered | (13) |
| Inoue et al,
2024 | 1 | Rathke's cleft
cyst | Negative | Transnasal
surgery | Recovered | (47) |
Pituitary abscesses are rare but serious
complications, often arising as secondary infections in the context
of underlying sellar lesions, including craniopharyngiomas. The
pathophysiology behind secondary pituitary abscesses is
multifactorial. Craniopharyngiomas, being locally invasive tumors,
may cause significant inflammatory responses, leading to tumor
necrosis and subsequent abscess formation. Additionally, the
disruption of vascular supply to the pituitary gland due to the
tumor's encroachment can compromise immune defenses, further
predisposing the patient to infection (45,48).
In the present case, the patient's craniopharyngioma exhibited
extensive inflammatory infiltration, which likely contributed to
the development of the abscess. A retrograde infection from
adjacent structures, such as the sphenoid sinus or the nasopharynx,
cannot be ruled out as a contributing factor. In the present case,
the MRI findings suggested a mixed cystic-solid lesion in the
suprasellar region, which was consistent with the craniopharyngioma
(13). While the role of
tumor-induced necrosis has been implicated in other reports, immune
dysfunction plays a significant role in increasing the risk of
secondary infections in these patients. In the present case, the
inflammatory changes seen in the craniopharyngioma likely
compromised local immune responses, predisposing the patient to the
development of an abscess.
Clinically, secondary pituitary abscesses often
present with symptoms related to the mass effect of the primary
lesion and signs of infection. The most common non-specific
symptoms include headache (91.7%) (8). Blurred vision and visual field
defects (58%) (49) are the second
most frequent symptoms, following headache. In addition to the mass
effect, inflammation of the optic nerve and optic chiasm due to the
abscess can also contribute to visual disturbances. Moreover,
pituitary hormone secretion may be impaired due to the involvement
of the pituitary gland, leading to hypopituitarism, with
panhypopituitarism being the most common manifestation. The extent
of pituitary dysfunction can reflect the severity of the abscess
(50). This feature can make it
difficult to differentiate from non-functioning pituitary adenomas;
however, the latter typically do not present with signs of
infection. If the lesion involves the pituitary stalk, polyuria
(50%) may occur, with a higher incidence than in pituitary adenomas
(51). The lack of typical
infectious symptoms aligns with the literature, where secondary
pituitary abscesses often lack clear signs of infection,
complicating the diagnosis (13).
Diagnosing secondary pituitary abscesses is often
challenging, particularly when they are associated with
pre-existing lesions, as demonstrated in the present case. The
initial clinical presentation of visual disturbances, headaches and
nausea can easily be misattributed to the primary craniopharyngioma
itself or to other more common causes of sellar mass effects.
Therefore, early suspicion and a comprehensive diagnostic approach
are crucial in identifying this rare complication. In the present
case, advanced imaging modalities, including MRI with contrast and
CT scans, were employed. Imaging by CT typically shows an enlarged
sella turcica and a low-density sellar mass, which does not
significantly aid in diagnosis. The typical MRI appearance is a
cystic or cystic-solid sellar mass that is hypointense or
isointense on T1-weighted images and hyperintense or isointense on
T2-weighted images. A characteristic finding is the rim enhancement
of the cystic mass after contrast administration (52,53).
Due to the restricted diffusion within the abscess cavity, it often
appears hyperintense on diffusion-weighted imaging, which can help
differentiate a pituitary abscess from other sellar lesions
(54). However, there are reported
cases that lack this feature (55,56).
According to reports, the most common causative
microorganisms in secondary pituitary abscesses are gram-positive
bacteria, including Staphylococcus and Streptococcus
species. However, as in the present case, numerous patients have
negative culture results whereby no pathogenic organisms are
identified from secretions. In >50% of cases, pathogens fail to
be isolated (57). This absence of
bacteria may be related to the use of antibiotics before surgery or
limitations of culture conditions (54-56).
Thus, metagenomic NGS (mNGS) is recommended for analyzing abscesses
and tissues obtained during surgery, as mNGS provides more accurate
information on the presence of pathogens. The mNGS platform can
perform high-throughput nucleic acid analysis of samples containing
microorganisms and match them to reference genomes to identify the
microbial species present and their relative abundance (57,58).
For the treatment of secondary pituitary abscesses,
surgical intervention is considered the preferred approach
(59). Surgery serves two primary
purposes: It alleviates the mass effect of the primary lesion and
promptly drains the infection, thereby preventing further
progression of the infection and minimizing the risk of permanent
neurological and pituitary dysfunction. Upon the identification of
symptoms suggestive of a pituitary abscess, empirical antibiotic
therapy should be initiated immediately to establish an optimal
window for surgical intervention. This early antibiotic treatment
is crucial in controlling infection and preparing the patient for
subsequent surgery.
Regarding surgical options, the endoscopic
transsphenoidal approach is generally the first choice due to its
ability to reduce the risk of infection spread and minimize damage
to critical structures such as the optic nerves. This minimally
invasive technique offers a relatively quick recovery time and
avoids the need for more invasive procedures. However, if the
pituitary abscess extends into the suprasellar region or if the
patient has contraindications for transnasal surgery (such as
anatomical anomalies or prior nasal surgery), a craniotomy may be
necessary for abscess drainage (60). Craniotomy, while effective, is
associated with a higher risk of abscess recurrence (7), and requires more extensive
postoperative care.
For the patient in the present case, the abscess had
significantly extended into the right frontal lobe, prompting the
decision to opt for craniotomy to ensure thorough and effective
drainage. During surgery, the abscess wall was excised completely
to minimize the risk of recurrence and repeated irrigation of the
surgical site was performed with saline and antibiotics to further
reduce the infection risk.
The timing of surgery is also critical. Procedures
should be avoided during the acute phase of infection or when the
patient presents with a high fever, as this can increase the risk
of bacteremia and sepsis. During this period, the patient should
receive appropriate antibiotic therapy and fluid support to manage
symptoms. The selection of antibiotics should be tailored to the
identified or suspected pathogen, with consideration for local
antibiotic resistance patterns (61). Emerging clinical guidelines
emphasize the importance of initiating broad-spectrum antibiotics
until culture or sequencing results are available, followed by a
more targeted regimen based on these findings (62).
Postoperatively, antibiotic therapy should continue
for 3-4 weeks, adjusted according to the results of culture or
sequencing. Although some patients may experience partial recovery
of pituitary function following surgery (32.3%) (7), hormone replacement therapy remains
essential. Long-term management is particularly important for
patients who had significant preoperative pituitary dysfunction, as
these individuals are at a high risk of developing permanent
hypopituitarism after surgery (59).
Usually, it is difficult to identify the primary
lesion in secondary pituitary abscesses based solely on the
patient's history and imaging before surgery. Only after obtaining
histopathological evidence during surgery can clinicians determine
the primary sellar lesion, although histopathology often does not
fully explain the source of the sellar infection. An early and
accurate diagnosis of a pituitary abscess, followed by timely
intervention, can lead to a better prognosis (63).
A comprehensive review of the literature highlights
that secondary pituitary abscesses are more commonly associated
with pituitary adenomas (64%) and Rathke's cleft cysts (24%) than
with craniopharyngiomas (12%). Despite the relatively low frequency
of craniopharyngioma-associated abscesses, this case illustrates
the significant differential diagnostic challenges that clinicians
face. Differential diagnoses for secondary pituitary abscesses
include other sellar lesions such as pituitary adenomas, Rathke's
cleft cysts and sellar metastases. Inflammatory conditions, such as
sarcoidosis or tuberculosis, should also be considered, as these
can mimic the clinical and radiological features of a pituitary
abscess (64). A detailed clinical
history, coupled with advanced imaging and microbiological workup,
is essential for distinguishing these entities.
The present case report had several limitations. The
lack of long-term follow-up is a significant limitation, as the
resolution of abscesses and recovery of pituitary function can
evolve over time. Additionally, the rarity of secondary pituitary
abscesses means that our understanding of the optimal treatment
regimen is still evolving. Further investigation, ideally through
multi-center studies, is necessary to establish consensus
guidelines for the management of this rare and challenging
condition.
In conclusion, the present case highlighted the rare
occurrence of a secondary pituitary abscess arising from a
craniopharyngioma, emphasizing the diagnostic and therapeutic
challenges associated with such cases. Given the overlapping
clinical and radiological features with other sellar lesions,
maintaining a high index of suspicion is crucial for early
identification. A multidisciplinary approach involving
neurosurgeons, endocrinologists and infectious disease specialists
is essential to ensure prompt diagnosis and tailored management.
The present literature review provided a more comprehensive
discussion of the pathophysiology, clinical implications and
management strategies for this rare entity. The importance of early
surgical intervention combined with appropriate antibiotic therapy
to optimize patient outcomes was underscored. Furthermore,
long-term follow-up is necessary to monitor endocrine function and
prevent recurrence. By detailing this case and analyzing the
existing literature, the present study aimed to enhance awareness
among clinicians regarding the potential for secondary pituitary
abscesses in patients with pre-existing sellar lesions, ultimately
contributing to improved diagnostic accuracy and patient care.
Acknowledgements
Not applicable.
Funding
Funding: This project was supported by the Key Research and
Development Plan of China (grant no. 2022YFB3204304).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RF was the principal investigator who contributed
substantially to the study design, provided the treatment plan, and
was responsible for the analysis and interpretation of the
patient's clinical data. RF also participated in drafting and
revising the manuscript and approved the final version. WW designed
the case study, performed the surgical procedure, contributed to
the clinical decision-making, and was involved in the manuscript
revision. RZ and YZ were responsible for the acquisition of key
medical images, including MRI and CT scans. WW and RF confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Beijing Tiantan Hospital (Beijing, China; approval no.
KY2024-062-02). Studies involving human participants followed
ethical guidelines established by the institutional and/or national
research committee and complied with the 1964 Helsinki Declaration
and its subsequent amendments.
Patient consent for publication
The patient provided written informed consent for
the publication of the case report and associated clinical
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao L, Guo X, Tian R, Wang Q, Feng M, Bao
X, Deng K, Yao Y, Lian W, Wang R and Xing B: Pituitary abscess:
Clinical manifestations, diagnosis and treatment of 66 cases from a
large pituitary center over 23 years. Pituitary. 20:189–194.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Safaee MM, Blevins L, Liverman CS and
Theodosopoulos PV: Abscess formation in a nonfunctioning pituitary
adenoma. World Neurosurg. 90:703.e15–703.e18. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mallereau CH, Todeschi J, Ganau M, Cebula
H, Bozzi MT, Romano A, Le Van T, Ollivier I, Zaed I, Spatola G, et
al: Pituitary abscess: A challenging preoperative diagnosis-A
multicenter study. Medicina (Kaunas). 59(565)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aranda F, García R, Guarda FJ, Nilo F,
Cruz JP, Callejas C, Balcells ME, González G, Rojas R and
Villanueva P: Rathke's cleft cyst infections and pituitary
abscesses: Case series and review of the literature. Pituitary.
24:374–383. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Altas M, Serefhan A, Silav G, Cerci A,
Coskun KK and Elmaci I: Diagnosis and management of pituitary
abscess: A case series and review of the literature. Turk
Neurosurg. 23:611–616. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stringer F, Foong YC, Tan A, Hayman S,
Zajac JD, Grossmann M, Zane JNY, Zhu J and Ayyappan S: Pituitary
abscess: A case report and systematic review of 488 cases. Orphanet
J Rare Dis. 18(165)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Agyei JO, Lipinski LJ and Leonardo J: Case
report of a primary pituitary abscess and systematic literature
review of pituitary abscess with a focus on patient outcomes. World
Neurosurg. 101:76–92. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang L, Yao Y, Feng F, Deng K, Lian W, Li
G, Wang R and Xing B: Pituitary abscess following transsphenoidal
surgery: The experience of 12 cases from a single institution. Clin
Neurol Neurosurg. 124:66–71. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vates GE, Berger MS and Wilson CB:
Diagnosis and management of pituitary abscess: A review of
twenty-four cases. J Neurosurg. 95:233–241. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Batra PS, Citardi MJ and Lanza DC:
Isolated sphenoid sinusitis after transsphenoidal hypophysectomy.
Am J Rhinol. 19:185–189. 2005.PubMed/NCBI
|
|
11
|
Caimmi D, Caimmi S, Labò E, Marseglia A,
Pagella F, Castellazzi AM and Marseglia GL: Acute isolated sphenoid
sinusitis in children. Am J Rhinol Allergy. 25:e200–e202.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang X, Yu G, Du Z, Tran V, Zhu W and Hua
W: Secondary pituitary abscess inside adenoma: A case report and
review of literature. World Neurosurg. 137:281–285. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
López Gómez P, Mato Mañas D, Bucheli
Peñafiel C, Rodríguez Rodríguez EM, Pazos Toral FA, Obeso Aguera S,
Viera Artiles J, Yange Zambrano G, Marco de Lucas E and Martín Láez
R: A rare case of a secondary pituitary abscess arising in a
craniopharyngioma with atypical presentation and clinical course.
Neurocirugia (Engl Ed). 33:99–104. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Awad AJ, Rowland NC, Mian M, Hiniker A,
Tate M and Aghi MK: Etiology, prognosis, and management of
secondary pituitary abscesses forming in underlying pituitary
adenomas. J Neurooncol. 117:469–476. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ciappetta P, Calace A, D'Urso PI and De
Candia N: Endoscopic treatment of pituitary abscess: Two case
reports and literature review. Neurosurg Rev. 31:237–246.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Whalley N: Abscess formation in a
pituitary adenoma. J Neurol Neurosurg Psychiatry. 15:66–67.
1952.PubMed/NCBI View Article : Google Scholar
|
|
17
|
De Villiers Hammann H: Abscess formation
in the pituitary fossa associated with a pituitary adenoma. J
Neurosurg. 13:208–210. 1956.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Obenchain TG and Becker DP: Abscess
formation in a Rathke's cleft cyst. Case report. J Neurosurg.
36:359–362. 1972.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Obrador S and Blazquez MG: Pituitary
abscess in a craniopharyngioma. Case report. J Neurosurg.
36:785–789. 1972.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Domingue JN and Wilson CB: Pituitary
abscesses. Report of seven cases and review of the literature. J
Neurosurg. 46:601–608. 1977.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zorub DS, Martinez AJ, Nelson PB and Lam
MT: Invasive pituitary adenoma with abscess formation: Case report.
Neurosurgery. 5:718–722. 1979.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gomez Perun J, Eiras J and Carcavilla LI:
[Intrasellar abscess into a cyst of the Rathke's pouch (authors'
translation)]. Neurochirurgie. 27:201–205. 1981.PubMed/NCBI(in French).
|
|
23
|
Holck S and Laursen H: Prolactinoma
coexistent with granulomatous hypophysitis. Acta Neuropathol.
61:253–257. 1983.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nelson PB, Haverkos H, Martinez AJ and
Robinson AG: Abscess formation within pituitary tumors.
Neurosurgery. 12:331–333. 1983.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bossard D, Himed A, Badet C, Lapras V,
Mornex R, Fisher G, Tavernier T and Bochu M: MRI and CT in a case
of pituitary abscess. J Neuroradiol. 19:139–144. 1992.PubMed/NCBI
|
|
26
|
Bognàr L, Szeifert GT, Fedorcsàk I and
Pàsztor E: Abscess formation in Rathke's cleft cyst. Acta Neurochir
(Wien). 117:70–72. 1992.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shanley DJ and Holmes SM: Salmonella
typhi abscess in a craniopharyngioma: CT and MRI.
Neuroradiology. 36:35–36. 1994.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jain KC, Varma A and Mahapatra AK:
Pituitary abscess: A series of six cases. Br J Neurosurg.
11:139–143. 1997.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Thomas N, Wittert GA, Scott G and Reilly
PL: Infection of a Rathke's cleft cyst: A rare cause of pituitary
abscess. Case illustration. J Neurosurg. 89(682)1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jadhav RN, Dahiwadkar HV and Palande DA:
Abscess formation in invasive pituitary adenoma: Case report.
Neurosurgery. 43:616–619. 1998.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sharma MC, Arora R, Mahapatra AK,
Sarat-Chandra P, Gaikwad SB and Sarkar C: Intrasellar
tuberculoma--an enigmatic pituitary infection: A series of 18
cases. Clin Neurol Neurosurg. 102:72–77. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kroppenstedt SN, Liebig T, Mueller W, Gräf
KJ, Lanksch WR and Unterberg AW: Secondary abscess formation in
pituitary adenoma after tooth extraction. Case report. J Neurosurg.
94:335–338. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang X, Li Q, Hu P, Cheng H and Huang G:
Two case reports of pituitary adenoma associated with Toxoplasma
gondii infection. J Clin Pathol. 55:965–966. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jaiswal AK, Mahapatra AK and Sharma MC:
Pituitary abscess associated with prolactinoma. J Clin Neurosci.
11:533–534. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hatiboglu MA, Iplikcioglu AC and Ozcan D:
Abscess formation within invasive pituitary adenoma. J Clin
Neurosci. 13:774–777. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dutta P, Bhansali A, Singh P, Kotwal N,
Pathak A and Kumar Y: Pituitary abscess: Report of four cases and
review of literature. Pituitary. 9:267–273. 2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Celikoglu E, Boran BO and Bozbuga M:
Abscess formation in Rathke's cleft cyst. Neurol India. 54:213–214.
2006.PubMed/NCBI
|
|
38
|
Takayasu T, Yamasaki F, Tominaga A, Hidaka
T, Arita K and Kurisu K: A pituitary abscess showing high signal
intensity on diffusion-weighted imaging. Neurosurg Rev. 29:246–248.
2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Salinas-Lara C, Rembao-Bojórquez D, de la
Cruz E, Márquez C, Portocarrero L and Tena-Suck ML: Pituitary
apoplexy due to mucormycosis infection in a patient with an ACTH
producing pulmonary tumor. J Clin Neurosci. 15:67–70.
2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Qi S, Peng J, Pan J, Lu Y and Fan J:
Secondary abscess arising in a craniopharyngioma. J Clin Neurosci.
16:1667–1669. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bakker NA and Hoving EW: A rare case of
sudden blindness due to a pituitary adenoma coincidentally infected
with methicillin-resistant Staphylococcus aureus (MRSA).
Acta Neurochir (Wien). 152:1079–1080. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kuge A, Sato S, Takemura S, Sakurada K,
Kondo R and Kayama T: Abscess formation associated with pituitary
adenoma: A case report: Changes in the MRI appearance of pituitary
adenoma before and after abscess formation. Surg Neurol Int.
2(3)2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kotani H, Abiru H, Miyao M, Kakimoto Y,
Kawai Y, Ozeki M, Tsuruyama T and Tamaki K: Pituitary abscess
presenting a very rapid progression: Report of a fatal case. Am J
Forensic Med Pathol. 33:280–283. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Muscas G, Iacoangeli F, Lippa L and
Carangelo BR: Spontaneous rupture of a secondary pituitary abscess
causing acute meningoencephalitis: Case report and literature
review. Surg Neurol Int. 8(177)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bhaisora KS, Prasad SN, Das KK and Lal H:
Abscess inside craniopharyngioma: Diagnostic and management
implications. BMJ Case Rep. 2018(bcr2017223040)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Coulden A, Pepper J, Juszczak A, Batra R,
Chavda S, Senthil L, Ayuk J, Phol U, Nagaraju S, Karavitaki N and
Tsermoulas G: Rathke's Cleft Cyst Abscess with a Very Unusual
Course. Asian J Neurosurg. 17:527–531. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Inoue E, Kesumayadi I, Fujio S, Makino R,
Hanada T, Masuda K, Higa N, Kawade S, Niihara Y, Takagi H, et al:
Secondary hypophysitis associated with Rathke's cleft cyst
resembling a pituitary abscess. Surg Neurol Int.
15(69)2024.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Han SJ, Rolston JD, Jahangiri A and Aghi
MK: Rathke's cleft cysts: Review of natural history and surgical
outcomes. J Neurooncol. 117:197–203. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Dalan R and Leow MKS: Pituitary abscess:
Our experience with a case and a review of the literature.
Pituitary. 11:299–306. 2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Liu F, Li G, Yao Y, Yang Y, Ma W, Li Y,
Chen G and Wang R: Diagnosis and management of pituitary abscess:
Experiences from 33 cases. Clin Endocrinol (Oxf). 74:79–88.
2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
St-Pierre GH, de Ribaupierre S, Rotenberg
BW and Benson C: Pituitary abscess: Review and highlight of a case
mimicking pituitary apoplexy. Can J Neurol Sci. 40:743–745.
2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang Z, Gao L, Zhou X, Guo X, Wang Q, Lian
W, Wang R and Xing B: Magnetic resonance imaging characteristics of
pituitary abscess: A review of 51 cases. World Neurosurg.
114:e900–e912. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Connor SEJ and Penney CC: MRI in the
differential diagnosis of a sellar mass. Clin Radiol. 58:20–31.
2003.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Taguchi Y, Yoshida K, Takashima S and
Tanaka K: Diffusion-weighted MRI findings in a patient with
pituitary abscess. Intern Med. 51(683)2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Huang KT, Bi WL, Smith TR, Zamani AA, Dunn
IF and Laws ER Jr: Intrasellar abscess following pituitary surgery.
Pituitary. 18:731–737. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kawano T, Shinojima N, Hanatani S, Araki
E, Mikami Y and Mukasa A: Atypical pituitary abscess lacking rim
enhancement and diffusion restriction with an unusual organism,
Moraxella catarrhalis: A case report and review of the
literature. Surg Neurol Int. 12(617)2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang Y, Cui P, Zhang HC, Wu HL, Ye MZ,
Zhu YM, Ai JW and Zhang WH: Clinical application and evaluation of
metagenomic next-generation sequencing in suspected adult central
nervous system infection. J Transl Med. 18(199)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Sherrod BA, Makarenko S, Iyer RR, Eli I,
Kestle JR and Couldwell WT: Primary pituitary abscess in an
adolescent female patient: Case report, literature review, and
operative video. Childs Nerv Syst. 37:1423–1428. 2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ovenden CD, Almeida JP, Oswari S and
Gentili F: Pituitary abscess following endoscopic endonasal
drainage of a suprasellar arachnoid cyst: Case report and review of
the literature. J Clin Neurosci. 68:322–328. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Oktay K, Guzel E, Yildirim DC, Aliyev A,
Sari I and Guzel A: Primary pituitary abscess mimicking meningitis
in a pediatric patient. Childs Nerv Syst. 37:3241–3244.
2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Walia R, Bhansali A, Dutta P,
Shanmugasundar G, Mukherjee KK, Upreti V and Das A: An uncommon
cause of recurrent pyogenic meningitis: Pituitary abscess. BMJ Case
Rep. 23(bcr0620091945)2010.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Lin L, Fang J, Li J, Tang Y, Xin T, Ouyang
N, Cai W, Xie L, Lu S and Zhang J: Metagenomic next-generation
sequencing contributes to the early diagnosis of mixed infections
in central nervous system. Mycopathologia. 189(34)2024.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Cabuk B, Caklılı M, Anık I, Ceylan S,
Celik O and Ustün C: Primary pituitary abscess case series and a
review of the literature. Neuro Endocrinol Lett. 40:99–104.
2019.PubMed/NCBI
|
|
64
|
Zhang X, Sun J, Shen M, Shou X, Qiu H,
Qiao N, Zhang N, Li S, Wang Y and Zhao Y: Diagnosis and minimally
invasive surgery for the pituitary abscess: A review of twenty nine
cases. Clin Neurol Neurosurg. 114:957–961. 2012.PubMed/NCBI View Article : Google Scholar
|