Introduction
Human telomeres are nucleoprotein structures
consisting of the repeating nucleotide sequence 5'-TTAGGG-3', along
with protein complexes, that interact with the DNA. They are
located at the end of eucaryotic chromosomes, and their main role
is to maintain the stability of chromosomes and their protection
from degradation throughout enzymatic decay (1). Telomeres have been found to be at
their maximum length after birth and as cells divide over time,
they gradually get shorter due to incomplete synthesis of DNA.
After 40-60 cell divisions, they reach a critically short length
known as Hayflick's limit, that triggers cellular senescence and
apoptosis (2,3). To avoid the shortening of telomeres,
human somatic cells produce the enzyme telomerase, which adds
nucleotides at the 3' end of the telomeres, and thus the telomeres
can maintain their length. This enzyme is active in numerous
somatic tissues during fetal and early neonatal development, but
its activity gradually decreases with age, resulting in
inactivation in the somatic cells of adults (4).
Telomere length (TL) is a biomarker of cellular
aging and genomic stability, and is influenced by genetic,
environmental, and physiological factors. TL is known to be
partially inherited and may reflect maternal health and stress
exposures. Preterm birth has been associated with adverse
intrauterine environments, including inflammation, oxidative
stress, and maternal metabolic conditions, all of which may affect
telomere dynamics (4).
Investigating the correlation of TL between mothers and their
neonates, especially comparing full-term and preterm births, may
provide insight into how maternal health and gestational age
influence early-life biological aging.
TL can differ significantly among individuals of the
same age, and this variability is noticeable from birth. During in
utero development, the fetus undergoes crucial phases of cellular
growth, differentiation, and maturation, that cause modifications
in telomere dynamics (5). These
alterations can have far-reaching consequences for an individual's
health and susceptibility to diseases throughout their life
(6). Emerging research suggests
that TL in neonates can also impact the development of age-related
diseases. Shorter telomeres have been associated with increased
risks of cardiovascular diseases, cancer, neurodegenerative
disorders, and other chronic conditions (7-10).
Therefore, it is highly probable that the TL of an individual as
they age is significantly shaped by their TL at birth and the
subsequent attrition of telomeres during early life (11).
The typical duration of a human pregnancy is usually
between 37 to 42 gestational weeks, during which the developing
fetus undergoes cell differentiation and organ maturation,
essential for successful survival outside the womb. In this case,
the TL that neonates are born with has been estimated to be 9 to
12,5 kilobases (12). When the
gestation period is disrupted and childbirth occurs before 37
weeks, childbirth is characterized as premature (13). Preterm birth is a multifactorial
phenomenon that, according to the WHO, ranged from 4 to 16% across
countries in 2020 (https://www.who.int/news-room/fact-sheets/detail/preterm-birth).
It is more prevalent among male infants, with ~55% of preterm
births occurring in boys (14),
and mortality rates are also higher among premature boys compared
with premature girls (15).
Multiple pregnancies, obesity, gestational diabetes mellitus (GDM),
alcohol use during pregnancy, and smoking are main maternal factors
that appear to affect preterm birth, and studies have indicated
that these factors can also affect embryonic TL throughout
pregnancy (16-20).
Previous research has revealed that higher maternal pre-pregnancy
body mass index (BMI) is associated with shorter in both mothers
and their infants, indicating increased oxidative stress and
accelerated cellular aging (11).
The purpose of the present study was to investigate
the correlation between neonatal TL and that of their mothers, and
to examine potential differences between preterm and full-term
neonates. Additionally, associations between maternal
characteristics, medical history, and pregnancy-related events with
neonatal TL was assessed.
Materials and methods
Patients and ethics approval
The present study was conducted at the Department of
Neonatology and the Neonatal Intensive Care Unit, University
Hospital of Heraklion (Heraklion, Greece), and at the Laboratory of
Toxicology, Medical School, University of Crete (Heraklion,
Greece). Ethics approval (approval no. 112/08.09.2021) for the
study was obtained from the Research Ethics Committee of the
University of Crete (Heraklion, Greece). Informed consent was
obtained from all participants for both their own participation and
that of their newborns. All participants completed a specialized
questionnaire concerning their medical history, habits during
pregnancy, and anthropometric characteristics. All samples
generated by the present study were anonymized, and personal data
were managed according to the EU General Data Protection Regulation
(GDPR; https://gdpr-info.eu/).
Blood samples were collected from 54 mothers and
their neonates hospitalized at the Department of Neonatology and
Neonatal Intensive Care Unit, University Hospital of Heraklion
(Heraklion, Greece), from October 2022 to February 2023. Genomic
DNA was extracted using the QIAamp DNA Blood Mini Kit (cat no.
51104; QIAGEN) according to the manufacturer's instructions. All
the samples were measured using a photometer and the necessary
dilutions were made in order to achieve a final DNA concentration
of 5 ng/µl in the working solution. Subsequently, quantitative PCR
was performed to determine the length of the telomeric ends. The
thermocycling conditions were as follows: Initial denaturation at
95˚C for 10 min, denaturation at 95˚C for 20 sec, annealing at 52˚C
for 20 sec, extension at 72˚C for 45 sec with 32 number of cycles
and hold at 20˚C. The total telomere length of the target sample
was calculated as follows: Reference sample telomere length x
2-ΔΔCq (21).
The 2X GoldNStart TaqGreen qPCR Master Mix (cat no.
MB6018a-1; ScienCell Research Laboratories, Inc.) was a
SYBR® Green dye-based qPCR master mix with a ‘hot-start’
property). The single copy reference (SCR) primer set recognizes
and amplifies a 100 bp-long region on rat chromosome 17, and serves
as a reference for data normalization. The kit used was the
Relative Human Telomere Length Quantification qPCR Assay kit and
the primers were part of the kit (cat. no. 8918; ScienCell Research
Laboratories, Inc.). The telomere length was calculated based on
the instructions of the kit.
Statistical analysis
Data mainly consisted of qualitative data and were
expressed as counts and frequencies. Numerical variables were
expressed as means and standard deviation. Pearson's χ2
was used to associate discrete (qualitative) data, while Pearson's
r or Spearman's ρ coefficient was used to correlate continuous
variables. Independent samples t-tests were applied to continuous
variables to examine possible differences between two groups, while
one-way analysis of variance (ANOVA) followed by LSD post-hoc test,
was applied for differences in more than two groups. IBM SPSS
Statistics 24.0 (IBM Corp.) was used for analysis, and an a=0.05
was set as the significance level.
Results
Maternal and neonatal characteristics
and TL correlations
A total of 54 neonates, resulting from single
pregnancies, were included in the present study. Demographic
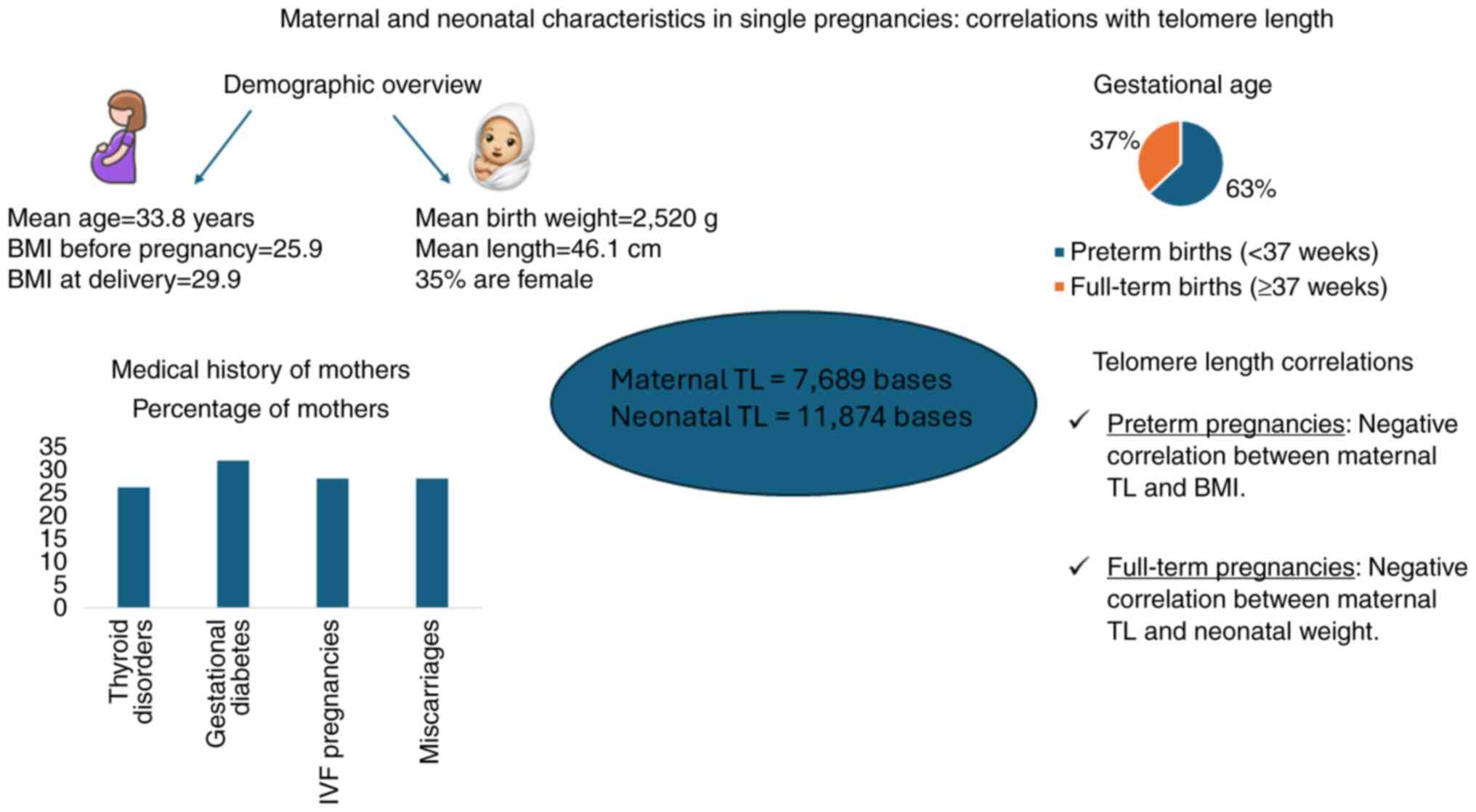
characteristics of participating mothers are presented in Table I. The mean maternal age was
33.8±6.1 years, ranging from 19 to 50 years. The mean BMI increased
from from 25.9±6.0 before pregnancy to 29.9±5.8 at delivery. The
medical history of the mothers is described in Table II. A total of 39 mothers (72.2%)
stated that they had not experienced a miscarriage in the past,
while the rest of them had at least one (27.8%). Thyroid disorders
were observed in 14 (25.9%) of the examined mothers. In addition,
17 (31.5%) of the mothers developed gestational diabetes. In
vitro fertilization (IVF) was performed for 27.8% of the total
pregnancies. Gestational age was #x003C;37 weeks in 63.0% of all
pregnancies (Table III).
 | Table IDemographic and somatometric
characteristics of participating mothers. |
Table I
Demographic and somatometric
characteristics of participating mothers.
| Variable | Mean | ± SD | Median | Min | Max |
|---|
| Age (years) | 33.8 | 6.1 | 33.5 | 19 | 50 |
| Height (cm) | 166.1 | 5.3 | 166.0 | 147 | 176 |
| Weight before
(kg) | 71.7 | 17.8 | 67.0 | 47 | 133 |
| Weight at delivery
(kg) | 82.5 | 16.8 | 78.5 | 58.0 | 128 |
| BMI before
(kg/m2) | 25.9 | 6.0 | 24.2 | 18.1 | 45.6 |
| BMI at delivery
(kg/m2) | 29.9 | 5.8 | 28.7 | 21.4 | 50.2 |
 | Table IIMedical history of the mothers. |
Table II
Medical history of the mothers.
| Maternal medical
history | n | Percentage (%) |
|---|
| Μiscarriage | 15 | 27.8 |
| Thyroid
disorders | 14 | 25.9 |
| Gestational
diabetes | 17 | 31.5 |
| Polycystic
ovaries | 6 | 11.1 |
| Cushing
syndrome | 1 | 1.9 |
| Hypertension | 1 | 1.9 |
 | Table IIIPregnancy characteristics. |
Table III
Pregnancy characteristics.
| Pregnancy-related
variables | N | Percentage (%) |
|---|
| IVF | 15 | 27.8 |
| Gestational
age | | |
|
Gestational
weeks #x003C;37 | 34 | 63.0 |
|
Gestational
weeks ≥37 | 20 | 37.0 |
The mean length of newborns was 46.1±5.4 cm, ranging
from 28 to 55 cm. The mean weight of the neonates was 2.520±842 g,
ranging from 600 to 4,180 g, and the mean BMI of the neonates was
11.4±1.9 kg/m2, ranging from 6.7 to 14.3
kg/m2. Only 19 (35.2%) of the neonates included were
females (Table IV). Notably, the
maternal mean TL was estimated at 7,689±1,528 bases, while the
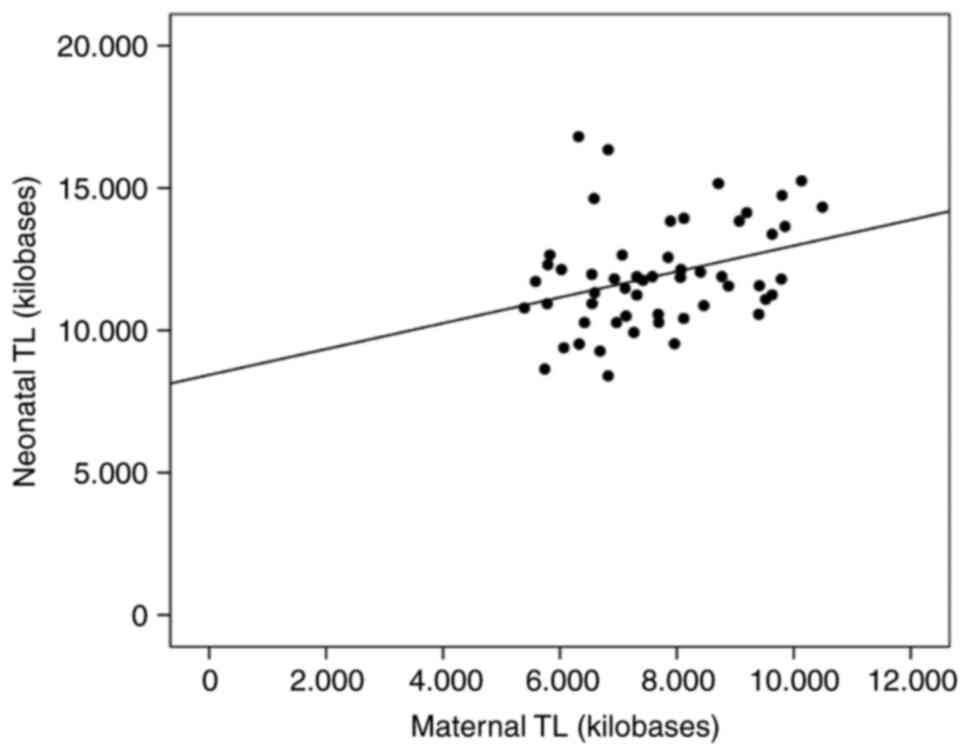
neonate TL was in the range of 11,874±1,787 bases. A weak to
moderate significant correlation was observed between maternal and
neonatal TL (Spearman's ρ=0.323; P=0.017) (Fig. 1).
 | Table IVCharacteristics of neonates. |
Table IV
Characteristics of neonates.
| At birth | Mean | Median | ± SD | Min | Max |
|---|
| Length (cm) | 46.1 | 47.0 | 5.4 | 28.0 | 55.0 |
| Birth weight
(g) | 2,520 | 2,590 | 842 | 600 | 4,180 |
| BMI
(kg/m2) | 11.4 | 11.6 | 1.9 | 6.7 | 14.3 |
Μaternal TL was negatively correlated with BMI
before pregnancy (r=-0.285; P=0.037), while a statistically
significant trend was also observed with maternal BMI at delivery
(r=0.251; P=0.067; 0.05#x003C;P#x003C;0.100) was observed.
Similarly, a trend towards significance was found between maternal
TL and weight before pregnancy (r=-0.229; P=0.096; 0.05#x003C;
P#x003C;0.100). No significant correlations were observed between
maternal TL and neonatal somatometrics and sex (Table V).
 | Table VCorrelation of maternal and neonatal
TL with maternal and neonatal somatometrics. |
Table V
Correlation of maternal and neonatal
TL with maternal and neonatal somatometrics.
| | Maternal TL | Neonatal TL |
|---|
| Somatometrics | r | P-value | r | P-value |
|---|
| Mother | | | | |
|
Age | 0.126 | 0.364 | 0.083 | 0.550 |
|
Height
(cm) | -0.077 | 0.582 | -0.165 | 0.234 |
|
Weight
before pregnancy (kg) | -0.229 | 0.096 | -0.123 | 0.375 |
|
Weight
during pregnancy (kg) | -0.179 | 0.178 | -0.087 | 0.516 |
|
BMI (before
pregnancy) | -0.285 | 0.037 | -0.091 | 0.515 |
|
BMI (at
delivery) | -0.251 | 0.067 | -0.002 | 0.990 |
| Neonate | | | | |
|
Height
(cm) | -0.053 | 0.709 | -0.060 | 0.673 |
|
Weight
(kg) | -0.037 | 0.793 | -0.127 | 0.368 |
|
BMI
(kg/m2) | 0.023 | 0.874 | -0.120 | 0.397 |
|
Sex | -0.088 | 0.525 | -0.004 | 0.979 |
Neonatal TL was not correlated with maternal age,
maternal somatometric measures, neonatal somatometric measures or
sex.
The effect of maternal smoking habits and diseases
on neonates TL are presented in Table
VI. There was no significant effect of smoking before pregnancy
(P=0.892), smoking during pregnancy (P=0.724), history of
miscarriage (P=0.488), type of conception (P=0.770), thyroid
disorders (hyperthyroidism, P=0.153 and hypothyroidism, P=0.971),
gestational diabetes (P=0.974) and gestational age at term
(P=0.867) on neonatal TL. No significant differences were found
between maternal TL and medical or gestational history.
 | Table VIAssociation between neonatal TL and
maternal smoking habits and medical history. |
Table VI
Association between neonatal TL and
maternal smoking habits and medical history.
| | | No | | Yes | |
|---|
| Neonate TL | n | Mean | SD | n | Mean | SD | P-value |
|---|
| Smoking before
pregnancy | 37 | 11.896 | 1.723 | 17 | 11.971 | 2.199 | 0.892 |
| Smoking during
pregnancy | 45 | 11.961 | 1.806 | 9 | 11.717 | 2.247 | 0.724 |
| Miscarriages | 39 | 12.123 | 1.972 | 15 | 11.698 | 1.603 | 0.488 |
| Thyroid
disorders | 40 | 11.992 | 2.038 | 14 | 11.715 | 1.285 | 0.637 |
|
Hyperthyroidism | 53 | 11.970 | 1.846 | 1 | 11.970 | 1.846 | 0.153 |
| Hypothyroidism | 41 | 11.925 | 2.057 | 13 | 11.903 | 1.119 | 0.971 |
| Gestational
diabetes | 37 | 11.958 | 1.915 | 17 | 11.837 | 1.805 | 0.974 |
| Full-term pregnancy
(≥37 weeks) | 34 | 11.953 | 1.871 | 20 | 11.864 | 1.900 | 0.867 |
| IVF conception | 38 | 11.954 | 1.978 | 15 | 11.784 | 1.661 | 0.770 |
Analysis between preterm and full-term
groups
This section presents the analysis of maternal and
neonate TL in relation to demographics, medical history and other
variables comparing preterm and full-term infants. In preterm
pregnancies #x003C;37 weeks, maternal TL showed a statistically
significant negative correlation with BMI before pregnancy
(r=-0.403; P=0.018) and BMI after pregnancy (r=-0.349; P=0.043). A
trend towards significance was observed between maternal TL and
pre-pregnancy weight (r=-0.313; P=0.072). No significant
associations were found between maternal TL and neonate variables
(P>0.100) in preterm pregnancies. Similarly, neonatal TL did not
show any significant correlations with maternal or neonatal
parameters (P>0.100).
In full-term pregnancies (gestational age, ≥37),
maternal TL was significantly correlated with neonatal weight after
delivery (r=-0.505; P=0.039), while a trend towards significance
was also observed between maternal TL with neonatal height
(0.05#x003C; P#x003C;0.100). No significant correlations were found
between neonatal TL and any of the measured maternal or neonatal
variables in full-term pregnancies (Table VII).
 | Table VIICorrelation of maternal and neonatal
TL with maternal somatometric variables in preterm and full-term
pregnancies. |
Table VII
Correlation of maternal and neonatal
TL with maternal somatometric variables in preterm and full-term
pregnancies.
| | Preterm pregnancy
(#x003C;37 weeks) | Full-term pregnancy
(≥37 weeks) |
|---|
| Variables | Maternal TL r | Neonatal TL r | Maternal TL r | Neonatal TL r |
|---|
| Mother | | | | |
|
Age
(year) | 0.202 | 0.018 | -0.049 | 0.185 |
|
Height
(cm) | 0.029 | -0.018 | -0.274 | -0.408a |
|
Weight
before pregnancy (kg) | -0.313a | -0.161 | -0.104 | -0.156 |
|
Weight
during pregnancy (kg) | -0.281 | -0.120 | -0.051 | 0.018 |
|
BMI before
pregnancy (kg/m2) | -0.403b | -0.166 | -0.143 | -0.051 |
|
BMI after
pregnancy (kg/m2) | -0.349b | -0.105 | -0.164 | 0.149 |
| Neonate | | | | |
|
Height
(cm) | 0.133 | -0.077 | -0.462a | -0.116 |
|
Weight
(g) | -0.048 | -0.132 | -0.505b | -0.308 |
|
BMI
(kg/m2) | 0.053 | 0.038 | 0.156 | -0.374 |
|
Sex | -0.253 | -0.103 | 0.230 | 0.142 |
In addition, no significant differences in maternal
and neonatal TL were observed between preterm and full-term
pregnancies based on history of miscarriage, smoking habits,
thyroid disorders, mode of conception and gestational diabetes
(Table VIII).
 | Table VIIIAssociation of maternal and neonatal
TL with smoking status, maternal medical history, and pregnancy
characteristics in preterm and full-term pregnancies. |
Table VIII
Association of maternal and neonatal
TL with smoking status, maternal medical history, and pregnancy
characteristics in preterm and full-term pregnancies.
| A, Preterm
pregnancy |
|---|
| | | Maternal TL | | Neonatal TL | |
|---|
| Variables | Yes/No | Mean | ± SD | P-value | Mean | ± SD | P-value |
|---|
| Abortions | No | 7.929 | 1.344 | 0.243 | 12.064 | 2.068 | 0.744 |
| | Yes | 7.334 | 1.548 | | 11.722 | 1.437 | |
| Smoking before
pregnancy | No | 7.694 | 1.429 | 0.772 | 12.029 | 1.784 | 0.885 |
| | Yes | 7.826 | 1.458 | | 11.795 | 2.125 | |
| Smoking during
pregnancy | No | 7.753 | 1.439 | >0.999 | 11.953 | 2.054 | 0.947 |
| | Yes | 7.660 | 1.442 | | 11.975 | 1.336 | |
| Thyroid
disorders | No | 7.832 | 1.435 | 0.645 | 11.945 | 2.054 | 0.673 |
| | Yes | 7.472 | 1.417 | | 11.975 | 1.336 | |
| Conception | Normal | 7.814 | 1.618 | 0.813 | 12.002 | 2.005 | 0.598 |
| | IVF | 7.791 | 1.457 | | 11.824 | 1.791 | |
| Gestational
diabetes | No | 7.984 | 1.355 | 0.204 | 12.075 | 1.952 | 0.817 |
| | Yes | 7.282 | 1.476 | | 11.730 | 1.774 | |
| B, Full-term
pregnancy |
| | | Maternal TL | | Neonatal TL | |
| Variables | Yes/No | Mean | ± SD | P-value | Mean | ± SD | P-value |
| Miscarriages | No | 7.477 | 1.291 | 0.622 | 12.220 | 1.874 | 0.179 |
| | Yes | 8.230 | 1.180 | | 11.033 | 1.846 | |
| Smoking before
pregnancy | No | 7.823 | 1.388 | 0.274 | 11.679 | 1.658 | 0.659 |
| | Yes | 7.000 | 941 | | 12.296 | 2.501 | |
| Smoking during
pregnancy | No | 7.557 | 1.403 | 0.765 | 11.973 | 1.873 | 0.616 |
| | Yes | 7.683 | 623 | | 11.247 | 2.359 | |
| Thyroid
disorders | No | 7.487 | 1.354 | 0.612 | 12.069 | 2.080 | 0.612 |
| | Yes | 7.842 | 1.245 | | 11.247 | 1.170 | |
| Pregnancy | Normal | 7.500 | 1.298 | 0.442 | 11.902 | 2.005 | 0.674 |
| | IVF | 8.263 | 1.623 | | 11.525 | 64 | |
| Gestational
diabetes | No | 7.542 | 1.240 | 0.933 | 11.787 | 1.913 | 0.612 |
| | Yes | 7.676 | 1.639 | | 12.094 | 2.064 | |
Discussion
The present study highlighted a weak to moderate,
positive and statistically significant correlation between maternal
and neonatal TL, irrespective of full-term or preterm pregnancy.
Some maternal features, particularly BMI before pregnancy, appeared
to be the determinant factor in maternal TL. Additionally, grouping
cases into preterm or full-term neonates influenced some of the
findings; however, no statistically significant associations were
observed between neonatal TL and maternal characteristics, medical
history, and pregnancy details (Fig.
2).
The mean maternal TL in the present study fell
within the reference range of 7 to 9 kilobases for women in their
30s (22), consistent with the
mean maternal age in our sample. For neonates, the TL was predicted
to fall within 8 to 11 kilobases (22). Most of the neonates examined were
preterm and the slightly higher mean TL, supports a previous
observation that preterm neonates tend to have longer telomeres
than those born at term (23).
A study involving 319 mother-newborn pairs found a
significant positive association between maternal and newborn TLs
(β=0.31; P#x003C;0.001), indicating that maternal TL is a predictor
of neonatal TL (24). Research has
shown that preterm infants have significantly longer telomeres than
their term-born counterparts. Specifically, TL was negatively
correlated with gestational age and birth weight in preterm infants
(25). In a cohort of African
American women, shorter maternal peripheral blood TL was associated
with an increased risk of preterm birth. Notably, for every 10-unit
decrease in the telomere-to-single-copy gene (T/S) ratio, the odds
of preterm birth increased by a factor of 2.664(26).
Previous studies did not directly correlate maternal
BMI with the TL of neonates, in contrast to most previous studies
(11,27). Indeed, prior research has
identified a negative correlation between maternal BMI and neonatal
TL (11). At the molecular level,
elevated maternal BMI may promote oxidative stress and inflammation
in the fetal environment, which could contribute to telomere
shortening during prenatal development (28-30).
Additionally, neonatal telomere shortening was more likely to occur
in infants born to mothers with higher anxiety scores, elevated
fasting blood glucose levels, lower levels of plasma insulin-like
growth factor-binding protein 3 (IGFBP-3) and vitamin B12, and who
actively smoke status pregnancy (27). However, there are studies which
support that a high maternal pre-pregnancy BMI is associated with
shorter maternal TL and TL in infants, indicating increased
oxidative stress and accelerated cellular aging (31,32).
The lack of association between maternal BMI and
neonatal TL contrasts with previous studies (11,27),
potentially due to the BMI distribution in this cohort, which
consisted primarily of normal-weight participants. Additionally,
the variability in qPCR protocols, along with factors such as
maternal stress, smoking, or nutrition, which can modulate TL
independently of BMI, may confound results. Furthermore, limited
statistical power and sample size may lack the statistical power to
detect subtle associations (33,34).
Pre-pregnancy maternal BMI was not correlated with
neonatal TL as expected but has been found to be related to preterm
birth, and the present study aligns with this indication (P=0.017)
(Fig. 1). Preterm deliveries
resulting from premature labor with cervical dilation or early
rupture of membranes are categorized as ‘spontaneous’, while those
that are induced or carried out via cesarean section, due to
maternal or fetal health concerns, are termed ‘indicated’ preterm
births. In the present study, the type of preterm delivery was not
provided. Most mothers who participated were categorized as
οverweight (BMI between 25 and 30; mean BMI, 26) prior to
pregnancy, which could also justify why a significant correlation
with neonatal TL was not observed. However, the sample did include
individuals from both the underweight and overweight categories,
with BMIs ranging from 18.1 to 45.6. Previous studies have found
that maternal overweight is more commonly associated with medically
indicated preterm births, whereas maternal underweight tends to
slightly increase the risk of spontaneous preterm labor (35). The exact mechanisms by which
abnormal maternal BMI affects neonatal TL are not fully understood,
although these effects may be linked to chronic inflammation and
oxidative stress during fetal development (30). Moving forward, this research is
planned to be expanded by categorizing participants into different
BMI groups. Through this approach, further exploration into how
maternal BMI may influence preterm deliveries is expected to be
conducted, possibly revealing novel insights into its effects on
fetal genotypic outcomes.
When mothers were examined separately, a moderate
negative correlation between pre-pregnancy maternal BMI and
maternal TL was observed (P=0.037). Previous research has examined
the correlation between maternal BMI and TL. For instance, adult
women with a BMI exceeding 30 kg/m2, had telomeres that
were on average, 240 base pairs shorter compared with women with a
BMI below 20 kg/m2, and this difference was equivalent
to an aging effect of ~8.8 years (36).
Finally, no evidence of a the relationship between
maternal pregnancy complications and neonatal TL was revealed in
the present study. This finding appears to be consistent with
recent data showing that maternal stress does not exert a
significant effect on infant TL of infants as determined by qPCR
analysis (37). Currently, the
results of the present study appear to contradict previous findings
supporting that maternal pregnancy complications can contribute to
neonatal telomere shortening (5,24,38-40).
The discrepancies among studies can be attributed to the small
sample size in previous studies. Indeed, numerous detrimental
variables (such as oxidative stress, inflammation, and other
genetic/epigenetic, immunological, physiological, lifestyle, and
environmental factors, including nutrition) have been shown to
result in an advanced aging trajectory and exacerbate placental
dysfunction, given that TL reflects the cumulative impact of
stressors (41,42). In addition, several pregnancy
complications, such as GDM, intrauterine growth restriction,
hypertensive disorders and preeclampsia, have been demonstrated to
be associated with prematurity and neonatal telomere alterations
while still in utero (43,44). In the majority of studies, GDM has
been associated with reduced neonatal telomeres (45-49),
although some other studies have found similar TLs (43,44,50)
or even longer ones in newborns compared with those from mothers
without GDM (51). Cardiovascular
conditions such as hypertension and preeclampsia have also been
associated with shorter telomeres in previous research, although
the mechanisms remain unclear (52). The small sample size in the present
study limited the ability to assess how pregnancy complications and
lifestyle factors (such as alcohol consumption and smoking) affect
neonatal TL, and future studies with larger participant pools are
required to address these gaps.
Even though a correlation between maternal and
neonatal TL was identified in the present study, it has several
limitations. Initially, qPCR was used to evaluate relatively
average TL, although this technique has been considered more
variable than the classically used terminal restriction fragment
analysis (53,54). Secondly, evaluating TL in neonates
represents only a snapshot of aging at birth, as limited data are
available on TL in embryos during pregnancy and childhood.
Indeed, the qPCR method was used to assess maternal
and neonatal TL, as only 200 µl of neonatal blood, obtained from
the complete blood count, was available for analysis. The collected
blood from preterm or full-term neonates was of invaluable and
utmost importance since neonate samples are unique, especially that
of preterm neonates. It is worth noting that the acquisition of
increased blood amounts can result in the reduction of neonatal
hematocrit (55). However, the
present study did not detect TL differences across chromosomes at a
single-cell level, which can be accomplished through metaphase
Q-FISH (56,57). The Q-FISH technique can provide an
overall analysis of TL distributions, providing measurements for
extremely short or extremely long telomeres at the chromosome level
(57). Despite this limitation,
the present study provides unique insight into the average TL of
maternal and full-term or preterm neonates. A limitation was also
noted due to the small sample size, which affects the statistical
power of sample. However, numerous studies have confirmed the
reproducibility of the qPCR method in neonatal samples (58-60).
Another challenge of the study was the limited
sample size. In most cases, there was also an insufficient number
of participants diagnosed with some of the examined disorders
within the timeframe of sample collection. Due to the shortcoming
of the sample size, a general overview of neonatal TL in relation
to maternal characteristics could not be provided. Therefore,
future studies with a more substantial and diverse participant pool
are encouraged to validate and strengthen the conclusions derived
from the present research.
In conclusion, the present study revealed a weak to
moderate, positive and statistically significant correlation
between maternal and neonatal TL, regardless of pregnancy duration.
No significant differences were observed between maternal TL and
maternal habits, medical history, or gestational history. However,
maternal BMI before pregnancy appears to have a decisive impact on
determining maternal TL. Future studies with a larger sample size
and the inclusion of additional parameters, such as socioeconomic
status, maternal stress levels, and environmental exposures, are
recommended to further clarify these associations.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
EV, EH, AT and DAS conceived the study. EV, MT and
PF acquired and interpreted the data of the study. EM, ZV, NA and
FM designed the study. AA, PI, SB, AM, NIP, MTV, TL, EV and AT
analyzed the data of the study and were major contributors in the
writing of the manuscript. AT and AA confirm the authenticity of
all the raw data. EV, EH and AT, provided final approval of the
version to be published. All authors have read and agreed to the
published version of the manuscript.
Ethics approval and consent to
participate
Ethics approval (approval no. 112/08.09.2021) for
the study was obtained from the Research Ethics Committee of the
University of Crete (Heraklion, Crete). Informed consent was
obtained from all participants for both their participation and
that of their newborns. All participants completed a specialized
questionnaire concerning their medical history, habits during
pregnancy, and anthropometric characteristics. All samples
generated by the present study were anonymized, and personal data
were managed according to the EU General Data Protection Regulation
(GDPR; https://gdpr-info.eu/).
Patient consent for publication
Not applicable.
Competing interests
DAS is the editor-in-chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Blackburn EH: Structure and function of
telomeres. Nature. 350:569–573. 1991.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Hayflick L and Moorhead PS: The serial
cultivation of human diploid cell strains. Exp Cell Res.
25:585–621. 1961.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bartlett Z: The hayflick limit. Embryo
Project Encyclopedia, 2014. http://embryo.asu.edu/handle/10776/8237.
|
|
4
|
Gomes NM, Shay JW and Wright WE: Telomere
biology in Metazoa. FEBS Lett. 584:3741–3751. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Entringer S, de Punder K, Buss C and
Wadhwa PD: The fetal programming of telomere biology hypothesis: An
update. Philos Trans R Soc Lond B Biol Sci.
373(20170151)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Entringer S, Buss C and Wadhwa PD:
Prenatal stress and developmental programming of human health and
disease risk: Concepts and integration of empirical findings. Curr
Opin Endocrinol Diabetes Obes. 17:507–516. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yeh JK and Wang CY: Telomeres and
telomerase in cardiovascular diseases. Genes (Basel).
7(58)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Okamoto K and Seimiya H: Revisiting
telomere shortening in cancer. Cells. 8(107)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Levstek T, Kozjek E, Dolžan V and Trebušak
Podkrajšek K: Telomere attrition in neurodegenerative disorders.
Front Cell Neurosci. 14(219)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gruber HJ, Semeraro MD, Renner W and
Herrmann M: Telomeres and age-related diseases. Biomedicines.
9(1335)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Martens DS, Plusquin M, Gyselaers W, De
Vivo I and Nawrot TS: Maternal pre-pregnancy body mass index and
newborn telomere length. BMC Med. 14(148)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Okuda K, Bardeguez A, Gardner JP,
Rodriguez P, Ganesh V, Kimura M, Skurnick J, Awad G and Aviv A:
Telomere length in the newborn. Pediatr Res. 52:377–381.
2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Samuel TM, Sakwinska O, Makinen K, Burdge
GC, Godfrey KM and Silva-Zolezzi I: Preterm birth: A narrative
review of the current evidence on nutritional and bioactive
solutions for risk reduction. Nutrients. 11(1811)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Haghighi L, Najmi Z, Barzegar SH and
Barzegar N: Twin's sex and risk of pre-term birth. J Obstet
Gynaecol. 33:823–826. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kent AL, Wright IM and Abdel-Latif ME: New
South Wales and Australian Capital Territory Neonatal Intensive
Care Units Audit Group. Mortality and adverse neurologic outcomes
are greater in preterm male infants. Pediatrics. 129:124–131.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Monden C, Pison G and Smits J: Twin peaks:
More twinning in humans than ever before. Hum Reprod. 36:1666–1673.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Buchanan TA, Xiang AH and Page KA:
Gestational diabetes mellitus: Risks and management during and
after pregnancy. Nat Rev Endocrinol. 8:639–649. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hawthorne G: Maternal complications in
diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol. 25:77–90.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
McCarthy FP, O'Keeffe LM, Khashan AS,
North RA, Poston L, McCowan LME, Baker PN, Dekker GA, Roberts CT,
Walker JJ and Kenny LC: Association between maternal alcohol
consumption in early pregnancy and pregnancy outcomes. Obstet
Gynecol. 122:830–837. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shah NR and Bracken MB: A systematic
review and meta-analysis of prospective studies on the association
between maternal cigarette smoking and preterm delivery. Am J
Obstet Gynecol. 182:465–472. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Factor-Litvak P, Susser E, Kezios K,
McKeague I, Kark JD, Hoffman M, Kimura M, Wapner R and Aviv A:
Leukocyte telomere length in newborns: Implications for the role of
telomeres in human disease. Pediatrics.
137(e20153927)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Colatto BN, de Souza IF, Schinke LAA,
Noda-Nicolau NM, da Silva MG, Morceli G, Menon R and Polettini J:
Telomere length and telomerase activity in foetal membranes from
term and spontaneous preterm births. Reprod Sci. 27:411–417.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Send TS, Gilles M, Codd V, Wolf I, Bardtke
S, Streit F, Strohmaier J, Frank J, Schendel D, Sütterlin MW, et
al: Telomere length in newborns is related to maternal stress
during pregnancy. Neuropsychopharmacology. 42:2407–2413.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vasu V, Turner KJ, George S, Greenall J,
Slijepcevic P and Griffin DK: Preterm infants have significantly
longer telomeres than their term born counterparts. PLoS One.
12(e0180082)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huang W, Han G, Taylor BD, Neal G, Kochan
K and Page RL: Maternal peripheral blood telomere length and
preterm birth in African American women: A pilot study. Arch
Gynecol Obstet. 311:1591–1598. 2025.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wei B, Shao Y, Liang J, Tang P, Mo M, Liu
B, Huang H, Tan HJJ, Huang D, Liu S and Qiu X: Maternal overweight
but not paternal overweight before pregnancy is associated with
shorter newborn telomere length: Evidence from Guangxi Zhuang birth
cohort in China. BMC Pregnancy Childbirth. 21(283)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Malti N, Merzouk H, Merzouk SA, Loukidi B,
Karaouzene N, Malti A and Narce M: Oxidative stress and maternal
obesity: Feto-placental unit interaction. Placenta. 35:411–416.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Myatt L: Placental adaptive responses and
fetal programming. J Physiol. 572:25–30. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Maugeri A, Magnano San Lio R, La Rosa MC,
Giunta G, Panella M, Cianci A, Caruso MAT, Agodi A and Barchitta M:
The relationship between telomere length and gestational weight
gain: Findings from the mamma & bambino cohort. Biomedicines.
10(67)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Barchitta M, Maugeri A, La Mastra C,
Favara G, La Rosa MC, Magnano San Lio R, Gholizade Atani Y, Gallo G
and Agodi A: Pre-pregnancy BMI, gestational weight gain, and
telomere length in amniotic fluid: a causal graph analysis. Sci
Rep. 8(14(1):23396)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Martens D. S..Plusquin M..Gyselaers W..De
Vivo I..Nawrot T. S: Maternal pre-pregnancy body mass index and
newborn telomere length. BMC Medicine. 14(1)(148)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lame-Jouybari AH, Fahami MS, Hosseini MS,
Moradpour M, Hojati A and Abbasalizad-Farhangi M: Association
between maternal prepregnancy and pregnancy body mass index and
children's telomere length: A systematic review and meta-analysis.
Nutr Rev. 83:622–635. 2025.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vahter M, Broberg K and Harari F:
Placental and cord blood telomere length in relation to maternal
nutritional status. J Nutr. 150:2646–2655. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu Y and Gao L: Preterm labor, a syndrome
attributed to the combination of external and internal factors.
Matern Fetal Med. 4:61–71. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Valdes AM, Andrew T, Gardner JP, Kimura M,
Oelsner E, Cherkas LF, Aviv A and Spector TD: Obesity, cigarette
smoking, and telomere length in women. Lancet. 366:662–664.
2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ämmälä AJ, Vitikainen EIK, Hovatta I,
Paavonen J, Saarenpää-Heikkilä O, Kylliäinen A, Pölkki P,
Porkka-Heiskanen T and Paunio T: Maternal stress or sleep during
pregnancy are not reflected on telomere length of newborns. Sci
Rep. 10(13986)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Salihu HM, King LM, Nwoga C, Paothong A,
Pradhan A, Marty PJ, Daas R and Whiteman VE: Association between
maternal-perceived psychological stress and fetal telomere length.
South Med J. 109:767–772. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Marchetto NM, Glynn RA, Ferry ML, Ostojic
M, Wolff SM, Yao R and Haussmann MF: Prenatal stress and newborn
telomere length. Am J Obstet Gynecol. 215:94.e1–e8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xu J, Ye J, Wu Y, Zhang H, Luo Q, Han C,
Ye X, Wang H, He J, Huang H, et al: Reduced fetal telomere length
in gestational diabetes. PLoS One. 9(e86161)2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Manna S, McCarthy C and McCarthy FP:
Placental ageing in adverse pregnancy outcomes: Telomere
shortening, cell senescence, and mitochondrial dysfunction. Oxid
Med Cell Longev. 2019(3095383)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Menon R, Boldogh I, Hawkins HK, Woodson M,
Polettini J, Syed TA, Fortunato SJ, Saade GR, Papaconstantinou J
and Taylor RN: Histological evidence of oxidative stress and
premature senescence in preterm premature rupture of the human
fetal membranes recapitulated in vitro. Am J Pathol. 184:1740–1751.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sultana Z, Maiti K, Dedman L and Smith R:
Is there a role for placental senescence in the genesis of
obstetric complications and fetal growth restriction? Am J Obstet
Gynecol. 218 (2S):S762–S773. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ferrari F, Facchinetti F, Saade G and
Menon R: Placental telomere shortening in stillbirth: A sign of
premature senescence? J Matern Fetal Neonatal Med. 29:1283–1288.
2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu S, Xu L, Cheng Y, Liu D, Zhang B, Chen
X and Zheng M: Decreased telomerase activity and shortened telomere
length in infants whose mothers have gestational diabetes mellitus
and increased severity of telomere shortening in male infants.
Front Endocrinol (Lausanne). 15(1490336)2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hjort L, Vryer R, Grunnet LG, Burgner D,
Olsen SF, Saffery R and Vaag A: Telomere length is reduced in 9- to
16-year-old girls exposed to gestational diabetes in utero.
Diabetologia. 61:870–880. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Garcia-Martin I, Penketh RJA, Janssen AB,
Jones RE, Grimstead J, Baird DM and John RM: Metformin and insulin
treatment prevent placental telomere attrition in boys exposed to
maternal diabetes. PLoS One. 13(e0208533)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pérez-López FR, López-Baena MT,
Ulloque-Badaracco JR and Benites-Zapata VA: Telomere length in
patients with gestational diabetes mellitus and normoglycemic
pregnant women: A systematic review and meta-analysis. Reprod Sci.
31:45–55. 2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Maeda S, Ohno K, Uchida K, Igarashi H,
Goto-Koshino Y, Fujino Y and Tsujimoto H: Intestinal
protease-activated receptor-2 and fecal serine protease activity
are increased in canine inflammatory bowel disease and may
contribute to intestinal cytokine expression. J Vet Med Sci.
76:1119–1127. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gilfillan C, Naidu P, Gunawan F, Hassan F,
Tian P and Elwood N: Leukocyte telomere length in the neonatal
offspring of mothers with gestational and pre-gestational diabetes.
PLoS One. 11(e0163824)2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gielen M, Hageman G, Pachen D, Derom C,
Vlietinck R and Zeegers MP: Placental telomere length decreases
with gestational age and is influenced by parity: A study of third
trimester live-born twins. Placenta. 35:791–796. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Tellechea M, Gianotti TF, Alvariñas J,
González CD, Sookoian S and Pirola CJ: Telomere length in the two
extremes of abnormal fetal growth and the programming effect of
maternal arterial hypertension. Sci Rep. 5(7869)2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kimura M, Stone RC, Hunt SC, Skurnick J,
Lu X, Cao X, Harley CB and Aviv A: Measurement of telomere length
by the Southern blot analysis of terminal restriction fragment
lengths. Nat Protoc. 5:1596–1607. 2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Aviv A, Hunt SC, Lin J, Cao X, Kimura M
and Blackburn E: Impartial comparative analysis of measurement of
leukocyte telomere length/DNA content by Southern blots and qPCR.
Nucleic Acids Res. 39(e134)2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Davenport P and Sola-Visner M: Hemostatic
challenges in neonates. Front Pediatr. 9(627715)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Gutierrez-Rodrigues F, Santana-Lemos BA,
Scheucher PS, Alves-Paiva RM and Calado RT: Direct comparison of
flow-FISH and qPCR as diagnostic tests for telomere length
measurement in humans. PLoS One. 9(e113747)2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Tsatsakis A, Tsoukalas D, Fragkiadaki P,
Vakonaki E, Tzatzarakis M, Sarandi E, Nikitovic D, Tsilimidos G and
Alegakis AK: Developing BIOTEL: A semi-automated spreadsheet for
estimating telomere length and biological age. Front Genet.
10(84)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Chen L, Tan KML, Gong M, Chong MFF, Tan
KH, Chong YS, Meaney MJ, Gluckman PD, Eriksson JG and Karnani N:
Variability in newborn telomere length is explained by inheritance
and intrauterine environment. BMC Med. 20(20)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wang C, Alfano R, Reimann B, Hogervorst J,
Bustamante M, De Vivo I, Plusquin M, Nawrot TS and Martens DS:
Genetic regulation of newborn telomere length is mediated and
modified by DNA methylation. Front Genet. 13(934277)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Daneels L, Martens DS, Arredouani S,
Billen J, Koppen G, Devlieger R, Nawrot TS, Ghosh M, Godderis L and
Pauwels S: Maternal vitamin D and newborn telomere length.
Nutrients. 13(2012)2021.PubMed/NCBI View Article : Google Scholar
|