Introduction
Primary salivary gland-type lung tumours are rare,
accounting for <1% of all primary lung carcinomas (1,2).
These tumours are thought to originate from glands in the submucosa
of the lower respiratory tract (3); therefore, they usually present as a
central space-occupying lung mass extending into the bronchus
(4). These tumours exhibit the
same histological variability as salivary gland tumours and are
therefore classified according to the World Health Organization
(WHO) criteria for salivary gland tumours (2). The most common histological subtype
of primary salivary gland lung tumours is mucoepidermoid carcinoma,
followed by adenoid cystic carcinoma (5). Primary pulmonary
epithelial-myoepithelial carcinoma (P-EMC) is a rare and much less
common tumour that has been recognized only within the last 2
decades (6), with only case
reports and small case series previously published in the
literature (4,6-20).
Morphologically and immunohistochemically, EMC is a low-grade
malignant tumour with biphasic epithelial and myoepithelial
morphology. The WHO classifies the tumour as ‘a malignant tumour
composed of variable proportions of two cell types, which typically
form duct-like structures. The biphasic morphology is represented
by an inner layer of duct-lining epithelial type cells and an outer
layer of clear myoepithelial cells (1). Many of these lesions are low-grade
malignant epithelial neoplasms but often pose diagnostic problems
because of their central location, circumscription, low-grade
cytological features and occasional focal similarity to other
salivary gland tumours, particularly in small endobronchial biopsy
samples (21). These tumours
usually have a good prognosis after complete surgical resection
with clear margins, but recurrence and metastasis have also been
reported (15,22,23).
To the best of our knowledge, ~50 cases of P-EMC
have been reported worldwide, as detailed in Table SI, but few publications concerning
primary P-EMC exist. Due to its rarity and unproven malignant
potential, the optimal treatment for P-EMC has not been
established. The present study reported a typical case of primary
P-EMC with diagnostic challenges and pitfalls associated with small
biopsy specimens of this rare tumor and reviewed the literature to
comprehensively analyse its clinical features, diagnosis and
appropriate treatment.
Case report
A 58-year-old nonsmoking female presented to Taihe
Hospital (Shiyan, China) after having experienced intermittent
haemoptysis for one month. The patient denied other respiratory
symptoms, including cough, expectoration, fever, night sweats,
fatigue, chest tightness and chest pain. The patient also denied
any history of cancer or relevant family history. Physical
examination indicated clear breath sounds on auscultation, with no
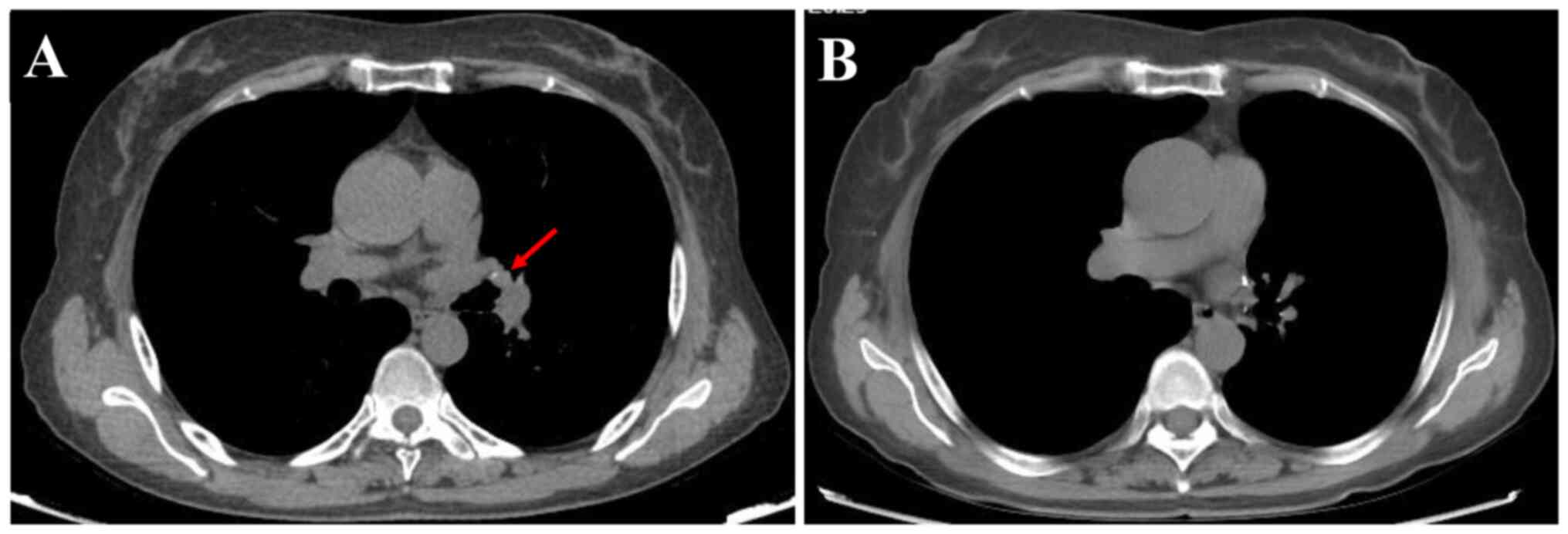
dry or wet rales in both lungs. Chest CT revealed a nodule in the
bronchial lumen of the left superior lobe with a maximum diameter
of 0.8 cm (Fig. 1A). The hilar and
mediastinal lymph nodes were not enlarged. Head and neck magnetic
resonance imaging, bone scintigraphy and whole-body CT scans with
contrast enhancement revealed no distant metastases or
lymphadenopathy. Serum tumour markers for general lung cancer
diagnosis were as follows: Neuron-specific enolase, 10.2 ng/ml
(normal range, 0-16.3 ng/ml); carcinoembryonic antigen, 4.1 µg/l
(normal range, 0-5 µg/l); Cyfra 21-1, 2.9 µg/l (normal range, 0-3.3
µg/l); squamous cell carcinoma antigen, 1.45 ng/ml (normal range,
0-2.7 ng/ml); and ferritin, 164 ng/ml (normal range, 30-400 ng/ml)
- all within normal limits.
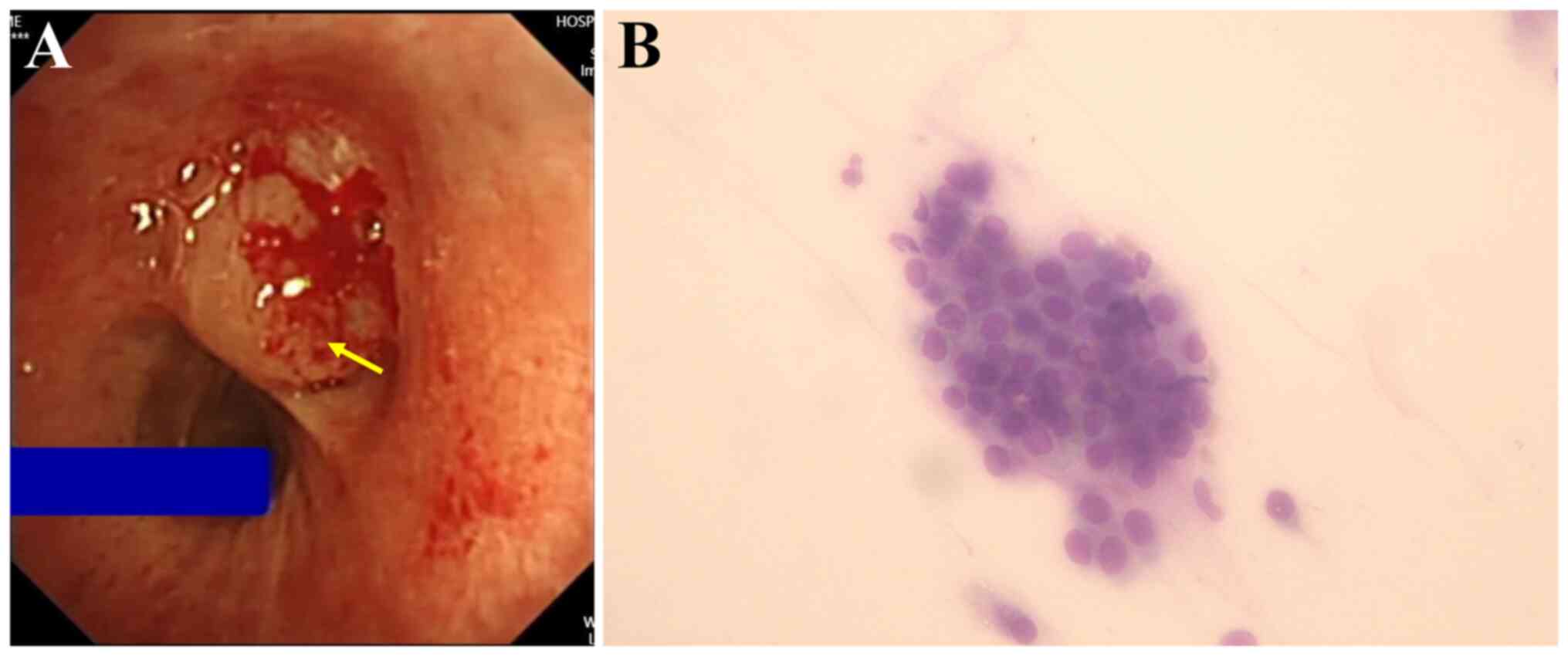
Bronchoscopy revealed a smooth neoplasm obstructing
the lumen at the entrance of the left upper lobe (Fig. 2A). Transbronchial forceps biopsy of
the neoplasm was conducted and rapid onsite evaluation (24) suggested non-small cell lung cancer
(Fig. 2B). Histopathology
indicated a probable diagnosis of mucoepidermoid carcinoma.
Positron emission tomography (PET)-CT scan can determine whether
there is a possibility of tumor metastasis. Given the patient's
financial constraints and the fact that PET-CT scan is not covered
by the local health policy (requiring full out-of-pocket payment),
the patient declined this procedure. Based on the absence of
abnormalities or metastases in the head and neck MRI, bone
scintigraphy and contrast-enhanced whole-body CT, along with
consensus by the multidisciplinary oncologic pulmonology board that
surgical resection was the most appropriate treatment,
video-assisted thoracoscopic (VATS) left upper lobectomy plus
sleeve resection of the left upper lobe bronchus were performed.
Intraoperative frozen section analysis could not definitively
diagnose the tumour, but gross examination of the resected mass
strongly suggested malignancy based on the mass's firm/rubbery
consistency, infiltrative borders (poorly circumscribed) and a
variegated (heterogeneous) cut surface showing mixtures of
grey-white tumour tissue and dense, glistening white sclerotic
areas. Consequently, lymph node dissection was performed at the
carinal, hilar, interlobar and Group 12 lymph nodes based on gross
examination suggested malignancy and the Chinese Society of
Clinical Oncology Non-small Cell Lung Cancer guidelines from
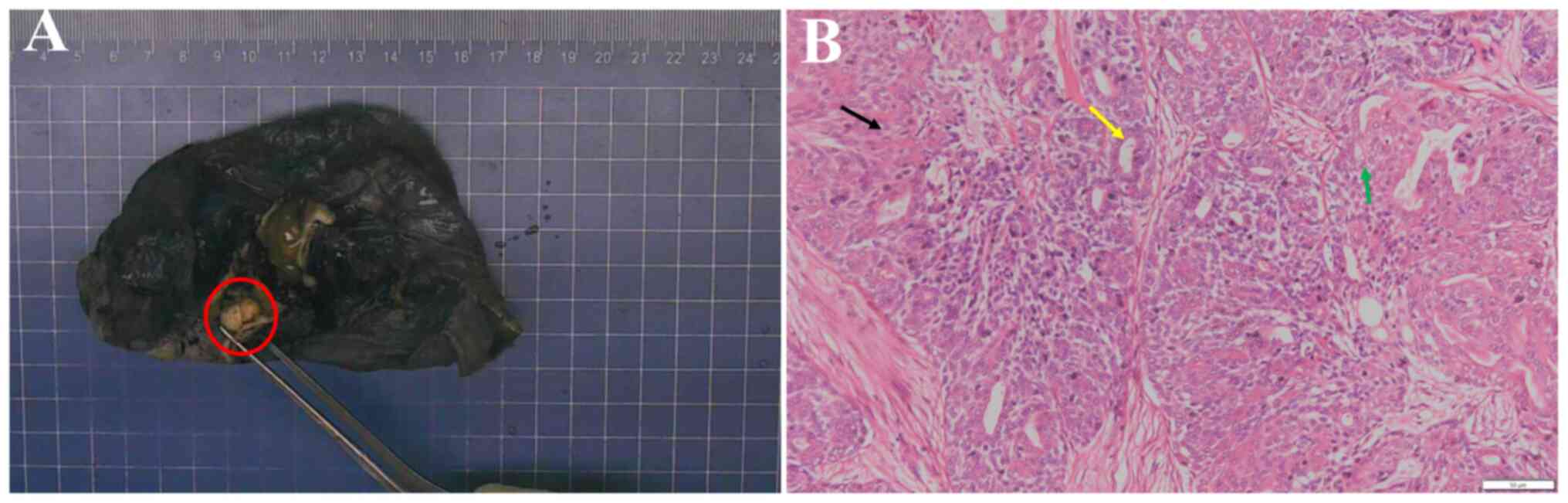
2024(25). Macroscopically, the
size of the resected upper lobe of the left lung was 11.5x8.5x4 cm
and a mass measuring 1.2x1x0.8 cm was observed 0.6 cm from the
bronchial stump (Fig. 3A). The cut
surface was grey-white, solid, firm and nodular, with clear
demarcation from the surrounding tissue, and the remaining lung
tissue was grey-red and soft.
The surgically resected samples were routinely fixed
in 10% neutral-buffered formalin, dehydrated, embedded in paraffin
and sectioned into 4-µm slices. Routine haematoxylin and eosin
staining was performed and the sections were imaged using a CX31
light microscope (Olympus Corp.). Histologically, the tumour
exhibited a biphasic pattern of inner ductal and outer
myoepithelial cells (Fig. 3B). At
low-power magnification, the tumour appeared well-defined but
unencapsulated, displaying a multinodular morphology with irregular
borders. Fibrosis bands were observed dissecting the tumour. The
inner layer consisted of a single row of cuboidal to columnar
epithelial cells and was characterized by dense to finely granular
cytoplasm surrounded by clear myoepithelial cells. The
myoepithelial cells were polygonal or spindle-shaped, with
abundant, clear and glycogen-rich cytoplasm. Myoepithelial cells
forming solid areas were focally observed and areas of squamous
differentiation were also present. These solid patterns blended
with more typical tubular patterns, making distinction between them
challenging. The luminal spaces contained eosinophilic material.
Minimal nuclear pleomorphism and a low mitotic rate were observed.
No metastatic cancer was found in the lymph nodes (0/11) and the
bronchial incisive margin was negative.
Immunohistochemistry (IHC) was performed on the
aforementioned surgical sections using the streptavidin-peroxidase
method. The sections were heated at 65˚C for 120 min and then
dewaxed by incubation with xylene for 3 min (six times). The
sections were rehydrated using a graded alcohol series and antigen
retrieval was performed using EDTA repair solution
(EDTA·2Na·2H2O, pH 9.0) with samples heated in a
pressure cooker to the boil and then kept warm for 20 min. The
sections were incubated with 3% H2O2 for 10
min at room temperature and then rinsed with PBS for 3 min to block
endogenous peroxidase activity. Sections were incubated with
primary antibodies against smooth muscle actin (SMA; cat. no.
Kit-0006; Fuzhou Maixin Biotech Co., Ltd.), SOX-10 (cat. no.
RMA-0726; Fuzhou Maixin Biotech Co., Ltd.), S-100 (cat. no.
Kit-0007; Fuzhou Maixin Biotech Co., Ltd.), CKp (cat. no. M3515;
Dako; Agilent Technologies, Inc.), Calponin (cat. no. MAB-0712;
Fuzhou Maixin Biotech Co., Ltd.), P40 (cat. no. RMA-1006; Fuzhou
Maixin Biotech Co., Ltd.), P63 (cat. no. MAB-0694; Fuzhou Maixin
Biotech Co., Ltd.), epithelial membrane antigen (EMA; cat. no.
KIT-0011; Fuzhou Maixin Biotech Co., Ltd.), CD117 (cat. no.
KIT-0029; Fuzhou Maixin Biotech Co., Ltd.), thyroid transcription
factor (TTF)-1 (cat. no. MAB-0599; Fuzhou Maixin Biotech Co.,
Ltd.), CK7 (cat. no. MAB-0828; Fuzhou Maixin Biotech Co., Ltd.) and
Ki-67 (cat. no. MAB-0542; Fuzhou Maixin Biotech Co., Ltd.) for 1 h
at room temperature. The sections were then incubated with the
EnVision detection system peroxidase/diaminobenzidine (DAB) and
rabbit/mouse secondary antibodies (cat. no. K5007; Dako; Agilent
Technologies, Inc.) for 30 min at room temperature. DAB from the
aforementioned secondary staining kit was added for detection. The
sections were counterstained with haematoxylin for 2 min and imaged
using a CX31 light microscope (Olympus Corp.). Microscopic
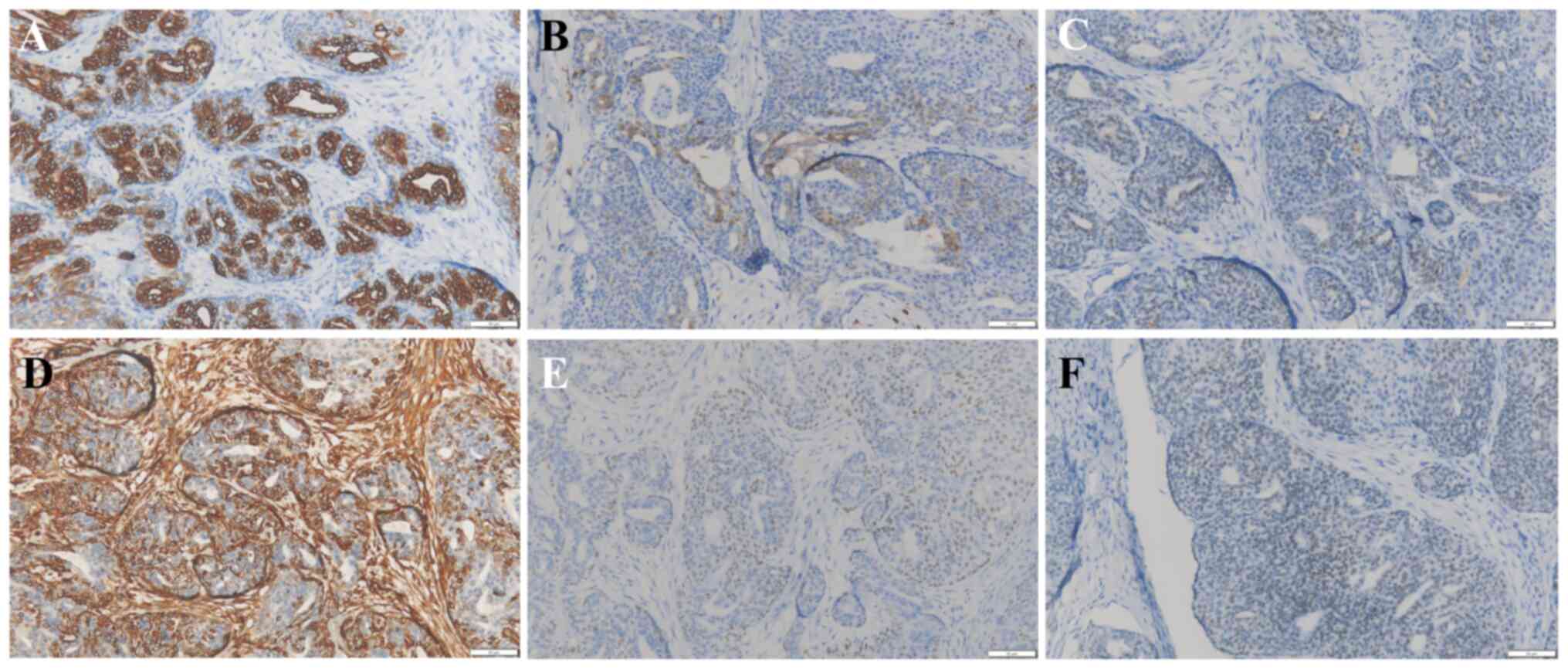
observation revealed the following immunohistochemical profile: CKp
(+), calponin (myoepithelium weak +), S-100 (myoepithelium +), SMA
(-), SOX-10 (myoepithelium +), P40 (myoepithelium +), P63
(myoepithelium +), EMA (ductal cell +), CK7 (ductal cell +), CD117
(ductal cell weak +), vimentin (myoepithelium +), TTF-1 (weak +)
and Ki-67 (15% +). Only critical markers for diagnosis were
preserved as images-specifically CK7, S-100, SOX10, VIM, P40 and
TTF-1 (Fig. 4) and images of the
remaining markers are missing.
Based on the above radiological, pathological and
immunostaining findings, the patient was diagnosed with P-EMC. The
patient did not receive any adjuvant chemotherapy and the patient
was discharged 5 days after the surgery with no complications. The
patient was referred to the pulmonary oncology clinic for
postoperative follow-up and underwent contrast-enhanced CT every 3
months within the first year after surgery, and every 6 months from
the second year. The patient was followed up for 5 years, until May
2025. During this period, the oncologist and thoracic surgeon were
responsible for performing the follow-up. A recent
contrast-enhanced chest CT scan (December 2024) revealed no
evidence of recurrence (Fig. 1B)
and the patient remained in good health. A close follow-up of every
1 to 2 years is ongoing.
Literature review
A comprehensive search of the PubMed (https://pubmed.ncbi.nlm.nih.gov/), Google Scholar
(https://scholar.nq69.top/) and Web of
Science databases (https://www.webofscience.com/) for primary P-EMC was
conducted to identify relevant studies published before January
1st, 2025, using the following terms: ‘primary’ AND
(‘tracheobronchial’ OR ‘bronchial’ OR ‘endobronchial’ OR ‘trachea’
OR ‘bronchus’ OR ‘lung’ OR ‘pulmonary’) AND
(‘epithelial-myoepithelial tumour’ OR ‘epithelial-myoepithelial
carcinoma’). Additionally, references from retrieved articles
meeting the inclusion criteria were manually searched. Exclusion
criteria comprised studies not written in English, metastatic
tumours or reports with insufficient data. The extracted data
included patient characteristics (geographic region, age, sex,
symptoms, smoking history, comorbidities, diagnostic modalities,
chest imaging, bronchoscopy, PET-CT, treatment, tumour size,
adjuvant chemotherapy, follow-up and survival). Since 2000, 50
global cases have been reported across 39 publications. After
applying exclusions-three cases for insufficient data, two
non-English reports and one metastatic tumour -44 unique cases met
the criteria for analysis. A total of 44 published cases of P-EMC
were reviewed with relatively complete clinical data reported from
2000 to 2025 (4,6,7,9,11-18,20,26-43),
with the following geographic distribution: The US (n=17), Japan
(n=6), the UK (n=6), China (n=4), South Korea (n=3), Australia
(n=1), Germany (n=1), Greece (n=1), India (n=1), Italy (n=1),
Portugal (n=1), Spain (n=1) and Turkey (n=1). The patients included
20 males and 24 females aged 7-83 years (mean, 55.8 years). A total
of 12 patients had a history of smoking, 22 were non-smokers and 10
had an undocumented smoking status. The tumour size ranged from
0.71 to 6 cm (mean, 2.82 cm), with 27 patients presenting with
endobronchial masses. Among the 10 patients who underwent PET-CT,
only 4 demonstrated normal 18F-fluorodeoxyglucose
(18F-FDG) uptake. Treatment modalities included surgical
resection in 37 patients (lobectomy, n=20; pneumonectomy, n=7;
wedge resection, n=6; segmentectomy, n=1; atypical resection, n=1;
and unspecified resection, n=2); bronchoscopic interventions in 4
patients (electrocautery snare, n=2; curative electrosurgery, n=1;
and laser ablation, n=1); and radiation with chemotherapy in 1
patient with nodal metastasis; while 2 patients received
undocumented treatment. Adjuvant chemotherapy was administered to
only 1 postoperative patient. Among the 44 patients, 32 had
follow-up data ranging from 2.5 to 96 months, while 12 had no
documented follow-up. Outcome analysis revealed no recurrence or
metastasis in 30 patients, death in 2, intrapulmonary metastasis in
1, loss to follow-up in 1 and unknown outcome in 10. Details of the
studies retrieved from the above-mentioned literature review are
provided in Table SI.
Discussion
The present case contributed to the existing
literature by reporting on the diagnostic challenges and pitfalls
associated with small biopsy specimens of this rare tumor and
demonstrating favorable outcomes with no recurrence or metastasis
during 5 years of long-term follow-up. The study underscores the
diagnostic pitfalls in clinical practice and provides evidence that
surgical resection can yield favorable clinical outcomes for this
rare tumor.
The most common salivary gland tumours in the head
and neck are benign, including pleomorphic adenoma, Warthin tumours
and basal cell adenoma, whereas the most common malignant tumours
are mucoepidermoid carcinoma (MEC) and adenoid cystic carcinoma
(ACC). However, salivary gland tumours located in the lungs are
mostly malignant, and the common types include ACC, MEC, EMC and
acinar cell carcinoma (1). EMC is
a rare salivary gland malignancy first described by Donath et
al (44) in 1972, accounting
for ~1% of salivary gland tumours; EMC in the pulmonary system is
even rarer, with only 50 cases reported globally between 2000 and
2025.
The literature review and current case indicate that
primary P-EMC is more prevalent in middle-aged and elderly
individuals, with women showing greater morbidity than men. The
maximum tumour diameter ranged from 0.71 to 6.0 cm (28,30)
and the maximum diameter of the resected mass from the patient of
the present study was 1.2 cm. Among the 44 reported cases of
primary P-EMC, 12 had a history of smoking, 22 had no smoking
history and 10 were undocumented. Therefore, it may be speculated
that the occurrence of primary P-EMC is not associated with
smoking, consistent with the conclusion of Goodwin et al
(45). According to Table SI, radiologically, most P-EMCs are
characterized as endobronchial masses, or bronchoscopy reveals
visible neoplasms or polyps in patients who usually present with
airway obstruction symptoms. However, patients with EMC located in
the lung parenchyma may have no obvious symptoms in the early
stages, and EMC is often detected during chest radiological
examinations. The diagnostic value of 18F-FDG-PET is
unclear; only for 4 cases, elevated glucose metabolism in EMC
lesions was reported (26,32,33,43);
by contrast, the majority of reports indicated normal
18F-FDG uptake (12,17,28,29,31,34).
The diagnosis of EMC depends on histopathology, and a duct-like
structure comprising epithelial and myoepithelial cells is the
typical pathological feature. These duct-like structures can be
categorised into two types: One with a large area with a typical
double-layer tubular structure-epithelial cells forming the inner
layer and myoepithelial cells forming the outer layer of the lumen;
the other type with only few atypical duct-like structures,
consisting of epithelial cells that form a single layer of
duct-like structure surrounded by large confluent myoepithelial
cells. IHC reveals CK positivity in inner epithelial cells, while
the outer myoepithelial cells stain positive for SMA and S-100.
These two pathological patterns may coexist in varying proportions
within the same tumour (6).
Compared with previously reported cases, the
pathological diagnosis presented in the current study poses
significant diagnostic challenges. Initial bronchoscopic biopsy
findings were suggestive of MEC, whereas definitive surgical
specimen pathology confirmed P-EMC. The reasons for the
misdiagnosis or diagnostic discrepancy in the present case are as
follows: Limitations of the small biopsy sample were evident, as
the bronchoscopic biopsy consisted solely of fragmented tissue
measuring 0.5x0.3x0.2 cm, precluding observation of the
characteristic biphasic tubular structure (inner ductal epithelium
plus outer myoepithelial layer) of EMC. The biopsy may have sampled
only an area of epidermoid or intermediate-type cells, mimicking
MEC morphology and leading to misdiagnosis. For instance, abundant
myoepithelial cells could be misinterpreted as myoepithelioma or
myoepithelial carcinoma, while predominant epithelial cells broaden
the differential to include mucinous cystadenocarcinoma, MEC,
mucinous adenoma, acinic cell carcinoma, myoepithelioma,
myoepithelial carcinoma, clear cell (‘sugar’) tumour, metastatic
EMC, and primary or metastatic clear cell carcinomas (6,7,10,30,46).
With respect to tumour heterogeneity, EMC frequently exhibits
regional variation in differentiation: Sampling a dedifferentiated
myoepithelial zone (lacking myoepithelial markers) or a ductal
epithelium-predominant region (expressing CK, P40) in small
biopsies readily leads to MEC misdiagnosis, whereas comprehensive
sampling of resection specimens reveals the typical biphasic
differentiation (6). A critical
diagnostic pitfall involves P40, which is expressed in
myoepithelial cells (particularly basaloid variants), indicating
that a small-biopsy P40(+) result likely represents the
myoepithelial layer of EMC rather than the squamous cells of MEC.
Nevertheless, this P40(+) finding was interpreted as a ‘squamous
differentiation’ marker of MEC. Morphologically, MECs and EMCs
share overlapping features, including solid areas, clear cell
changes and squamoid cells. The absence of definitive EMC biphasic
structures or MEC mucinous cells in limited biopsies creates
diagnostic uncertainty. Finally, while MAML2 rearrangement
demonstrates high specificity for MEC (particularly low- to
intermediate-grade), where positivity supports MEC and negativity
favours EMC, this test was omitted due to the patient's financial
constraints, necessitating reliance on limited immunohistochemistry
for the initial diagnosis.
In general, VATS resection emerged as the most
prevalent therapeutic approach, with lobectomy predominating
(54.1%, n=20), followed by pneumonectomy (18.9%, n=7), wedge
resection (16.2%, n=6) and segmentectomy (2.7%, n=1). Of note,
Hagmeyer et al (11)
reported good outcomes in a young patient who underwent segmental
tracheal resection, with no recurrence or metastasis at the
24-month follow-up. Bronchoscopic interventions were reported in 4
patients (9.1%), including electrocautery snare (n=2), curative
electrosurgery (n=1) and laser ablation therapy (n=1). Among these,
Chao et al (27) reported
successful curative electrosurgery for an endobronchial mass in the
distal left main bronchus of a 43-year-old woman, demonstrating
disease-free survival at the 6-month follow-up. Regrettably, the
remaining three bronchoscopically managed patients were lost to
follow-up or lacked outcome documentation. All patients treated
bronchoscopically and the patient who underwent segmentectomy had
sub-2 cm lesions (≤2 cm), suggesting that bronchoscopic management
is a viable option for small, endobronchially confined tumours,
whereas segmentectomy has favourable outcomes for similarly sized
nonmetastatic lesions. By contrast, lobectomy cases predominantly
involve lesions >2 cm (4,6,7,12,14,18,20,26,30,33,39,40,42).
Among the 6 patients who underwent pneumonectomy, the maximum
diameters of the lesions in 4 patients were 2.7, 4.2, 4.5 and 6 cm,
while the sizes in 2 patients were not recorded. Among the 6
patients who underwent wedge resection, the maximum lesion size was
4 cm and the minimum was 0.8 cm. The patient of the present study
(tumour dimensions: 1.2x1.0x0.8 cm) underwent VATS left upper
lobectomy with bronchial sleeve resection and mediastinal lymph
node dissection due to initial diagnostic pitfalls on bronchoscopic
biopsy. While excellent outcomes with 5-year recurrence-free
survival were achieved, retrospective analysis suggested that
definitive preoperative diagnosis of low-grade malignant P-EMC
might have permitted parenchymal-sparing approaches, such as
segmentectomy or wedge resection, as feasible alternatives.
Of note, the biological characteristics of P-EMC and
salivary EMC are have remained to be fully elucidated. However,
previous reports have shown that the recurrence or metastasis
interval of salivary EMC is relatively long, averaging 5 years
(47) and 15 years (48), respectively. The longest reported
follow-up period to date is 96 months (4). Therefore, the recurrence and
metastasis of P-EMC require further investigation through extended
follow-up. Although typical EMC is generally considered a low-grade
malignancy with a favourable clinical prognosis, numerous studies
have shown that P-EMC with high-grade transformation is aggressive,
with a poor prognosis and a high rate of distant metastasis
(18,30,49).
Tajima et al (18) reported
that Ki-67 and cyclin D1 were key molecules associated with the
high-grade transformation of EMC, suggesting a poor prognosis and
the need for close follow-up. Overall, three studies have reported
the use of NGS to detect mutations associated with primary P-EMC.
Nakashima et al (31)
reported on a 54-year-old female patient with P-EMC in the right
middle lobe. NGS detected no mutations in the Kirsten rat
sarcoma viral oncogene homolog or epidermal growth factor
receptor (EGFR) genes. Sharma et al (17) reported a case of P-EMC in a
38-year-old man presenting with a 3-cm diameter homogeneous mass in
the lower lobe of the right lung. Genetic testing for mutations in
EGFR, anaplastic lymphoma kinase, ROS proto-oncogene
1 tyrosine kinase, B-Raf proto-oncogene, serine/threonine
kinase and mesenchymal-epithelial transition factor was
performed, but no mutations were found. Charles et al
(30) reported on a 72-year-old
female patient with P-EMC with focal high-grade transformation.
Mutations in DNMT3A, APC, STAT3 and
KDM5C were detected. Despite these detected gene mutations,
the patient was still treated with a left pneumonectomy along with
mediastinal lymph node dissection. Based on this literature review,
no specific gene testing is generally recommended for detecting
particular mutations associated with P-EMC, including for guiding
treatment strategies. The clinical significance of DNMT3A,
APC, STAT3 and KDM5C mutations in the
diagnosis, prognosis and treatment of P-EMC requires further
investigation to be established.
In conclusion, primary P-EMC is a low-grade
malignant tumour that most commonly occurs in the tracheobronchial
tree. Histologically, a duct-like structure composed of epithelial
and myoepithelial cells provides evidence for a definite diagnosis.
Based on the present case and a literature review, for
endobronchial EMC lesions confined to the bronchus and measuring
<2 cm without metastasis, bronchoscopic intervention or VATS
segmentectomy are viable therapeutic alternatives; for
nonmetastatic central lesions >2 cm, lobectomy or wedge
resection should be considered to avoid the more traumatic
pneumonectomy as much as possible.
Supplementary Material
Clinical data of previously reported
cases of primary P-EMC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding authors.
Authors' contributions
DC, JW and FW were involved in the conception/design
of the study. DC and ZZ drafted the manuscript and performed data
acquisition and analysis/interpretation for the study. ZZ and YZ
made contributions to the interpretation of the data for the work
and critically revised the manuscript for important intellectual
content. YW, YZ and TR acquired data and collected pathological and
surgical information of the patient. ZZ and JW assisted in updating
patient follow-up information and the literature search. FW, DC and
ZZ confirm the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Taihe Hospital (approval no., KS2025KS027) and performed in
accordance with the principles of Good Clinical Practice following
the Tri-Council guidelines.
Patient consent for publication
Written informed consent was obtained from the
patient for anonymized information (e.g. medical records/case
information and images) to be published in this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 World health organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Skálová A, Hyrcza MD and Leivo I: Update
from the 5th Edition of the World Health organization
classification of head and neck tumors: Salivary glands. Head Neck
Pathol. 16:40–53. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Moran CA: Primary salivary gland-type
tumors of the lung. Semin Diagn Pathol. 12:106–122. 1995.PubMed/NCBI
|
|
4
|
Fulford LG, Kamata Y, Okudera K, Dawson A,
Corrin B, Sheppard MN, Ibrahim NB and Nicholson AG:
Epithelial-myoepithelial carcinomas of the bronchus. Am J Surg
Pathol. 25:1508–1514. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhu F, Liu Z, Hou Y, He D, Ge X, Bai C,
Jiang L and Li S: Primary salivary gland-type lung cancer:
Clinicopathological analysis of 88 cases from China. J Thorac
Oncol. 8:1578–1584. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nguyen CV, Suster S and Moran CA:
Pulmonary epithelial-myoepithelial carcinoma: A clinicopathologic
and immunohistochemical study of 5 cases. Hum Pathol. 40:366–373.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Doganay L, Bilgi S, Ozdil A, Yoruk Y,
Altaner S and Kutlu K: Epithelial-myoepithelial carcinoma of the
lung. A case report and review of the literature. Arch Pathol Lab
Med. 127:e177–e180. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Naso JR and Roden AC: Recent developments
in the pathology of primary pulmonary salivary gland-type tumours.
Histopathology. 84:102–123. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shen C, Wang X and Che G: A rare case of
primary peripheral epithelial myoepithelial carcinoma of lung: Case
report and literature review. Medicine (Baltimore).
95(e4371)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wilson RW and Moran CA:
Epithelial-myoepithelial carcinoma of the lung: Immunohistochemical
and ultrastructural observations and review of the literature. Hum
Pathol. 28:631–635. 1997.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hagmeyer L, Tharun L, Schäfer SC, Hekmat
K, Büttner R and Randerath W: First case report of a curative wedge
resection in epithelial-myoepithelial carcinoma of the lung. Gen
Thorac Cardiovasc Surg. 65:535–538. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jindani R, Lopez MA, Miquel TP and Sylvin
E: Robotic resection of pulmonary epithelial myoepithelial
carcinoma: A case report. Thorac Cardiovasc Surg Rep. 10:e42–e44.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Konoglou M, Cheva A, Zarogoulidis P,
Porpodis K, Pataka A, Mpaliaka A, Papaiwannou A, Zarogoulidis K,
Kontakiotis T, Karaiskos T, et al: Epithelial-myoepithelial
carcinoma of the trachea-a rare entity case report. J Thorac Dis. 6
(Suppl 1):S194–S199. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mori M, Hanagiri T, Nakanishi R, Ashikari
S, Yasuda M and Tanaka F: Primary epithelial-myoepithelial
carcinoma of the lung with cavitary lesion: A case report. Mol Clin
Oncol. 9:315–317. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nishihara M, Takeda N, Tatsumi S,
Kidoguchi K, Hayashi S, Sasayama T, Kohmura E and Hashimoto K:
Skull metastasis as initial manifestation of pulmonary
epithelial-myoepithelial carcinoma: A case report of an unusual
case. Case Rep Oncol Med. 2011(610383)2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Patterson DT, Halverson Q, Williams S,
Bishop JA, Ochoa CD and Styrvoky K: Bronchoscopic management of a
primary endobronchial salivary epithelial-myoepithelial carcinoma:
A case report. Respir Med Case Rep. 30(101083)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sharma S, Tayal A, Khatri S, Mohapatra SG
and Mohanty SK: Primary pulmonary epithelial-myoepithelial
carcinoma: Report of a rare and under-diagnosed low-grade
malignancy. J Cancer Res Ther. 18:795–800. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tajima S, Aki M, Yajima K, Takahashi T,
Neyatani H and Koda K: Primary epithelial-myoepithelial carcinoma
of the lung: A case report demonstrating high-grade
transformation-like changes. Oncol Lett. 10:175–181.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang H, Zhang Y, Wang B, Wei J, Ji R, Dong
L and Jiang X: Epithelial-myoepithelial carcinoma of the parotid
gland with primary lung cancer: A rare case report. Medicine
(Baltimore). 99(e22483)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang G, Wang R, Qiao X and Li J: Primary
epithelial-myoepithelial carcinoma of the lung presenting as
multiple synchronous lesions. Ann Thorac Surg. 115:e17–e19.
2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
J.W.E. L. Barnes, P. Reichart, and D.
Sidransky, Eds., ‘Epithelial myoepithelial carcinoma,’ in World
Health Organization Classification of Tumors, Pathology and
Genetics of Head and Neck Tumors, 2005.
|
|
22
|
Song DH, Choi IH, Ha SY, Han KM, Han J,
Kim TS, Kim J and Kim H: Epithelial-myoepthelial carcinoma of the
tracheobronchial tree: The prognostic role of myoepithelial cells.
Lung Cancer. 83:416–419. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Muslimani AA, Kundranda M, Jain S and Daw
HA: Recurrent bronchial epithelial-myoepithelial carcinoma after
local therapy. Clin Lung Cancer. 8:386–388. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang H, Wei N, Tang Y, Wang Y, Luo G, Ren
T, Xiong T, Li H, Wang M and Qian X: The utility of rapid on-site
evaluation during bronchoscopic biopsy: A 2-year respiratory
endoscopy central experience. Biomed Res Int.
2019(5049248)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen S, Wang Z and Sun B: Chinese Society
of Clinical Oncology Non-small Cell Lung Cancer (CSCO NSCLC)
guidelines in 2024: Key update on the management of early and
locally advanced NSCLC. Cancer Biol Med. 22:191–196.
2025.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cha YJ, Han J, Lee MJ, Lee KS, Kim H and
Zo J: A rare case of bronchial epithelial-myoepithelial carcinoma
with solid lobular growth in a 53-year-old woman. Tuberc Respir Dis
(Seoul). 78:428–431. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chao TY, Lin AS, Lie CH, Chung YH, Lin JW
and Lin MC: Bronchial epithelial-myoepithelial carcinoma. Ann
Thorac Surg. 83:689–691. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim CH, Jeong JS, Kim SR and Lee YC:
Endobronchial epithelial-myoepithelial carcinoma of the lung.
Thorax. 73:593–594. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
McCracken D, Wieboldt J, Sidhu P and
McManus K: Endobronchial laser ablation in the management of
epithelial-myoepithelial carcinoma of the trachea. Respir Med Case
Rep. 16:151–153. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Charles R, Murray S, Gray E and Hu J:
Pulmonary epithelial-myoepithelial carcinoma (P-EMC) with focal
high grade transformation: Molecular and cytologic findings. Diagn
Cytopathol. 50:E156–E162. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Nakashima Y, Morita R, Ui A, Iihara K and
Yazawa T: Epithelial-myoepithelial carcinoma of the lung: A case
report. Surg Case Rep. 4(74)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Arif F, Wu S, Andaz S and Fox S: Primary
epithelial myoepithelial carcinoma of lung, reporting of a rare
entity, its molecular histogenesis and review of the literature.
Case Rep Pathol. 2012(319434)2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cho SH, Park SD, Ko TY, Lee HY and Kim JI:
Primary epithelial myoepithelial lung carcinoma. Korean J Thorac
Cardiovasc Surg. 47:59–62. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Westacott LS, Tsikleas G, Duhig E, Searle
J, Kanowski P, Diqer M and Binder J: Primary
epithelial-myoepithelial carcinoma of lung: A case report of a rare
salivary gland type tumour. Pathology. 45:420–422. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Huang HC, Zhao L, Cao LX, Meng G, Wang YJ
and Wu M: Primary salivary gland tumors of the lung: Two cases date
report and literature review. Respir Med Case Rep.
32(101333)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Doxtader EE, Shah AA, Zhang Y, Wang H,
Dyhdalo KS and Farver C: Primary salivary gland-type tumors of the
tracheobronchial tree diagnosed by transbronchial fine needle
aspiration: Clinical and cytomorphologic features with
histopathologic correlation. Diagn Cytopathol. 47:1168–1176.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shiina Y, Yoshimura M and Tauchi S:
Pulmonary epithelial-myoepithelial carcinoma;report of a case.
Kyobu Geka. 73:708–711. 2020.PubMed/NCBI(In Japanese).
|
|
38
|
Muro M, Yoshioka T, Idani H, Sasaki H,
Asami S, Nojima H, Kubo S, Kurose Y, Hirata M, Yamashita T, et al:
Epithelial-myoepithelial carcinoma of the lung. Kyobu Geka.
63:220–223. 2010.PubMed/NCBI
|
|
39
|
Rosenfeld A, Schwartz D, Garzon S and
Chaleff S: Epithelial-myoepithelial carcinoma of the lung: A case
report and review of the literature. J Pediatr Hematol Oncol.
31:206–208. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Muñoz G, Felipo F, Marquina I and Del Agua
C: Epithelial-myoepithelial tumour of the lung: a case report
referring to its molecular histogenesis. Diagn Pathol.
6(71)2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pelosi G, Fraggetta F, Maffini F, Solli P,
Cavallon A and Viale G: Pulmonary epithelial-myoepithelial tumor of
unproven malignant potential: Report of a case and review of the
literature. Mod Pathol. 14:521–526. 2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chang T, Husain AN, Colby T, Taxy JB,
Welch WR, Cheung OY, Early A, Travis W and Krausz T: Pneumocytic
adenomyoepithelioma: A distinctive lung tumor with epithelial,
myoepithelial, and pneumocytic differentiation. Am J Surg Pathol.
31:562–568. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Morgado S, Santos AF and Nogueira F:
Primary pulmonary epithelial-myoepithelial carcinoma: A case report
and comprehensive literature review of a rare lung neoplasm.
Cureus. 17(e80188)2025.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Donath K, Seifert G and Schmitz R:
Diagnosis and ultrastructure of the tubular carcinoma of salivary
gland ducts. Epithelial-myoepithelial carcinoma of the intercalated
ducts. Virchows Arch A Pathol Pathol Anat. 356:16–31.
1972.PubMed/NCBI(In German).
|
|
45
|
Goodwin CR, Khattab MH, Sankey EW, Crane
GM, McCarthy EF and Sciubba DM: Epithelial-myoepithelial carcinoma
metastasis to the thoracic spine. J Clin Neurosci. 24:143–146.
2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Miura K, Harada H, Aiba S and Tsutsui Y:
Myoepithelial carcinoma of the lung arising from bronchial
submucosa. Am J Surg Pathol. 24:1300–1304. 2000.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Luna MA, Ordonez NG, Mackay B, Batsakis JG
and Guillamondegui O: Salivary epithelial-myoepithelial carcinomas
of intercalated ducts: A clinical, electron microscopic, and
immunocytochemical study. Oral Surg Oral Med Oral Pathol.
59:482–490. 1985.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Cho KJ, el-Naggar AK, Ordonez NG, Luna MA,
Austin J and Batsakis JG: Epithelial-myoepithelial carcinoma of
salivary glands. A clinicopathologic, DNA flow cytometric, and
immunohistochemical study of Ki-67 and HER-2/neu oncogene. Am J
Clin Pathol. 103:432–437. 1995.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Costa AF, Altemani A and Hermsen M:
Current concepts on dedifferentiation/high-grade transformation in
salivary gland tumors. Patholog Res Int.
2011(325965)2011.PubMed/NCBI View Article : Google Scholar
|