1. Introduction
Over the past decade, a number of studies have shown
that women with endometriosis are at increased risk for developing
various diseases as comorbidities, including asthma (1), various types of cancer, such as
ovarian and breast cancer, as well as cutaneous melanoma (2), cardiovascular and psychiatric
diseases (3,4), hypothyroidism and fibromyalgia
(1). In addition, other studies
have reported an association between different autoimmune diseases,
including autoimmune thyroid disorder, Crohn's disease and
ulcerative colitis, Sjogren's syndrome (SS), systemic lupus
erythematosus (SLE), multiple sclerosis, ankylosing spondylitis
(AS), coeliac disease and rheumatoid arthritis (RA), and an
increased risk for endometriosis (5-9).
Endometriosis is a common yet enigmatic
inflammatory, multifactorial, estrogen-dependent gynecological
condition with an unknown etiology, affecting up to 10% of women of
reproductive age worldwide and 9 million women in the USA (10). Endometriosis is characterized by
the presence of ectopic endometrial tissue (endometrial glands and
stroma) on other organs, predominantly external to the uterine
cavity and most commonly in the pelvic cavity, due to the ectopic
localization of endometrial cells. Endometriosis can appear as
peritoneal lesions, ovarian endometriotic cysts and deeply
infiltrative endometrial lesions (11). Furthermore, endometriosis is
associated with chronic pelvic pain, dysmenorrhea, dyspareunia,
heavy or irregular menstrual bleeding, urinary tract symptoms,
subfertility and infertility in 30% of patients (11,12).
Endometriosis-associated gene polymorphisms, epigenetic
modifications (such as methylation, acetylation, phosphorylation
and modifications to the histone proteins associated with DNA
packaging in chromatin), proinflammatory factors (including
cytokines like IL-1β, IL-6, IL-8 and TNF-α, as well as
prostaglandins), hormonal factors (including an imbalance between
estrogen and progesterone) and environmental factors (including
organic pollutants, polyhalogenated aromatic hydrocarbons, tobacco,
heavy metals and pesticides) contribute to the development of the
disease and trigger its specific clinical symptoms (11,12).
Nowadays, a large amount of evidence indicates that dysregulation
of the immune system leads to chronic inflammatory response in the
ectopic endometrium, thus resulting in the subsequent development
of endometriosis (13,14).
According to previous studies, women with
endometriosis are at a higher risk for developing a concomitant
autoimmune disease, considering the deregulation of the immune
system observed in these patients (2,4,5,10,12).
In this context, both proinflammatory and mitogenic proteins from
endometriotic lesions, including cytokines (RANTES, IL-1, IL-6,
TNF-a) and VEGF, as well as associated activated immune cells, such
as pelvic macrophages and lymphocytes, can cause an imbalance in
the immune system, which contributes to the enhanced inflammatory
reaction that is associated with the development of endometriosis
(15). Accordingly, functional
abnormalities in almost all types of immune cells have been
identified in endometriosis, including neutrophils, macrophages,
natural killer (NK) cells, B and T lymphocytes, as well as
dendritic cells (DCs) (13).
Moreover, a series of anti-nuclear, anti-phospholipid and
anti-endometrial antibodies are produced in endometriosis (14). This link of endometriosis with
aberrant immune responses and the subsequent development of
autoimmune diseases has led to the suggestion that endometriosis
itself may be classified as a typical autoimmune disorder (14,16).
Alternatively, another concept suggests that an underlying abnormal
immune response may contribute to the development of endometriosis
(5).
Recently, Chen et al (17) attempted to clarify whether patients
with endometriosis have a higher risk of developing
antiphospholipid syndrome (APS), by conducting a retrospective
cohort study. It was demonstrated, for the first time to the best
of our knowledge, that patients with endometriosis have a higher
risk (2.84-fold) of developing subsequent APS compared with women
without endometriosis (17).
However, the existing information refers only to epidemiological
and clinical aspects of this detected association. APS is a
systemic autoimmune disease characterized by the occurrence of
vascular or arterial thromboses at different localizations,
including the brain, heart, lungs, kidneys and limbs, obstetric
complications in women of childbearing age including miscarriages,
recurrent fetal deaths and premature birth, and the presence of
circulating antiphospholipid (aPL) antibodies (18). As an autoimmune disease, APS is
developed by an interplay of genetic and antigenic factors, while
specific autoantibodies are present and used for its diagnosis,
such as lupus anticoagulant, anti-cardiolipin (aCL) antibodies,
anti-β2 glycoprotein-I (β2GPI) antibodies and
anti-phosphatidylserine/prothrombin antibodies (19,20).
The genetic predisposition of APS has been demonstrated by studies
in monozygotic twins, gene-association and genome-wide association
studies (GWAS) as well as whole-exome sequencing (WES) approaches
(21-25).
Importantly, APS can occur as a single disorder where there is no
clinical or laboratory evidence of another underlying disease,
called ‘primary APS’ (PAPS), but it can also be associated with
another autoimmune disease, and referred to in this case as
‘secondary APS’ (26).
Consequently, APS may, in part, share a common mechanism for
disease development or progression with other autoimmune or
immunity-related diseases.
The recent data regarding the increased risk of
subsequent APS in women with endometriosis posed an interesting
question concerning the putative role of shared genetic factors in
the co-occurrence of both conditions (17). Notably, our previous studies have
focused on the delineation of the genetic components that are
involved in the co-occurrence of endometriosis with various
autoimmune diseases, including SLE (27), RA (7), AS (8), SS (9), as well as psoriasis and psoriatic
arthritis (28). In the present
review, prompted by the findings of Chen et al (17), the present review sought to
delineate the genetic basis of the co-occurrence of endometriosis
with APS by systematically searching the literature for genes
involved in the development of both diseases, aiming to shed light
on the functional significance of the shared polymorphisms and
unravel the underlying molecular and pathogenetic mechanisms of
these diseases.
2. Shared genetic loci associated with
endometriosis and APS
In the current review, a potential shared genetic
background regarding the co-occurrence of endometriosis and APS was
investigated. To the best of our knowledge, the present review is
the first study in the literature focusing on the genetic basis of
the subsequent development of APS in patients with endometriosis.
The present review included case-control, GWAS, whole-genome
sequencing and WES studies, as well as systematic reviews and
cross-sectional studies. Notably, case report series, editorial
letters, expert opinions and conference abstracts were excluded.
The articles included were written in the English language and
published from 2000-2025. Five electronic databases were used,
namely PubMed (https://pubmed.ncbi.nlm.nih.gov/), PubMed Central
(https://pmc.ncbi.nlm.nih.gov/), Google
Scholar (https://scholar.google.com/), Web of
Science (https://www,webofscience.com/wos/) and MEDLINE
(https://www.nlm.nih.gov/medline/medline_home.html/).
The keywords used for the search were as follows: ‘Endometriosis’,
‘antiphospholipid syndrome’, ‘antiphospholipid antibodies’,
‘genetics’, ‘gene polymorphisms’, ‘association studies’ and ‘GWAS’.
Two authors worked individually to extract the data following the
specified criteria and no disagreements arose during this
process.
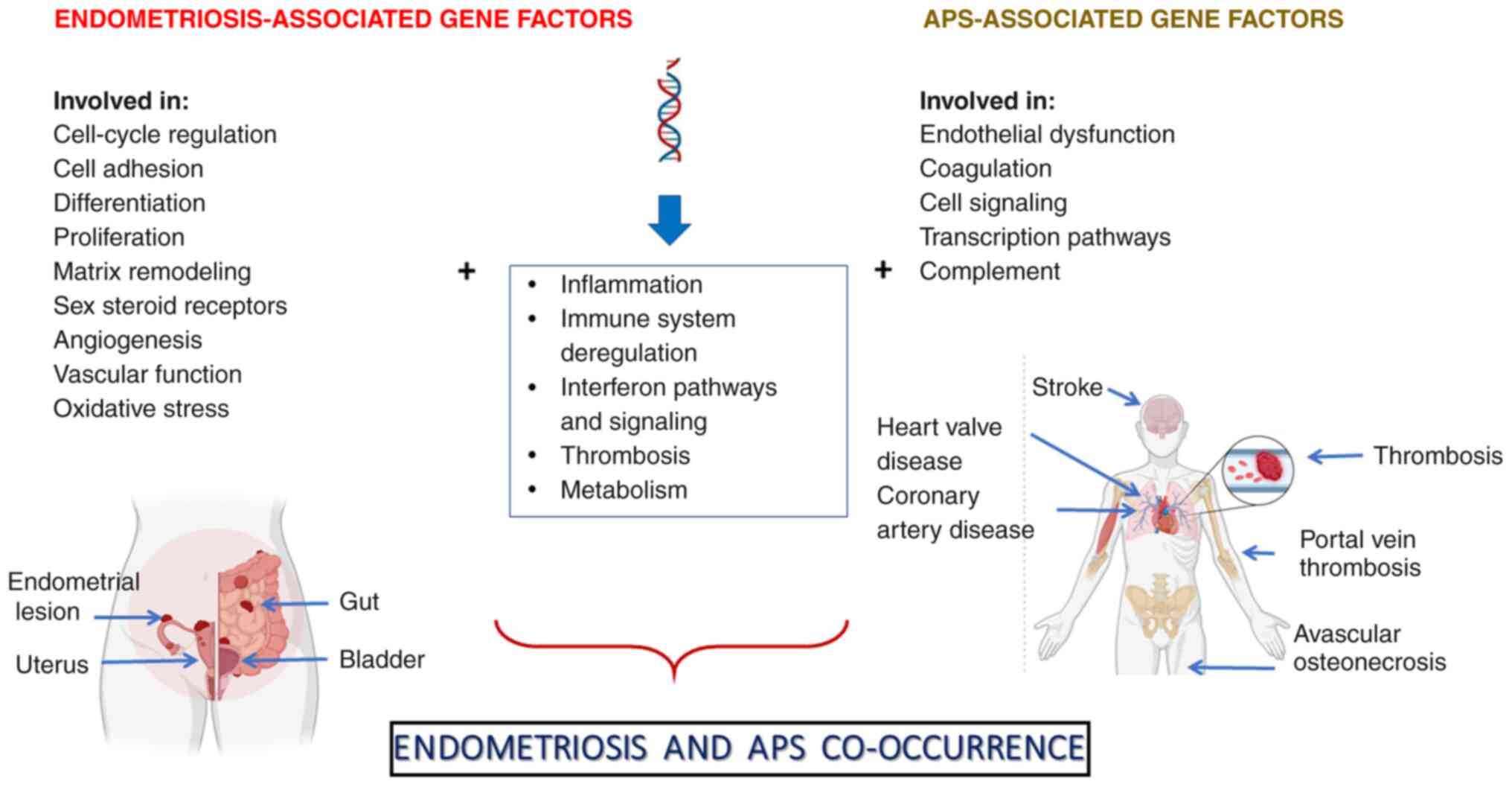
Accordingly, certain genes were identified as
potential risk factors for developing endometriosis and APS, which
are involved in inflammatory and/or interferon (IFN)-inducible
processes, immune system dysregulation and thrombosis (Fig. 1). The identified gene-related risk
factors included the human leukocyte antigen (HLA)-DQB1*0301
(29,30) and HLA-DQA1*0301 alleles
(29,31), the 4G/5G polymorphism of the serpin
family E member 1 (SERPINE1) gene [encoding plasminogen
activator inhibitor type 1 (PAI-1) protein] (32,33),
methylenetetrahydrofolate reductase (MTHFR) rs1801133
(34,35), signal transducer and activator of
transcription 4 (STAT4) rs7574865 (36,37)
and toll-like receptor 4 (TLR4) rs4986790 (38,39)
single nucleotide polymorphisms (SNPs). The shared gene
polymorphisms and the function of the respective genes are
demonstrated in Table I.
 | Table IOverview of genetic polymorphisms
associated with both endometriosis and APS, as confirmed by
gene-association and/or genome-wide association studies. |
Table I
Overview of genetic polymorphisms
associated with both endometriosis and APS, as confirmed by
gene-association and/or genome-wide association studies.
| Endometriosis- and
APS- associated gene | Single nucleotide
polymorphism database ID | Function | (Refs.) |
|---|
|
HLA-DQB1*0301 | N/A | Presents peptides
derived from extracellular proteins | (29,30) |
|
HLA-DQA1*0301 | N/A | Presents peptides
derived from extracellular proteins | (29,31) |
|
SERPINE1 | N/A | A serine protease
inhibitor, involved in fibrinolysis | (32,33) |
| MTHFR | rs1801133 | A key regulatory
enzyme in folate and homocysteine metabolism | (34,35) |
| STAT4 | rs7574865 | A transcription
factor involved in T helper 17 differentiation and monocyte
activation | (36,37) |
| TLR4 | rs4986790 | A transmembrane
protein, member of the TLR family | (38,39) |
3. Biological mechanisms relative to the
co-occurrence of endometriosis and APS
Polymorphisms in genes involved in
immune responses (major histocompatibility complex-associated
genes)
The HLA genes encode proteins that bind
antigen peptides and present them to T cells, and also play key
roles in the immune response by interacting with the complement
system and restricting the recognition of antigenic peptides by T
cells and their involvement in cellular immunity (40). The HLA-DQB1*0301
allele of the HLA class II gene DQB1 has been associated
with increased susceptibility for both endometriosis and APS
(29,30). The association between
endometriosis and the genes encoding the protein components of the
HLA system has not yet been fully elucidated. However, considering
that the suppression of cellular immunity in the peritoneal space
causes endometriosis, it has been hypothesized that particular
HLA gene alleles may signal these cells to decrease their
cytotoxic properties (30). A
notably higher frequency of the HLA-DQB1*0301 allele
has been observed in patients with endometriosis, but the
underlying mechanism of this association has not been fully
clarified yet (30,41). The DQB1*0301 allele has also
been associated with the development of APS, as well as with the
presence of aCL antibodies in PAPS (29). In fact, it has been suggested that
DQB1*0301 among other HLA alleles, such as
HLA-DRB1*04, DRB1*07, DRB1*1302, DRw53,
DQA1*0102 and DQA1*0201, determines the
susceptibility to the production of aPL antibodies, which are
responsible for the clinical manifestations of APS (29). Notably, HLA-DQA1*0301 allele
was also found to be associated with both endometriosis and APS
(29,31); this allele is associated with an
increased production of β2GPI antibodies in patients with APS, thus
leading to thrombotic events and pregnancy morbidity (42).
Polymorphism in a gene involved in
thrombosis
The SERPINE1 gene (coding for PAI-1) encodes
a monomeric glycoprotein that is a fast-acting inhibitor of
plasminogen activation, belonging to the serine protease inhibitor
superfamily and regulating the fibrinolytic pathway (32). Genetic alterations of
SERPINE1 have been considered as risk factors for the
development of various diseases, including thrombosis (43). A common insertion/deletion SNP in
the SERPINE1 gene, located 675 bp upstream from the start
codon, induces the generation of two alleles containing either 4 or
5 sequential guanosines, with individuals carrying homozygosity in
the deletion allele (4G/4G) having increased gene transcriptional
activity and markedly elevated plasma PAI-1 protein activity
compared with those in 5G/5G individuals (44,45).
The 4G/5G polymorphism in the promoter region of SERPINE1
has been associated with the development of endometriosis and APS
(32,33). Particularly, the 4G/4G and the
4G/5G genotypes, as well as the 4G allele of this polymorphism of
the SERPINE1 gene, were found to be associated with an
increased susceptibility for endometriosis. Furthermore, the 4G
allele was also associated with hypofibrinolysis, which was
observed at a notably higher frequency in women with endometriosis
compared with that in women without endometriosis (32). Notably, it has been suggested that
persistence of a fibrin matrix as a result of hypofibrinolysis
could mediate the initiation of endometriotic lesions in the
peritoneal cavity (32). Moreover,
the presence of the 4G allele has been associated with higher
plasma levels of PAI-1 and increased risk for thrombosis in
patients with PAPS, compared with those in healthy controls
(33).
Polymorphism in a metabolism-related
gene
The MTHFR gene encodes the 77-kDa MTHFR
protein, which catalyzes the irreversible reduction of 5-10-MTHF to
5-methylTHF, thus representing an essential enzyme in folate and
homocysteine metabolism (46) and
playing an important role in DNA methylation and synthesis
(46). The missense MTHFR
rs1801133 (Ala222Val, C677T) SNP, located in exon 4, has been shown
to be associated with both endometriosis and APS (29,30).
The risk allele ‘T’ influences the enzymatic activity by producing
a thermally unstable protein, which prevents optimal functioning of
the enzyme at temperatures >37˚C (46) and leads to elevated plasma levels
of homocysteine. In endometriosis, elevated homocysteine, along
with oxidative stress, may contribute to impaired blood flow and
reduced fertility (47). Notably,
the increased homocysteine levels in combination with vascular
inflammation and injury may lead to the development of
cardiovascular disease, which is a known comorbidity of
endometriosis (3,48). It is worth noting that the
accumulation of high levels of cytotoxic homocysteine in the
circulating blood may lead to damage of the inner wall of blood
vessels. Consequently, the coagulation cascade reactions can be
activated and the risk for thrombosis and ultimately APS is
increased (34).
Polymorphisms in genes associated with
IFN pathways and signaling
The STAT4 gene is located on chromosome
2q32.2-2q32-3(49), and encodes a
transcription factor that is expressed in activated peripheral
blood monocytes, macrophages, myeloid cells, DCs and T cells, and
plays pivotal roles in the differentiation and proliferation of
both T helper (Th) 1 and Th17 cells (49,50).
Combined evidence based on gene association studies, GWAS and
meta-analyses has shown an unambiguous association between
STAT4 rs7574865 SNP and various autoimmune diseases, as well
as chronic inflammatory diseases (51). It has been suggested that this
variant of STAT4 may be involved in the regulation of the
balance between the IL-12 and IL-23 effect, thus leading to
inflammatory diseases through the dysregulation of the Th1 vs. Th17
differentiation (51). In
addition, in silico studies have highlighted the regulatory
function of this SNP in gene expression of STAT4,
considering its location near both distal enhancers and an
important genome regulatory factor, CCCTC-binding factor, which
represents a highly conserved zinc finger protein with key roles in
genome organization and gene expression, influencing both gene
activation and repression (52).
In endometriosis, an increased frequency of the TT
genotype of the rs7574865 (G/T) SNP has been observed in women
suffering from minimal or mild endometriosis compared with that in
women without endometriosis (36).
Furthermore, it has been suggested that this SNP may impair either
the gene expression or mRNA splicing of the STAT4 gene,
thereby playing an important role in IFN signaling (36). Furthermore, the role of the
rs7574865 SNP may be strengthened, considering both the strong
association of the T allele with Th1 responses and the association
between Th1 responses and deep infiltration in pelvic endometriosis
(53). Notably, a positive
association between the T allele of STAT4 rs7574865 and APS
has been reported; moreover, this association was still observed
after the stratification of patients with APS by the clinical
manifestations of this disorder, including thrombosis or obstetric
complications (37).
TLRs are pattern recognition receptors (PRRs), which
form a family of transmembrane protein molecules that play a
crucial role in the regulation of immune and inflammatory responses
(54). TLRs activate cellular
signaling pathways to induce immune-response genes, including genes
encoding cytokines, chemokines and other immune mediators, and
represent a host defense mechanism against infections and tissue
damage, leading to the secretion of various inflammatory cytokines
(55). TLR4, a type I
transmembrane glycoprotein, is a PRR that mainly recognizes the
lipopolysaccharide (LPS) of Gram-negative bacteria as well as
structures from fungal and mycobacterial pathogens, and activates
innate immunity through the production of cytokines, such as IL-1,
IL-6, IL-8, TNF-α and MIP (56,57).
TLR4 is composed of three domains, an extracellular, a
transmembrane and a cytoplasmic domain, and was found to be
involved in the pathogenesis of infectious (i.e. gram-negative
infections, periodontitis and septic shock), inflammatory (obesity,
liver and kidney disease, cardiovascular and neurodegenerative
diseases) and autoimmune diseases (SLE, RA, MS, SSc, PS, SS and
APS) (45,58).
The TLR4 rs4986790 SNP results in an aspartic
acid to glycine substitution at position 299 (D299G), which alters
the formation of the TLR4/myeloid differentiation factor 2 complex
(59). This SNP has been
associated with the development of both endometriosis and APS
(25,38). In particular, the involvement of
TLR4 in endometriosis has been linked to its regulatory role in the
activation of immune and inflammatory responses (60). TLR4 is expressed in endometrial and
endometriotic epithelial cells in women with endometriosis, and
alterations in its expression levels or function have been detected
in the endometrium and pelvic environment (38,60).
The risk allele ‘G’ of the rs4986790 SNP was found to affect the
function of TLR4. Particularly, the ‘G’ allele of the TLR4
(A896G) rs4986790 SNP was found to result in hypo-responsiveness of
the receptor, thus resulting in peritoneal inflammation as
suggested by Latha et al (38). Under this condition, the molecular
microenvironment may become favorable for the endometrial cells to
adhere to the pelvic peritoneum, thus leading to the initiation of
endometriosis (38). Previous
findings based on molecular dynamics simulations showed that the
rs4986790 SNP may abrogate the stability of the hexamer complex
that is crucial for LPS recognition and antibacterial immune
response, being necessary to dimerize the cytoplasmic domain of the
TLR4 protein and ultimately result in compromised TLR4 signaling
(61). Notably, an eight-fold
increase of endometriosis risk was seen in women who are carriers
of the GG genotype (38).
Regarding APS, elevated levels of TLR4 in the cell surface of
peripheral blood mononuclear cells, as well as in monocytes, were
observed among patients with PAPS compared with those in healthy
controls (62). As suggested
previously, the activation of the TLR pathway and TLR4 reactivity
can lead to vascular thrombosis in patients with PAPS (45). Pierangeli et al (39) suggested that, in patients with APS,
the risk allele ‘G’ of the rs4986790 SNP confers a higher
susceptibility to prothrombotic endothelial activation mediated by
aPL antibodies.
4. Conclusions and future perspectives
To the best of our knowledge, the present review is
the first study attempting to delineate the genetic basis of the
co-occurrence of endometriosis and APS. Although only a few shared
gene polymorphisms for these disorders were identified, the present
review highlighted a number of loci involved in inflammatory and/or
IFN-inducible processes, immune system deregulation and thrombosis.
Genetic factors are important in the development of both
endometriosis and APS, as has been demonstrated previously by the
familiar occurrence of these health conditions and their
association with various gene polymorphisms. The identification of
further genetic factors associated with these diseases is of
emerging interest and may help to better delineate the mechanisms
leading to their clinical association. These results are important
not only for future genetic and clinical studies, but also for
directing future function/structure studies on the molecular cause
of endometriosis and APS. Moreover, the increasing number of
disease-associated variants may contribute to the development and
validation of gene risk scores for both diseases, thus improving
the risk assessment and tailored management of these disorders.
Further exploration of immune cell signatures and/or
cytokine profiles has emphasized the pivotal role of immune
dysregulation in the immune-mediated mechanisms leading to
endometriosis and APS. The specific immune cell signature of APS is
characterized by activated neutrophils and an enhanced type I IFN
response, while the involvement of other types of immune cells,
including DCs, T and B cells, monocytes and NK cells has been also
reported in the pathogenesis of APS (63,64).
In endometriosis, distinct immune cells signatures have been found
in the peritoneal cavity, including increased numbers of
CD8+ T cells and activated NK cells, along with
follicular Th cells. By contrast, M2 macrophages and resting mast
cells were observed at low levels in eutopic endometria. Moreover,
activated CD4+ T and B cells form part of the immune
signatures associated with endometriosis (65,66),
while neutrophils, macrophages, NK cells and DCs also serve a role
in endometriosis (13). Cytokine
profiles in APS often show an imbalance, with increased levels of
pro-inflammatory cytokines, such as TNF-α and IL-6, and potentially
decreased levels of anti-inflammatory cytokines, such as IL-10.
Furthermore, notably elevated levels of IL-7, IL-8, IL-10, IL-16,
IL-17 and IL-12/IL-23p40 and decreased levels of IL-22 and IL-31
have been detected in patients with APS compared with those in
healthy controls (67,68). In women with endometriosis,
elevated levels of pro-inflammatory cytokines are frequently
observed in both peritoneal fluid and serum. Pro-inflammatory
cytokines, such as IL-1β, IL-6, IL-8 and TNF-α, have been found to
be increased in the peritoneal fluid of women with endometriosis,
while some anti-inflammatory cytokines, such as IL-10 and IL-33,
may also appear at elevated levels in endometriosis. IL-25 (also
known as IL-17E), IL-33 and thymic stromal lymphopoietin, which are
involved in Th2 cell development, are increased in the peripheral
blood, endometrium and peritoneal fluid of patients with
endometriosis compared with women without endometriosis.
Furthermore, women with advanced endometriosis (stage 3-4) exhibit
markedly increased levels of IL-1α and IL-6 in the endometrial
fluid compared with those in women without endometriosis (69,70).
In recent years, genomic and epigenomic research in
endometriosis and APS has grown (11,12,21,23,25,71-73),
driven by novel high-throughput technologies that may identify new
potential signatures and therapeutic targets for further
exploration in detail. Notably, Murrin et al (74) performed a systematic analysis of
the contribution of genetics to multimorbidity based on
representative primary care data on 72 chronic diseases, involving
individuals aged ≥65 years from two large primary-care databases.
All diseases were compared between each other in pairs. The study
concluded that most pairs of chronic conditions show evidence of a
shared genetics background and shared pathogenetic mechanisms.
Therefore, it was suggested that the identified patterns of shared
genetics may provide a foundation for future multimorbidity
research. Importantly, the shared genetic polymorphisms have
potential implications for therapeutic interventions and a better
clinical management of the patients, as further analysis of gene
expression patterns and future advanced pharmacogenomic approaches
may refine personalized treatment strategies for women with both
conditions (74).
In a retrospective cohort study of 50,078 women with
endometriosis conducted by Chen et al (17), patients showed a notably increased
risk of APS regardless of the presence or absence of SLE. This is
an interesting but unexpected finding, taking into account that
some of the shared gene polymorphisms identified in the present
study, such as the MTHFR rs1801133 and STAT4
rs7574865 SNPs also represent SLE-associated risk factors (27,75-77).
Notably, STAT4 rs7574865, apart from its association with
the co-occurrence of endometriosis and APS, has been also
associated with the co-occurrence of endometriosis with SLE, RA, AS
and SS (78).
There are a few limitations to be considered in the
present study. Firstly, there is a definite epidemiological lack of
replication studies thus far in the literature, concerning the
development of APS in women with endometriosis in different racial
and/or ethnic populations. Secondly, there is a lack of information
in the literature about the role of the already identified gene
polymorphisms in women with endometriosis and APS of different
ethnic origins. This is an important point, taking into account the
differential level of contribution of the various polymorphisms
involved in the development of these disorders according to the
ancestry of the populations under examination (71,79).
In this framework, in a previous investigation, we showed that the
availability of a number of endometriosis-associated SNPs (302 SNPs
were examined) provided the opportunity for the study of this
condition through SNP genomic disease genomic ‘grammar’ (DGG).
Thus, the genomic pedigree of endometriosis was identified using
its DGG through the reported SNPs for five major groups of the
world human population, including Europeans, Africans, Americans,
East Asians and South Asians, and notable ‘key’ genetic targets of
endometriosis were revealed with the evidence of population-based
heterogeneity (71). Thirdly, the
search strategy was restricted to the English language only;
therefore, there is a possibility of excluding eligible studies
published in other languages. Furthermore, the source of the
inter-individual susceptibility to endometriosis or APS does not
lie exclusively in genetics, considering that epigenetics
mechanisms, such as methylation, acetylation, phosphorylation and
modifications to the histone proteins associated with DNA packaging
in chromatin, or others that have been analyzed in APS, are now
widely believed to participate also in the susceptibility to these
diseases (70,80,81).
Regarding APS, hypomethylation of ETS1 and EMP2 genes
in APS neutrophils, downregulation of miR-19b and miR-20a, as well
as significant reduction in methylation of the IL-8 promoter
and significantly increased methylation of the tissue factor
(F3) gene, have been reported so far (80,81).
Thus, the role of potential shared epigenetic factors has to be
investigated in future studies, considering that this issue was
beyond the scope of the present preliminary study.
Despite the high number of environmental exposure
factors identified for endometriosis and APS, no common factors
have been reported so far. Notably, most endometriosis-associated
environmental factors refer to endocrine disruptors and organic
pollutants, such as polyhalogenated aromatic hydrocarbons, tobacco
smoking and exposure to heavy metals, pesticides and organohalogen
compounds (82,83). By contrast, the environmental
triggers for APS refer to bacteria, viruses, parasites, vaccines
and drugs (such as antiepileptic, antipsychotic, antihypertensive,
antiarrhythmic drugs and antibiotics), as well as other factors
that are potentially capable of inducing a variety of pathogenic
aPL antibodies, and causing thrombosis, pregnancy loss and APS
through a range of mechanisms (84).
In conclusion, the observed association between
endometriosis and APS suggests that clinicians and scientists
conducting research on endometriosis need to be aware of potential
subsequent APS when endometriosis is diagnosed. Thus, as suggested
recently, clinicians should review thoroughly the symptoms and
assess risk factors for APS when evaluating women with
endometriosis (17). A better
functional characterization of the disease-associated genes may
lead to the detection of new therapeutic targets for pharmaceutical
intervention and ultimately result in improved therapeutic
management for the patients by designing and producing monoclonal
antibodies targeting selected genes, specific inhibitors or
mimicking molecules. Notably, transcriptomic profiling and analysis
have allowed for improved understanding of the mechanisms
underlying endometriosis and have created opportunities for drug
repurposing (85). As an example,
fenoprofen, an uncommonly prescribed NSAID, was the top therapeutic
candidate for further investigation, which was tested in an
established rat model of endometriosis and successfully alleviated
endometriosis-associated vaginal hyperalgesia (85). In the same framework, the
identification of altered transcriptome signatures in samples from
patients with APS and analysis using bioinformatics tools have
significantly enhanced the understanding of APS pathology and led
to the identification of potential therapeutic targets related to
the role of the IFN pathway in this disease (81). Particularly, the upregulation of
genes associated with IFN pathways suggested the potential for
targeted therapeutic interventions aimed at modulating the IFN
signaling pathway in patients with APS (81). Moreover, the distinct gene
signatures associated with different thrombosis types and disease
manifestations suggested the possibility of further tailored
treatment strategies based on the patient's thrombotic profile
(81). Therefore, integrating
clinical and molecular data using high-throughput technologies, in
combination with the employment of computational and bioinformatics
tools, can advance precision medicine for patients with
endometriosis and APS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MIZ, TBT and GNG designed the current study,
searched the literature and drafted the manuscript. MIZ, BCT, GB
and DAS analyzed and organized the data. TBT, BCT, GB and DAS
critically revised the manuscript. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
MIZ, TBT, BCT, GB and GNG declare that they have no
competing interests. DAS is the Editor-in-Chief for the journal,
but had no personal involvement in the reviewing process, or any
influence in terms of adjudicating on the final decision, for this
article.
References
|
1
|
Sinaii N, Cleary SD, Ballweg ML, Nieman LK
and Stratton P: High rates of autoimmune and endocrine disorders,
fibromyalgia, chronic fatigue syndrome and atopic diseases among
women with endometriosis: a survey analysis. Hum Reprod.
17:2715–2724. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kvaskoff M, Mu F, Terry KL, Harris HR,
Poole EM, Farland L and Missmer SA: Endometriosis: A high-risk
population for major chronic diseases? Hum Reprod Update.
21:500–516. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rafi U, Ahmad S, Bokhari SS, Iqbal MA, Zia
A, Khan MA and Roohi N: Association of inflammatory
markers/cytokines with cardiovascular risk manifestation in
patients with endometriosis. Mediators Inflamm.
2021(3425560)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Surrey ES, Soliman AM, Johnson SJ, Davis
M, Castelli-Haley J and Snabes MC: Risk of developing comorbidities
among women with endometriosis: A retrospective matched cohort
study. J Women's Health (Larchmt). 27:1114–1123. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shigesi N, Kvaskoff M, Kirtley S, Feng Q,
Fang H, Knight JC, Missmer SA, Rahmioglu N, Zondervan KT and Becker
CM: The association between endometriosis and autoimmune diseases:
A systematic review and meta-analysis. Hum Reprod Update.
25:486–503. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yin Z, Low HY, Chen BS, Huang KS, Zhang Y,
Wang YH, Ye Z and Wei JCC: Risk of ankylosing spondylitis in
patients with endometriosis: A population-based retrospective
cohort study. Front Immunol. 13(877942)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zervou MI, Vlachakis D, Papageorgiou L,
Eliopoulos E and Goulielmos GN: Increased risk of rheumatoid
arthritis in patients with endometriosis: Genetic aspects.
Rheumatology (Oxford). 61:4252–4262. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zervou MI, Papageorgiou L, Vlachakis D,
Spandidos DA, Eliopoulos E and Goulielmos GN: Genetic factors
involved in the co-occurrence of endometriosis with ankylosing
spondylitis (review). Mol Med Rep. 27(96)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zervou MI, Tarlatzis BC, Grimbizis GF,
Spandidos DA, Niewold TB and Goulielmos GN: Association of
endometriosis with Sjögren's syndrome: Genetic insights (review).
Int J Mol Med. 53(20)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
As-Sanie S, Mackenzie SC, Morrison L,
Schrepf A, Zondervan KT, Horne AW and Missmer SA: Endometriosis: A
review. JAMA. 334:64–78. 2025.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Symons LK, Miller JE, Kay VR, Marks RM,
Liblik K, Koti M and Tayade C: The immunopathophysiology of
endometriosis. Trends Mol Med. 24:748–762. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zondervan KT, Becker CM, Koga K, Missmer
SA, Taylor RN and Viganò P: Endometriosis. Nat Rev Dis Primers.
4(9)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Abramiuk M, Grywalska E, Małkowska P,
Sierawska O, Hrynkiewicz R and Niedźwiedzka-Rystwej P: The role of
the immune system in the development of endometriosis. Cells.
11(2028)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Eisenberg VH, Zolti M and Soriano D: Is
there an association between autoimmunity and endometriosis?
Autoimmun Rev. 11:806–814. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lebovic DI, Mueller MD and Taylor RN:
Immunobiology of endometriosis. Fertil Steril. 75:1–10.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Matarese G, De Placido G, Nikas Y and
Alviggi C: Pathogenesis of endometriosis: Natural immunity
dysfunction or autoimmune disease? Trends Mol Med. 9:223–228.
2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen Z, Cui R, Wang SI, Zhang H, Chen M,
Wang Q, Tong Q, Wei JCC and Dai SM: Increased risk of subsequent
antiphospholipid syndrome in patients with endometriosis. Int J
Epidemiol. 54(dyae167)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Clark KEN and Giles I: Antiphospholipid
syndrome. Medicine. 46:118–125. 2018.
|
|
19
|
Sciascia S, Amigo MC, Roccatello D and
Khamashta M: Diagnosing antiphospholipid syndrome: ‘Extra-criteria’
manifestations and technical advances. Nat Rev Rheumatol.
13:548–560. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rodríguez-García V, Ioannou Y,
Fernández-Nebro A, Isenberg DA and Giles IP: Examining the
prevalence of non-criteria anti-phospholipid antibodies in patients
with anti-phospholipid syndrome: A systematic review. Rheumatology
(Oxford). 54:2042–2050. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Celia AI, Galli M, Mancuso S, Alessandri
C, Frati G, Sciarretta S and Conti F: Antiphospholipid syndrome:
Insights into molecular mechanisms and clinical manifestations. J
Clin Med. 13(4191)2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ravindran V, Rajendran S and Elias G:
Primary antiphospholipid syndrome in monozygotic twins. Lupus.
22:92–94. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lopez-Pedrera C, Barbarroja N,
Patiño-Trives AM, Collantes E, Aguirre MA and Perez-Sanchez C: New
biomarkers for atherothrombosis in antiphospholipid syndrome:
Genomics and epigenetics approaches. Front Immunol.
10(764)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zubrzycka A, Zubrzycki M, Perdas E and
Zubrzycka M: Genetic, epigenetic, and steroidogenic modulation
mechanisms in endometriosis. J Clin Med. 9(1309)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Barinotti A, Radin M, Cecchi I, Foddai SG,
Rubini E, Roccatello D, Sciascia S and Menegatti E: Genetic factors
in antiphospholipid syndrome: Preliminary experience with whole
exome sequencing. Int J Mol Sci. 21(9551)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gómez-Puerta JA and Cervera R: Diagnosis
and classification of the antiphospholipid syndrome. J Autoimmun.
48-49:20–25. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zervou MI, Matalliotakis M and Goulielmos
GN: Comment on ‘Risk of systemic lupus erythematosus in patients
with endometriosis: A nationwide population-based cohort study’.
Arch Gynecol Obstet. 305:543–544. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Goulielmos GN, Tarlatzi TB, Grimbizis GF,
Spandidos DA, Niewold TB, Bertsias G, Tarlatzis BC and Zervou MI:
Co-occurrence of endometriosis with psoriasis and psoriatic
arthritis: Genetic insights (review). Exp Ther Med.
30(171)2025.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Domenico Sebastiani G, Minisola G and
Galeazzi M: HLA class II alleles and genetic predisposition to the
antiphospholipid syndrome. Autoimmun Rev. 2:387–394.
2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ishii K, Takakuwa K, Kashima K, Tamura M
and Tanaka K: Associations between patients with endometriosis and
HLA class II; the analysis of HLA-DQB1 and HLA-DPB1 genotypes. Hum
Reprod. 18:985–989. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zong L, Pan D, Chen W, He Y, Liu Z, Lin J
and Xu A: Comparative study of HLA-DQA1 and HLA-DRB1 allele in
patients with endometriosis and adenomyosis. Zhonghua Yi Xue Yi
Chuan Xue Za Zhi. 19:49–51. 2002.PubMed/NCBI(In Chinese).
|
|
32
|
Bedaiwy MA, Falcone T and Casper RF:
Plasminogen activator inhibitor (PAI) and thrombin activatable
fibrinolysis inhibitor (TAFI) Polymorphisms in women with
endometriosis. Fertil Steril. 84 (Suppl 1):S189–S190. 2005.
|
|
33
|
Aisina RB, Mukhametova LI, Ostryakova EV,
Seredavkina NV, Patrushev LI, Patrusheva NL, Reshetnyak TM, Gulin
DA, Gershkovich KB, Nasonov EL and Varfolomeyev SD: Polymorphism of
the plasminogen activator inhibitor type 1 gene, plasminogen level
and thromboses in patients with the antiphospholipid syndrome.
Biochem Suppl Ser B Biomed Chem. 7:1–15. 2013.
|
|
34
|
Sayfutdinovich KZ, Shakarimovna AD,
Rudolfovna KT and Erkinovna ZN: Association of MTHFR and MTRR genes
with the development of antiphospholipid syndrome in pregnant women
of Uzbek population. Eur Sci Rev. 7-8:85–87. 2016.
|
|
35
|
Delli Carpini G, Giannella L, Di Giuseppe
J, Montik N, Montanari M, Fichera M, Crescenzi D, Marzocchini C,
Meccariello ML, Di Biase D, et al: Homozygous C677T
methylenetetrahydrofolate reductase (MTHFR) polymorphism as a risk
factor for endometriosis: A retrospective case-control study. Int J
Mol Sci. 24(15404)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bianco B, Fernandes RFM, Trevisan CM,
Christofolini DM, Sanz-Lomana CM, de Bernabe JV and Barbosa CP:
Influence of STAT4 gene polymorphisms in the pathogenesis of
endometriosis. Ann Hum Genet. 83:249–255. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Horita T, Atsumi T, Yoshida N, Nakagawa H,
Kataoka H, Yasuda S and Koike T: STAT4 single nucleotide
polymorphism, rs7574865 G/T, as a risk for antiphospholipid
syndrome. Ann Rheum Dis. 68:1366–1367. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Latha M, Vaidya S, Movva S, Chava S,
Govindan S, Govatati S, Banoori M, Hasan Q and Kodati VL: Molecular
pathogenesis of endometriosis; Toll-like receptor-4 A896G (D299G)
polymorphism: A novel explanation. Genet Test Mol Biomarkers.
15:181–184. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pierangeli SS, Vega-Ostertag ME, Raschi E,
Liu X, Romay-Penabad Z, De Micheli V, Galli M, Moia M, Tincani A,
Borghi MO, et al: Toll-like receptor and antiphospholipid mediated
thrombosis: In vivo studies. Ann Rheum Dis. 66:1327–1333.
2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Carey BS, Poulton KV and Poles A: Factors
affecting HLA expression: A review. Int J Immunogenet. 46:307–320.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ali AM, Nader MI, Al-Ghurabi BH and
Mohammed AK: Association of HLA class II alleles (DRB1 and DQB1) in
Iraqi women with endometriosis. Iraqi J Biotechnol. 15:42–50.
2016.
|
|
42
|
Ioannidis JP, Tektonidou MG,
Vlachoyiannopoulos PG, Stavropoulos-Giokas C, Spyropoulou-Vlachou
M, Reveille JD, Arnett FC and Moutsopoulos HM: HLA associations of
anti-beta2 glycoprotein I response in a Greek cohort with
antiphospholipid syndrome and meta-analysis of four ethnic groups.
Hum Immunol. 60:1274–1280. 1999.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mansilha A, Araújo F, Severo M, Sampaio
SM, Toledo T, Henriques I and Albuquerque R: The association
between the 4G/5G polymorphism in the promoter of the plasminogen
activator inhibitor-1 gene and deep venous thrombosis in young
people. Phlebology. 20:48–52. 2005.
|
|
44
|
Dossenbach-Glaninger A, van Trotsenburg M,
Dossenbach M, Oberkanins C, Moritz A, Krugluger W, Huber J and
Hopmeier P: Plasminogen activator inhibitor 1 4G/5G polymorphism
and coagulation factor XIII Val34Leu polymorphism: Impaired
fibrinolysis and early pregnancy loss. Clin Chem. 49:1081–1086.
2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Islam MA, Khandker SS, Alam F, Kamal MA
and Gan SH: Genetic risk factors in thrombotic primary
antiphospholipid syndrome: A systematic review with bioinformatic
analyses. Autoimmun Rev. 17:226–243. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Raghubeer S and Matsha TE:
Methylenetetrahydrofolate (MTHFR), the one-carbon cycle, and
cardiovascular risks. Nutrients. 13(4562)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Guedes T, Santos AA, Vieira-Neto FH,
Bianco B, Barbosa CP and Christofolini DM: Folate metabolism
abnormalities in infertile patients with endometriosis. Biomark
Med. 16:549–557. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Luo Z, Lu Z, Muhammad C, Chen Y, Chen Q,
Zhang J and Song Y: Associations of the MTHFR rs1801133
polymorphism with coronary artery disease and lipid levels: A
systematic review and updated meta-analysis. Lipids Health Dis.
17(191)2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wu S, Wang M, Wang Y, Zhang M and He JQ:
Polymorphisms of the STAT4 gene in the pathogenesis of
tuberculosis. Biosci Rep. 38(BSR20180498)2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Korman BD, Kastner DL, Gregersen PK and
Remmers EF: STAT4: Genetics, mechanisms, and implications for
autoimmunity. Curr Allergy Asthma Rep. 8:398–403. 2008.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Liang YL, Wu H, Shen X, Li PQ, Yang XQ,
Liang L, Tian WH, Zhang LF and Xie XD: Association of STAT4
rs7574865 polymorphism with autoimmune diseases: A meta-analysis.
Mol Biol Rep. 39:8873–8882. 2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bravo-Villagra KM, Muñoz-Valle JF,
Baños-Hernández CJ, Cerpa-Cruz S, Navarro-Zarza JE, Parra-Rojas I,
Aguilar-Velázquez JA, García-Arellano S and López-Quintero A: STAT4
gene variant rs7574865 is associated with rheumatoid arthritis
activity and anti-CCP levels in the western but not in the southern
population of Mexico Mexico. Genes (Basel). 15(241)2024.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Podgaec S, Dias Junior JA, Chapron C, de
Oliveira RM, Baracat EC and Abrão MS: Th1 and Th2 ummune responses
related to pelvic endometriosis. Assoc Med Bras (1992). 56:92–98.
2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Medzhitov R and Janeway C Jr: Innate
immunity. N Engl J Med. 343:338–344. 2000.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ozato K, Tsujimura H and Tamura T:
Toll-like receptor signaling and regulation of cytokine gene
expression in the immune system. BioTechniques. 33 (Suppl):S66–S75.
2002.PubMed/NCBI
|
|
56
|
Poltorak A, Smirnova I, He X, Liu MY, Van
Huffel C, McNally O, Birdwell D, Alejos E, Silva M, Du X, et al:
Genetic and physical mapping of the Lps locus: Identification of
the toll-4 receptor as a candidate gene in the critical region.
Blood Cells Mol Dis. 24:340–355. 1998.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801.
2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kim HJ, Kim H, Lee JH and Hwangbo C:
Toll-like receptor 4 (TLR4): New insight immune and aging. Immun
Ageing. 20(67)2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Yamakawa N, Ohto U, Akashi-Takamura S,
Takahashi K, Saitoh S, Tanimura N, Suganami T, Ogawa Y, Shibata T,
Shimizu T and Miyake K: Human TLR4 polymorphism D299G/T399I alters
TLR4/MD-2 conformation and response to a weak ligand monophosphoryl
lipid A. Int Immunol. 25:45–52. 2013.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Khan KN, Kitajima M, Fujishita A,
Nakashima M and Masuzaki H: Toll-like receptor system and
endometriosis. J Obstet Gynaecol Res. 39:1281–1292. 2013.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Anwar MA and Choi S: Structure-activity
relationship in TLR 4 mutations: Atomistic molecular dynamics
simulations and residue interaction network analysis. Sci Rep.
7(43807)2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Benhamou Y, Bellien J, Armengol G,
Brakenhielm E, Adriouch S, Iacob M, Remy-Jouet I, Le Cam-Duchez V,
Monteil C, Renet S, et al: Role of Toll-like receptors 2 and 4 in
mediating endothelial dysfunction and arterial remodeling in
primary arterial antiphospholipid syndrome. Arthritis Rheum.
66:3210–3220. 2014.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Palli E, Kravvariti E and Tektonidou MG:
Type I interferon signature in primary antiphospholipid syndrome:
Clinical and laboratory associations. Front Immunol.
10(487)2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Tang KT, Chen HH, Chen TT, Bracci NR and
Lin CC: Dendritic cells and antiphospholipid syndrome: An updated
systematic review. Life (Basel). 11(801)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Wu XG, Chen JJ, Zhou HL, Wu Y, Lin F, Shi
J, Wu HZ, Xiao HQ and Wang W: Identification and validation of the
signatures of infiltrating immune cells in the eutopic endometrium
endometria of women with endometriosis. Front Immunol.
12(671201)2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Ahn SH, Khalaj K, Young SL, Lessey BA,
Koti M and Tayade C: Immune-inflammation gene signatures in
endometriosis patients. Fertil Steril. 106:1420–1431.e7.
2016.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Hubben AK, Bazeley P, Swaidani S, Alarabi
A, Kulkarni PP, Shim YJ, Palihati M, Barnard J and Mccrae KR:
Cytokine profiling in antiphospholipid syndrome demonstrates
persistent immune dysregulation and a procoagulant phenotype.
Blood. 142 (Suppl 1)(S5400)2023.
|
|
68
|
Yan H, Li B, Su R, Gao C, Li X and Wang C:
Preliminary study on the imbalance between Th17 and regulatory T
cells in antiphospholipid syndrome. Front Immunol.
13(873644)2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Llarena NC, Richards EG, Priyadarshini A,
Fletcher D, Bonfield T and Flyckt RL: Characterizing the
endometrial fluid cytokine profile in women with endometriosis. J
Assist Reprod Genet. 37:2999–3006. 2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Oală IE, Mitranovici MI, Chiorean DM,
Irimia T, Crișan AI, Melinte IM, Cotruș T, Tudorache V, Moraru L,
Moraru R, et al: Endometriosis and the Role of Pro-Inflammatory and
Anti-Inflammatory Cytokines in Pathophysiology: A narrative review
of the literature. Diagnostics (Basel). 14(312)2024.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Papageorgiou L, Andreou A, Zervou M,
Vlachakis D, Goulielmos GN and Eliopoulos E: A global population
genomic analysis shows novel insights into the genetic
characteristics of endometriosis. World Acad Sci J. 5(12)2023.
|
|
72
|
Goulielmos GN, Matalliotakis M,
Matalliotaki C, Eliopoulos E, Matalliotakis I and Zervou MI:
Endometriosis research in the-omics era. Gene.
741(144545)2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Zouein J, Naim N, Spencer DM and Ortel TL:
Genetic and genomic associations in antiphospholipid syndrome: A
systematic review. Autoimmun Rev. 24(103712)2025.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Murrin O, Mounier N, Voller B, Tata L,
Gallego-Moll C, Roso-Llorach A, Carrasco-Ribelles LA, Fox C, Allan
LM, Woodward RM, et al: A systematic analysis of the contribution
of genetics to multimorbidity and comparisons with primary care
data. EBioMedicine. 113(105584)2025.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Remmers EF, Plenge RM, Lee AT, Graham RR,
Hom G, Behrens TW, de Bakker PIW, Le JM, Lee HS, Batliwalla F, et
al: STAT4 and the risk of rheumatoid arthritis and systemic lupus
erythematosus. N Engl J Med. 357:977–986. 2007.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zhou HY and Yuan M: MTHFR polymorphisms
(rs1801133) and systemic lupus erythematosus risk: A meta-analysis.
Medicine (Baltimore). 99(e22614)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Chen L, Huang Z, Liao Y, Yang B and Zhang
J: Association between tumor necrosis factor polymorphisms and
rheumatoid arthritis as well as systemic lupus erythematosus: A
meta-analysis. Braz J Med Biol Res. 52(e7927)2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Zervou MI and Goulielmos GN: Comment on:
Enhancing current guidance for psoriatic arthritis and its
comorbidities: Recommendations from an expert consensus panel.
Rheumatology (Oxford). 64:904–905. 2025.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Uthman I and Khamashta M: Ethnic and
geographical variation in antiphospholipid (Hughes) syndrome. Ann
Rheum Dis. 64:1671–1676. 2005.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Weeding E, Coit P, Yalavarthi S, Kaplan
MJ, Knight JS and Sawalha AH: Genome-wide DNA methylation analysis
in primary antiphospholipid syndrome neutrophils. Clin Immunol.
196:110–116. 2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
López-Pedrera C, Cerdó T, Jury EC,
Muñoz-Barrera L, Escudero-Contreras A, Aguirre MA and Pérez-Sánchez
C: New advances in genomics and epigenetics in antiphospholipid
syndrome. Rheumatology (Oxford). 63 (SI):SI14–SI23. 2024.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Caporossi L, Capanna S, Viganò P, Alteri A
and Papaleo B: From environmental to possible occupational exposure
to risk factors: What role do they play in the etiology of
endometriosis? Int J Environ Res Public Health.
18(532)2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Matta K, Lefebvre T, Vigneau E, Cariou V,
Marchand P, Guitton Y, Royer AL, Ploteau S, Le Bizec B, Antignac JP
and Cano-Sancho G: Associations between persistent organic
pollutants and endometriosis: A multiblock approach integrating
metabolic and cytokine profiling. Environ Int.
158(106926)2022.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Martirosyan A, Aminov R and Manukyan G:
Environmental triggers of autoreactive responses: Induction of
antiphospholipid antibody formation. Front Immunol.
10(1609)2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Oskotsky TT, Bhoja A, Bunis D, Le BL, Tang
AS, Kosti I, Li C, Houshdaran S, Sen S, Vallvé-Juanico J, et al:
Identifying therapeutic candidates for endometriosis through a
transcriptomics-based drug repositioning approach. iScience.
27(109388)2024.PubMed/NCBI View Article : Google Scholar
|