Introduction
Stress urinary incontinence (SUI) is defined as the
involuntary loss of urine during physical exertion or effort
(1). The prevalence of SUI has
been reported to be as high as 49%, but varies depending on the
study population and the diagnostic criteria used (2,3). The
incidence of this dysfunction becomes more common with age, with
~25% of young women, 44-57% of middle-aged and postmenopausal
women, and 75% of elderly women reporting some involuntary urine
loss (4). SUI adversely impacts
the physical and mental health of patients and also represents a
notable medical and economic burden on individuals and healthcare
systems (5-7).
Currently, the recommended first-line treatment for
SUI is ≥3 months of supervised exercise focused on the pelvic floor
muscles (PFMs), known as PFM training (PFMT) (3,6).
PFMT involves the patient regularly performing repeated voluntary
PFM contractions, with sufficient progression in exercise intensity
and duration to produce a training effect on the muscles. Surface
electromyography (sEMG) is considered a useful tool for the
real-time monitoring of PFM contractions and the assessment of PFM
function via the detection of PFM motor unit action potentials
(3). The validity of sEMG in the
assessment of the bioelectrical activity of PFMs, as well as
muscles that act as synergistically with the PFMs, has been
demonstrated in numerous studies (8-10).
Electrical signals from these muscles reflect the recruitment of
motor units during muscle contraction, and a positive association
has been observed between muscle strength and the activation of
motor units (3). The electrical
activity of the PFMs is typically measured using an internal
vaginal probe with a rigid two-channel electrode, which is widely
used in clinical practice due to its simplicity, speed and ease of
operation. However, sEMG cannot determine precisely which specific
muscles are being measured, as it captures the cumulative activity
from all muscles that contact the electrode plates. Furthermore,
the suboptimal probe-muscle interface, traditional two-channel
electrode structure, and lack of accurate evaluation methods hinder
the accurate measurement of muscle activity under specific
conditions, such as when two electrodes are placed on opposite
sides of the PFMs (one on the left and the other on the right, or
one on the top and the other on the bottom) inside the vagina
(11). To address these
challenges, the present study evaluated a novel airbag-type
stretchable/inflatable electrode array (ASEA) device for sEMG
signal acquisition in PFM analysis. As previously reported, the
ASEA device can inflate to conform with varying vaginal anatomies,
while closely following muscle movement, thereby providing a stable
probe-muscle interface with notable biological adaptability
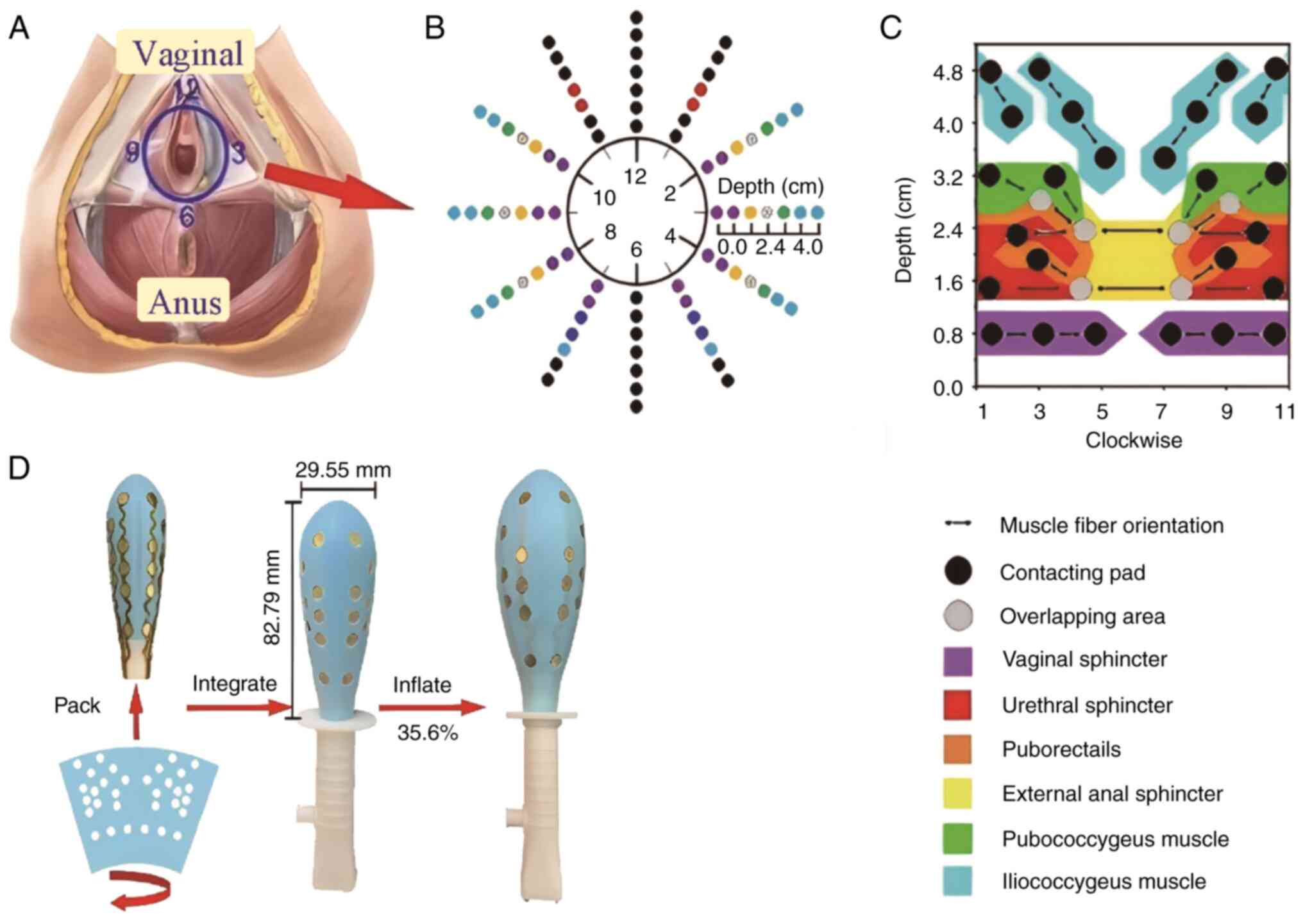
(12). The ASEA comprises 24
bipolar electrode pairs, which map to 10 PFM regions and are
approximately aligned with the orientation of muscle fibers. These
regions include the urethral sphincter (US), vaginal sphincter
(VS), external anal sphincter (EAS) and levator ani muscles (LAMs),
which consist of the puborectalis (PR), pubococcygeus (PC) and
iliococcygeus (IC) muscles. Electrode placement along the muscle
fibers improves the accuracy of signal acquisition, and dividing
the sEMG signals by region makes it possible to pinpoint the
activity of specific muscle regions.
Several key parameters are hypothesized to
comprehensively evaluate the regional muscles closely associated
with SUI. These parameters, calculated on the basis of regional
functional data include: i) Pre-baseline, referred to as the
anterior resting potential (ARP); ii) maximum voluntary contraction
(MVC), also known as rapid contraction potential; iii) tonic
contraction potential (TCP); iv) endurance contraction potential
(ECP); and v) post-baseline, referred to as the posterior resting
potential (PRP). The aim of the present study was to evaluate the
regional characteristics and spatial precision of PFM sEMG signals
during rest and contraction using the ASEA device in postmenopausal
women with SUI compared with healthy controls.
Materials and methods
Ethics statement
Ethics approval for the present study was obtained
from The Ethics Committee of the Women's Hospital, School of
Medicine, Zhejiang University (Hangzhou, China; approval nos.
IRB-20220008-R and IRB-20240381-R). All participants provided
written informed consent to participate in the study, and the
ethics committee approved the consent procedure.
Study design
The study was designed as a retrospective data
analysis. The data were originally sampled by the same research
team during a prior study focusing on the effect of whole-body
vibration on PFM activity.
Participants
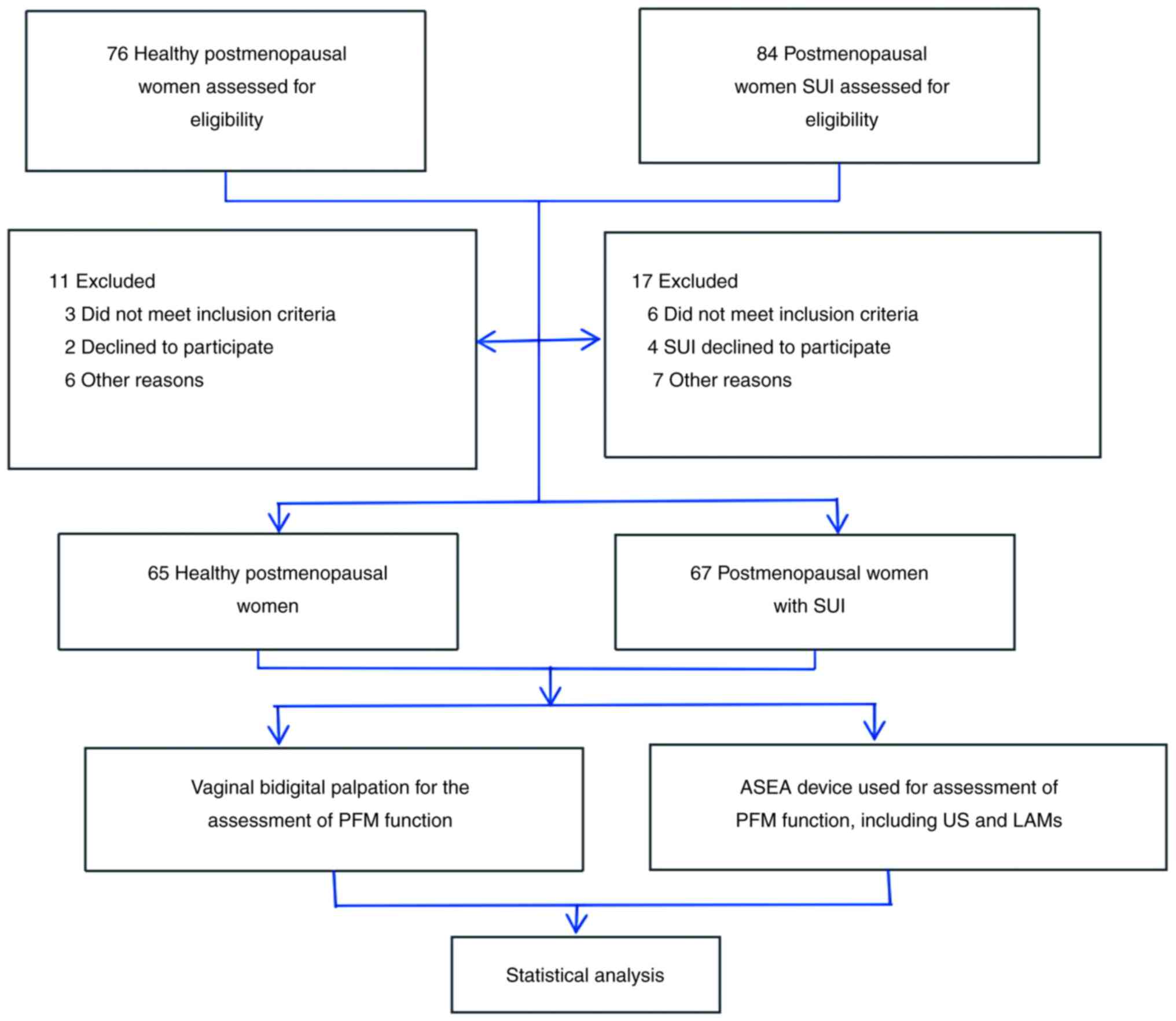
For the purpose of the present study, two groups
were established as follows: i) A control group comprising healthy
postmenopausal women with no history of pelvic floor dysfunction
(n=65); and ii) an SUI group, comprising postmenopausal women with
SUI, recruited from the Women's Hospital, School of Medicine,
Zhejiang University (n=67). The participants were all female and
recruited between August 2022 and September 2023 (Fig. 1 and Table I). The inclusion criteria for the
postmenopausal women with SUI were as follows: i) Clinical
diagnosis of SUI; ii) a sexual history; and iii) menopausal for ≥1
year. The inclusion criteria for the postmenopausal women without
SUI: i) a sexual history; and ii) menopausal for ≥1 year. Since the
ASEA device is a vaginal probe, its insertion requires a history of
sexual activity. In China, performing vaginal procedures on
individuals without prior sexual experience is considered ethically
inappropriate; therefore, only individuals with a sexual history
were included. The exclusion criteria were the same for both groups
and were as follows: i) Acute reproductive organ inflammation; ii)
presence of a cardiac pacemaker; iii) malignant tumors; iv) history
of pelvic radiotherapy; v) pelvic floor surgery during the previous
6 months; vi) any condition or symptom that could interfere with
the implementation of the study or the interpretation of the
results; and vii) concurrent participation in another clinical
trial. In total, 76 participants were initially screened for
inclusion in the control group; however, 11 participants were then
excluded due to not meeting the inclusion criteria (3 cases),
declining to participate (2 cases), bad comprehension and
cooperative degree (3 cases), malignant tumors (1 case) and missing
follow-up appointments (2 cases). In total, 84 participants were
initially screened for inclusion in the SUI group; however, 17
participants were then excluded due to not meeting the inclusion
criteria (6 cases), declining to participate (4 cases), bad
comprehension and cooperative degree (3 cases) and missing
follow-up appointments (4 cases).
 | Table IDemographics of participants. |
Table I
Demographics of participants.
| | Group | |
|---|
| Characteristics | SUI (n=67) | Control (n=65) | t-value | Z-value | P-value |
|---|
| Age, years | 59.03±5.90 | 57.38±6.01 | 1.59 | - | 0.115 |
| Body mass index,
kg/m2 | 23.68±3.16 | 23.01±2.46 | 1.37 | - | 0.175 |
| Waist-to-hip
ratio | 0.91±0.60 | 0.89±0.06 | 1.85 | - | 0.067 |
| Years after
menopause | 7 (4,10) | 6 (3,10.5) | - | 0.79 | 0.428 |
| Parity | 3 (2,3) | 2 (2,4) | - | 1.31 | 0.189 |
| Gravidity | 1 (1,2) | 1 (1,2) | - | 0.60 | 0.546 |
| Vaginal delivery | 1 (1,2) | 1 (1,2) | - | 0.15 | 0.878 |
| Cesarean section | 0 (0,0) | 0 (0,0) | - | 1.14 | 0.253 |
Procedures and data collection.
Vaginal bidigital palpation for the assessment of PFM function
PFM function was assessed by vaginal bidigital
palpation following the PERFECT scheme (13,14).
During the examination, the participants were instructed how to
properly contract their PFMs and asked to perform a maximal
contraction. All participants were assigned a PFM testing score of
M0-M5 using the modified Oxford grading system: i) M0, no
contraction; ii) M1, flicker; iii) M2, weak; iv) M3, moderate; v)
M4, good; and vi) M5, strong (14).
ASEA device for the assessment of PFM
function. Introduction of the ASEA device
The ASEA is an airbag-type, inflatable vaginal probe
designed to maintain tight contact and a stable interface between
its electrodes and the PFMs. As previously described, it has 24
bipolar electrode pairs, along with 32 contact pads, which are
positioned in alignment with the muscle fibers and cover 10
distinct PFM regions, thereby enabling the acquisition of
high-quality sEMG signals (12).
The structure of the ASEA and the physical positioning of the
contact pads on the muscles are shown in Fig. 2. The ASEA records electrical
potential distributions and corresponding frequency information for
all major vaginal muscles, including the US, VS and EAS, as well as
the PR, PC and IC muscles of the LAM group. Details of the
fabrication process, the arrangement of the electrodes (electrical
contacts) and clinical reliability assessments of the ASEA have
been described previously (15),
and have demonstrated that the accuracy and stability of the ASEA
are improved compared with those of conventional PFM probes.
Use of the ASEA device for the assessment of PFM
function. To ensure consistency, all assessments were performed
by a single therapist, experienced in pelvic floor rehabilitation.
The patients were first asked to adopt a supine position with
slightly bent knees and a neutral hip position, and then the ASEA
probe was inserted into the vagina and the patients were instructed
to activate their PFMs, which was controlled with electromyographic
biofeedback. The PFM activity was measured using the novel ASEA
probe (patent no. ZL 202210927100.3; China). Patients were first
instructed to squeeze their PFMs freely to acclimate to the test
probe, while the therapist monitored the abdomen, perineum, hip and
gluteal muscles to ensure isolated activation of the PFMs.
Subsequently, the ASEA probe was carefully inserted into the vagina
and its proper placement confirmed before any measurements were
recorded.
The protocol of all measurements of PFM activity
consisted of the assessment of the following elements: i)
Pre-baseline, also known as the ARP, where a 10-sec measurement of
PFM activity at rest was taken before the functional tests; ii)
MVC, also termed ‘flick contractions’, consisting of a 10-sec
measurement while participants performed short, quick contractions
of the PFMs; iii) TCP, comprising 5 repetitions of 10-sec
contractions, during which participants attempted to contract and
hold the PFMs for 10 sec; iv) ECP, where participants attempt to
hold a PFM contraction for 60 sec; and v) post-baseline, also known
as the PRP, consisting of a 10-sec measurement of the PFM at rest
after the functional tests.
Statistical analysis
Statistical analysis was performed using SPSS 26.0
(IBM Corporation). The distributions of baseline characteristics in
the SUI and control groups were compared using unpaired t-tests or
Wilcoxon rank-sum tests. To determine the optimal cut-off level for
sEMG activation of the PFMs for use in the diagnosis of SUI,
receiver operating characteristic (ROC) curve analysis was
performed and the area under the curve (AUC), 95% confidence
intervals (CIs), sensitivity and specificity were calculated.
P<0.05 was considered to indicate a statistically significant
result.
Results
Demographic and clinical
characteristics
In total, 132 postmenopausal women were included in
the present study, with 67 participants in the SUI group and 65 in
the control group. In the SUI group, the median age was 58 years
and the age range was 48-74 years, while in the control group, the
median age was 57 years and the age range was 46-78 years. The
demographic and clinical characteristics of the participants are
presented in Table I. No
statistically significant difference was identified between the two
groups for age, BMI, waist-to-hip ratio, parity, gravidity and mode
of delivery.
PFM function assessment by vaginal
bidigital palpation
All patients in each group were assigned an initial
PFM testing score of M0-M5 according to the modified Oxford grading
system (Table II). Notably, the
scores in the SUI group were significantly lower compared with
those in the control group.
 | Table IIPelvic floor muscle functional
assessment by vaginal bidigital palpation. |
Table II
Pelvic floor muscle functional
assessment by vaginal bidigital palpation.
| | Group | |
|---|
| Type of muscle
fiber | SUI (n=67) | Control (n=65) | Z-value | P-value |
|---|
| Type I | 1 (1,2) | 2 (1,3) | 4.48 | <0.001 |
| Type II | 1 (1,2) | 2 (1.5,3) | 4.53 | <0.001 |
Comparison of sEMG results between the
SUI and control groups
Multi-channel sEMG signals were successfully
acquired from all participants. Differences in the sEMG activity of
the PFMs between the SUI and control groups were determined using
unpaired t-tests (Table III).
The PFM activity measurements included ARP, MVC, TCP, ECP and PRP.
In the resting state, the pre-baseline and post-baseline
assessments of PFM activity, namely the ARP and PRP, exhibited no
statistically significant differences between the two groups,
except the ARP US and PR pre-baselines muscles. The ARP of US in
SUI was significantly lower compared with the control, while the
ARP of PR was significantly higher compared with the control.
However, the activation amplitudes of the US, PR and PC muscles in
the SUI group during flick, tonic and endurance contractions were
significantly reduced compared with those in the control group
(Fig. S1).
 | Table IIISurface electromyography results of
the urethral sphincter and levator ani muscles in postmenopausal
women. |
Table III
Surface electromyography results of
the urethral sphincter and levator ani muscles in postmenopausal
women.
| | | Levator ani
muscles |
|---|
| | Urethral
sphincter | Puborectalis | Pubococcygeus | Iliococcygeus |
|---|
| Parameter | SUI, µV | Control, µV | P-value | SUI, µV | Control, µV | P-value | SUI, µV | Control, µV | P-value | SUI, µV | Control, µV | P-value |
|---|
| Anterior resting
potential | 9.12±6.37 | 16.33±31.60 | 0.014 | 52.92±122.67 | 10.44±7.30 | 0.017 | 11.48±9.37 | 13.86±15.36 | 0.447 | 19.61±50.47 | 14.24±17.32 | 0.518 |
| Maximum voluntary
contraction | 43.4±43.11 | 142.88±495.68 | 0.031 | 45.64±48.39 | 127.19±388.94 | 0.014 | 41.76±41.91 | 58.14±73.70 | 0.017 | 59.22±80.31 | 64.5±81.11 | 0.345 |
| Tonic contraction
potential | 24.74±29.00 | 47.51±98.40 | 0.038 | 25.75±30.54 | 48.51±92.66 | 0.009 | 22.36±17.68 | 39.64±70.73 | 0.021 | 30.61±40.85 | 33.5±52.39 | 0.657 |
| Endurance
contraction potential | 23.64±24.19 | 51.17±116.60 | 0.017 | 23.28±17.65 | 52.92±122.67 | 0.002 | 23.22±16.26 | 36.86±60.42 | 0.041 | 29.75±23.12 | 29.51±19.68 | 0.636 |
| Posterior resting
potential | 12.43±20.68 | 19.97±65.12 | 0.464 | 11.56±8.17 | 14±13.71 | 0.514 | 11.01±6.73 | 12.99±13.64 | 0.904 | 14.23±12.35 | 13.37±12.87 | 0.224 |
Predictive capability of the sEMG
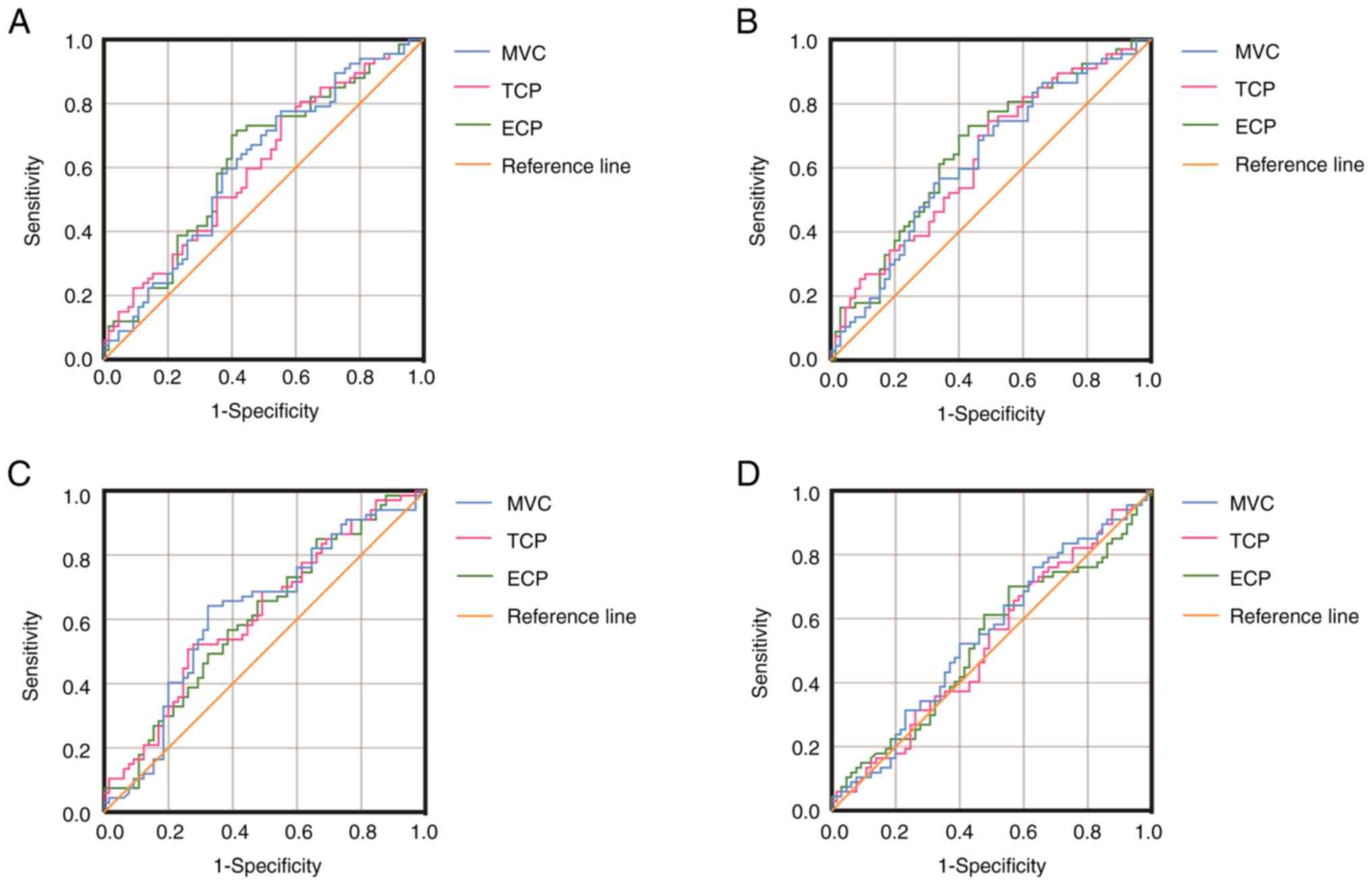
ROC curves were constructed separately for each sEMG
parameter (Fig. 3) to evaluate
their predictive performance for SUI. The optimum predicted
cut-offs (µV) for the US were: MVC, 48.55 (AUC, 0.61; 95% CI,
0.51-0.71; sensitivity, 76.1%; specificity, 46.2%); TCP, 25.79
(AUC, 0.61; 95% CI, 0.51-0.70; sensitivity, 77.6%; specificity,
44.6%); and ECP, 23.02 (AUC, 0.62; 95% CI, 0.53-0.72; sensitivity,
70.1%; specificity, 60.0%). For the PR, the optimum predicted
cut-offs (µV) were: MVC, 34.48 (AUC, 0.62; 95% CI, 0.53-0.72;
sensitivity, 55.2%; specificity, 67.7%); TCP, 26.96 (AUC, 0.63; 95%
CI, 0.54-0.73; sensitivity, 74.6%; specificity, 50.8%); and ECP,
26.20 (AUC, 0.65; 95% CI, 0.56-0.75; sensitivity, 70.1%;
specificity, 60.0%). Finally, for the PC, the optimum predicted
cut-offs (µV) were: MVC, 35.05 (AUC, 0.62; 95% CI, 0.52-0.72;
sensitivity, 64.2%; specificity, 67.7%); TCP, 17.36 (AUC, 0.62; 95%
CI, 0.52-0.71; sensitivity, 50.7%; specificity, 73.8%); and ECP,
34.68 (AUC, 0.60; 95% CI, 0.51-0.70; sensitivity, 85.1%;
specificity, 33.8%). Notably, no significant difference in sEMG
results for the IC muscles was observed between the SUI and control
groups.
Discussion
To the best of our knowledge, the present study is
the first to objectively quantify regional differences in PFM
function between postmenopausal women with and without SUI using
both vaginal digital palpation and an intravaginal physiology-based
airbag-type electrode array probe. Firstly, vaginal digital
palpation was used to evaluate PFMs according to the modified
Oxford grading system, revealing that the scores of the SUI group
were lower than those of the control group. Although vaginal
digital palpation has certain limitations, such as subjectivity,
poor reliability and low sensitivity for the detection of small
changes in pressure, it is still recommended by the International
Urogynecological Association and International Continence Society
for the evaluation of PFMs (1). In
the present study, vaginal digital palpation clearly identified
that the PFM strength of postmenopausal women with SUI was lower
compared with that of postmenopausal women without this condition.
Furthermore, previous studies by Ignácio et al (16) and Yang et al (17) endorse the routine clinical use of
digital palpation examination for PFM evaluation. The findings of
the present study also support vaginal digital palpation as a
useful and simple strategy for the evaluation of PFM function.
Several studies have reported the value and
application of sEMG in the evaluation of PFMs in cases of pelvic
floor dysfunction (18-20).
However, conventional sEMG signals reflect the overall activity of
the PFMs, and do not provide region-specific data. In the present
study, a novel ASEA probe was used to collect sEMG signals from
different PFM regions based on anatomical positioning, including
the US, VS, EAS and LAMs. The majority of existing vaginal probes
can record only 1-4 channels of sEMG signals using custom
configurations (21,22), and their rigid structure cannot
adapt to anatomical variability of the vaginal structure, leading
to reduced accuracy. In addition, differences in impedance, muscle
depth and muscle fiber orientation hinder valid comparisons between
groups without signal normalization. By contrast, the inflatable
probe used in present study is able to adapt to diverse vaginal
anatomies and align with specific muscle regions, which is not
possible using existing technologies. Therefore, the present study
describes a novel evaluation method for the accurate examination of
PFMs.
Regarding specific regional PFM abnormalities and
insufficiencies, significant differences in muscle strength were
observed between the two groups based on sEMG results, particularly
during flick, tonic and endurance contractions. In the resting
state, as assessed by the pre- and post-baseline measurements, most
PFMs exhibited no significant differences between the SUI and
control groups, except the US muscles in the SUI group was lower,
while the PR muscles in the SUI group was higher compared with the
control. However, a statistically significant reduction in the
activity of the US, PR and PC muscles was observed during flick,
tonic and endurance contractions in the SUI group compared with the
control group.
The pathogenesis of SUI is associated with
anatomical abnormalities involving the urethra, urinary bladder and
urogenital diaphragm. Insufficiency of the US and vesicourethral
ligament, as well as weakening of the muscle-fascial structures of
the whole pelvic floor, impairs normal urinary continence.
Falah-Hassani et al (21)
highlighted the potential role of routine clinical EMG examination
in the evaluation of US insufficiency. The ASEA device used in the
present study provides tight contact and a stable interface between
the electrode units and PFMs, enabling the collection of
high-quality sEMG signals from specific muscle regions. Notably,
the mean sEMG values in the US of the SUI group were significantly
lower compared with those in the control group. Although US
insufficiency has attracted increasing attention (21,23)
its functional assessment remains challenging. The present study
describes a promising method for the evaluation of US function and
supports the involvement of US insufficiency in the pathogenesis of
SUI.
DeLancey (24)
described the ‘hammock’-like structure formed by the continuity of
the intrapelvic fascia, vaginal wall and LAMs, which helps to
maintain urethral stability and urethral closure pressure during
increases in abdominal pressure. The LAMs are considered a
functional unit that supports the pelvic organs by lifting them in
the transverse plane and compresses the urethra against the
anterior vagina in the mid-sagittal plane to aid in urethral
closure. Damage or dysfunction of the LAMs is a recognized
contributor to SUI (23). Various
approaches are available for the evaluation of LAM structure and
function (21,25,26),
and intravaginal dynamometry has been proposed to be the most
direct approach for measuring the force-generating capacity of the
LAMs (21). However, existing
intravaginal devices only record the combined force of all PFMs,
which may not precisely reflect the activity of individual PFMs. By
contrast, the ASEA device used in the present study provides
separate signals from the PR, PC and IC muscles.
Notably, the sEMG activity of the LAMs, specifically
the PR and PC muscles, in the SUI group was lower compared with
that in the control group. The present study demonstrates that LAM
insufficiency is associated with the pathogenesis of SUI, and
suggests that the PR and PC muscles, located near the center of the
PFMs, play a key role in SUI pathogenesis. This suggests that
dysfunction in specific LAM regions, rather than impairment of the
whole muscle group, contributes to the pathogenesis of SUI.
In the present study, ROC curves were constructed
for certain sEMG test results to evaluate the predictive ability of
each variable for SUI. Optimum cut-off points were determined based
on MVC, TCP and ECP measurements of the US and LAMs, and the
corresponding AUC, 95% CI, sensitivity and specificity were
assessed. In a previous study by Ptaszkowski et al (18), ‘quick flicks’, ‘contractions’ and
‘static hold’ were used to assess PFM activity in women with SUI.
Sensitivities ranging from 60 to 70% were obtained, which are
similar to those in the present study. However, the specificities
were reported to range from 90 to 94%, which were notably higher
than those in the present study. It must be noted the PFM
activities in the previous study were based on the mean functional
activity of all PFMs, while the present study collected signals
from specific PFMs, including the US, PR and PC. These findings
suggest that measuring sEMG activity in targeted PFMs may serve as
a valuable tool in the diagnosis of SUI.
Based on the aforementioned analyses, the present
study suggests a point-to-point evaluation and targeted treatment
approach for the US and LAM regions to improve muscle function.
Coordinated training may be used to increase the functional
association between the US, LAMs and other muscles. The approach
used in the present study may be more accurate than traditional PFM
evaluation and treatment methods. However, additional clinical
research is required to verify its effectiveness.
Several factors are associated with an increased
risk of female SUI, including pregnancy, multiple vaginal
deliveries, menopause, obesity and pelvic surgeries such as
hysterectomy (27,28). Although vaginal delivery is
considered an independent high-risk factor for PFM injury, the
present study did not find a significant association. However, a
previous study suggests that the number of vaginal deliveries is a
risk factor for SUI, with one vaginal delivery being associated
with a lower risk of SUI than multiple deliveries (29). Furthermore, a long-term study
reported no association of stress or urgency urinary incontinence
with the mode of delivery in women aged ≥50 years (30). The present study may not have found
a significant association of vaginal delivery with SUI due to the
low number of multiparous women among the participants. However, a
variety of factors may be involved, and further verification with a
larger and more diverse population, including both postpartum and
menopausal women, is necessary.
To conclude, in postmenopausal women with SUI, the
novel ASEA device revealed that the functional activities of
specific US and LAMs, particularly the PR and PC, were lower than
those in postmenopausal women without pelvic floor dysfunction.
This suggests that US defects and dysfunctional LAMs play an
important role in the pathogenesis of SUI in postmenopausal women.
In addition, the ability to assess the US and LAMs separately may
support the development of a reliable and optimized treatment
strategy for the precise rehabilitation of PFMs.
Supplementary Material
Comparison of surface electromyography
results for PFMs between postmenopausal women with or without SUI.
Comparison of (A) MVC, (B) TCP and (C) ECP in the US, PR, PC and IC
muscles. PFMs, pelvic floor muscles; SUI, stress urinary
incontinence; MVC, maximum voluntary contraction; TCP, tonic
contraction potential; ECP, endurance contraction potential; US,
urethral sphincter; PR, puborectalis; PC, pubococcygeus; IC,
Iliococcygeus; sEMG, surface electromyography.
*P<0.05 as indicated.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the 4+X Clinical Research
Project of the Women's Hospital, School of Medicine, Zhejiang
University (No. ZDFY2021-4X102) and the Major Health and Health
Issues in Zhejiang Province Technology Plan Project of National
Health Commission Scientific Research Fund (grant no.
WKJ-ZJ-2507).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZZ was responsible for conceptualization, software,
visualization and writing the original draft of the manuscript. QC
and SH contributed to methodology and validation. ZZ, QC and SH
performed the formal analysis and reviewed and edited the
manuscript. WL performed the investigation. SW was responsible for
data curation and collecting electrical signals. ZX conceived and
designed the study, analyzed and interpretated the data, reviewed
and edited the final manuscript, and supervised the research. ZZ
and ZX confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki and approved by the Ethics Committee of the
Women's Hospital, School of Medicine, Zhejiang University (approval
nos. IRB-20220008-R and IRB-20240381-R). All participants provided
written informed consent for participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nambiar AK, Arlandis S, Bø K,
Cobussen-Boekhorst H, Costantini E, de Heide M, Farag F, Groen J,
Karavitakis M, Lapitan MC, et al: European association of urology
guidelines on the diagnosis and management of female non-neurogenic
lower urinary tract symptoms. Part 1: Diagnostics, overactive
bladder, stress urinary incontinence, and mixed urinary
incontinence. Eur Urol. 82:49–59. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kobashi KC, Albo ME, Dmochowski RR,
Ginsberg DA, Goldman HB, Gomelsky A, Kraus SR, Sandhu JS, Shepler
T, Treadwell JR, et al: Surgical treatment of female stress urinary
incontinence: AUA/SUFU guideline. J Urol. 198:875–883.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hagen S, Elders A, Stratton S, Sergenson
N, Bugge C, Dean S, Hay-Smith J, Kilonzo M, Dimitrova M,
Abdel-Fattah M, et al: Effectiveness of pelvic floor muscle
training with and without electromyographic biofeedback for urinary
incontinence in women: Multicentre randomised controlled trial.
BMJ. 371(m3719)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Abufaraj M, Xu T, Cao C, Siyam A, Isleem
U, Massad A, Soria F, Shariat SF, Sutcliffe S and Yang L:
Prevalence and trends in urinary incontinence among women in the
United States, 2005-2018. AM J Obstet Gynecol. 225:166.e1–166.e12.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhu L, Lang J, Liu C, Han S, Huang J and
Li X: The epidemiological study of women with urinary incontinence
and risk factors for stress urinary incontinence in China.
Menopause. 16:831–836. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dumoulin C, Morin M, Danieli C, Cacciari
L, Mayrand MH, Tousignant M and Abrahamowicz M: Urinary
Incontinence and Aging Study Group. Group-based vs individual
pelvic floor muscle training to treat urinary incontinence in older
women: A randomized Clinical trial. JAMA Intern Med. 180:1284–1293.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vaughan CP and Markland AD: Urinary
incontinence in women. Ann Intern Med. 172:ITC17–ITC32.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bharucha AE and Lacy BE: Mechanisms,
evaluation, and management of chronic constipation.
Gastroenterology. 158:1232–1249.e3. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Worman RS, Stafford RE, Cowley D,
Prudencio CB and Hodges PW: Evidence for increased tone or
overactivity of pelvic floor muscles in pelvic health conditions: A
systematic review. Am J Obstet Gynecol. 228:657–674.e9.
2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brækken IH, Stuge B, Tveter AT and Bø K:
Reliability, validity and responsiveness of pelvic floor muscle
surface electromyography and manometry. Int Urogynecol J.
32:3267–3274. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ballmer C, Eichelberger P, Leitner M,
Moser H, Luginbuehl H, Kuhn A and Radlinger L: Electromyography of
pelvic floor muscles with true differential versus faux
differential electrode configuration. Int Urogynecol J.
31:2051–2059. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang S, Dong S, Li W, Cen J, Cen J, Zhu H,
Fu C, Jin H, Li Y, Feng X, et al: Physiology-based stretchable
electronics design method for accurate surface electromyography
evaluation. Adv Sci. 8(2004987)2021.
|
|
13
|
da Silva JB, de Godoi Fernandes JG,
Caracciolo BR, Zanello SC, de Oliveira Sato T and Driusso P:
Reliability of the PERFECT scheme assessed by unidigital and
bidigital vaginal palpation. Int Urogynecol J. 32:3199–3207.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Laycock J and Jerwood D: Pelvic floor
muscle assessment: The PERFECT scheme. Physiotherapy. 87:631–642.
2001.
|
|
15
|
Wang S, Yang L, Jiang H, Xia J, Li W,
Zhang Z, Zhang S, Jin H, Luo J, Dong S, et al: Multifunctional
evaluation technology for diagnosing malfunctions of regional
pelvic floor muscles based on stretchable electrode array probe.
Diagnostics (Basel). 13(1158)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ignácio Antônio F, Bø K, Pena CC, Bueno
SM, Mateus-Vasconcelos ECL, Fernandes ACNL and Ferreira CHJ:
Intravaginal electrical stimulation increases voluntarily pelvic
floor muscle contractions in women who are unable to voluntarily
contract their pelvic floor muscles: A randomised trial. J
Physiother. 68:37–42. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang X, Zhu L, Li W, Sun X, Huang Q, Tong
B and Xie Z: Comparisons of electromyography and digital palpation
measurement of pelvic floor muscle strength in postpartum women
with stress urinary incontinence and asymptomatic parturients: A
cross-sectional study. Gynecol Obstet Invest. 84:599–605.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ptaszkowski K, Malkiewicz B, Zdrojowy R,
Paprocka-Borowicz M and Ptaszkowska L: The prognostic value of the
surface electromyographic assessment of pelvic floor muscles in
women with stress urinary incontinence. J Clin Med.
9(1967)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Albaladejo-Belmonte M, Nohales-Alfonso FJ,
Tarazona-Motes M, De-Arriba M, Alberola-Rubio J and Garcia-Casado
J: Effect of BoNT/A in the surface electromyographic
characteristics of the pelvic floor muscles for the treatment of
chronic pelvic pain. Sensors (Basel). 21(4668)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jórasz K, Truszczyńska-Baszak A and Dąbek
A: Posture correction therapy and pelvic floor muscle function
assessed by sEMG with intravaginal electrode and manometry in
female with urinary incontinence. Int J Environ Res Public Health.
20(369)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Falah-Hassani K, Reeves J, Shiri R,
Hickling D and McLean L: The pathophysiology of stress urinary
incontinence: A systematic review and meta-analysis. Int Urogynecol
J. 32:501–552. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chill HH, Martin LC, Abramowitch SD and
Rostaminia G: Quantifying the effect of an endo-vaginal probe on
position of the pelvic floor viscera and muscles. Int Urogynecol J.
34:2399–2406. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang X, Wang X, Gao Z, Li L, Lin H, Wang
H, Zhou H, Tian D, Zhang Q and Shen J: The anatomical pathogenesis
of stress urinary incontinence in women. Medicina (Kaunas).
59(5)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
DeLancey JO: Structural support of the
urethra as it relates to stress urinary incontinence: The hammock
hypothesis. Am J Obstet Gynecol. 170:1713–1723. 1994.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sheng Y, Liu X, Low LK, Ashton-Miller JA
and Miller JM: Association of pubovisceral muscle tear with
functional capacity of urethral closure: Evaluating maternal
recovery from labor and delivery. Am J Obstet Gynecol.
222:598.e1–598.e7. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Miller JM, Low LK, Zielinski R, Smith AR,
DeLancey JO and Brandon C: Evaluating maternal recovery from labor
and delivery: Bone and levator ani injuries. Am J Obstet Gynecol.
213:188.e1–188.e11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ptak M, Ciećwież S, Brodowska A,
Starczewski A, Nawrocka-Rutkowska J, Diaz-Mohedo E and Rotter I:
The effect of pelvic floor muscles exercise on quality of life in
women with stress urinary incontinence and its relationship with
vaginal deliveries: A randomized trial. Biomed Res Int.
2019(5321864)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lugo T, Leslie SW, Mikes BA and Riggs J:
Stress Urinary Incontinence. In: StatPearls. StatPearls Publishing,
Treasure Island, FL, 2025.
|
|
29
|
Blomquist JL, Carroll M, Muñoz A and Handa
VL: Pelvic floor muscle strength and the incidence of pelvic floor
disorders after vaginal and cesarean delivery. AM J Obstet Gynecol.
222:62.e1–62.e8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tähtinen RM, Cartwright R, Vernooij RWM,
Rortveit G, Hunskaar S, Guyatt GH and Tikkinen KAO: Long-term risks
of stress and urgency urinary incontinence after different vaginal
delivery modes. AM J Obstet Gynecol. 220:181.e1–181.e8.
2019.PubMed/NCBI View Article : Google Scholar
|