Obesity is a chronic lifestyle disease and the fifth
leading cause of death globally (1). While obesity is often associated with
an inability to adopt healthy habits (1), there are various other factors that
can affect fat metabolism and contribute to the development of
obesity, including genetic, environmental, pharmacological,
behavioral and sociocultural factors (2,3). For
example, certain drugs, excessive calorie consumption, low levels
of physical activity and endocrinological conditions can all
contribute to the development of obesity (4).
Obesity is typically diagnosed by determining the
body mass index (BMI), which the World Health Organization defines
as a basic weight-for-height indicator used to classify individuals
as underweight, normal weight, overweight or obese. BMI
(kg/m2) is calculated by dividing body weight in
kilograms by the square of height in meters (5). In adults, a diagnosis of overweight
is made when the BMI is 25.0-29.9 kg/m2, and obesity is
diagnosed when the BMI is ≥30 kg/m2 (6). Higher BMI values are associated with
increased mortality. Specifically, every 5-unit increase in BMI
above 25 kg/m2 has been shown to increase overall
mortality by 29%, vascular mortality by 41% and diabetes-related
mortality by 21% (6,7). In patients with obesity, a weight
loss of 5-15% of body weight is recommended to significantly
ameliorate comorbid medical conditions (8).
White adipose tissue primarily consists of
fat-storing cells known as adipocytes, which store energy from food
as triglycerides. In addition to adipocytes, this tissue also
contains a variety of immune cells, including lymphocytes,
macrophages and fibroblasts (9).
Obesity can lead to adipose tissue dysfunction and disrupt the
distribution and activity of immune cells, resulting in local and
systemic inflammation (10). This
inflammation is driven by the activation of inflammatory signaling
pathways and the increased expression of inflammatory receptors
(9). Insulin resistance and
elevated inflammatory markers are associated with visceral fat
accumulation in obesity (11).
Hyperglycemia, hypertension and dyslipidemia are the hallmarks of
metabolic syndrome, which frequently coexists with central obesity
(12). It has been demonstrated
that individuals with type 2 diabetes mellitus (T2DM) and
cardiovascular disease (CVD) who are obese and have metabolic
syndrome have a high risk of mortality (13).
The homeostatic and hedonic pathways play key roles
in the regulation of food and energy intake in humans. When energy
stores are low, the homeostatic pathway, which is controlled by the
hypothalamus and brain stem, promotes food intake (14). By contrast, the hedonic pathway
stimulates food intake during relative energy abundance by engaging
the reward or motivational components of the brain, promoting the
consumption of appetizing foods even when energy demands are low
(15). Overeating has been
reported to increase sympathetic nervous system activity, and
altered sympathetic nervous system regulation is associated with
obesity (16). By contrast,
fasting lowers the amount of food consumed and reduces sympathetic
nervous system activity (17).
Obesity is associated with reduced serotonin signaling in the
hypothalamus, which weakens the negative feedback that normally
limits food intake and thereby leads to overeating (18). Additionally, it has been suggested
that an overindulgence in appetizing foods is associated with a
reduction in dopamine signaling (19). Ghrelin, which is also known as the
hunger hormone, normally decreases in response to food consumption;
however, this regulation is impaired by obesity (20). The reduced activity of anorexigenic
hormones such as glucagon-like peptide-1 (GLP-1), peptide
tyrosine-tyrosine (PYY) and cholecystokinin has been linked to
increased food consumption in patients with obesity (21). Leptin promotes the synthesis of
anorexigenic peptides and counterbalances the effects of ghrelin;
however, leptin signaling is compromised in overweight individuals,
which results in overeating (21).
Anti-obesity medications (AOMs) are drugs are recommended by the
U.S. National Institutes of Health for individuals with a BMI of
≥30 kg/m2 who also have comorbidities such as diabetes,
hypertension, dyslipidemia or sleep apnea (22). According to the Asia-Pacific
Obesity Treatment Guidelines, AOMs should be considered for
individuals with a BMI of ≥25 kg/m2 who also have at
least one weight-related comorbidity (23).
Obesity, among other chronic illnesses, is
associated with poor outcomes in diseases such as COVID-19(24). A study found that when age, sex,
hypertension and diabetes are taken into account, obesity is
independently associated with inferior outcomes in patients with
COVID-19; specifically, compared with patients with a normal BMI,
patients with an elevated BMI had a greater risk of death and
intubation (25). A key
characteristic of the chronic inflammatory state promoted by
obesity is the aberrant activation of leukocytes that infiltrate
adipocytes and increase the release of proinflammatory cytokines
(26). This proinflammatory
environment is considered to impair lymphocyte function, thereby
increasing the risk of severe COVID-19 in obese individuals
(27).
GLP-1 was identified in the early 1980s as a
proglucagon cleavage product generated in intestinal L cells
(28). GLP-1 is a key incretin
hormone, as it is a gut-derived peptide typically released
following the consumption of glucose or a mixed meal and increases
the glucose-stimulated release of insulin at physiological plasma
concentrations (29). In 2005, a
new class of pharmacological agents, known as GLP1RAs, was
introduced for the treatment of T2DM (30). These drugs increase insulin
secretion and suppress hunger, both of which are critical in the
management of T2DM and obesity. In addition, drugs such as
tirzepatide, which target both GLP-1 receptors and other receptors
involved in energy regulation, have been developed and exhibit
potential for use in glycemic management and weight-loss strategies
(31). GLP1RAs have been
demonstrated to support weight loss, and since GLP-1 maintains its
insulinotropic efficacy in individuals with T2DM, GLP-1 may
contribute to the pharmacotherapy of this illness (32). In addition, GLP1RAs that are
resistant to degradation by dipeptidyl peptidase (DPP)-4 have
prolonged activity and target accessible receptors throughout the
body (33). Due to their limited
absorption and rapid breakdown when taken orally, most GLP1RAs are
administered subcutaneously (34).
However, several orally administered GLP1Ras are currently in
clinical development (35).
GLP1RAs are classified into two categories:
Long-acting, including albiglutide, exenatide extended release,
dulaglutide and liraglutide, and short acting, including
lixisenatide, exenatide immediate release and liraglutide, as
summarized in Table I. While the
mechanisms by which GLP1RAs lower food intake and aid weight loss
have not been fully elucidated, GLP1RAs are known to directly
interact with GLP-1 receptors in the circumventricular organs of
the hypothalamus, the hindbrain and certain regions of the brain
adjacent to ventricles that are considered to promote satiety and
weight loss (36). Notably, two
GLP1RAs, liraglutide and semaglutide, have been shown to access the
area postrema, subfornical organ and median eminence; by contrast,
the ability of dulaglutide and albiglutide, which are relatively
large molecules, to access to these regions may be limited
(37,38). Both mono-agonists targeting GLP-1
alone, and poly-agonists targeting multiple receptors, have been
developed as a result of the abundant research and development
efforts triggered by the successful outcomes of early GLP-1-based
medicines (39). Notably,
liraglutide and semaglutide have been evaluated in advanced
clinical trials (40) and approved
for clinical use. However, despite these advances, efforts to
develop safe and efficient therapies for obesity and T2DM are
ongoing, as demonstrated by the continuous growth of the
pharmacological research pipeline (41) (Table
I).
Liraglutide is a homolog of human GLP-1 that
includes a palmitic acid side chain and a lysine-to-arginine
substitution at position 34(42).
These modifications slow the rate of degradation by DPP and
increase its absorption period (42). Liraglutide has a half-life of 13 h,
and a bioavailability of 55% after a daily subcutaneous (s/c)
injection (43). As a GLP-1
analog, liraglutide delays the emptying of the stomach, reduces
appetite, inhibits glucagon secretion and enhances insulin
secretion in a glucose-dependent manner (44). Liraglutide was first approved for
the treatment of T2DM in 2010, and subsequently approved by the
U.S. Food and Drug Administration (FDA) for the treatment of adult
obesity at doses <3.0 mg in 2014, and for the treatment of
juvenile obesity in 2020. The effect of liraglutide on obesity in
adults was evaluated in five double-blind placebo-controlled
clinical studies as part of the Satiety and Clinical
Adiposity-Liraglutide Evidence trial (45,46).
The results showed a substantial variation in the effect of
liraglutide, with the majority of participants not achieving a
large (≥10%) weight loss. However, 33.1% of the participants
achieved a ≥10% weight loss with liraglutide compared with 10.6% in
the placebo group (46).
Nevertheless, both liraglutide and semaglutide have been shown to
improve glycemic management and support weight loss (47).
Semaglutide is the most recently approved GLP1RA
since these agents first entered the market in 2005(48). It has a very long half-life, which
can reach 183 h and significantly exceeds the half-lives of other
GLP1RAs (49). This GLP-1 analog,
with a high binding affinity for albumin, was developed to enable
weekly s/c GLP1RA administration (50). As a longer-acting GLP1RA,
semaglutide (C187H291N45O59) shares 94% structural similarity with
native GLP-1(51). Adults and
elderly patients with renal, hepatic or cardiovascular conditions
can safely use semaglutide (50).
Semaglutide is available in two forms: Rybelsus®,
comprising once-daily oral tablets, and Ozempic®, which
is a once-weekly s/c injection (13). In one study, semaglutide was found
to degrade into six distinct metabolites, with the parent molecule
accounting for 82.6% of the plasma concentration of the drug
(48). The same study also
reported that semaglutide was metabolized via β-oxidation of its
fatty acid side chain and proteolytic cleavage of its peptide
backbone; in addition, plasma metabolite concentrations decreased
over time, and only the parent substance was detectable in plasma
28 days after administration. However, the effects of the
metabolites on the efficacy of semaglutide and the side-effect
profile are unknown (48).
Lixisenatide and exenatide are short-acting GLP1RAs.
When injected, they produce brief peaks in plasma drug
concentrations, interspersed with intervals of barely detectable
concentrations. As a result, the pharmacodynamic profiles of these
compounds include ‘resting’ periods, when GLP-1 receptors are not
activated, and periods of several hours when effective medication
concentrations are present in the circulation. On the other hand,
the sharply delayed gastric emptying caused by the quick rise in
plasma levels of these short-acting receptor agonists significantly
reduces postprandial glucose excursions after breakfast (for
exenatide and lixisenatide) and before dinner (for exenatide)
(30).
Some GLP1RAs have been created by fusing modified
GLP-1 or GLP-1 segments to large proteins such as the
immunoglobulin (Ig) Fc fragment (dulaglutide and efpeglenatide) or
albumin (albiglutide). These compounds decay slowly, with
half-lives of ~1 week. After s/c injection, they reach their
effective circulation concentrations very quickly, thereby
decreasing plasma glucose levels soon after the start of treatment
(57). Weekly, biweekly and
monthly dosing of albiglutide has been shown to reduce glycated
hemoglobin (HbA1c) levels to a similar extent, but weekly treatment
causes the least noticeable changes in fasting plasma glucose
levels (58). Improvements in
fasting blood glucose levels have been noted as early as 2 weeks
after the initiation of albiglutide treatment (58). Although evidence suggests that
natural GLP-1 can access the central nervous system (CNS) through
regions lacking a normal blood-brain barrier (59), the large size of the albiglutide
molecule, primarily due to its two human albumin components,
suggests that it may not have the same CNS effects as smaller
GLP1RAs. Nonetheless, albiglutide treatment has been shown to
induce a small reduction in body weight, which may be the result of
the medication exerting central effects (60).
Dulaglutide consists of two GLP-1 analog peptides,
modified with amino acid substitutions that prevent degradation by
DPP-4, connected via short peptides to a modified human IgG4-Fc
heavy chain, which decreases immunogenicity and cytotoxicity while
enhancing the stability of the molecule (61). Due to its high molecular weight of
59.7 kDa, dulaglutide has limited renal clearance. In addition, it
has a half-life of ~4 days and a time-to-peak concentration of ~70
h, both of which support its use as a weekly medication and
increases patient compliance (62). Large-scale, long-term randomized
trials have been conducted, and are ongoing, to evaluate the
viability of dulaglutide as a therapeutic agent for the treatment
of T2DM (63). It has been
demonstrated that weekly doses of 0.05-8.0 mg dulaglutide for 5
weeks can reduce HbA1c levels by 0.2-1.2% (63). However, in a clinical trial, only
individuals who received the two highest weekly doses of 5 and 8 mg
demonstrated significant mean reductions in body weight of 2.5 and
2.0 kg, respectively (64).
Amylin, also known as islet amyloid polypeptide, is
a peptide hormone co-secreted with insulin that centrally controls
satiety pathways and thus regulates food intake (65). It activates certain receptors, such
as calcitonin gene-related peptide receptors (66). Amylin has been demonstrated to
influence the hedonic pathway, for example, by reducing the
activity of neural circuits that mediate the rewarding aspects of
food consumption, although it primarily affects energy metabolism
by increasing satiety (67). The
clinical use of human amylin as an anti-obesity drug is hampered by
its tendency to aggregate, which results in pancreatic islet death
(68). However, rat-derived amylin
analogs, such as pramlintide, have been developed as diabetic
treatments that improve glycemic management and provide modest but
notable weight loss (65).
Pramlintide was approved by the U.S. FDA in 2005 as an adjunct to
insulin or oral medications for individuals with T1DM and T2DM
(69). However, the effects of
pramlintide on body weight and food consumption are not limited to
individuals with diabetes. Research has indicated that moderate
weight loss can be achieved with pramlintide, regardless of
diabetic status (67). In a
preclinical trial, pramlintide was found to induce notable weight
loss and alleviate leptin resistance when paired with the leptin
analog metreleptin (70). As a
result of these findings, amylin has become a focal point for
research into the management of obesity. Several amylin-based
medications have been shown to be safe and effective in humans,
such as cagrilintide, which is a long-acting amylin receptor
agonist (71). Cagrilintide was
designed by making structural modifications to amylin to improve
its potency, solubility and duration of action, as well as reducing
its propensity to form amyloid fibrils (71). Cagrilintide has been found to
significantly reduce body weight in clinical trials (72), particularly when paired with
semaglutide (66). Notably, the
development of dual-acting amylin and calcitonin receptor agonists
is a promising area in obesity pharmacotherapy, as human amylin
receptor subtypes include calcitonin receptors complexed with
receptor activity-modifying proteins (73).
Due to the relatively weak insulinotropic effect
observed in patients with T2DM, in addition to the preclinical data
on GIP receptor (GIPR) agonism in mice with diet-induced obesity,
the use of GIPR agonists (GIPRAs) for the treatment of T2DM and
obesity was initially viewed with skepticism (74). However, long-acting GIPRAs have
been shown to reduce body weight in obese mice by signaling through
the CNS to act on GIPR-expressing neurons and cells in the
hindbrain and hypothalamus (75).
When acylated GIP was administered centrally to obese mice, they
exhibited weight loss and a reduction in food intake. Peripheral
injection results in weight loss in both wild-type and GLP1R
knockout mice; however, in CNS GIPR knockout animals, the reduction
in body weight was less notable than that in wild-type mice
(76). These findings challenge
the hypothesis that GIP promotes obesity and support the suggestion
that long-acting GIPRAs help to regulate body weight and blood
sugar levels.
In addition to promoting weight loss and appetite
control, GIPRAs have been shown to provide metabolic benefits
beyond those of GLP1RAs by acting through GLP-1
receptor-independent pathways (62). In preclinical models, GIP was shown
to counteract the emetic effects of GLP1RAs, whereas in individuals
with T2DM, GLP1RAs were found to restore the insulinotropic action
of GIP (77). Furthermore, GIPRAs
increase the storage capacity of adipocytes, thereby preventing
adipocyte lipid spillover and aberrant lipid build-up in other
tissues (78). Compounds with dual
GLP1RA/GIPRA activity have been developed, and it has been
suggested that even when the specific contribution of the GIPRA to
weight loss is unclear, it may promote weight loss by modulating
GLP-1 signaling (79). To the best
of our knowledge, tirzepatide is considered the most effective dual
GLP1RA/GIPRA. Tirzepatide has five-fold greater efficacy at the
human GIPR receptor compared with that at the GLP-1 receptor
(31). In addition, the half-life
of tirzepatide is ~117 h (31).
The U.S. FDA and the European Medicine Agency approved tirzepatide
for the treatment of T2DM based on data from the SURPASS clinical
trial (80).
The L cells of the gastrointestinal (GI) tract
produce oxyntomodulin and PYY, with a number of these cells
co-secreting both hormones (81).
Specifically, PYY and oxyntomodulin are released simultaneously
during the postprandial period (82). In addition to acting locally to
improve digestion, these hormones act as signalling molecules when
their blood levels increase, relaying information about the
postprandial shift in energy status to the brain. While
oxyntomodulin reduces gastric acid output and retards stomach
emptying (83), PYY delays
pancreatic and gallbladder secretions, slows stomach emptying and
increases ileal absorption (84).
Oxyntomodulin is a 37-amino-acid peptide that consists of glucagon
(29 amino acids) with an octapeptide C-terminal extension; it is
derived from the post-translational processing of proglucagon,
which is encoded by the glucagon gene and expressed in the
intestine (85). Prohormone
convertases 1 and 2 cleave proglucagon to generate various products
in a tissue-dependent manner (86). For example, glucagon is the main
product in the pancreas and glicentin, and GLP-1 and GLP-2 are
produced in the gut and brain. Oxyntomodulin and glicentin are also
products of proglucagon processing, and significant levels of
oxyntomodulin can be detected in the human distal intestine
(87). The tyrosine residues at
the C- and N-termini of the 36-amino acid PYY give this peptide its
name. PYY belongs to the same family as neuropeptide Y and
pancreatic polypeptide, and all of these peptides have a
distinctive U-shaped fold in their tertiary structure (88). PYY is most abundant in the rectum,
followed by the colon and ileum (89). Certain neurons in the CNS, such as
those in the hypothalamus, exhibit PYY immunoreactivity (90). The amount of PYY released from the
GI system correlates with calorie intake, with levels peaking 1-2 h
after a meal and remaining elevated for up to 6 h (88).
A promising strategy for combating cardiometabolic
disorders, including obesity and T2DM, is to reduce food intake
while boosting energy expenditure (91). However, although current
pharmaceutical approaches to accomplish these outcomes have
involved a combination of multiple receptor agonists, no safe
energy-using solution has yet made it to the clinic (92). Recently, Sass et al
(93) identified a genetic
approach for the discovery of novel candidate anti-obesity drugs by
activating NK2Rs in mice. This strategy was able to reduce appetite
without the side effects associated with earlier treatments. Mice
with diet-induced obesity received s/c injections of neurokinin A
(NKA), an NK2R ligand, twice daily for 9 days. These mice exhibited
improvements in insulin tolerance, along with decreased food
intake, body weight and inguinal and epididymal white adipose
tissue. In the study, a longer-acting version of the NK2R ligand
was created by covalently attaching a 16-carbon fatty acid
conjugate of γ-glutamic acid, which is the same moiety used in the
GLP1RA liraglutide, to the Lys2 residue of native NKA. This
modification extended the blood retention of the peptide from
minutes to hours, likely through albumin binding (93). Targeting energy expenditure is
particularly important given the reduction in the average basal
metabolic rate over the last 30 years (94). Although agonism of the glucagon
receptor has emerged as a promising approach for increasing
catabolic metabolism, an elevated heart rate, hepatic glucose
production and concerns about lean mass loss, might restrict its
application, especially for T2DM (95). Furthermore, most GLP-based drugs
cause undesirable side effects such as nausea, stomach motility
issues, and disorders of insulin and glucagon release (91).
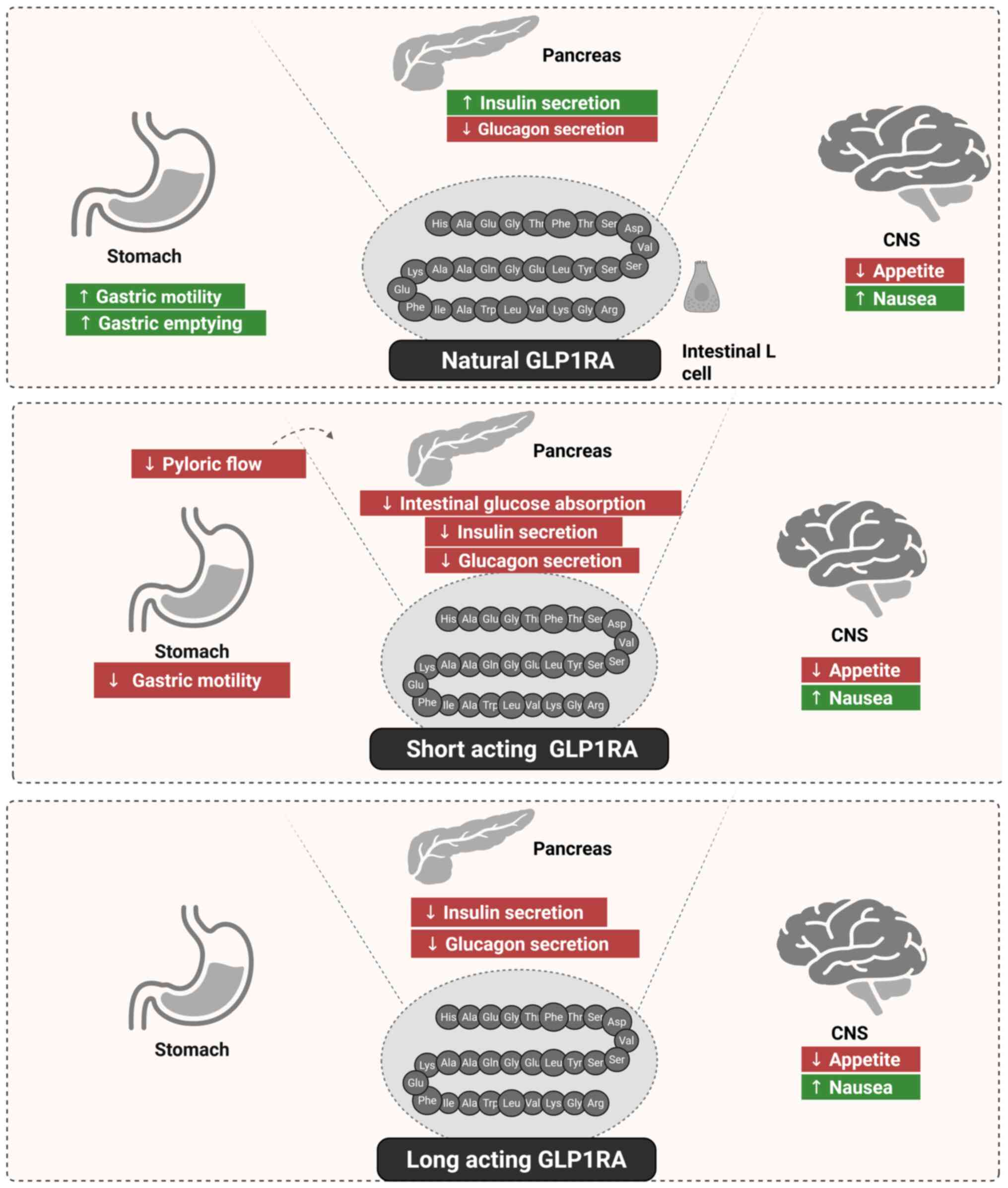
GLP-1 is secreated by specialised enteroendocrine
cells, termed L cells, in the GI tract. The secretion of GLP-1
directly stimulates the pancreas to increase insulin secretion and
decrease glucagon secretion. It also suppresses stomach motility;
delays stomach emptying and decreases hunger by acting on the CNS.
However, GLP-1 may also cause nausea (96). Short-acting GLP1RAs have been shown
to decrease transpyloric flow and suppress stomach motility
(97). These effects result in
reduced postprandial insulin secretion, delayed intestinal glucose
absorption, nausea induction and appetite suppression. Furthermore,
short-acting GLP1RAs appear to directly influence glucagon
secretion and the CNS. By contrast, long-acting GLP1RAs have been
found to affect the pancreas by directly stimulating insulin
secretion and suppressing glucagon secretion through the paracrine
production of somatostatin. These compounds also decrease appetite
and may cause nausea through their effects on the CNS (98) (Fig.
1).
Saxenda (liraglutide) is an FDA-approved GLP1RA for
chronic weight management in adults. It reduces the rate of stomach
emptying, increases satiety and reduces appetite, all of which
contribute to decreased calorie intake and, consequently, weight
loss (46). Clinical research has
shown that when is paired with a lower-calorie diet and greater
physical activity, Saxenda can lead to a weight loss ranging from 5
to 10% (46). In one randomized
controlled trial, after 1 year, individuals who received Saxenda
lost an average of 8% more of their body weight than those who
received a placebo (99) (Table II).
Mounjaro (tirzepatide) is a dual GIPRA/GLP1RA. Due
to its dual actions, Mounjaro not only reduces appetite but also
enhances insulin sensitivity and glucose regulation. It has been
suggested that its use may lead to superior weight loss and
glucose-lowering effects compared with those achieved with
single-hormone treatments, such as those targeting the GLP-1R alone
(100). A study found that
patients receiving high doses of Mounjaro experienced a weight loss
of up to 22.5% of their initial body weight over 72 weeks, along
with significant improvements in blood HbA1c levels, a marker of
long-term blood glucose control. The efficacy achieved was
comparable to that of bariatric surgery (101) (Table II).
Ozempic (semaglutide) is another GLP1RA that
stimulates insulin release, suppresses hunger and slows stomach
emptying. Ozempic, which was first licensed for the treatment of
T2DM, has also been demonstrated to induce significant weight-loss
effects, prompting the creation of a higher-dose version (Wegovy)
exclusively for the induction of weight loss (50). Studies have shown that Ozempic can
lead to a 10-15% reduction in body weight, particularly at higher
doses (102,103). In a randomized clinical trial,
participants who received Ozempic lost significantly more weight
than those in the placebo group (103). Ozempic has also been found to
improve HbA1c levels and other markers of metabolic health, making
it highly effective for both diabetes management and weight loss
(104) (Table II).
At present, to the best of our knowledge, no drugs
have been authorized for the treatment of non-monogenic,
non-syndromic obesity in children <12 years of age. Liraglutide
has been demonstrated to help obese adults and adolescents lose
weight, but its efficiency and safety in children have not been
established (105). In one study,
liraglutide treatment for 56 weeks combined with lifestyle
modifications led to a higher reduction in BMI among obese children
aged 6 to <12 years than was achieved with placebo plus
lifestyle modifications (105).
However, another study raised uncertainty about the efficacy of
anti-obesity drugs in children and teenagers (106). In a comprehensive weight
management clinic, however, semaglutide has shown promise as a safe
and effective weight loss aid for patients aged 10-18 years
(107).
Osteoarthritis (OA) is a degenerative joint disease
closely associated with obesity, as excess body weight increases
the mechanical stress on joints and exacerbates systemic
inflammation. Given that obesity is a modifiable risk factor for
OA, the use of AOMs to promote weight has been observed to improve
joint health by reducing both load and inflammation (108). Studies have shown that a 5-10%
weight loss achieved using AOMs such as Saxenda and Ozempic
significantly reduces joint load and alleviates OA symptoms,
particularly in weight-bearing joints such as the knees (109). Load reduction has been associated
with improvements in pain, function and quality of life in patients
with OA (110). As previously
reviewed, a number of studies have investigated how AOM-induced
weight loss affects bone density, and have indicated that rapid
weight reduction may raise the risk of fractures by decreasing bone
density, which a potentially serious side effect for obese patients
with OA (111). However, GLP1RA
use may reduce systemic inflammation. Specifically, the use of
Saxenda has been linked to reductions in the level of C-reactive
protein, an inflammatory marker associated with OA progression
(46).
GLP1RAs and dual-action GIPRA/GLP1RA compounds
primarily act on the digestive system by slowing gastric emptying
and enhancing satiety, leading to reduced calorie intake (112). However, side effects impacting
the GI tract, gallbladder and pancreas have been reported.
Regarding GI side effects, a notable proportion of patients
experience nausea, vomiting, diarrhoea and constipation when taking
GLP1RAs such as semaglutide and liraglutide. However, these effects
are dose-dependent and typically diminish over time (113). Clinical trial data indicate that
Mounjaro is associated with a higher incidence of nausea than other
AOMs, likely due to its dual mechanism of action (114). The rapid weight loss induced by
AOMs has also been linked to an increased risk of gallstone
formation. Specifically, a study has shown that GLP1RAs, including
Ozempic, can reduce gallbladder motility, potentially leading to
cholelithiasis (103). Although
definitive evidence is lacking, a study has indicated that the risk
of pancreatitis may be elevated among patients who use GLP1RAs,
particularly those with a history of pancreatic disorders (115). However, further research is
required to confirm existing findings.
AOMs such as Saxenda and Ozempic act on appetite
regulation centers in the hypothalamus by mimicking GLP-1, thereby
reducing hunger and increasing satiety. These actions contribute to
lower calorie intake and facilitate weight loss (50). A preliminary study has suggested
that GLP1RAs may have positive effects on mood and could provide
benefits to patients with mood disorders, such as depression
(116). However, these effects
are likely secondary to weight loss and overall health
improvements, and further investigation in this area is warranted
(117).
GLP1RAs have been demonstrated to lower the risk of
cardiovascular events in individuals with T2DM and obesity.
Specifically, the LEADER and SUSTAIN-6 trials (116) found that liraglutide and
semaglutide were associated with reductions in major adverse
cardiovascular events, such as heart attack and stroke. The
findings suggest that AOMs may confer cardiovascular protection by
lowering blood pressure, improving lipid profiles and reducing
systemic inflammation (118). In
addition, small reductions in both systolic and diastolic blood
pressure have been associated with AOM-induced weight loss.
However, some patients receiving GLP1RAs have exhibited an increase
in resting heart rate (101), and
more research is necessary to determine the therapeutic importance
of this result.
It is well established that GLP1RAs increase insulin
sensitivity and reduce blood glucose levels, making them
particularly effective for use in the management of T2DM. It is
also notable that the improved glucose regulation achieved with
GLP1RAs also mitigates diabetes-related complications such as
neuropathy and nephropathy (115). The weight loss associated with
AOMs typically involves a preferential loss of visceral fat, which
is strongly associated with the risk of metabolic disorders and
CVDs. As a result, AOMs are considered to improve metabolic health
and lower systemic inflammation by decreasing visceral adiposity
(110).
Exenatide was the first GLP1RA to receive approved,
which occurred in 2005 in the USA, followed by liraglutide in 2010
and lixisenatide in 2016. Another GLP1RA, albiglutide, was approved
in 2014 in Europe, but was taken off the market due to economic
considerations (119). In its
latest recommendations for the management of chronic coronary
syndrome, the European Society of Cardiology suggested as a class
1, level A recommendation that GLP1RAs can be used to treat T2DM
patients with CVD (120).
Similarly, the American Diabetes Association recommends the use of
GLP1RAs to treat individuals who are at high risk for CVD (121). This recommendation also includes
the use of sodium-glucose transport protein 2 inhibitors to treat
patients with heart failure or chronic renal disease (119). Regarding substances of abuse
other than food, the effects of GLP1RAs on alcohol consumption in
laboratory animals, such as male rats, mice and non-human primates
have been investigated (122,123). In addition, it has been observed
that systemic exenatide injection inhibits cocaine-conditioned
location preference in mice (124,125). Exenatide or liraglutide
administration has also been shown to lessen the psychomotor
stimulant effects of amphetamine in rats and mice, as evidenced by
changes in locomotor activity (126). Furthermore, in several tests,
liraglutide attenuated the harmful effects of amphetamine on
cognitive function in rats (127).
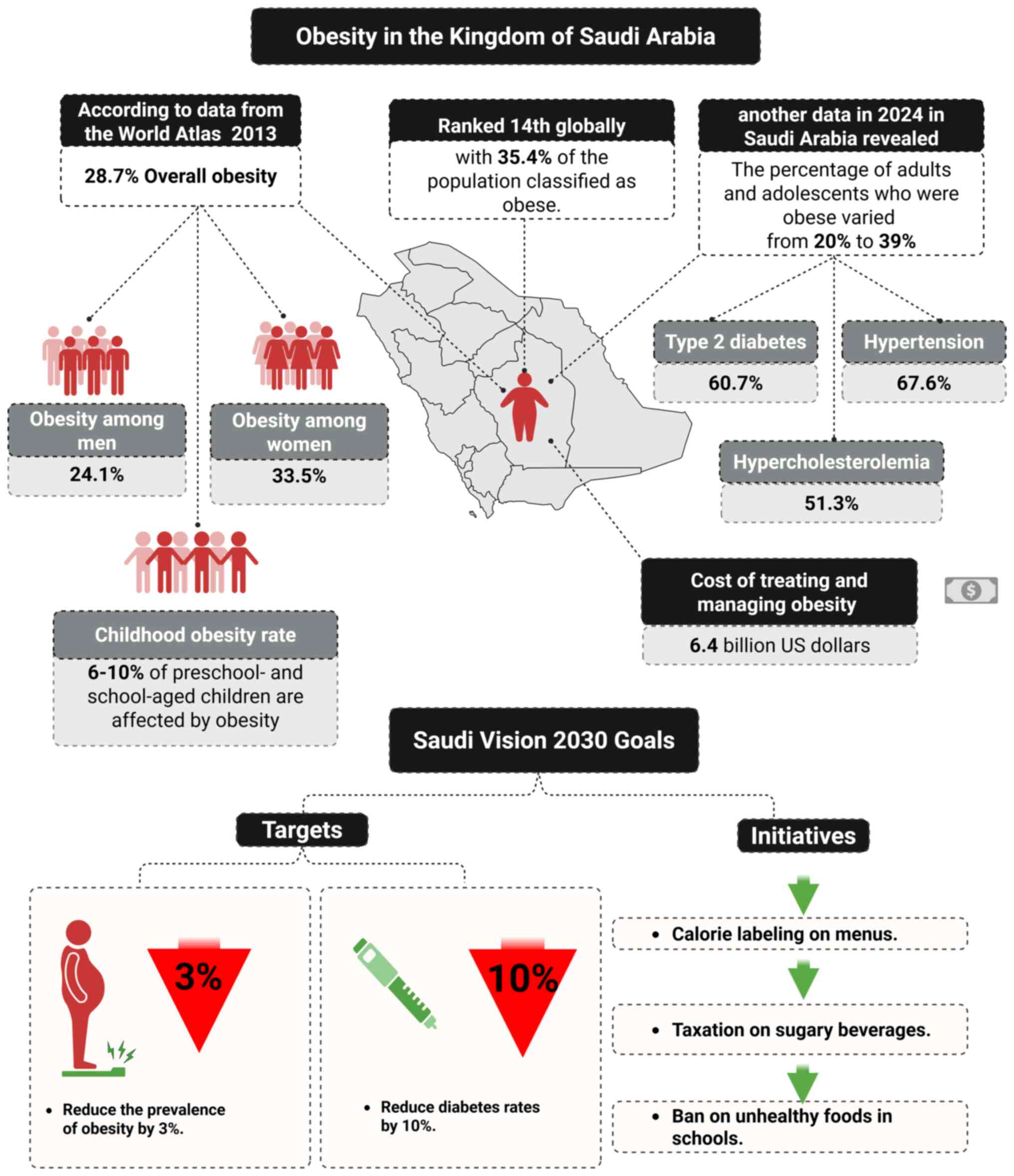
A nationwide survey conducted between 1995 and 2000
indicated that 35.6% of the Saudi population were obese (128). Additionally, a subsequent
systematic review of obese and overweight adults in Middle Eastern
countries from 2000 to 2020 indicated that the prevalence of
obesity in the KSA was 24.95%, (129), and 31.80% of individuals in the
KSA were overweight (130).
According to the World Atlas, 35.4% of the population of the KSA is
obese, making it the 14th most obese nation in the world (131). In addition, a national study
performed in 2013 found that the overall prevalence of obesity in
the KSA was 28.7%, with 33.5% of women and 24.1% of men being obese
(132). Furthermore, a Survey of
Health Information in the KSA (131) reported that childhood obesity was
increasing in the KSA and affected 6-10% of preschool- and
school-aged children. Therefore, both adult and childhood obesity
are major national public health issues (131). Data reported in 2024 indicate
that 20% of adults and 39% of adolescents are obese, with T2DM
(60.7%), hypertension (67.6%) and hypercholesterolemia (51.3%)
being the most commonly reported obesity-related conditions.
Additionally, an association between obesity and obesity-related
comorbidities was established, indicating an increased risk of
comorbidity as BMI rises, and the cost of treating and managing
obesity in the KSA was estimated to be $6.4 billion (133).
Obesity is a major issue in the KSA. Previous
research has shown an increase in overweight and obesity rates in
the KSA, which are major risk factors for other disorders,
including diabetes, obstructive sleep apnoea, hyperlipidaemia and
OA (134). The direct healthcare
costs of managing overweight- and obesity-related conditions was
estimated to be $3.8 billion in 2019, accounting for 4.3% of the
total healthcare spending in the KSA (135). In addition, the medical cost of
reduced productivity while at work (presenteeism) or absent from
work (absenteeism) due to weight-related health issues was
estimated to be $15.5 billion in 2019, representing 0.9% of the
gross domestic product. Therefore, these findings indicate that
obesity and excess weight pose a significant financial burden in
the KSA (135).
The KSA aims to reduce the prevalence of obesity by
3% and diabetes by 10% by 2030. To help consumers make improved
choices, measures such as mandatory calorie labeling on menus in
all food service facilities have been implemented. In addition, as
part of a strategy to manage obesity, the implementation of a 50%
tax on sugar-sweetened beverages and a 100% levy on energy drinks
introduced in 2017 has resulted in the sales of carbonated drinks
dropping by 35%. A number of mandatory and optional measures have
also been implemented to encourage businesses to reduce the sugar,
fat and salt content of their food and beverage products (136). Furthermore several school-based
policies have been established with the aim of preventing obesity.
For example, a wide range of products are banned from canteens,
including sweets, chips, soft drinks and fried food (137). Priority is now also being given
to child-focused interventions and the promotion of physical
activity in all schools. In 2017, the KSA first permitted physical
education in girls' schools and launched ‘Rashaqa’, a promising
program implemented in boys' and girls' schools as part of broader
efforts to address obesity by improving dietary habits and
promoting physical activity (136,137) (Fig.
2).
The rising incidence of obesity, a global health
concern associated with numerous complications, necessitates
creative solutions. The history of pharmacological obesity
treatment is long and encompasses notable setbacks. Large-scale,
long-term clinical trials in diverse individuals with obesity are
costly to conduct and challenging to justify given previous
failures. As part of Saudi Vision 2030, a number of measures are
being used to lessen the negative effects of obesity on diet and
health. The most commonly used AOMs include compounds targeting
GLP-1 and amylin, with emerging compounds under investigation
targeting GIP, oxyntomodulin and PYY. Promising new strategies in
development include stable PYY analogs, improved amylin analogs and
NK2R-targeting agents, which may offer more precise and efficient
treatments for the treatment of obesity, particularly in
individuals with T2DM. Saxenda, Mounjaro and Ozempic have exhibited
significant effects on weight loss and T2DM. Childhood obesity is
also a global public health concern; however, only a limited number
of AOMs are currently approved to manage this condition under
medical supervision. In addition, AOMs have been observed to have
positive benefits on cardiovascular risk factors and joint health.
Preclinical research suggests that the activation of GPL-1Rs may
reduce alcohol and cocaine abuse. Finally, ongoing clinical
research aims to ascertain whether certain AOMs could be as
effective as bariatric surgery. In conclusion, ongoing advances in
pharmacological development may help to address current challenges
and achieve superior outcomes in the treatment of obesity.
Not applicable.
Funding: No funding was received.
Not applicable.
AA and SSA were responsible for conception and
design. AA, SSA and EHE performed data collection and/or
processing, and searched the literature. SSA, EHE, AA, SMA and OD
wrote and proofread the review. All authors read and approved the
final version of the manuscript. Data authentication was not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Safaei M, Sundararajan EA, Driss M,
Boulila W and Shapi'i A: A systematic literature review on obesity:
Understanding the causes & consequences of obesity and
reviewing various machine learning approaches used to predict
obesity. Comput Biol Med. 136(104754)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Konttinen H: Emotional eating and obesity
in adults: The role of depression, sleep and genes. Proc Nutr Soc.
79:283–289. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tappia PS and Defries D: Prevalence,
consequences, causes and management of obesity. In: Pathophysiology
of Obesity-Induced Health Complications. Springer, Heidelberg,
pp3-22, 2020.
|
|
4
|
Omer T: The causes of obesity: An in-depth
review. Adv Obes Weight Manag Control. 10:90–94. 2020.
|
|
5
|
Franco M, Orduñez P, Caballero B, Tapia
Granados JA, Lazo M, Bernal JL, Guallar E and Cooper RS: Impact of
energy intake, physical activity, and population-wide weight loss
on cardiovascular disease and diabetes mortality in Cuba,
1980-2005. Am J Epidemiol. 166:1374–1380. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Popoviciu MS, Păduraru L, Yahya G,
Metwally K and Cavalu S: Emerging role of GLP-1 agonists in
obesity: A comprehensive review of randomised controlled trials.
Int J Mol Sci. 24(10449)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lebovitz HE and Banerji MA: Point:
Visceral adiposity is causally related to insulin resistance.
Diabetes Care. 28:2322–2325. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Crowley N, Arlinghaus KR and Myers ES:
Dietary management of overweight and obesity. In: Lifestyle
Medicine. 3rd edition. CRC Press, pp483-489, 2019.
|
|
9
|
Hotamisligil GS: Inflammation and
metabolic disorders. Nature. 444:860–867. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Maurizi G, Della Guardia L, Maurizi A and
Poloni A: Adipocytes properties and crosstalk with immune system in
obesity-related inflammation. J Cell Physiol. 233:88–97.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Herman MA, Peroni OD, Villoria J, Schön
MR, Abumrad NA, Blüher M, Klein S and Kahn BB: A novel ChREBP
isoform in adipose tissue regulates systemic glucose metabolism.
Nature. 484:333–338. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Grundy SM: Metabolic complications of
obesity. Endocrine. 13:155–165. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mahapatra MK, Karuppasamy M and Sahoo BM:
Semaglutide, a glucagon like peptide-1 receptor agonist with
cardiovascular benefits for management of type 2 diabetes. Rev
Endocr Metab Disord. 23:521–539. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lutter M and Nestler EJ: Homeostatic and
hedonic signals interact in the regulation of food intake. J Nutr.
139:629–632. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ferrario CR, Labouèbe G, Liu S, Nieh EH,
Routh VH, Xu S and O'Connor EC: Homeostasis meets motivation in the
battle to control food intake. J Neurosci. 36:11469–11481.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Welle S and Campbell RG: Stimulation of
thermogenesis by carbohydrate overfeeding. Evidence against
sympathetic nervous system mediation. J Clin Invest. 71:916–925.
1983.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Young JB and Landsberg L: Suppression of
sympathetic nervous system during fasting. Obes Res. 5:646–649.
1997.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hesse S, van de Giessen E, Zientek F,
Petroff D, Winter K, Dickson JC, Tossici-Bolt L, Sera T, Asenbaum
S, Darcourt J, et al: Association of central serotonin transporter
availability and body mass index in healthy Europeans. Eur
Neuropsychopharmacol. 24:1240–1247. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Blum K, Thanos PK and Gold MS: Dopamine
and glucose, obesity, and reward deficiency syndrome. Front
Psychol. 5(919)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
English PJ, Ghatei MA, Malik IA, Bloom SR
and Wilding JPH: Food fails to suppress ghrelin levels in obese
humans. J Clin Endocrinol Metab. 87(2984)2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
El-Haschimi K, Pierroz DD, Hileman SM,
Bjørbæk C and Flier JS: Two defects contribute to hypothalamic
leptin resistance in mice with diet-induced obesity. J Clin Invest.
105:1827–1832. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Apovian CM, Aronne LJ, Bessesen DH,
McDonnell ME, Murad MH, Pagotto U, Ryan DH and Still CD: Endocrine
Society. Pharmacological management of obesity: An endocrine
society clinical practice guideline. J Clin Endocrinol Metab.
100:342–362. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Obesity WHOJIAftSo, International Obesity
Task Force TA-Ppro and treatment i: Regional Office for the Western
Pacific, 2000.
|
|
24
|
Goyal P, Choi JJ, Pinheiro LC, Schenck EJ,
Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, et
al: Clinical characteristics of Covid-19 in New York city. N Engl J
Med. 382:2372–2374. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nakeshbandi M, Maini R, Daniel P,
Rosengarten S, Parmar P, Wilson C, Kim JM, Oommen A, Mecklenburg M,
Salvani J, et al: The impact of obesity on COVID-19 complications:
A retrospective cohort study. Int J Obes (Lond). 44:1832–1837.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Haupt TH, Kallemose T, Ladelund S,
Rasmussen LJ, Thorball CW, Andersen O, Pisinger C and Eugen-Olsen
J: Risk factors associated with serum levels of the inflammatory
biomarker soluble urokinase plasminogen activator receptor in a
general population. Biomark Insights. 9:91–100. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Frasca D, Reidy L, Cray C, Diaz A, Romero
M, Kahl K and Blomberg BB: Influence of obesity on serum levels of
SARS-CoV-2-specific antibodies in COVID-19 patients. PLoS One.
16(e0245424)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bell GI, Sanchez-Pescador R, Laybourn PJ
and Najarian RCJN: Exon duplication and divergence in the human
preproglucagon gene. Nature. 304:368–371. 1983.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gutniak M, Orskov C, Holst JJ, Ahrén B and
Efendic S: Antidiabetogenic effect of glucagon-like peptide-1
(7-36) amide in normal subjects and patients with diabetes
mellitus. N Engl J Med. 326:1316–1322. 1992.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Drucker DJ, Buse JB, Taylor K, Kendall DM,
Trautmann M, Zhuang D and Porter L: DURATION-1 Study Group.
Exenatide once weekly versus twice daily for the treatment of type
2 diabetes: A randomised, open-label, non-inferiority study.
Lancet. 372:1240–1250. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Coskun T, Sloop KW, Loghin C,
Alsina-Fernandez J, Urva S, Bokvist KB, Cui X, Briere DA, Cabrera
O, Roell WC, et al: LY3298176, a novel dual GIP and GLP-1 receptor
agonist for the treatment of type 2 diabetes mellitus: From
discovery to clinical proof of concept. Mol Metab. 18:3–14.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nauck MA, Heimesaat MM, Orskov C, Holst
JJ, Ebert R and Creutzfeldt W: Preserved incretin activity of
glucagon-like peptide 1 [7-36 amide] but not of synthetic human
gastric inhibitory polypeptide in patients with type-2 diabetes
mellitus. J Clin Invest. 91:301–307. 1993.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Holst JJ: Glucagon-like peptide-1: Are its
roles as endogenous hormone and therapeutic wizard congruent? J
Intern Med. 291:557–573. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kim HS and Jung CH: Oral semaglutide, the
first ingestible glucagon-like peptide-1 receptor agonist: Could it
be a magic bullet for type 2 diabetes? Int J Mol Sci.
22(9936)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Saxena AR, Gorman DN, Esquejo RM, Bergman
A, Chidsey K, Buckeridge C, Griffith DA and Kim AM: Danuglipron
(PF-06882961) in type 2 diabetes: A randomized, placebo-controlled,
multiple ascending-dose phase 1 trial. Nat Med. 27:1079–1087.
2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xia L, Shen T, Dong W, Su F, Wang J, Wang
Q, Niu S and Fang Y: Comparative efficacy and safety of 8 GLP-1RAs
in patients with type 2 diabetes: A network meta-analysis. Diabetes
Res Clin Pract. 177(108904)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gabery S, Salinas CG, Paulsen SJ,
Ahnfelt-Rønne J, Alanentalo T, Baquero AF, Buckley ST, Farkas E,
Fekete C, Frederiksen KS, et al: Semaglutide lowers body weight in
rodents via distributed neural pathways. JCI Insight.
5(e133429)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Secher A, Jelsing J, Baquero AF,
Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL,
Pyke C, Raun K, et al: The arcuate nucleus mediates GLP-1 receptor
agonist liraglutide-dependent weight loss. J Clin Invest.
124:4473–4488. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Brandt SJ, Müller TD, DiMarchi RD, Tschöp
MH and Stemmer K: Peptide-based multi-agonists: A new paradigm in
metabolic pharmacology. J Intern Med. 284:581–602. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wadden TA, Tronieri JS, Sugimoto D, Lund
MT, Auerbach P, Jensen C and Rubino D: Liraglutide 3.0 mg and
intensive behavioral therapy (IBT) for obesity in primary care: The
SCALE IBT randomized controlled trial. Obesity (Silver Spring).
28:529–536. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Müller TD, Blüher M, Tschöp MH and
DiMarchi RD: Anti-obesity drug discovery: Advances and challenges.
Nat Rev Drug Discov. 21:201–223. 2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lin CH, Shao L, Zhang YM, Tu YJ, Zhang Y,
Tomlinson B, Chan P and Liu Z: An evaluation of liraglutide
including its efficacy and safety for the treatment of obesity.
Expert Opin Pharmacother. 21:275–285. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kela R, Khunti K and Davies MJ:
Liraglutide for type 2 diabetes mellitus. Expert Opin Biol Ther.
11:951–959. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gallwitz B: Glucagon-like peptide-1
analogues for Type 2 diabetes mellitus: Current and emerging
agents. Drugs. 71:1675–1688. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Le Roux CW, Astrup A, Fujioka K, Greenway
F, Lau DCW, Van Gaal L, Ortiz RV, Wilding JPH, Skjøth TV, Manning
LS, et al: 3 Years of liraglutide versus placebo for type 2
diabetes risk reduction and weight management in individuals with
prediabetes: A randomised, double-blind trial. Lancet.
389:1399–1409. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pi-Sunyer X, Astrup A, Fujioka K, Greenway
F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R,
Jensen CB, et al: A randomized, controlled trial of 3.0 mg of
liraglutide in weight management. N Engl J Med. 373:11–22.
2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Grill HJ: A role for GLP-1 in treating
hyperphagia and obesity. Endocrinology. 161(bqaa093)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hall S, Isaacs D and Clements JN:
Pharmacokinetics and clinical implications of semaglutide: A new
glucagon-like peptide (GLP)-1 receptor agonist. Clin Pharmacokinet.
57:1529–1538. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Christou GA, Katsiki N, Blundell J,
Fruhbeck G and Kiortsis DN: Semaglutide as a promising antiobesity
drug. Obes Rev. 20:805–815. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wilding JPH, Batterham RL, Calanna S,
Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran
MTD, Wadden TA, et al: Once-weekly semaglutide in adults with
overweight or obesity. N Engl J Med. 384:989–1002. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Knudsen LB and Lau J: The discovery and
development of liraglutide and semaglutide. Front Endocrinol
(Lausanne). 10(155)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
ElSayed NA, Aleppo G, Aroda VR, Bannuru
RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D,
Johnson EL, et al: 9. Pharmacologic approaches to glycemic
treatment: Standards of care in diabetes-2023. Diabetes Care. 46
(Suppl 1):S140–S157. 2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lau J, Bloch P, Schäffer L, Pettersson I,
Spetzler J, Kofoed J, Madsen K, Knudsen LB, McGuire J, Steensgaard
DB, et al: Discovery of the once-weekly glucagon-like peptide-1
(GLP-1) analogue semaglutide. J Med Chem. 58:7370–7380.
2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Buckley ST, Bækdal TA, Vegge A, Maarbjerg
SJ, Pyke C, Ahnfelt-Rønne J, Madsen KG, Schéele SG, Alanentalo T,
Kirk RK, et al: Transcellular stomach absorption of a derivatized
glucagon-like peptide-1 receptor agonist. Sci Transl Med.
10(eaar7047)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Aroda VR, Rosenstock J, Terauchi Y,
Altuntas Y, Lalic NM, Morales Villegas EC, Jeppesen OK,
Christiansen E, Hertz CL and Haluzík M: PIONEER 1 Investigators.
PIONEER 1: Randomized clinical trial of the efficacy and safety of
oral semaglutide monotherapy in comparison with placebo in patients
with type 2 diabetes. Diabetes Care. 42:1724–1732. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wharton S, Blevins T, Connery L,
Rosenstock J, Raha S, Liu R, Ma X, Mather KJ, Haupt A, Robins D, et
al: Daily oral GLP-1 receptor agonist orforglipron for adults with
obesity. N Engl J Med. 389:877–888. 2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Davies M, Pieber TR, Hartoft-Nielsen ML,
Hansen OK, Jabbour S and Rosenstock J: Effect of oral semaglutide
compared with placebo and subcutaneous semaglutide on glycemic
control in patients with type 2 diabetes: A randomized clinical
trial. JAMA. 318:1460–1470. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Reusch JE, Rosenstock J, Bush MA, Yang F
and Stewart MW: Albiglutide, a long-acting GLP-1 receptor agonist,
improves glycemia in type 2 diabetes: Time-course analysis. In:
American Diabetes Association, pA123, 2009.
|
|
59
|
Ørskov C, Poulsen SS, Mørten M and Holst
JJ: Glucagon-like peptide I receptors in the subfornical organ and
the area postrema are accessible to circulating glucagon-like
peptide I. Diabetes. 45:832–835. 1996.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Rosenstock J, Reusch J, Bush M, Yang F and
Stewart M: Albiglutide Study Group. Potential of albiglutide, a
long-acting GLP-1 receptor agonist, in type 2 diabetes: A
randomized controlled trial exploring weekly, biweekly, and monthly
dosing. Diabetes Care. 32:1880–1886. 2009.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Glaesner W, Vick AM, Millican R, Ellis B,
Tschang SH, Tian Y, Bokvist K, Brenner M, Koester A, Porksen N, et
al: Engineering and characterization of the long-acting
glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein.
Diabetes Metab Res Rev. 26:287–296. 2010.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhang Q, Delessa CT, Augustin R, Bakhti M,
Colldén G, Drucker DJ, Feuchtinger A, Caceres CG, Grandl G, Harger
A, et al: The glucose-dependent insulinotropic polypeptide (GIP)
regulates body weight and food intake via CNS-GIPR signaling. Cell
Metab. 33:833–844.e5. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Barrington P, Chien JY, Tibaldi F,
Showalter HD, Schneck K and Ellis B: LY2189265, a long-acting
glucagon-like peptide-1 analogue, showed a dose-dependent effect on
insulin secretion in healthy subjects. Diabetes Obes Metab.
13:434–438. 2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Umpierrez G, Blevins T, Rosenstock J,
Cheng C, Anderson JH and Bastyr EJ III: EGO Study Group. The
effects of LY2189265, a long-acting glucagon-like peptide-1
analogue, in a randomized, placebo-controlled, double-blind study
of overweight/obese patients with type 2 diabetes: The EGO study.
Diabetes Obes Metab. 13:418–425. 2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Hollander P, Maggs DG, Ruggles JA, Fineman
M, Shen L, Kolterman OG and Weyer C: Effect of pramlintide on
weight in overweight and obese insulin-treated type 2 diabetes
patients. Obes Res. 12:661–668. 2004.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Enebo LB, Berthelsen KK, Kankam M, Lund
MT, Rubino DM, Satylganova A and Lau DCW: Safety, tolerability,
pharmacokinetics, and pharmacodynamics of concomitant
administration of multiple doses of cagrilintide with semaglutide
2·4 mg for weight management: A randomised, controlled, phase 1b
trial. Lancet. 397:1736–1748. 2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Boyle CN, Lutz TA and Le Foll C:
Amylin-its role in the homeostatic and hedonic control of eating
and recent developments of amylin analogs to treat obesity. Mol
Metab. 8:203–210. 2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Ling W, Huang YM, Qiao YC, Zhang XX and
Zhao HL: Human amylin: From pathology to physiology and
pharmacology. Curr Protein Pept Sci. 20:944–957. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Day C: Amylin analogue as an antidiabetic
agent. Br J Diabetes Vasc Dis. 5:151–154. 2005.
|
|
70
|
Ravussin E, Smith SR, Mitchell JA,
Shringarpure R, Shan K, Maier H, Koda JE and Weyer C: Enhanced
weight loss with pramlintide/metreleptin: An integrated
neurohormonal approach to obesity pharmacotherapy. Obesity (Silver
Spring). 17:1736–1743. 2009.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Kruse T, Hansen JL, Dahl K, Schäffer L,
Sensfuss U, Poulsen C, Schlein M, Hansen AMK, Jeppesen CB,
Dornonville de la Cour C, et al: Development of cagrilintide, a
long-acting amylin analogue. J Med Chem. 64:11183–11194.
2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Lau DCW, Erichsen L, Francisco AM,
Satylganova A, le Roux CW, McGowan B, Pedersen SD, Pietiläinen KH,
Rubino D and Batterham RL: Once-weekly cagrilintide for weight
management in people with overweight and obesity: A multicentre,
randomised, double-blind, placebo-controlled and active-controlled,
dose-finding phase 2 trial. Lancet. 398:2160–2172. 2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Sidrak WR, Kalra S and Kalhan A: Approved
and emerging hormone-based anti-obesity medications: A review
article. Indian J Endocrinol Metab. 28:445–460. 2024.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Kaneko K, Fu Y, Lin HY, Cordonier EL, Mo
Q, Gao Y, Yao T, Naylor J, Howard V, Saito K, et al: Gut-derived
GIP activates central Rap1 to impair neural leptin sensitivity
during overnutrition. J Clin Invest. 129:3786–3791. 2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Mroz PA, Finan B, Gelfanov V, Yang B,
Tschöp MH, DiMarchi RD and Perez-Tilve D: Optimized GIP analogs
promote body weight lowering in mice through GIPR agonism not
antagonism. Mol Metab. 20:51–62. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Adriaenssens AE, Biggs EK, Darwish T,
Tadross J, Sukthankar T, Girish M, Polex-Wolf J, Lam BY, Zvetkova
I, Pan W, et al: Glucose-dependent insulinotropic polypeptide
receptor-expressing cells in the hypothalamus regulate food intake.
Cell Metab. 30:987–996.e6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Borner T, Workinger JL, Tinsley IC, Fortin
SM, Stein LM, Chepurny OG, Holz GG, Wierzba AJ, Gryko D, Nexø E, et
al: Corrination of a GLP-1 receptor agonist for glycemic control
without emesis. Cell Rep. 31(107768)2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Samms RJ, Coghlan MP and Sloop KW: How may
GIP enhance the therapeutic efficacy of GLP-1? Trends Endocrinol
Metab. 31:410–421. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Holst JJ and Rosenkilde MM: GIP as a
therapeutic target in diabetes and obesity: Insight from incretin
co-agonists. J Clin Endocrinol Metab. 105:e2710–e2716.
2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Rosenstock J, Wysham C, Frías JP, Kaneko
S, Lee CJ, Fernández Landó L, Mao H, Cui X, Karanikas CA and Thieu
VT: Efficacy and safety of a novel dual GIP and GLP-1 receptor
agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A
double-blind, randomised, phase 3 trial. Lancet. 398:143–155.
2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Taylor R, Beveridge D and Fuller PJ:
Expression of ileal glucagon and peptide tyrosine-tyrosine genes.
Response to inhibition of polyamine synthesis in the presence of
massive small-bowel resection. Biochem J. 286:737–741.
1992.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Anini Y, Fu-Cheng X, Cuber JC, Kervran A,
Chariot J and Roz C: Comparison of the postprandial release of
peptide YY and proglucagon-derived peptides in the rat. Pflugers
Arch. 438:299–306. 1999.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Schjoldager BT, Baldissera FG, Mortensen
PE, Holst JJ and Christiansen J: Oxyntomodulin: A potential hormone
from the distal gut. Pharmacokinetics and effects on gastric acid
and insulin secretion in man. Eur J Clin Invest. 18:499–503.
1988.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Hoentjen F, Hopman WP and Jansen JB:
Effect of circulating peptide YY on gallbladder emptying in humans.
Scand J Gastroenterol. 36:1086–1091. 2001.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Tang-Christensen M, Vrang N and Larsen PJ:
Glucagon-like peptide containing pathways in the regulation of
feeding behaviour. Int J Obes Relat Metab Disord. 25 (Suppl
5):S42–S47. 2001.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Holst JJ: Glucagon-like peptide 1 (GLP-1):
An intestinal hormone, signalling nutritional abundance, with an
unusual therapeutic potential. Trends Endocrinol Metab. 10:229–235.
1999.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Baldissera FG and Holst JJ:
Glucagon-related peptides in the human gastrointestinal mucosa.
Diabetologia. 26:223–228. 1984.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Glover I, Haneef I, Pitts J, Wood S, Moss
D, Tickle I and Blundell T: Conformational flexibility in a small
globular hormone: X-ray analysis of avian pancreatic polypeptide at
0.98-A resolution. Biopolymers. 22:293–304. 1983.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Adrian TE, Ferri GL, Bacarese-Hamilton AJ,
Fuessl HS, Polak JM and Bloom SR: Human distribution and release of
a putative new gut hormone, peptide YY. Gastroenterology.
89:1070–1077. 1985.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Ekblad E and Sundler F: Distribution of
pancreatic polypeptide and peptide YY. Peptides. 23:251–261.
2002.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Christoffersen BØ, Sanchez-Delgado G, John
LM, Ryan DH, Raun K and Ravussin E: Beyond appetite regulation:
Targeting energy expenditure, fat oxidation, and lean mass
preservation for sustainable weight loss. Obesity (Silver Spring).
30:841–857. 2022.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Finan B, Yang B, Ottaway N, Smiley DL, Ma
T, Clemmensen C, Chabenne J, Zhang L, Habegger KM, Fischer K, et
al: A rationally designed monomeric peptide triagonist corrects
obesity and diabetes in rodents. Nat Med. 21:27–36. 2015.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Sass F, Ma T, Ekberg JH, Kirigiti M, Ureña
MG, Dollet L, Brown JM, Basse AL, Yacawych WT, Burm HB, et al: NK2R
control of energy expenditure and feeding to treat metabolic
diseases. Nature. 635:987–1000. 2024.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Speakman JR, de Jong JMA, Sinha S,
Westerterp KR, Yamada Y, Sagayama H, Ainslie PN, Anderson LJ, Arab
L, Bedu-Addo K, et al: Total daily energy expenditure has declined
over the past three decades due to declining basal expenditure, not
reduced activity expenditure. Nat Metab. 5:579–588. 2023.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Hauser AS, Attwood MM, Rask-Andersen M,
Schiöth HB and Gloriam DE: Trends in GPCR drug discovery: New
agents, targets and indications. Nat Rev Drug Discov. 16:829–842.
2017.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Brown JC, Mutt V and Pederson RA: Further
purification of a polypeptide demonstrating enterogastrone
activity. J Physiol. 209:57–64. 1970.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Buse JB, Rosenstock J, Sesti G, Schmidt
WE, Montanya E, Brett JH, Zychma M and Blonde L: LEAD-6 Study
Group. Liraglutide once a day versus exenatide twice a day for type
2 diabetes: A 26-week randomised, parallel-group, multinational,
open-label trial (LEAD-6). Lancet. 374:39–47. 2009.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Meier JJ: GLP-1 receptor agonists for
individualized treatment of type 2 diabetes mellitus. Nat Rev
Endocrinol. 8:728–742. 2012.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Astrup A, Rössner S, Van Gaal L, Rissanen
A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF and Lean ME:
NN8022-1807 Study Group. Effects of liraglutide in the treatment of
obesity: A randomised, double-blind, placebo-controlled study.
Lancet. 374:1606–1616. 2009.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Jastreboff AM, Aronne LJ and Stefanski A:
Tirzepatide once weekly for the treatment of obesity. Reply. N Engl
J Med. 387:1434–1435. 2022.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Frías JP, Davies MJ, Rosenstock J, Pérez
Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X and Brown K:
SURPASS-2 Investigators. Tirzepatide versus semaglutide once weekly
in patients with type 2 diabetes. N Engl J Med. 385:503–515.
2021.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Garvey WT, Batterham RL, Bhatta M, Buscemi
S, Christensen LN, Frias JP, Jódar E, Kandler K, Rigas G, Wadden
TA, et al: Two-year effects of semaglutide in adults with
overweight or obesity: The STEP 5 trial. Nat Med. 28:2083–2091.
2022.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Chao AM, Tronieri JS, Amaro A and Wadden
TA: Clinical insight on semaglutide for chronic weight management
in adults: Patient selection and special considerations. Drug Des
Devel Ther. 16:4449–4461. 2022.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Davies MJ, D'Alessio DA, Fradkin J, Kernan
WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ and Buse
JB: Management of hyperglycemia in type 2 diabetes, 2018. A
consensus report by the American diabetes association (ADA) and the
European association for the study of diabetes (EASD). Diabetes
Care. 41:2669–2701. 2018.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Torbahn G, Lischka J, Brown T, Ells LJ,
Kelly AS, Wabitsch M and Weghuber D: Anti-obesity medication in the
management of children and adolescents with obesity: Recent
developments and research gaps. Clin Endocrinol (Oxf). 102:51–61.
2025.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Torbahn G, Jones A, Griffiths A, Matu J,
Metzendorf MI, Ells LJ, Gartlehner G, Kelly AS, Weghuber D and
Brown T: Pharmacological interventions for the management of
children and adolescents living with obesity-An update of a
Cochrane systematic review with meta-analyses. Pediatr Obes.
19(e13113)2024.PubMed/NCBI View Article : Google Scholar
|
|
107
|
van Boxel EJ, Rahman S, Lai K, Boulos N
and Davis N: Semaglutide treatment for children with obesity: An
observational study. Arch Dis Child. 109:822–825. 2024.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Nedunchezhiyan U, Varughese I, Sun AR, Wu
X, Crawford R and Prasadam I: Obesity, inflammation, and immune
system in osteoarthritis. Front Immunol. 13(907750)2022.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Jensen MD, Ryan DH, Apovian CM, Ard JD,
Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF,
et al: 2013 AHA/ACC/TOS guideline for the management of overweight
and obesity in adults: A report of the American college of
cardiology/American heart association task force on practice
guidelines and the obesity society. Circulation. 129 (Suppl
2):S102–S138. 2014.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Ryan DH and Yockey SR: Weight loss and
improvement in comorbidity: Differences at 5, 10, 15%, and over.
Curr Obes Rep. 6:187–194. 2017.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Roseti L, Desando G, Cavallo C, Petretta M
and Grigolo B: Articular cartilage regeneration in osteoarthritis.
Cells. 8(1305)2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Moiz A, Filion KB, Tsoukas MA, Yu OH,
Peters TM and Eisenberg MJ: Mechanisms of GLP-1 receptor
agonist-induced weight loss: A review of central and peripheral
pathways in appetite and energy regulation. Am J Med: Jan 31, 2025
(Epub ahead of print).
|
|
113
|
Petrongolo G: Liraglutide and
cardiovascular outcomes in diabetes (LEADER)-2016. In: Top Articles
in Primary Care. Russell J and Skolnik NS (eds). Springer
International Publishing, Cham, pp91-94, 2023.
|
|
114
|
Bhagavathula AS, Vidyasagar K and Tesfaye
W: Efficacy and safety of tirzepatide in patients with type 2
diabetes mellitus: A systematic review and meta-analysis of
randomized phase II/III trials. Pharmaceuticals (Basel).
14(991)2021.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Davies MJ, Bergenstal R, Bode B, Kushner
RF, Lewin A, Skjøth TV, Andreasen AH, Jensen CB and DeFronzo RA:
NN8022-1922 Study Group. Efficacy of liraglutide for weight loss
among patients with type 2 diabetes: The SCALE diabetes randomized
clinical trial. JAMA. 314:687–699. 2015.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Westermeier F and Fisman EZ: Correction:
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) and
cardiometabolic protection: Historical development and future
challenges. Cardiovasc Diabetol. 24(118)2025.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Cefalu WT, Kaul S, Gerstein HC, Holman RR,
Zinman B, Skyler JS, Green JB, Buse JB, Inzucchi SE, Leiter LA, et
al: Cardiovascular outcomes trials in type 2 diabetes: Where do we
go from here? Reflections from a diabetes care editors' expert
forum. Diabetes Care. 41:14–31. 2018.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Marso SP, Daniels GH, Brown-Frandsen K,
Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR,
Ravn LS, et al: Liraglutide and cardiovascular outcomes in type 2
diabetes. N Engl J Med. 375:311–322. 2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Helmstädter J, Keppeler K, Küster L,
Münzel T, Daiber A and Steven S: Glucagon-like peptide-1 (GLP-1)
receptor agonists and their cardiovascular benefits-The role of the
GLP-1 receptor. Br J Pharmacol. 179:659–676. 2022.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Knuuti J, Wijns W, Saraste A, Capodanno D,
Barbato E, Funck-Brentano C, Prescott E, Storey R, Deaton C,
Cuisset T, et al: 2019 ESC Guidelines for the diagnosis and
management of chronic coronary syndromes: The Task Force for the
diagnosis and management of chronic coronary syndromes of the
European SOCIETY OF CARDIOlogy (ESC). Eur Heart J. 41:407–477.
2020.
|
|
121
|
American Diabetes Association. 9.
Pharmacologic approaches to glycemic treatment: Standards of
medical care in diabetes-2020. Diabetes Care. 43 (Suppl
1):S98–S110. 2020.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Marty VN, Farokhnia M, Munier JJ, Mulpuri
Y, Leggio L and Spigelman I: Long-acting glucagon-like peptide-1
receptor agonists suppress voluntary alcohol intake in male wistar
rats. Front Neurosci. 14(599646)2020.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Klausen MK, Thomsen M, Wortwein G and
Fink-Jensen A: The role of glucagon-like peptide 1 (GLP-1) in
addictive disorders. Br J Pharmacol. 179:625–641. 2022.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Graham DL, Erreger K, Galli A and Stanwood
GD: GLP-1 analog attenuates cocaine reward. Mol Psychiatry.
18:961–962. 2013.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Egecioglu E, Engel JA and Jerlhag E: The
glucagon-like peptide 1 analogue, exendin-4, attenuates the
rewarding properties of psychostimulant drugs in mice. PLoS One.
8(e69010)2013.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Erreger K, Davis AR, Poe AM, Greig NH,
Stanwood GD and Galli A: Exendin-4 decreases amphetamine-induced
locomotor activity. Physiol Behav. 106:574–578. 2012.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Chaves Filho AJM, Cunha NL, de Souza AG,
Soares MV, Jucá PM, de Queiroz T, Oliveira JVS, Valvassori SS,
Barichello T, Quevedo J, et al: The GLP-1 receptor agonist
liraglutide reverses mania-like alterations and memory deficits
induced by D-amphetamine and augments lithium effects in mice:
Relevance for bipolar disorder. Prog Neuropsychopharmacol Biol
Psychiatry. 99(109872)2020.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Al-Nozha MM, Arafah MR, Al-Maatouq MA,
Khalil MZ, Khan NB, Al-Marzouki K, Al-Mazrou YY, Abdullah M,
Al-Khadra A, Al-Harthi SS, et al: Hyperlipidemia in Saudi Arabia.
Saudi Med J. 29:282–287. 2008.PubMed/NCBI
|
|
129
|
Okati-Aliabad H, Ansari-Moghaddam A,
Kargar S and Jabbari N: Prevalence of obesity and overweight among
adults in the Middle East countries from 2000 to 2020: A systematic
review and meta-analysis. J Obes. 2022(8074837)2022.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Alshaikh A, Aljedai A, Alfadda A,
Alrobayan A, Bawahab A, Ouf SA, Sultan A, Alhozali A, Bawazeer M,
Sheshah E, et al: Clinical practice guideline for the management of
overweight and obesity in adults in Saudi Arabia. Int J Clin Med.
13:590–649. 2022.
|
|
131
|
World Health Organization: Saudi Arabia
health profile 2015. World Health Organization. Regional Office for
the Eastern Mediterranean, 2016.
|
|
132
|
Memish ZA, El Bcheraoui C, Tuffaha M,
Robinson M, Daoud F, Jaber S, Mikhitarian S, Al Saeedi M, AlMazroa
MA, Mokdad AH and Al Rabeeah AA: Obesity and associated
factors-Kingdom of Saudi Arabia, 2013. Prev Chronic Dis.
11(E174)2014.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Al-Omar HA, Alshehri A, Alqahtani SA,
Alabdulkarim H, Alrumaih A and Eldin MS: A systematic review of
obesity burden in Saudi Arabia: Prevalence and associated
co-morbidities. Saudi Pharm J. 32(102192)2024.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Bin Horaib G, Al-Khashan HI, Mishriky AM,
Selim MA, Alnowaiser N, Binsaeed AA, Alawad AD, Al-Asmari AK and
Alqumaizi K: Prevalence of obesity among military personnel in
Saudi Arabia and associated risk factors. Saudi Med J. 34:401–407.
2013.PubMed/NCBI
|
|
135
|
Malkin JD, Baid D, Alsukait RF, Alghaith
T, Alluhidan M, Alabdulkarim H, Altowaijri A, Almalki ZS, Herbst
CH, Finkelstein EA, et al: The economic burden of overweight and
obesity in Saudi Arabia. PLoS One. 17(e0264993)2022.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Alluhidan M, Alsukait R, Alghaith T,
Shekar M and Herbst C: Overweight and obesity in Saudi Arabia:
Consequences and solutions. World Bank Publications, Washington,
DC, 2022.
|
|
137
|
Aldubayan K and Murimi M: Compliance with
school nutrition policy in Saudi Arabia: A quantitative study. East
Mediterr Health J. 25:230–238. 2019.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Gerich J, Fonseca V and Alvarado-Ruiz R:
Monotherapy with GLP-1 receptor agonist, Lixisenatide,
significantly improves glycaemic control in type 2 diabetic
patients. In: Diabetologia. Springer, New York, NY, 2010.
|
|
139
|
Sanofi-Aventis UJB, NJ, Sanofi-Aventis US
LLC: LLC: Adlyxin (Lixisenatide)[Prescribing Information],
2016.
|
|
140
|
LP APJW, DE: AstraZeneca Pharmaceuticals
LP: Imfinzi (durvalumab) injection, for intravenous use
[prescribing information], 2017.
|
|
141
|
Derosa G, Franzetti IG, Querci F, Carbone
A, Ciccarelli L, Piccinni MN, Fogari E and Maffioli P: Exenatide
plus metformin compared with metformin alone on β-cell function in
patients with Type 2 diabetes. Diabet Med. 29:1515–1523.
2012.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Htike ZZ, Zaccardi F, Papamargaritis D,
Webb DR, Khunti K and Davies MJ: Efficacy and safety of
glucagon-like peptide-1 receptor agonists in type 2 diabetes: A
systematic review and mixed-treatment comparison analysis. Diabetes
Obes Metab. 19:524–536. 2017.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Chun E, Siojo A, Rivera D, Reyna K, Legere
H, Joseph R and Pojednic R: Weight loss and body composition after
compounded semaglutide treatment in a real world setting. Diabetes
Obes Metab. 27:1536–1543. 2025.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Kalra S: Choosing appropriate
glucagon-like peptide 1 receptor agonists: A patient-centered
approach. Diabetes Ther. 5:333–340. 2014.PubMed/NCBI View Article : Google Scholar
|
|
145
|
GlaxoSmithKline G: Tanzeum (albiglutide)
prescribing informtion, 2015.
|
|
146
|
Geiser JS, Heathman MA, Cui X, Martin J,
Loghin C, Chien JY and de la Peña A: Clinical pharmacokinetics of
dulaglutide in patients with type 2 diabetes: Analyses of data from
clinical trials. Clin Pharmacokinet. 55:625–634. 2016.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Montanya E and Sesti G: A review of
efficacy and safety data regarding the use of liraglutide, a
once-daily human glucagon-like peptide 1 analogue, in the treatment
of type 2 diabetes mellitus. Clin Ther. 31:2472–2488.
2009.PubMed/NCBI View Article : Google Scholar
|
|
148
|
O'Neil PM, Birkenfeld AL, McGowan B,
Mosenzon O, Pedersen SD, Wharton S, Carson CG, Jepsen CH, Kabisch M
and Wilding JPH: Efficacy and safety of semaglutide compared with
liraglutide and placebo for weight loss in patients with obesity: A
randomised, double-blind, placebo and active controlled,
dose-ranging, phase 2 trial. Lancet. 392:637–649. 2018.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Onge ES, Miller SA and Motycka C:
Liraglutide (Saxenda®) as a treatment for obesity. Food Nutr Sci.
7:227–235. 2016.
|
|
150
|
Chavda VP, Ajabiya J, Teli D, Bojarska J
and Apostolopoulos V: Tirzepatide, a new era of dual-targeted
treatment for diabetes and obesity: A mini-review. Molecules.
27(4315)2022.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Cai W, Zhang R, Yao Y, Wu Q and Zhang J:
Tirzepatide as a novel effective and safe strategy for treating
obesity: A systematic review and meta-analysis of randomized
controlled trials. Front Public Health. 12(1277113)2024.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Feier CVI, Vonica RC, Faur AM, Streinu DR
and Muntean C: Assessment of thyroid carcinogenic risk and safety
profile of GLP1-RA semaglutide (Ozempic) therapy for diabetes
mellitus and obesity: A systematic literature review. Int J Mol
Sci. 25(4346)2024.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Yang XD and Yang YY: Clinical
pharmacokinetics of semaglutide: A systematic review. Drug Des
Devel Ther. 18:2555–2570. 2024.PubMed/NCBI View Article : Google Scholar
|