Introduction
Treatment options for wide-necked anterior
communicating artery (ACoA) aneurysms include open surgical
clipping and endovascular embolization. While surgical clipping
offers robust aneurysm occlusion, endovascular techniques are
increasingly preferred due to lower complication rates and
favorable clinical outcomes (1).
Despite these advantages, wide-necked ACoA aneurysms present
technical challenges during endovascular intervention, primarily
due to the risk of coil protrusion into the parent vessel.
Therefore, adjunctive techniques such as stent-assisted or
balloon-assisted coiling are frequently used. Stent-assisted
coiling effectively prevents coil protrusion, improving the
completeness and durability of embolization (2). However, the need for antiplatelet
therapy following stent placement may increase the risk of
rebleeding in patients with ruptured aneurysms (3,4).
Other techniques, such as balloon remodeling and
triple-microcatheter techniques, have been employed to mitigate
coil protrusion (5,6). However, each has inherent
limitations. Balloon remodeling in ACoA aneurysms requires
temporary flow arrest, increasing the risk of ischemia,
particularly in patients with a unilaterally dominant A1 segment.
Furthermore, the small caliber and limited luminal space of the
anterior cerebral arteries render triple-microcatheter techniques
technically challenging (7). In
the present study, a modified technique using dual microcatheter
and guidewire support is described to address these limitations and
provide a less complex, potentially safer alternative. This method
offers vessel protection and enables successful coil embolization
without adjunctive devices, thereby minimizing stent-related risks
and procedural complexity.
Case report
A 36-year-old female presented with the sudden onset
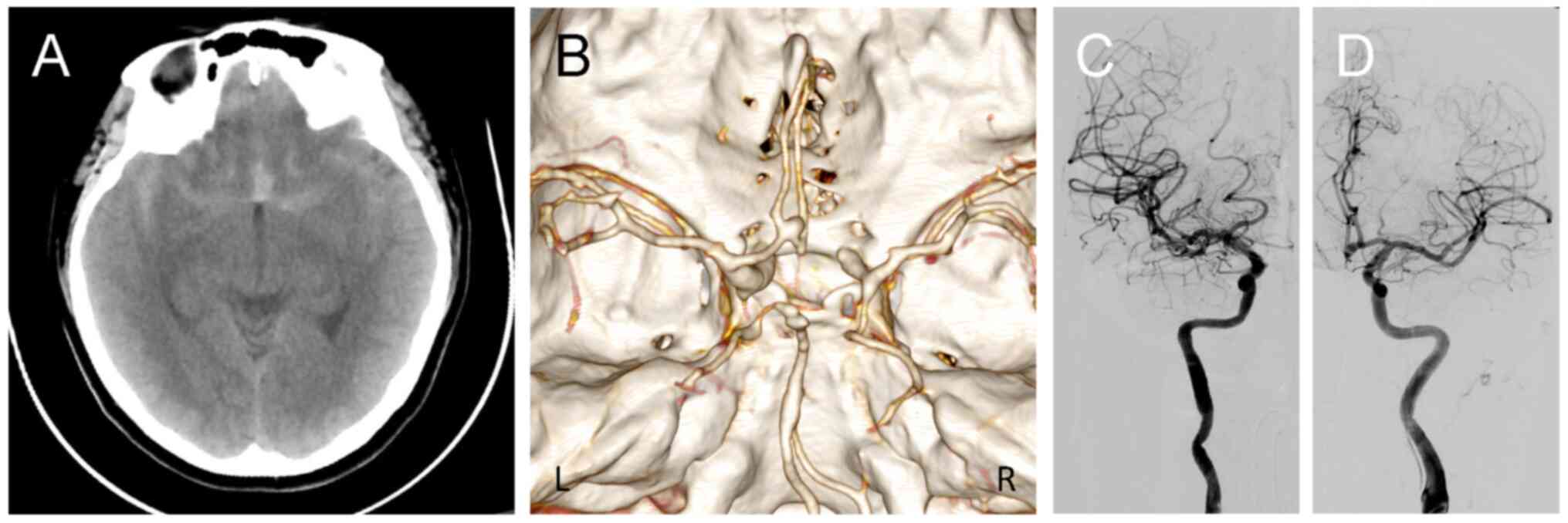
of a severe headache. Non-contrast computed tomography (CT)
performed at a local hospital in September 2024 revealed
subarachnoid hemorrhage, predominantly in the interhemispheric
fissure, with a modified Fisher grade of 2 (Fig. 1A). CT angiography further
demonstrated an ACoA aneurysm (Fig.
1B).
Following the initial evaluation, the patient was
transferred to Beijing Anzhen Nanchong Hospital of Capital Medical
University & Nanchong Central Hospital (Nanchong, China) later
that day. Upon admission, neurological examination was unremarkable
and the patient was classified as World Federation of Neurological
Surgeons Grade 1. After a discussion of treatment options, the
patient declined surgical clipping and opted for endovascular
intervention. Diagnostic cerebral angiography confirmed an ACoA
aneurysm predominantly supplied by the left anterior cerebral
artery (Fig. 1C and D). Three-dimensional (3D) rotational
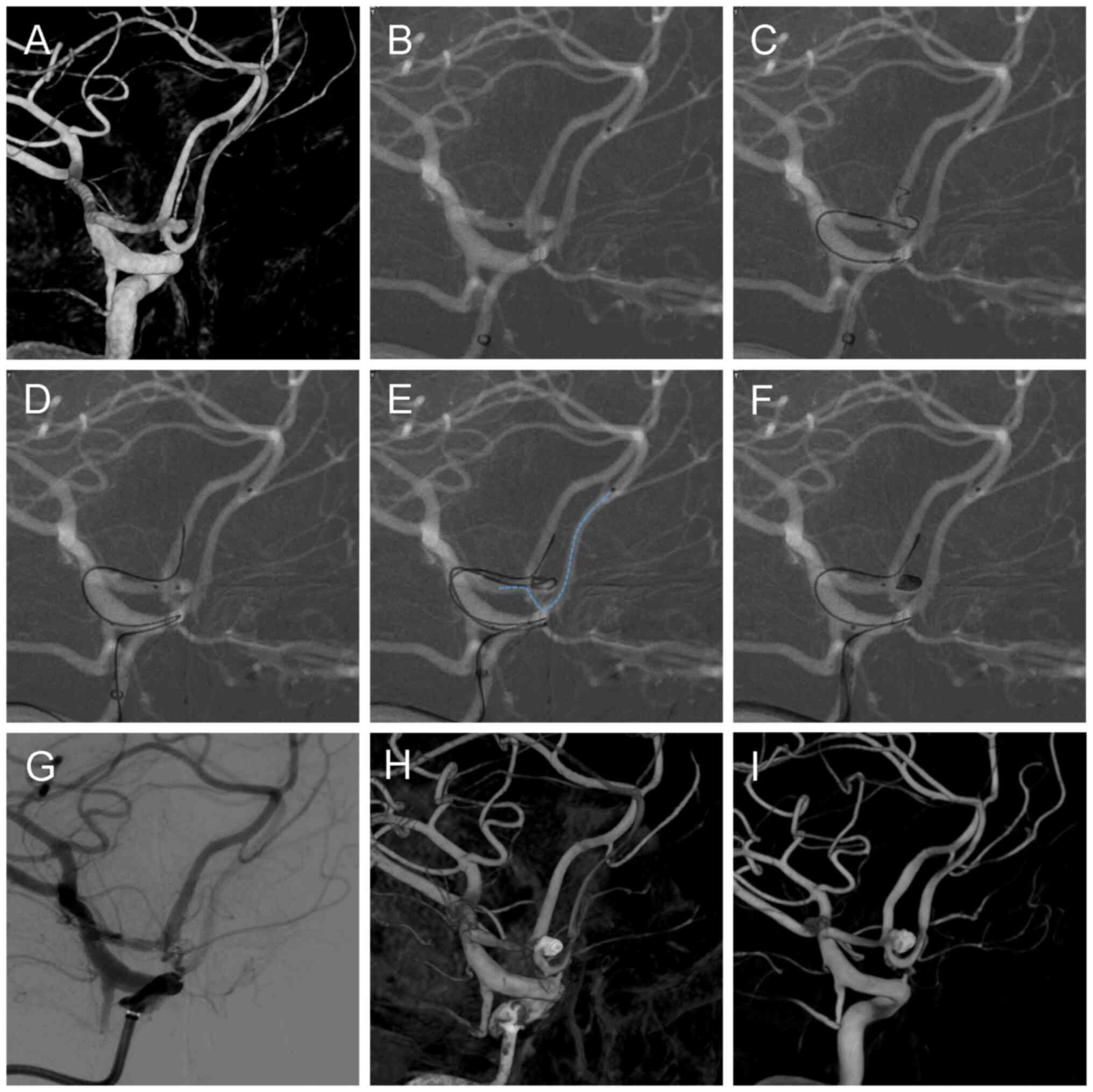
angiography revealed a wide-necked aneurysm measuring ~4x3x3 mm
(width x depth x height), with a dome-to-neck ratio of 1.2
(Fig. 2A). No pretreatment with
dual antiplatelet agents was administered. The procedure was
performed under general anesthesia with continuous nimodipine
infusion at 5 ml/h. A right 8F femoral sheath (Terumo Corporation)
was inserted and a 6F 115-cm intracranial support catheter
(Tonbridge Medical Technology Co., Ltd.) was advanced into the
distal internal carotid artery at the C4 segment. Following
vascular access, systemic heparinization was initiated with a 5,000
U bolus, followed by maintenance at 1,000 U/h.
Due to the left-sided dominance of the ACoA
aneurysm's inflow and its morphology, stent deployment, if
required, would necessitate crossing from the ACoA to the
contralateral (right) A2 segment to cover the aneurysm neck
adequately. To prepare for this contingency, a Synchro 14 guidewire
(Stryker Neurovascular) was used to advance a Headway 17
microcatheter (MicroVention, Inc.) into the distal right A2
segment, positioning it for potential deployment of a Neuroform
Atlas stent (Stryker Neurovascular) (Fig. 2B). A second Headway 17
microcatheter (MicroVention, Inc.) was introduced into the aneurysm
sac for coil embolization. However, during deployment of the
initial coil, protrusion into the left A2 segment occurred despite
multiple attempts (Fig. 2C). To
address this coil protrusion, a Synchro 14 guidewire (Stryker
Neurovascular) was inserted through the y-connector hemostasis
valve (Merit Medical Systems, Inc.) of the stent microcatheter and
navigated through the intracranial support catheter to a position
distal to the left A2 segment (Fig.
2D). The guidewire tip was shaped ~1.5 cm from its distal end
to optimize its conformation to the aneurysm neck, forming a stable
‘guardrail’ configuration at the aneurysm orifice. This Y-shaped
configuration of the guidewire and microcatheter enabled stable
coil framing without protrusion (Fig.
2E), leading to complete aneurysm occlusion without stent
deployment (Fig. 2F). Given the
instability typically associated with aneurysms of this
configuration, strict adherence to a defined withdrawal sequence
was essential to maintain coil stability (8). After confirming final coil
detachment, the distal tip of the coil pusher was kept slightly
extended beyond the microcatheter tip to facilitate the release of
the coil tail and prevent displacement. The guidewire was then
carefully withdrawn, followed by the gradual withdrawal of the
coiling microcatheter. Finally, the stent microcatheter was
withdrawn at a slow and controlled pace. Continuous digital
subtraction angiography (DSA) was performed throughout the
withdrawal process to monitor coil mass stability and ensure no
displacement or protrusion.
Post-embolization 2D (Fig. 2G) and 3D (Fig. 2H) rotational angiography
demonstrated satisfactory aneurysm occlusion, with patent bilateral
A2 segments and no evidence of distal thromboembolic complications.
The operative procedure is documented in Video S1.
The patient was discharged one week postoperatively
without hemiparesis or cognitive deficits and resumed routine
activities, including work. The modified Rankin Scale score was 0.
At the six-month follow-up, DSA demonstrated stable coil placement
with continued patency of the bilateral anterior cerebral arteries
(Fig. 2I).
Discussion
Stent-assisted coiling for ruptured aneurysms
presents significant challenges compared to coil embolization
alone. The requirement for dual antiplatelet therapy increases the
risk of post-procedural rebleeding, particularly in cases involving
external ventricular drains (4).
Alternative endovascular strategies, including
multi-catheter and balloon-assisted techniques, have their own
limitations. Multiple microcatheter techniques frequently require
removing previously placed catheters, necessitating upsizing a 7F
or 8F access sheath. This approach escalates procedural complexity,
duration and cost. Furthermore, the small caliber of the anterior
cerebral arteries renders multi-catheter navigation technically
challenging or unfeasible. When balloon remodeling fails to achieve
adequate neck control, the lack of readily deployable stent bailout
heightens the risk of coil protrusion (9).
The documented dual-catheter technique involves an
adjunctive microcatheter that facilitates partial aneurysm neck
occlusion to reduce coil migration and enhance framing (8). However, our initial experience with
this technique revealed critical limitations: The stent
microcatheter prevented coil protrusion into the right anterior
cerebral artery A2 segment but not into the left. Based on the
aforementioned technical enhancements, a combined microcatheter and
guidewire technique was developed and implemented in the present
study to overcome the challenges associated with treating ruptured
wide-necked ACoA aneurysms. This novel approach, developed in our
study, modifies the adjunctive microcatheter technique by
incorporating a guidewire for enhanced support. This technique
employs the strategic combination of guidewire and microcatheter
placement to establish a ‘Y’-shaped configuration that functions as
a dual-support ‘physical barrier’. This configuration thereby
facilitates stable coil framing within the anterior communicating
artery aneurysm while preventing coil protrusion into the parent
vessel. The contrast between our initial and refined approaches
demonstrates the technique's evolution: While the auxiliary method
failed due to incomplete neck coverage, the microcatheter-guidewire
combination achieved complete neck sealing and procedural
efficacy.
This technology boasts broad clinical applicability,
accommodating a wide range of patient populations, and is generally
not constrained by the inherent disease severity or coexisting
comorbidities. However, procedural success and safety remain
contingent upon individual intracranial vascular anatomy and
pathophysiology. Several technical challenges may arise. Primarily,
long-distance microcatheter navigation through cerebral vasculature
frequently diminishes torque transmission and pushability,
compromising tactile feedback. This is particularly pronounced in
tortuous vessels, necessitating intermediate catheter deployment
for optimal maneuverability. Furthermore, simultaneous operation of
two microcatheters and one micro guidewire reduces intraluminal
space, predisposing to entanglement and knotting. Mitigation
strategies include avoiding complex microcatheter shaping and
sequential delivery with microcatheter positioning preceding
microwire advancement. Additionally, patients with compromised
cerebrovascular architecture, particularly those with
atherosclerotic burden, vessel tortuosity or significant stenosis,
demonstrate heightened susceptibility to thromboembolic
complications and plaque disruption during manipulation (10-13).
Risk mitigation encompasses prophylactic anticoagulation and
precision navigation under continuous fluoroscopic monitoring
(14).
Of note, there are currently no specific training
programs or simulation methods dedicated to mastering the present
technique, to the best of our knowledge. This procedure may have a
certain learning curve and appear somewhat complex for beginners.
However, experienced neurointerventional physicians may be able to
acquire proficiency in this technique quickly and efficiently due
to their foundational skills and experience with similar
procedures.
This technique demonstrates several distinct
advantages, as demonstrated in the present case. First, it
eliminates the need to upsize the access sheath, allowing the
procedure to be completed via a standard 6F system. This allows for
further advancement of a guidewire to the A2 segment of the
anterior cerebral artery without removing pre-existing long
sheaths, intracranial support catheters and microcatheters. This
minimizes catheter exchanges, thereby reducing procedural steps and
mitigating the risk of vascular injury associated with repeated
device manipulation. Second, it provides optimal support to both A2
segments of the anterior cerebral arteries, thereby minimizing the
risk of coil protrusion and facilitating successful embolization
without requiring adjunctive devices such as stents or balloons.
Third, compared to balloon remodeling, this technique better
preserves cerebral perfusion throughout the procedure (5).
However, certain limitations and risks must be
acknowledged. Despite the enhanced stability afforded by this
technique, the potential for coil migration or incomplete aneurysm
occlusion persists. In such scenarios, stent deployment remains a
viable contingency option to ensure coil retention and prevent
protrusion. Furthermore, compared to stent-assisted coiling, coil
embolization alone shows superior immediate occlusion rates and
better prevents rebleeding in ruptured aneurysms. However, coil
embolization alone may be associated with higher recurrence rates
over time (15,16). The absence of extended follow-up is
a limitation of the present study. Extended follow-up is essential
to confirm the long-term stability and efficacy of the
treatment.
In conclusion, ruptured wide-necked bifurcation
aneurysms, such as those of the ACoA, carry a substantial risk of
coil protrusion when treated with coil embolization alone. This
report details an improved technique employing dual microcatheter
and guidewire support via a 6F access system to embolize select
ruptured wide-necked ACoA aneurysms, obviating the need for
adjunctive devices. In the presented case, this technique proved
safe and effective. However, stent deployment remains a viable
bailout strategy in select complex cases.
Supplementary Material
Supplementary Data.
Coil embolization of an anterior
communicating artery aneurysm using combined microcatheter and
guidewire technique.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HT performed the surgery as the lead surgeon. HT and
ZL were involved in the conception and design of the study, were
responsible for the project and provided final approval of the
manuscript. ZL, CC and YZ collected, acquired and interpreted the
data. HT, MS and ZL wrote the manuscript. HT, ZL and MS critically
revised the article. HT and MS were responsible for creating and
assembling the figures. HT and ZL edited the supplementary video.
HT and ZL verified the authenticity of the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient and their family for the proposed treatment.
Patient consent for publication
Written consent was obtained from the patient and
family for the publication of the patient's case information and
images. All identifying patient information has been removed to
ensure confidentiality.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sattari SA, Shahbandi A, Lee RP, Feghali
J, Rincon-Torroella J, Yang W, Abdulrahim M, Ahmadi S, So RJ, Hung
A, et al: Surgery or endovascular treatment in patients with
anterior communicating artery aneurysm: A systematic review and
meta-analysis. World Neurosurg. 175:31–44. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kocur D, Ślusarczyk W, Przybyłko N,
Bażowski P, Właszczuk A and Kwiek S: Stent-Assisted endovascular
treatment of anterior communicating artery aneurysms-literature
review. Pol J Radiol. 81:374–379. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Proust F, Martinaud O, Gérardin E, Derrey
S, Levèque S, Bioux S, Tollard E, Clavier E, Langlois O, Godefroy
O, et al: Quality of life and brain damage after microsurgical clip
occlusion or endovascular coil embolization for ruptured anterior
communicating artery aneurysms: Neuropsychological assessment. J
Neurosurg. 110:19–29. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhao B, Tan X, Yang H, Zheng K, Li Z,
Xiong Y and Zhong M: AMPAS study group. Stent-assisted coiling
versus coiling alone of poor-grade ruptured intracranial aneurysms:
A multicenter study. J Neurointerv Surg. 9:165–168. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moon K, Albuquerque FC, Ducruet AF,
Crowley RW and McDougall CG: Balloon remodeling of complex anterior
communicating artery aneurysms: Technical considerations and
complications. J Neurointerv Surg. 7:418–424. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cho YD, Rhim JK, Kang HS, Park JJ, Jeon
JP, Kim JE, Cho WS and Han MH: Use of triple microcatheters for
endovascular treatment of wide-necked intracranial aneurysms: A
single center experience. Korean J Radiol. 16:1109–1118.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chung EJ, Shin YS, Lee CH, Song JH and
Park JE: Comparison of clinical and radiologic outcomes among
stent-assisted, double-catheter, and balloon-assisted coil
embolization of wide neck aneurysms. Acta Neurochir (Wien).
156:1289–1295. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Muralidharan V, Travali M, Cavallaro TL,
Tomarchio L, Corsale G, Cosentino F, Politi MA and Cristaudo C:
Micro-catheter assisted coiling (MAC) A mid-path between simple and
assisted coiling techniques in treating ruptured wide neck
aneurysms and immediate post procedure outcomes. J Cerebrovasc Sci.
9:3–8. 2021.
|
|
9
|
Ladner TR, He L, Davis BJ, Froehler MT and
Mocco J: Simultaneous stent expansion/balloon deflation technique
to salvage failed balloon remodeling. BMJ Case Rep.
2015(bcr2014011600)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim SH, Nam TM, Lee SH, Jang JH, Kim YZ,
Kim KH, Kim DH and Lee CH: Association of aortic arch calcification
on chest X-ray with procedural thromboembolism after coil
embolization of cerebral aneurysm. J Clin Neurosci. 99:373–378.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ogata A, Suzuyama K, Ebashi R, Takase Y,
Inoue K, Masuoka J, Yoshioka F, Nakahara Y and Abe T: Association
between extracranial internal carotid artery tortuosity and
thromboembolic complications during coil embolization of anterior
circulation ruptured aneurysms. Acta Neurochir (Wien).
161:1175–1181. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Park CK, Shin HS, Choi SK, Lee SH and Koh
JS: Clinical analysis and surgical considerations of
atherosclerotic cerebral aneurysms: Experience of a single center.
J Cerebrovasc Endovasc Neurosurg. 16:247–253. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yin Z, Zhang Q, Zhao Y, Lu J, Ge P, Xie H,
Wu D, Yu S, Kang S, Zhang Q, et al: Prevalence and procedural risk
of intracranial atherosclerotic stenosis coexisting with unruptured
intracranial aneurysm. Stroke. 54:1484–1493. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ihn YK, Shin SH, Baik SK and Choi IS:
Complications of endovascular treatment for intracranial aneurysms:
Management and prevention. Interv Neuroradiol. 24:237–245.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hong Y, Wang YJ, Deng Z, Wu Q and Zhang
JM: Stent-assisted coiling versus coiling in treatment of
intracranial aneurysm: A systematic review and meta-analysis. PLoS
One. 9(e82311)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nabizadeh F, Valizadeh P and Balabandian
M: Stent-assistant versus non-stent-assistant coiling for ruptured
and unruptured intracranial aneurysms: A meta-analysis and
systematic review. World Neurosurg X. 21(100243)2024.PubMed/NCBI View Article : Google Scholar
|